Abstract
The Itapessoca Estuarine Complex is characterized by a history of intense economic activities developed on the banks of its tributary rivers, often exercised without any control or planning. In order to relate natural and/or anthropic events to the sediment age, radiometric dating method was performed by determining excess 210Pb in the sediment using the proportional gas flow technique. To investigate possible disturbances in recent sedimentation processes in studied area, 20 surfaces sediment samples and 2 sediment cores were collected for determination of concentrations of the chemical elements. Al, Ca, Fe, K, Mg, Mn, Ni, Pb, Si, Sr, Ti and Zn concentrations were determined by the energy-dispersive X-ray fluorescence (EDXRF) technique. Through the enrichment factors and ratios among some elements associated with geochronological data, it was possible identify geochemical changes in sedimentation of sampled points, with increase of minerals associated with fine fractions and decrease of the proportions of elements associated with coarse fractions. The radiometric dating reveals that Pb enrichment and the exacerbated increase of Ca and Sr enrichment factors are strongly related to the anthropic activity in the region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries are recognized for their biological richness and as important sources of nutrients and shelter for different animal and plant species (Whitfield & Elliott, 2011). These environments are under constant anthropogenic threat: half the world’s population living in cities with more than 100,000 inhabitants is located within a radius of up to 100 km from a coast (Barragán & Andrés, 2015). In Brazil, 26.6% of the population live in coastal zone cities as a result of historical factors relating to the way the territory was occupied, causing impacts on the marine biome and survival of its species (IBGE, 2011). The estuaries are subject to anthropic disturbances such as mining and pollution (Davis et al., 2000). Due their intense natural physical–chemical variability, however, anthropic alterations are difficult to detect in these environments and they can be confused with natural stress (Elliot & Quintino, 2007).
The Itapessoca Estuarine Complex is part of Itamaracá Estuarine Ecosystem, one of most important ecosystems of the Pernambuco State coast, an ecological unit of environmental and socioeconomic significance due to its considerable biodiversity and the activities developed in region (Medeiros et al., 2001). However, the occupation and soil use caused by anthropic activities have produced significant changes in the environment (Oliveira, 2017). Tourism, fishing, sugarcane culture, shrimp farming, sand mining and limestone mining are the main local economic activities (Barreto et al., 2011; CPRH, 2003). Artisanal fishing has played an important role in the survival of several traditional fishing communities in region, with a predominance of estuarine fishing over marine fishing, since the northern coast concentrates most of the estuarine areas of Pernambuco state (CPRH, 2003). Although sugarcane monoculture has advanced toward the mangrove environment, shrimp farming and mining are among the most damaging activities, due to the suppression of native vegetation coverage on large scale (Oliveira, 2017). Open pit sand mining has created large craters where shrimp farms have been implemented to reuse the mined areas (Oliveira, 2017). Unfortunately, this mining reclamation method has caused major environmental impacts such as deforestation and chemical pollution (Oliveira, 2017). Besides deforestation, urban and industrial development have altered the chemical properties in the water column, sedimentation patterns and caused metal contamination (Lacerda et al., 2006).
A set of analytical techniques can help in understanding the environment status. Sediment core geochronology has been used to understand environmental evolution in coastal areas, providing natural or anthropic records of sedimentation patterns (Joshi & Ku, 1979). The energy-dispersive X-ray fluorescence (EDXRF) is an effective technique for the determination of chemical elements in different environmental matrices (Fernández et al., 2017), without needing sample digestion (Marguí et al., 2005). Multielemental chemical analysis by EDXRF has been employed to investigate metal concentrations in estuarine sediments (Olsen et al., 1978; Barcellos et al., 2017). The variation in concentrations of chemical elements in a sediment profile can be related to both natural and anthropogenic factors. The geochemical normalization technique using a conservative element enables the detection of anomalies in the concentration of these elements, facilitating the identification of anthropogenic contamination (Loring, 1991). Different conservative chemical elements have been used as normalizers, usually Al (Loring, 1991) and Fe (Blomqvist et al., 1992). The ratios between chemical elements quantified by different techniques can be used for several purposes, including acting as indicators of ecological processes (Lopez et al., 2006).
Although there are many studies of anthropic impact on the region, this present work has not been preceded by other studies that diagnosing the behaviour of chemical elements (metals and metalloids) over time in the Estuarine Ecosystem of Itamaracá. The objective of this study was to investigate the distribution and evolution of metal concentrations at several sampling points in the Itapessoca Estuarine Complex as well as the eventual implications of these on the geochemistry of the local sediments.
Materials and methods
Study area
The Itapessoca Estuarine Complex (7°40′S/34°50′W) is located on the northern coast of the state of Pernambuco, in Brazil, about 70 km from the capital, Recife, and approximately 10 km from the border of the state of Paraíba. The study area is under the influence of the As climate (Köppen-Geiger classification), or tropical Atlantic, with winter rains and annual precipitation average of 1800 mm. The average temperatures of the area oscillate between 24 °C in July and August and 27 °C in February, with low annual thermal amplitude (CPRH, 2003).
