Abstract
This research was conducted to determine the concentration of heavy metals (Cu, Pb, and Ni) in the sediments as well as the gill and muscle tissue of Siganus javus and two species of algae (Padina australis and Sargassum vulgare) collected from the Persian Gulf coasts of Bushehr province, which were studied using standard laboratory methods. The general form and trend of metal uptake at different stations in the gill and muscle tissue was Cu > Ni > Pb. The results of the study of metal uptake in both algae showed that the uptake of all three metals was higher in Padina species (Pb ˂ Cu ˂ Ni). The estimated daily intake (EDI), estimated weekly intake (EWI), allowable fish consumption rate limit (CRlim), and the target hazard quotients (THQ) for the consumption of this fish were also calculated. It was found that the concentration of heavy metals in the edible parts of the fish did not exceed the permissible limits proposed by the WHO, MAFF, JECFA, and NHMRC for human consumption, but the Ni concentration was higher than standard. The consumer risk indexes for non-cancerous diseases due to all metals were lower than standard. Also, the total risk index (HI) in this study was 0.065.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The study of aquatic habitats provides us with the knowledge of their biological and non-biological characteristics and a more comprehensive understanding of their ecological structure, which can help us with better conservation, as well as sustainable exploitation and management of such areas (Kamaruzzaman et al., 2011). Rapid development of industry and agriculture increases the pollution of aquatic ecosystems, especially rivers and lakes, with heavy metals. Heavy metals enter the rivers through polluted waters and eventually reach the seas and lakes (Yi et al., 2017). Heavy metals are carried from natural and synthetic sources into the bodies and tissue of organisms and accumulate there, which increases their toxic danger (Khatri & Tyagi, 2015). Unlike other organic pollutants, metals are not destroyed or eliminated in the ecosystem but accumulate in sediments or living organisms and eventually enter the human body as part of a food chain. Direct transmission of heavy metals from sediments to aquatic organisms is the main transmission route for many aquatic species. Copper and nickel are very low in the body, but these metals and a number of other heavy metals can have adverse effects on human health and wildlife behaviors because when they exceed the acceptable limits, they might lead to slower growth, renal failure, and reproductive disorders (Ahmed et al., 2019; Hosseini et al., 2013). Fish are the best organisms for assessing the pollution of aquatic environments by toxic elements. Fish play an important role in the transfer of metal pollutants from the marine ecosystem to the sea since they are in direct contact with the sediments of the seafloor which are the site of final accumulation of heavy metals; moreover, fish are important owing to their role in the human diet (Balcıoğlu, 2016). Sediments actually serve as a reservoir for the accumulation of heavy metals in aquatic ecosystems and often store up to 99% of metals in them (Laxmi Priya et al., 2011). Siganus javus of the family of Siganidae lives in the waters of the Persian Gulf and the Oman Sea around rocky areas and coral reefs. This fish moves in small shoals, feeding on seaweed (El-Sayed et al., 1995). Siganus javus is one of the most widely consumed fish in the region and is mostly sold in Southeast Asian and Arab markets (Sadeghi, 2001). Padina algae belong to the group of brown algae and are widely found in tropical and temperate coastal areas in intertidal and subtidal zones. About 6 species of Padina have been identified so far on the northern coasts of the Persian Gulf (Amini et al., 2013). Sargassum algae are brown algae which grow mainly in the intertidal and subtidal zones as well as in shallow water areas and on rocky beds. The accumulation of pollutants is not the same in different types of tissue (muscle, gill, liver). Since muscles are the most edible part of fish, the potential risks of muscle contamination are of great concern to humans, and research on their pollutants is of great importance despite the muscles’ low potential for concentration of metals compared to gills and liver (Neves et al., 2015). Therefore, it is important to measure the amount of heavy metal absorption in determining the general health status and in protecting the marine environment. Many researchers use health risk assessment methods to determine the risk of consuming polluted materials for humans, and the calculation of risk indices can help human health decision-makers and authorities (Varol & Sünbül, 2018). Many previous studies have been carried out on algae as bioindicators for determining heavy metal contamination in water. Ali et al.’s study (2017) on the brown algae of Red Sea in Sudan and in Iran and Dadolahi et al.’s research (2010) on 13 dominant species of algae (including 3 species of green algae, 4 species of brown algae, and 6 species of red algae on the coasts of Bushehr) are two examples of this type of research. Therefore, the aim of this study was to assess the uptake of heavy metals (Cu, Pb, and Ni) in coastal sediments, gill and muscle tissue of fish and two species of Padina australis and Sargassum vulgare as bioindicators in order to help protect the marine environment and assess the risk for the fish consumers.
Materials and methods
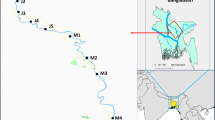
The sampling of fish, algae, and sediments was carried out on the shores of Bushehr province (north of the Persian Gulf). For this purpose, 60 fish, 30 sediment, and 60 algae samples were collected from the Bushehr province coasts.
Sampling and preparation of chemical digestion of fish tissue
Samples were harvested with special fish cages by local fishermen to investigate the accumulation of heavy metals in the muscle tissue and gills of fish along the entire coasts of Bushehr. Samples were transferred to the University Research Laboratory (1:1) in baskets containing crushed ice. The fish biometric indices of weight in grams and total length in centimeters were measured by scales and a calibrated board, respectively. To perform chemical digestion, 10 g of muscle tissue and 10 g of gills were weighed and dried for 72 h in the oven at 50 to 55 °C. Then, 10 ml of concentrated nitric acid was added to 10 g of each sample and was placed on the hotplate at 95 °C for 3 h for complete digestion. After cooling completely, the specimens were filtered with Whatman filter paper 42, increased to 25 ml with distilled water, and placed in sterile capped tubes for injection into the atomic absorption unit (Moopam, 2010).
Sampling and preparation of chemical digestion of algae
After being collected, the specimens of algae from the coasts of Bushehr were transferred to the Research Laboratory using polyethylene containers. They were then rinsed twice with distilled water and completely dried at 80 °C. For preliminary digestion of algae samples, 10 ml of 65% nitric acid was added to 1 g of powdered algae and kept at room temperature for at least 12 h. Then, the samples were placed on a hotplate at 140 °C for 5 h for complete digestion. After digestion, 10 ml of 10% nitric acid was added to it. After filtration with Whatman filter paper 42, the solution was increased to 25 ml with double-distilled water and kept in the refrigerator at less than 4 °C before injection into the atomic absorption unit (Rajendran et al., 1993).
Calculating daily and weekly absorption
Fish are generally consumed in fresh weight. For this reason, metal accumulation should also be calculated in terms of wet weight by multiplying the amounts of metals in the fish muscles by the correction factor of 0.2 (UNEP, 1984). Con in WW: 1 − (the amount of moisture in the fish’s muscle/100) × Con in DW. In order to determine the amounts of contaminants absorbed through the nutrient per kilogram of body weight per day (EDI = estimated daily intake) and per week (EWI = estimated weekly intake), the US Environmental Protection Agency’s proposed method was used in Formulas 1 and 2 (Norouzi, 2020):
C = the average heavy metal concentration in fish (μg/g)
MSD = rate of daily fish consumption (32.57 g day−1 according to the EPA standard)
MSW = rate of weekly fish consumption (228 g week−1 according to the EPA standard)
BW = the average body weight (adults 70 kg, children 14.5 kg).
Calculating allowable fish consumption rate
One of the most important methods of determining fish consumption limits is the method proposed by the US Environmental Protection Agency (EPA) (USEPA, 2000). In this method, based on the amount of metals in edible fish tissue and using a reference dose (RfD) according to Formula 3, the maximum acceptable amount of fish and fishery products to be consumed within a specific period can be calculated (Pinzón-Bedoya et al., 2020):
CRlim = maximum allowable fish consumption rate (g/day)
RfD = reference dose (mg/kg/day)
Risk Calculation − Risk Index (THQ = Target Hazard Quotients)
The potential hazard is the ratio of the concentration of an element to the maximum concentration of the element that does not cause problems in the body. Formula 4, presented by the EPA, was used to calculate the probability of people being at risk of non-cancerous diseases. According to this index, if the number is higher than one, it indicates a high risk of non-cancerous diseases (USEPA, 2009), while an index number of less than one indicates no adverse effect on consumers. The total hazard indicator (HI) was obtained using Formula 5 (Chien et al., 2002; Dee et al., 2019):
THQ = target hazard quotient
HI = hazard index
EFr = exposure frequency (365 days per year)
ED = total exposure time (exposure duration) (70 years)
FIR = daily fish ingestion rate (32.5 g/day according to the EPA standard)
ATn = average exposure time (70 years × 365 days)
One-way ANOVA was used for data analysis, and the Pearson correlation test was used to determine the relationship between factors with a 95% confidence level.