Five geological units make up the northern coast of Pernambuco state: Barreiras Formation, Beberibe Formation, Gramame Formation, Marinha Farinha Formation and Itamaracá Formation, belonging to Paraíba Sedimentary Basin (Barbosa et al., 2003; CPRH, 2003). The Gramame and Maria Farinha deposits have been intensively exploited by the cement industry for limestone to use as raw material (CPRH, 2003). Solid waste from mining and limestone processing is commonly disposed in landfills or dumped directly onto the land without any process or treatment (IPEA, 2011).
The Gramame Formation is the first carbonatic unit in the marine domain and occurs on the Itamaracá Formation (Oliveira, 1940). This unit dates back to the Maastrichtian period (Beurlen, 1967), is fossiliferous and has alternating deposits of limestones and marls on a shallow carbonate platform (Barbosa et al., 2007) that directly reflect the environmental changes associated with the marine phase (Westphal, 2006). The Maria Farinha Formation is composed of marine limestones and thick strips of marl in its lower portion, with dolomitic fossiliferous limestones in its upper portion (Beurlen, 1967). This formation displays sedimentary deposits characteristic of a regressive phase, from the cretaceous-tertiary age, related to the evolutionary process of the Paraíba Basin and the expansion of the South Atlantic Ocean (Mabesoone, 1967).
The Gramame Formation is the most representative unit of the carbonate sequence of the Itamaracá sheet, and its best exposures are contained in the Itapessoca Estuarine Complex, especially on Itapessoca Island and in the community of Tejucupapo, municipality of Goiana (Valença & Souza, 2017). On the island of Itapessoca, municipality of Goiana, portland cement is manufactured from limestones of the Gramame and Maria Farinha Formations, combined with clays of the Barreiras Formation, sands of the Beberibe Formation and Plio-Pleistocene deposits (Valença & Souza, 2017).
Sampling
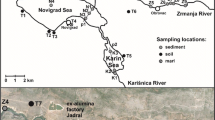
Initially, a bathymetric survey of the area was done, where sediment samples were collected from the surface down to 20 cm deep, at different points distributed in the estuary, using a Van-Veen grab sampler. Sediment core samples were collected at 2 points in the study area (Fig. 1) using the pushcore method. The accessibility of the estuarine areas and the feasibility of penetrating the polyethylene tubes, both with diameter 75 mm, were the criteria used to choose sample points. The abrupt increase in depth made it impossible to sample sediment core at some points. This is because the region is located in geological faults areas, with grabens or tectonic pits, and the estuarine system of Itapessoca is considered a ria estuary, of tectonic origin (Silva et al., 2011; Topan & Lima Filho, 2017).
The collection points of superficial samples and sediment cores were executed in intertidal banks, divided in sectors: sector A — situated on the Catuama margins, where the superficial samples were collected from P6 to P10 and C1 sediment core; sector B — opposite margin, on the Itapessoca Island, where the superficial samples were collected from P1 to P5 and C2 sediment core; and sector C — situated on the Itapessoca Island, its banks bathed by the Santa Cruz Channel, where the points P11 to P20 of the superficial samples were collected. Seventy-six centimetres was recovered for C1 and 52 cm for C2. The samples were transported and conditioned at the Environmental Monitoring Service of the Regional Center for Nuclear Sciences of the Northeast (SEAMB/CRCN-NE). Subsequently, they were thawed and, with the aid of a Makita circular saw, longitudinal cuts were made in the tubes. The samples were visually described and sectioned into 1–2-cm layers, depending on the texture and sedimentary structures (clay layers allowed thicker cuts), totalling 56 samples. The samples were dried at 40 °C until they reached a constant weight, and then, they were crushed to a particle size of less than 80 µm.
As a differentiation and classification factor of surface and subsurface sediments, a visual description of the collected samples in terms of texture (sandy/coarse and mud/fine), sedimentary structures and concentration of marine biogenic indicators (biodetritic carbonate — CaCO3) was made before the maceration of the sampled material.
Analytical procedures
Samples of 1 g sediment and reference materials were transferred to polyethylene tubes and sealed with specific polypropylene films for analysis of EDXRF. Measurements were performed on Shimadzu ED-720 instrument, previously calibrated, with specific setting up samples (SUS) and A750 calibration standards.
For the quality assessment of the analytical procedure, two reference materials were used: IAEA-SL-1 “Trace and Minor Elements in Lake Sediments” and SRM1646a — “Estuarine Sediment”, produced by the National Institute of Standards and Technology (NIST). The samples and reference materials were subjected to an atmospheric vacuum less than 30 Pa to measure the fluorescence coming from the sample for 300 s, with a maximum Dead Time of 36%.
To increase detection sensitivity, different filters have been used for background reduction and interference peak filtering, facilitating the detection of target peaks (Shimadzu Corporation, 2006). Usually four or five filters are needed to cover the entire element range (Shimadzu Corporation, 2006). The elements As, Cu, Fe, Ga, Mn, Pb, Zn, Sr and Ti were determined with a Mo filter and voltage adjusted to 50 kV. The Al, Ca, K, Mg and Si elements were measured with an Al filter and 15 kV tension. Ba, Ni and V elements were measured with Ti filter at 50 kV. The concentrations were expressed in milligrams per kilogram. Details on the method can be found in Fernández et al. (2017).
For the validation of the analytical results the Z-score was calculated, which takes into consideration the difference between the obtained and certified values of the reference material, divided by the expected standard deviation, and the En Number, which contemplates the expanded analytical uncertainties obtained and certified, in such a way that the limits adopted varied from −2 to 2 for Z-score and −1 to 1 for the En Number, respectively, at a 95% confidence level.
Zinc, nickel and lead concentration values were compared with the Canadian Council of Ministers of the Environment (2001) guidelines for marine and freshwater sediments. Threshold effect levels (TELs) and probable effect levels (PELs) guideline choice is justified by information on degree of sediment contamination based on toxicological studies. TEL and PEL values are used to relate chemical concentrations of a given element to its biological effects: (a) concentration values below the TEL suggest minimal effect range, with rare occurrence of adverse effects; (b) the range of values between TEL and PEL indicates possible adverse effects; and (c) values above the PEL indicate likely occurrence of frequent adverse effects.
Geochronology
The geochronology had been previously performed by the unsupported (excess) 210Pb activity method, adapted from Godoy et al. (1998), with the 210Pb radionuclide being used as a geochronological marker for determining sedimentation rates of coastal environment (Wanderley et al., 2014). 210Pb radionuclide was determined by the proportional gas flow technique, through the Canberra proportional gas flow counter, model Tennelec Series 5 XLB, a gaseous ionization detector device used to measure ionizing radiation particles (Knoll, 2010). The activity concentration of 210Pb was determined from its decay product, 210Bi, after reaching secular equilibrium. Only the beta activity of 210Bi was measured because the filter paper used to cover the sample filters retains the low-energy beta particles of 210Pb and the alpha ingrown particles of 210Pb (Albuquerque et al., 2019). Sedimentation rates of 0.84 ± 0.07 cm year−1 and 1.03 ± 0.22 cm year−1 were determined for sediment cores 1 and 2, respectively, and reported by Albuquerque et al. (2019), where further details on radiometric dating can be found.
Geochemical normalization and ratios between chemical elements
The enrichment factor (EF) in metals is an indicator used to assess the presence and intensity of deposition of anthropogenic contaminants in soil and is calculated by normalizing a metal concentration in the soil higher than the concentration of a reference element (Barbieri, 2016). The enrichment factor is expressed as follows: EF = (Metal/RE)soil/(Metal/RE)background, where, RE is the value of metal, adopted as reference element (Barbieri, 2016). The reference element is a stable element in the soil, which is characterized by the absence of vertical mobility and/or degradation phenomena (Barbieri, 2016). The chosen constituent should also be associated with finer particles, and its concentration should not be anthropogenically altered (Ackerman, 1980). Some elements are widely used, such as Al and Fe, for example (Barbieri, 2016). Aluminium considered a conservative element and an important constituent of clay minerals, widely used successfully (Balls et al., 1997; Sinex & Wright, 1988). Fe (Iron) is not a matrix element because of its geochemistry similar to that of many trace elements in oxic and anoxic environment (Barbieri et al., 2014); however, it has been successfully used by many scientists working with estuarine sediments (Emmerson et al., 1997; Lee et al., 1998; Liu et al., 2003).
Enrichment factor values of 0.5 ≤ EF ≤ 1.5 suggest that the trace metal concentration may come entirely from natural weathering processes (Zhang & Liu, 2002). EFs above 1.5, however, indicate that a significant proportion of these elements originated from anthropogenic processes (Garcia-Ordiales et al., 2014). Enrichment factor values < 3 indicate little or poor enrichment, 3–5 moderate enrichment, 5–10 severe enrichment, 25–50 very severe and > 50 indicate extremely severe enrichment (Szefer et al., 1998). For the calculation of the enrichment factor, the reference values proposed by Turekian and Wedepohl (1961) were used.