Results and discussion
Fish
The Siganus javus fish caught in various stations of Bushehr’s coasts were 100.48 ± 15.67 g in average weight and 18.59 ± 0.75 cm in length. The difference in weight was significant (P ˂ 0.05), but the difference in length was not significant (P ˃ 0.05). The general form and trend of metal uptake in the gill and muscle tissue was Cu > Ni > Pb. It is important to note that the non-significance of some metals in fish tissue depends on different metabolic activities and physiological changes over time, which may lead to a negative correlation between the fish size and the amount of metals in its muscle (Fard et al., 2015). The results of analyzing metal accumulation in the gill and muscle tissue of Siganus javus were compared with those of other species in the Persian Gulf based on international standards (Table 1).
The results of this comparison showed that the concentrations of lead and copper were lower than normal (but on the borderline), and the concentration of nickel was higher than normal by international standards. Various factors are responsible for the high amount of metal accumulation in the gill and muscle tissue of Siganus javus. Fishing boats as well as urban, agricultural, and domestic wastewater are some major contributors to the contamination of water resources (Luoma & Rainbow, 2008). The other major source of heavy metals is the anti-corrosion and anti-clogging compounds and paint used to protect the body of ships and boats against algae and barnacles, with about 15 to 30% of this paint being composed of some metals such as zinc, copper, and lead (Keshavarzi et al., 2015). In addition, the physiological differences and locations of different types of tissue in fish can affect the bioaccumulation of each metal. Levels of metal accumulation are controlled by physical factors such as severity of contamination, exposure time, water pH, alkalinity, and temperature, while biological factors such as size, age, feeding habits, and reproductive cycle are the main factors for controlling the degree of contamination in fish (Milošković et al., 2016; Mziray & Kimirei, 2016). The Persian Gulf is one of the most important sites in Iran that hosts a variety of benthic and pelagic species. The benthic species live and feed in the sediments, while the pelagic ones reside in water columns and surface layers (Naccari et al., 2015). In general, the Persian Gulf is one of the areas surrounded by large deserts of the world and receives a high volume of sediments each year, especially in cold seasons when wind speeds are higher. On the other hand, as the wind speed increases, waves are created, thereby increasing the potential for the water bed sediments to mix with the water columns and the living creatures in these areas. These conditions provide grounds for increasing concentrations of pollutants such as heavy metals found in sediments and fish and can lead to higher levels of contamination (Gelsleichter et al., 2020). Due to the fact that Siganus javus is a pelagic kind of fish and lives in surface areas, the sources of water contamination have caused an increase in the accumulation of metals in its tissue. Siganus javus has a vegetarian diet and omnivorous behavior and is capable of utilizing low levels of food in the aquatic environment (Boonyaratpalin, 1997). It seems that the high concentration of metals in the gill tissue is due to the mixing of elements with the gill mucosa, which makes it impossible to completely move the elements from the gill hinge when preparing the tissue for testing (Heath, 2018). In dealing with metals, gills apply four mechanisms: reducing the water uptake, detoxifying the metals with metallothionein proteins, protecting cellular structures by binding to proteins, and excreting metals (Yilmaz, 2009). Since different species accumulate metals and eliminate toxic elements in different ways, it is important to sample different species (Koide et al., 2016).