Additionally, the following elemental ratios were calculated: Mg/Ca, Mn/Sr, Al/Fe, Ti/Fe, Si/Fe, Mn/Fe and Fe/Ca determined in the samples of the sediment cores. The Mg/Ca ratio was used to distinguish sediments originating in limestone taken from the different formations of the Paraiba basin, as proposed by Nascimento-Silva et al. (2011). The Mn/Sr ratio was used to distinguish sediments from carbonates of different degrees of diagenetic alteration (Nagarajan et al., 2008), on the assumption that the proportions between both reflect the carbonate characteristics of the source area where Mn/Sr < 2 ratios did not indicate diagenetic alteration; the sediments would have originated from predominantly detritus carbonates. Mn/Sr > 2 ratios confirm diagenetic change (Jacobsen & Kaufman, 1999). For surface sediments, only the Mg/Ca and Mn/Sr ratios were used.
Some minerals are associated with sediments with different granulometries. Aluminium and iron, for example, are associated with fine particles of sediment (Araújo et al., 2002; Biscaye, 1965). Fe and Al have also been used as river proxies for sediment transport (Nizou et al., 2010). In this regard, the Ti/Fe ratio consists of an indicator of sediment provenance (Konfirst et al., 2011), since iron (Fe) and titanium (Ti) represent chemical proxies of the contribution of terrestrial material as they are associated with essentially clay minerals and siliceous components, respectively (Arz et al., 1998). Si is used as a proxy for the contribution of terrestrial sediments (Blanchet et al., 2007; Kleiven et al., 2007) and in studies of provenance (Frenz et al., 2009). The Mn/Fe ratio is used as an indirect measure of the redox potential of sediment, since Mn can be mobilized under suboxic conditions, separating from Fe during diagenesis (Marsh et al., 2007). Constant Mn/Fe ratios indicate oxic conditions and Mn/Fe peaks occur during suboxic diagenesis (Thomson et al., 1993). The Fe/Ca ratio was used as a proxy for sediment provenance, considering that these two elements reflect marine production and terrestrial input, respectively (Nizou et al., 2010).
Statistical analysis
The sediment dataset consists of 11 variables in 20 surface samples and 56 samples of C1 (32 samples) and C2 (24 samples) cores. Descriptive statistics for the surface samples and the control samples were developed, as well as multivariate analysis with the STATISTICA (v.10) software support. Factorial analysis was used to reduce the dimensions of the data and to identify concentration patterns of measured elements in superficial sediment samples. The factors were calculated by the principal factor method and rotated by Varimax method (Kaiser, 1958) in order to maximize the loadings of some variables of different factors for the production of simple structures (Thurstone, 1947) more easily interpreted. The Kaiser (1960) rule was used for the choice of factors — the factors with eigenvalues greater than 1 being chosen. The communality is the proportion of each variable’s variance that can be explained by the factors and can be defined as the sum of squared factor loadings for the variables (Field, 2009). Communalities were estimated by squared multiple correlation (SMC) of loadings for the corresponding variables. According to Hair et al. (2006) in factor analysis, a variable needs to have a large proportion of common variance to function well. A variable that does not present specific variance would have a communality of 1, while a variable that does not share variance with any other variable would have a communality value of 0. Generally, the communality must have a minimum value of 0.5 to be considered satisfactory. Simple linear regressions between the concentrations of the chemical normalizing element candidate metals (Al and Fe) and other metals were adjusted, where the highest adjusted R2 values were used to choose of the normalizing element. All graphics were made with STATISTICA (v.10), Origin (v.8.0) and Inkscape software.
Results and discussion
Figure 2 shows the validation of the analytical procedure with En number values for the analytical portions of the SRM-1646a and IAEA-SL-1 certificates. Fe, K, Mn, Ni, Pb, Sr, Ti and Zn values are at the 95% significance level (−1 < En < 1) of both certificates, while Al, Ca, Mg and Si are not certified for IAEA-SL1. The elements As, Cu, Ga and V are below the minimum detectable concentration (MDC).
Surface sediment
According to Table 1, the average of the concentrations of the some elements in sector B (Itapessoca Island) is close to an arithmetic mean between the concentrations of sediments collected in sectors A and C. Despite this, the sampling system does not allow to us state that sector B is a mixing zone of marine and terrestrial materials. The average concentrations of zinc and lead found in all surface sediment samples are below the TEL values (124 mg kg−1 and 30.2 mg kg−1, respectively), although the maximum values of Pb concentrations (24 mg kg−1) in the sectors B and C are close to the TEL in some points. Considering only the maximum values, the surface sediments reveal that sector A had the lowest concentrations of the elements. Sector B showed the highest maximum concentration of elements commonly associated with fine particles, such as Al, Fe, Mn and trace metals Ni, Zn and Pb (Kouassi et al., 2018). For the nickel element, all concentrations of were below the TEL and PEL values — 18 mg kg−1 and 35.9 mg kg−1, respectively, except for the maximum concentration value (19 mg kg−1), which was higher than the TEL value in C2. In contrast, sector C showed higher maximum concentration for Ti and Si elements, commonly associated with coarse sediment fractions, together with Ca, Sr, K and Mg associated with marine sediment.