Algae
The Persian Gulf, with a depth of 40 m, is a shallow and semi-enclosed sea connected to the Indian Ocean through the Strait of Hormuz (Fard et al., 2015; Hosseini et al., 2016). Over the past few decades, rapid industrial development and population growth in the Persian Gulf states have led to a significant increase in heavy metals in aquatic ecosystems (Pourkerman et al., 2017). In the Persian Gulf area, the Moussa river and Mahshahr Port, there are two coastal ecosystems that host the fauna and some unique plants. These ecosystems receive large amounts of pollution from municipal and agricultural effluents, petrochemicals, and large transportation ports. The results of the study of metal uptake in both genera of algae showed that the uptake of all three metals was higher in Padina species. The metal uptake behavior was Pb < Cu < Ni in Padina species and Pb < Ni < Cu in Sargassum species. There are two major pathways for the biosorption of heavy metals within plants. The first is the entry of pollutants through the extracellular space and the cell wall, which is reversible and occurs at a high speed. The second is through the cell membrane, which is slow, energy consuming, and irreversible. Because copper takes the first path and lead the second path to penetrate into the plant tissue and accumulate there, the highest and lowest adsorption rates were related to copper and lead in Sargassum species. The high levels of nickel and copper in the algae are due to the high consumption of fossil fuels for industrial and transportation purposes in the Persian Gulf. Urban wastewater can also be a contributing factor (Dadolahi et al., 2010). High amounts of heavy metals travel toward the coasts through surface running waters, dry land, and the land surrounding industries. Temperature changes and solar energy also have a considerable effect on the bioaccumulation of metals because a decrease in the light and heat of the sun or high temperature stresses without efficient transpiration makes for a reduction of in plants’ metabolic activity (Mahan & Burke, 2015), which leads to further accumulation of metals in them (Strezov & Nonova, 2005).
BSAF (biosediment accumulation factor) was used to compare the capability of heavy metal accumulation in the studied algae. This indicator shows the ratio of heavy metal accumulation in algae and related sediments. The results of this index are shown in Table 2.
In Table 3, the amount of heavy metal uptake in wet weight fish muscles and the reference dose, as well as the estimated daily intake (EDI) and weekly intake (EWI) rates of metal in adults and children and the maximum allowable weekly intake (μg/g/70 kg/week), are shown. Table 3 also shows the risk level and allowable fish consumption rate limit (CRlim). According to these results, the amounts of heavy metals in the fish muscles were lower than the heavy metal consumption limits allowed by international standards. The calculated values of the risk of contracting non-cancerous diseases due to the consumption of all three metals were less than 1, indicating that eating Siganus javus had no adverse effect on consumers. Finally, the total hazard index (HI) in this study was below 1 (0.064).
According to the results of the Pearson correlation test, there was a direct relationship between the amount of nickel in the sediments with its content in both fish tissue and two species of algae. Furthermore, a significant positive relationship was found between the amount of copper adsorbed in the sediments and the amount of lead metal adsorbed in Sargassum algae (Table 4). Most well-known analyses indicate that most sources of metal contamination are related to crude oil. Due to the oil-related activities in the studied area over the past 50 years, it appears that these metals in fish originate in oil contaminants such as oil leakage or discharge in the sea (Osuji & Onojake, 2004).
Conclusions
The results of this study showed that considering the relevant standards, the accumulation of metals in the sediments was harmless to the environment. Also, it was found that the concentrations of copper and lead (but not nickel) in the gill and muscle tissue of Siganus javus caught in Bushehr waters were below the permissible limit based on international standards. Moreover, considering the risk assessment of the human consumption of fish, it seems that the consumption of this fish caught off the coast of Bushehr currently does not pose a serious threat to consumers. However, there are some considerations for the consumption of this fish by pregnant women and children. Since fish consumption patterns are not the same all over Iran, the fishing community and even people living in coastal areas may frequently consume seafood. Although the consumption of fish does not pose a health risk to the consumers, caution should be exercised by pregnant women and children in eating fish because fetuses, infants, and children under 10 are sensitive. Moreover, the risk indexes for adults’ and children’s bodies weight are 70 kg and 14.5 kg, respectively. Clearly, the optimal intake rate also varies for overweight and underweight people. The increasing number of pollutant sources in the studied coasts seems to be related to industrialization and people’s activities for expanding their businesses, which have caused the decay of the aquatic ecosystems (Rajeshkumar et al., 2018; Hu et al., 2010; Padmini & Geetha, 2007; Huang, 2000). Because of the variability in diet, marine fish have a high level of metal accumulation in their tissue. Also, the data show that metal pollution is increasing in the studied region. For this reason, special attention should be paid to the safety of seafood in an effort to increase the per capita fish consumption and to change people’s consumption behavior.
Data availability
Data supporting Tables 1–4, are publicly available in Marine Data Archive repository, as part of this record: http://mda.vliz.be/directlink.php?fid=VLIZ_00001041_60f2bbdbe4a7e893968028
References
Abdolahpur Monikh, F., et al. (2013). The relationship between heavy metal (Cd Co, Cu, Ni and Pb) levels and the size of benthic, benthopelagic and pelagic fish species, Persian Gulf. Bulletin of Environmental Contamination and Toxicology, 90(6), 691–696.
Ahmed, A. S., et al. (2019). Bioaccumulation of heavy metals in some commercially important fishes from a tropical river estuary suggests higher potential health risk in children than adults. PLoS One, 14(10), e0219336.
Akhbarizadeh, R., et al. (2018). Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environmental Pollution, 232, 154–163.
Ali, A. Y., et al. (2017). Brown algae (Phaeophyta) for monitoring heavy metals at the Sudanese Red Sea coast. Applied Water Science, 7(7), 3817–3824.
Amini, F., et al. (2013). Ribulose-1, 5-bisphosphate carboxylase/oxygenase gene sequencing in taxonomic delineation of Padina species in theNorthern Coast of the Persian Gulf,(IRAN). 4(13), 47–57.
Balcıoğlu, E. B. (2016). Potential effects of polycyclic aromatic hydrocarbons (PAHs) in marine foods on human health: A critical review. Toxin Reviews, 35(3–4), 98–105.
Bastami, K. D., et al. (2015). Bioaccumulation and ecological risk assessment of heavy metals in the sediments and mullet Liza klunzingeri in the northern part of the Persian Gulf. Marine Pollution Bulletin, 94(1–2), 329–334.
Boonyaratpalin, M. (1997). Nutrient requirements of marine food fish cultured in Southeast Asia. Aquaculture, 151(1–4), 283–313.
Chien, L. -C., et al. (2002). Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Science of the Total Environment, 285(1–3), 177–185.
Dadolahi, A., et al. (2010). Seasonal variations of nickel, cadmium, copper, lead in the dominant macroscopic algae off the coast of Bushehr province (north coast of Persian Gulf). Journal of Marine Science and Technology., 9(4), 65-75P.
Dee, K. H., et al. (2019). Health risk assessment of heavy metals from smoked Corbicula fluminea collected on Roadside Vendors at Kelantan (p. 2019). BioMed research international.
El-Sayed, A. F. M., et al. (1995). Effects of stocking density and feeding levels on growth rates and feed utilization of rabbitfish Siganus canaliculatus. Journal of the World Aquaculture Society, 26(2), 212–216.
FAO. (1983). Compilation of legal limits for hazardous substances in fish and fishery products. Food and Agriculture Organization of the United Nations, Rome, 464, 465–410.
Fard, N. J. H., et al. (2015). Determination of mercury and vanadium concentration in Johnius belangerii (C) fish in Musa estuary in Persian Gulf. Marine Pollution Bulletin, 97(1–2), 499–505.
Gelsleichter, J., et al. (2020). Elevated accumulation of the toxic metal mercury in the critically endangered oceanic whitetip shark Carcharhinus longimanus from the northwestern Atlantic Ocean. Endangered Species Research, 43, 267–279.
Gholamhosseini, A., et al. (2021). Bioaccumulation of metals in marine fish species captured from the northern shores of the Gulf of Oman, Iran. Regional Studies in Marine Science, 41, 101599.
Huang, Y. (2000). The water quality of Lake Taihu and its protection. Research on lake sciences, 239–246.
Heath, A. G. (2018). Water pollution and fish physiology. CRC Press.
Hosseini, M., et al. (2013). Mercury levels in selected tissues of blue crab Thalamita prymna (Portunidae) from Musa Estuary, of the Persian Gulf. Journal of the Persian Gulf, 4(14), 33–38.