Factorial analysis supported the identification of intercorrelation patterns between the variables, allowing a geochemical characterization of the sediment samples. Three factors were extracted, explaining 82.1% of the variance. The results of the factorial analysis for the three factors solution can be seen in Table 2.
The factor 1, denominated “fine sediments”, presents high positive load for Al (0.89), Fe (0.99) and Zn (0.91) and high negative load for Si (−0.81), denoting the presence of two groups: Al, K, Fe, Mn, Mg and Zn, usually associated with fine sediment particles, and Si, associated with coarse particles (quartz). Negative correlations between Fe–Si and Al–Si were also reported by Delgado et al. (2010). Factorial loads represent higher proportions of fine sediments in sector B (Itapessoca) at points P1, P2, P3 and P4, as seen in Fig. 3. In sectors A and C, the fine fractions tend to be proportionally smaller, contrasting with an increase in the detritus fractions of Si, especially in sector C, located in an area under the influence of the Rio Botafogo and the ocean.
Factor 2 distinguishes sediments based on the contraposition between quartz (Si) and carbonates (Ca), two chemically distinct groups, exhibiting negative correlation for metalloid Si (−0.51), high positive factorial load for Ca (0.90) associated with carbonates and high load for K (0.97). This is because the distributions of K and Ca are commonly related to drainage basin lithology, which explains a higher percentage of K associated mainly with sedimentary feldspar (Araújo et al., 1998). The increasing tendency of Ca at estuary points flowing into the sea is a result of the sediment mixing with materials originating from the adjacent marine platform, including biogenic material. Oliveira et al. (2017) observed an import of marine biogenic sandy gravel sediments, which are brought by the high tide to the lower Itapessoca estuary, through the discharge of the system (Barra de Catuama), at each tidal cycle. However, factor values suggest that higher factorial loads associated with sector B (P1 to P5) may be justified by the limestone deposits, abundant in the region (Beurlen, 1967) and outcropping in the areas immediately adjacent to the sediment core C2 and sampling stations B. Thus, factor 2 has been called “marine sediments”, whose elements are associated with both shallow marine detritus and the carbonate depositional system, also of marine origin.
Factor 3, named “coarse sediments”, explains the distribution of higher granulation particles of the surface sediment, considering the higher load values for Ti, usually associated with heavier mineral particles (Araújo et al., 2002). For factor 3, Si presents a charge very close to zero (−0.02), denoting little representativeness in this factor, probably associated with the minor influence of siliciclastic of the Gramame Formation (Barbosa et al., 2007). The higher factor loads of Ti in samples collected in sector C, more specifically at points P11 to P15, indicate a greater marine influence concerning the composition of this sediment, directly related to the closer proximity to the system outflow (Barra de Catuama) in relation to the collection stations of A and B, probably due to the presence of ilmenite (FeTiO3) and rutile (TiO2), common in the region (Valença & Souza, 2017). Ilmenite can be transformed into leucoxene due to the loss of iron in its structure during the natural weathering process, implying a progressive enrichment of titanium (Deysel, 2007), which justifies lower iron loads for the factor. For the other points, the factor 3 values reflect a mobilization of heavy minerals, with a tendency to accumulate non-resuspended materials at points less influenced by the estuarine hydrodynamics. The higher loads for the Mn element, on the other hand, suggest that this sediment is related to the limestone from the Gramame Formation, whose carbonates have high levels of Mn (Nascimento-Silva et al., 2011).
Sediment core — geochemical normalization, concentrations and ratios between chemical elements
Geochemical normalization
Considering the significance of the adjusted determination coefficients, Fe was best for the normalization of most elements. Al showed slightly better correlation only with Ni, Pb and Si (Table 3).
The chemical element concentrations in the profiles and enrichment factor
The results of the elements Al, Ca, Fe, K, Mg, Mn, Ni, Pb, Si, Sr, Ti and Zn concentrations in analytes of the two cores are summarized in Table 4. Higher concentrations for the elements Fe, Al and Si and lower concentrations for Mn, Pb, Zn and Ni are observed. It is noted that, in general, the C2 core presents higher concentrations for Mn, Fe, Ni, Pb, Al, Sr and Ca, considering the maximum values, which denote greater relationship with limestone, given the proximity with outcrops of local carbonate source rocks. Differently, the C1 sediment core presents higher maximum values of Si, which denotes its greater association with siliceous terrigenous sediments. Thin to thick silica sand palimpsests are the main components of the unconsolidated sediments that make up the coastal plains, beaches and estuarine-lagunar channels of Pernambuco state (Manso et al., 2018).
The enrichment factor ranges for the metals and metalloids analysed in C1 were as follows: Mn 0.29–0.49 (mean 0.39), Ni 0.37–0.52 (mean 0.88), Zn 0.65–1.15 (mean 0.85), Pb 0.96–2. 80 (mean 1.67), Sr 0.75–5.12 (mean 1.67), Ti 1.91–2.98 (mean 2.35), Mg 0.77–1.41 (mean 1.01), Al 0.89–1.41 (mean 1.19), Si 1.15–2.02 (mean 1.53), K 0.65–0.98 (mean 0.8) and Ca 0.69–6.22 (mean 2.52). For C2 are Mn 0.32–0.45 (mean 0.39), Ni 0.56–1.43 (mean 0.98), Zn 0.76–1.08 (mean 0.92), Pb 0.96–3.14 (mean 1.68), Sr 2.05–5.49 (mean 3.76), Ti 1.89–2. 29 (mean 2.05), Mg 0.66–1.38 (mean 0.93), Al 1.03–1.51 (mean 1.32), Si 0.99–1.54 (mean 1.22), K 0.55–0.84 (mean 0.67) and Ca 2.32–6.32 (mean 4.24) (Fig. 4). K, Mn and Zn metals are unenriched in both C1 and C2 — the zinc concentrations between 20 and 55 mg kg−1 in both controls imply little enrichment, with values below TEL values (124 mg kg−1). Mg and Ni reveal poor enrichment (Figs. 4 and 5), not anthropogenic. For the sediment cores, there is a difference in the distribution table between elements associated with fine and coarse sediment fractions: in general, the C1 core has more Si enrichment and less Al enrichment compared to the C2 core.
Although the poor enrichment (< 1.5) of the aluminium element in C1 and C2 cores indicates origin in natural processes, the highest peaks occur in the 1960s: 1963 (EF 1.41) in C1 and 1969 (1.49) in C2 (Fig. 5). In general, Ti and Si present little enrichment and a slight tendency towards impoverishment, with little variation along the profiles, although there were some oscillations. In C1, the most prominent peak of Ti occurred in 1963 (EF 2.98) and in C2, in 2009 (EF 2.29). For Si, there was a peak from 1952 (EF 2) in C1. In C1, enrichment peaks for Ca, Mg, Mn and Si elements are noticeable in 1934. After 1934, there was a decrease in the enrichment factors, with subsequent enrichment of the elements Al, Ca, K, Mg, Mn, Si and Sr in 1952. In C2, there was an increase in the enrichment factors of the elements Al, Ca, Mg, Sr, Ti and Zn in 1969. After that, the enrichment of the other elements did not accompany the enrichment of Ca and Sr — the Ca, which presented low enrichment until 1982, started to present severe enrichment in 1994. The Sr, in turn, was little enriched until 1986, starting to be severely enriched from 2005.
The Ca and Sr concentrations were the most enriched in both cores (Figs. 4 and 5). In C1, the Ca enrichment occurs from 1966 (EF 1.15), with peaks in 1979 (EF 5.35), 1984 (EF 6.22) and 1996 (6.10). Since then, there has been a retreat and the severe enrichment has been moderate. In C2, Ca has shown little enrichment since 1966 (EF 2.32), but moderate enrichment in 1982 (EF 3.15). From 1994, the enrichment has been severe (EF 5.06), with highest peaks in 1996 (EF 6.32) and 2005 (EF 6.07). Unlike C1, the behaviour of Ca in C2 does not show significant attenuation and the enrichment remains severe up to the most recent layers. The behaviour of the Sr element follows the behaviour of the Ca element in both cores. The persistence of the severe enrichment of Ca and Sr in C2 may justify the higher rate of sedimentation of this core, collected in a place strongly influenced by limestone mining for Portland cement manufacture, whose industrial plant is installed in the Itapessoca island, officially in operation since 1951 (Brazil, 1951), fact that justifies the behaviour of some elements around 1950.