Hosseini, M., et al. (2016). Mercury contamination in some marine biota species from Khuzestan shore, Persian Gulf. Toxicology and Industrial Health, 32(7), 1302–1309.
Hu, L., et al. (2010). Effects on water quality following water transfer in Lake Taihu, China. Ecological Engineering, 36(4), 471–481.
Jiang, Z., et al. (2018). Metal concentrations and risk assessment in water, sediment and economic fish species with various habitat preferences and trophic guilds from Lake Caizi, Southeast China. Ecotoxicology and Environmental Safety, 157, 1–8.
JECFA. (1982). Evaluation of certain food additives and contaminants, twenty-sixth report of the joint FAO/WHO expert committee on food additives. WHO Technical Report Series, No. 683, World Health Organization, Geneva.
JECFA. (2000). Evaluation of certain food additives and contaminants, fifty-third report of the joint FAO/WHO expert committee on food additives. WHO Technical Report Series, No. 896, World Health Organization, Geneva.
Kamaruzzaman, B., et al. (2011). Bioaccumulation of heavy metals in horseshoe crabs Tachypleus gigas) from Pekan, Pahang, Malaysia. Research Journal of Environmental Toxicology, 5(3), 222.
Keshavarzi, B., et al. (2015). A GIS-based approach for detecting pollution sources and bioavailability of metals in coastal and marine sediments of Chabahar Bay, SE Iran. Geochemistry, 75(2), 185–195.
Keshavarzi, B., et al. (2018). Heavy metal contamination and health risk assessment in three commercial fish species in the Persian Gulf. Marine Pollution Bulletin, 129(1), 245–252.
Khatri, N., & Tyagi, S. (2015). Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Frontiers in Life Science, 8(1), 23–39.
Koide, S., et al. (2016). Bioaccumulation of chemical warfare agents, energetic materials, and metals in deep-sea shrimp from discarded military munitions sites off Pearl Harbor. Deep Sea Research Part II: Topical Studies in Oceanography, 128, 53–62.
Laxmi Priya, S., et al. (2011). Bioaccumulation of heavy metals in mullet (Mugil cephalus) and oyster (Crassostrea madrasensis) from Pulicat lake, south east coast of India. Toxicology and Industrial Health, 27(2), 117–126.
Luoma, S. N., & Rainbow, P. S. (2008). Metal contamination in aquatic environments: science and lateral management. Cambridge, New York.
MAFF. (1995). surveillance of non-radioactive contaminants in the aquatic environment and activities regulating the disposal of wastes at sea, 1993. Directorate of Fisheries Research, Lowestoft, Aquatic Environment Monitoring Report,(44), 114.
Mahan, J. R., & Burke, J. J. (2015). Active management of plant canopy temperature as a tool for modifying plant metabolic activity. American Journal of Plant Sciences, 6(01), 249.
Maher, W. (1986). Trace metal concentrations in marine organisms from St. Vincent Gulf, South Australia. Water, Air, and Soil Pollution, 29(1), 77–84.
Milošković, A., et al. (2016). Non avoidance of hydrocarbon laden sediments juvenile flatfishes. Netherlands Journal of the Sea Research, 361–367.
Moopam, R. (2010). Manual of oceanographic observations and pollutant analysis methods. ROPME. Kuwait, 220p. (3rd)
Mziray, P., & Kimirei, I. A. (2016). Bioaccumulation of heavy metals in marine fishes (Siganus sutor, Lethrinus harak, and Rastrelliger kanagurta) from Dar es Salaam Tanzania. Regional Studies in Marine Science, 7, 72–80.
Naccari, C., et al. (2015). Toxic metals in pelagic, benthic and demersal fish species from Mediterranean FAO zone 37. Bulletin of Environmental Contamination and Toxicology, 95(5), 567–573.
Neves, D., et al. (2015). Ingestion of microplastics by commercial fish off the Portuguese coast. Marine Pollution Bulletin, 101(1), 119–126.
Norouzi, M. (2020). Evaluating the accumulation and consumption hazard risk of heavy metals in the fish muscles of species living in the waters of the Persian Gulf, Iran. Pollution, 6(4), 849–862.
NRC(National Research Council). (1989). Recommended dietary allowances.Washington DC (pp. 241–244).