For toxic metal Pb, some samples showed concentrations below the detection limit of the method; however, the remaining samples (5 samples in C1 and 9 samples in C2) were sufficient to identify enrichment in both cores. In C1, all layers analysed showed poor Pb enrichment, with higher values in recent layers and all concentrations below TEL values. Barcellos et al. (2017) found Pb concentrations with similar enrichment (EF < 3) in deposits collected from the Capibaribe River estuary, which is about 45 km from Itapessoca Island and attributed the geogenic origin to Pb enrichment. In C2, in the 12-cm-deep layer, which corresponds to the year 1999 (Albuquerque et al., 2019), the concentration of Pb exceeds the TEL reference value (30.2 mg kg−1), reaching 40 mg kg−1 of Pb. The layer at 18 cm in C2 (1990) (Albuquerque et al., 2019) also presents values above the TEL limits, with 33 mg kg−1 Pb. The moderate enrichment of Pb in C2 since 1978 could be related to sugarcane cultivation, because the application of fertilizers and pesticides contributes to the increase of toxic metals in the soil and sediments, such as Cr, Zn and Pb (Yadav et al., 2009). It should be noted, however, that (1) the sugarcane monoculture in the region has been going on since the sixteenth century (Schwartz, 2005); (2) curiously, it is observed that Pb in C1 has been enriched since 1927. In these circumstances, it cannot be safely said that sugarcane monoculture is responsible for the enrichment of Pb, because the import and use of organosynthetic pesticides in Brazil took place around 1950 (Moragas & Schneider, 2003). The region also has old calcination furnaces — vestiges of lime production for civil construction, built by Portuguese and Dutch colonialists in the seventeenth century (Santos & Oliveira, 2015); however, this fact does not justify the enrichment of Pb in the 1920s, being more likely that enrichment in this period is associated with the urban development of the Recife Metropolitan Region (RMR). In C2, it was only possible to identify Pb enrichment from 1978 onwards. Albuquerque et al. (2019) reported a peak Hg increase in sediment core collected on the Itapessoca island around 1980. Although there is some relationship between Pb and Hg enrichment in the environment due to the proximity of events, geochronological evidence indicates a higher relationship between Pb enrichment and the cement industry — in C1, it was possible to identify an increase in Pb enrichment from 20 mg kg−1 in 1927, to 27 mg kg−1 in 1948 (increase of 35%), a bit before the official start of operation of industry-scale limestone mining and cement manufacturing. The cement industry can contaminate the soil through emissions of particulate matter released by calcination furnaces, with the emission of toxic metals originating from the burning of raw materials and mainly fuels used in the clinker production process (Freitas & Nóbrega, 2014) — up to 1993 Pb was used as a gasoline additive in Brazil (Ministério do Meio Ambiente, 1993). Part of the Pb retained and accumulated in old diagenetic processes, associated with local sources, for example, the carbonate sediments themselves (Barcellos et al., 2017), may have been made available through burning. Cement dust released by industry may contain toxic metals such as lead, chromium, nickel and mercury (Baby et al., 2008). Holban et al. (2015) report that the air pollutants generated by the cement industry produce significant pollution: they found a concentration of 41.9 mg kg−1 of Pb in a soil sample near a cement plant. A similar value has found in the present work, of 40 mg kg−1 in C2 (Itapessoca Island).
The concentration of the analysed elements is subject to local hydrology, because salinity and total suspended solids (TSS) tend to be lower during the rainy season (Albuquerque et al., 2019). Despite the higher contribution of fine sediment and higher dilution of the elements, in the rainy season, under appropriate hydrodynamic conditions, the fine sediments are enriched and precipitated, enriching the deep sediments (Xavier et al., 2016). It is conceivable that the concentration of many elements is associated with the cycles and scales of limestone/cement production. In this context, Albuquerque et al. (2019) noted that the decrease in mercury (Hg) concentration along with the release of this element into the environment was associated with a decrease in the operation of mining activities on Itapessoca Island, where suspended sediments and residual organic matter from mining would act as the adsorption medium for Hg.
Ratios between chemical elements
The superficial sediments had low Mg/Ca ratios (0.008 to 0.13). This means that the relations between Ca and Mg are more compatible with the ratios present in carbonates from the Gramame Formation (0.02 to 0.09), indicating that it is more exploited in the region than the carbonates from the Itamaracá Formation (around 0.5), for example (Nascimento-Silva et al., 2011). The mean Mg/Ca ratios were within the range of the Gramame Formation (0.02–0.09) for both cores; however, in C2, the Mg/Ca ratios are closer: in C1 0.062 to 0.63 (mean 0.22) and in C2, 0.06 to 0.14 (mean 0.09). The increase in the Mg/Ca ratio found in C1 reveals a sharp peak in 1948, reinforcing the possibility that limestone mining on the island of Itapessoca began before the concession for cement production in the 1950s, also corroborated by the more enriched contents of Pb. This is possible considering the existence of the installation phase before the operation and marketing stages of the limestone/cement production chain. The decline of the Mg/Ca ratio from the 1960s in C1 and C2 cores seems to be related to the enrichment of Ca and Sr caused by limestone mining. The results of Mn/Sr ratios indicated that all samples in the C1 and C2 cores had Mn/Sr < 2 ratios, not indicating diagenetic alteration, as mentioned by Jacobsen and Kaufman (1999). The Mg/Ca and Mn/Sr ratios are shown in Fig. 6. The Mn/Sr and Mg/Ca ratios indirectly point to a decrease in Fe (most visible in C1), considering that Ca is a typical anti-Correlate of Fe and other earth elements (MacLeod et al., 2001).