Osuji, L. C., & Onojake, C. M. (2004). Trace heavy metals associated with crude oil: A case study of Ebocha-8 Oil-spill-polluted site in Niger Delta, Nigeria. Chemistry & Biodiversity, 1(11), 1708–1715.
Padmini, E., & Geetha, B. (2007). A comparative seasonal pollution assessment study on Ennore Estuary with respect to metal accumulation in the grey mullet, Mugil cephalus. Oceanological and Hydrobiological Studies, 36(4), 91–103.
Pinzón-Bedoya, C. H., et al. (2020). Assessment of potential health risks associated with the intake of heavy metals in fish harvested from the largest estuary in Colombia. International Journal of Environmental Research and Public Health, 17(8), 2921.
Pourkerman, M., et al. (2017). Evaluation of metal contamination in the Mand River delta, Persian Gulf. Marine Pollution Bulletin, 119(2), 261–267.
Rajeshkumar, S., et al. (2018). Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere, 191(2018), 626–638.
Rajendran, K., et al. (1993). Levels of trace metals (Mn, Fe, Cu and Zn) in some Indian seaweeds. Marine Pollution Bulletin, 26(5), 283–285.
Sadeghi, N. (2001). Morphological and biological characteristics of southern Iranian fishes (the Persian Gulf and Oman Sea). Naghshe Mehr.
Sadough Niri, A., et al. (2012). Quantitative analysis of heavy metals in muscle, liver and gill tissues of Euryglossa orientalis in Northern Persian Gulf waters. Iranian Scientific Fisheries Journal, 21(1), 147–160.
Strezov, A., & Nonova, T. (2005). Environmental monitoring of heavy metals in Bulgarian Black Sea green algae. Environmental Monitoring and Assessment, 105(1), 99–110.
Tuzen, M. (2009). Toxic and essential trace elemental contents in fish species from the Black Sea, Turkey. Food and Chemical Toxicology, 47(8), 1785–1790.
USEPA. (2011). Exposure Factors Handbook. U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-09/052F.
UNEP. (1984). Sampling of selected marine organisms and sample preparation for trace metal analysis reference metal for marine Pollution Studies.
USEPA. (2009). Risk-based Concentration Table Environmental Protection Agency. Philadelphia PA.
USEPA, U. (2000). Risk-based concentration table.In:United States Environmental Protection Agency Philadelphia, PA.
USEPA (US Environmental Protection Agency). (2002). Supplemental guidance for developing soil screening levels for superfund sites OSWER 9355. Office of Emergency and Remedial Response Washington.
Varol, M., & Sünbül, M. R. (2018). Multiple approaches to assess human health risks from carcinogenic and non-carcinogenic metals via consumption of five fish species from a large reservoir in Turkey. Science of the Total Environment, 633, 684–694.
WHO. (1996). Health criteria othersupporting information. (2nd ed., Vol. 2). In Guidelines for drinking waterquality. Geneva.
World Health Organization. (1989). Heavy metals-environmental aspects. Environment Health Criteria. WHO No.85.
Yi, Y., et al. (2017). Health risk assessment of heavy metals in fish and accumulation patterns in food web in the upper Yangtze River, China. Ecotoxicology and Environmental Safety, 145, 295–302.
Yilmaz, F. (2009). The comparison of heavy metal concentrations (Cd, Cu, Mn, Pb, and Zn) in tissues of three economically important fish (Anguilla anguilla, Mugil cephalus and Oreochromis niloticus) inhabiting Koycegiz Lake-Mugla (Turkey). Turkish Journal of Science & Technology, 4(1).
Acknowledgements
Special appreciation is due to Dr. Mohammad-Taghi Ronagh for his accompaniment for field sampling.
Funding
This project was carried out at Khorramshahr University Laboratory and funded by the Khorramshahr University of Marine Science and Technology, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saadatmand, M., Dadolahi-Sohrab, A., Tavani, M.B. et al. Monitoring heavy metal contamination on the Iranian coasts of the Persian Gulf using biological indicators: risk assessment for the consumers. Environ Monit Assess 194, 83 (2022). https://doi.org/10.1007/s10661-022-09755-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-09755-6