The higher concentration of Fe in C2 denotes terrestrial origin associated with mining (Fig. 7). The Al/Fe ratio increases slightly in the C1core in mid-1950. In C2, the Al/Fe ratio increases quite sharply, with a more evident increase in the 1980s. The Si/Fe ratio in C1 shows many oscillations, but with slight depletion over the years in C1, with an increase in the Al/Fe ratio. In summary, the Ti/Fe, Si/Fe ratios indicate an increase in the Ti and Si ratios in C2 and a slight depletion in C1. The Al/Fe ratio indicates an increase in the proportion of aluminium in both ratios, although there is a more marked increase in C2 from 1965 onwards, when there is also an increase in the Si/Fe ratio in C2. Considering the above, it can be concluded that there is a decrease in the proportion of denser elements associated with thicker sediment, an increase in the proportion of elements associated with finer fractions of sediment and a lower proportion of quartz sand in C1. In C2, the availability of silica components from mining and limestone processing waste on the island of Itapessoca may explain the increased proportion of Al, Ti and Si in C2. Clays are one of the largest impurities in carbonated rock: alumina, silica and iron oxides in the form of clay minerals are the most common (Sampaio & Almeida, 2005). This also includes the leaching of exhausted mines. One should also consider the proximity of outcrops of the carbonate source rock and the resulting natural contribution of limestone sediments from the Gramame Formation to C2. The terrestrial material and marine carbonates reason expressed by Fe/Ca indicates an increase in the proportion of Ca in the two cores; however, there is a sharp drop in the Fe/Ca ratio in C1, from a peak in 1952. The Mn/Fe ratio shows an almost stationary behaviour along the C1 and C2 profiles, with peaks revealing suboxic conditions; however, the diagenetic formation of carbonates still depends on available metabolizable organic matter (Coleman et al., 1993) and on concentrations of dissolved oxygen and sulphate ions in the interstitial water of the pores (Curtis, 1987). The concentrations of Fe (in mg kg−1) and the Fe/Ca, Al/Fe, Mn/Fe, Si/Fe and Ti/Fe ratios over time can be seen in Fig. 7.
As mentioned, the Mn/Sr, Mg/Ca and Fe/Ca ratios indicate an increase in the proportion of these elements in the two cores, again suggesting the influence of mining on local sedimentation through the greater contribution especially of Ca and Sr. The availability of these and other elements through the loading of suspended particulate material (MPS), total suspended solids (TSS) and consequent accumulation of sediment in the study area seems to have favoured, for example, the advancement of the mangrove swamp, generating positive feedback on account of the mangrove's own nutrient retaining nature (Tam & Wong, 1993). Pélage et al. (2019) reached similar conclusions and found general growth in the mangrove system attributed to increased salinity. The high rates of sedimentation observed in both cores (C1: 0.84 ± 0.07 cm year−1 and C2: 1.03 ± 0.22 cm year−1) when compared, for example, with the rate of the mid and low Capibaribe estuaries 70 km south, 0.55 cm year−1 and 0.44 cm year−1, respectively (Barcellos et al., 2017; Xavier et al., 2016), corroborate these geochemical indications, which may be related to the high mobility and morphosedimentary dynamics of intertidal banks, associated with the expansion of the fringe of mangroves adjacent to C1 and C2 cores.
Conclusion
The analysis of superficial sediments and sediment cores suggests the influence of both natural and anthropic components in the sedimentation process. The geochemical composition of the superficial sediments enabled the identification of three factors related to finer terrestrial material, coarser sediments and calcareous/carbonate sediments of marine origin. The variations in the distributions of elements such as Al, Fe and Si seem to reflect different granulometric fractions, since Si is the main constituent of coarse particles (quartz), while Al and Fe compose the enrichment of clay minerals. The multielementary approach also allowed the identification of the variations in the concentrations of the elements for the two deposits, signalling geochemical changes in the sedimentation of the points sampled in the Itapessoca Estuarine Complex. This assertion is supported by radiometric dating, which identified a higher sedimentation rate in C2, a strong indication of the influence of cement industry and limestone mining, officially in operation since the 1950s. The quantitative analysis of the elements in the sediment cores in association with radiometric dating enabled the identification of a change in the element ratios associated with fine fractions and greater sediment granulation, with an increase in elements associated with fine fractions and associated with carbonates from mining.
These ratios between the chemical elements which reinforce the results of the enrichment factors assist in the understanding of two important issues involving the geochemistry of local sedimentation: (1) recent sediments have their origin mostly in the region's own carbonate system, especially the Gramame Formation; (2) there is a clear increase in the proportion of elements associated with carbonates in periods very close to the official start of the operational phase of limestone mining and cement manufacturing. From the evidence, it can be concluded that man-made activities related to mining and cement manufacturing have disturbed local sedimentation, leading to increased concentrations of the toxic metal lead in the region.
Data availability
The data supporting the conclusions of this study are available upon reasonable request to the corresponding author.
References
Ackerman, F. (1980). A procedure for correcting the grain size effect in heavy metal analyses of estuarine and coastal sediments. Environmental Technology Letters, 1, 518–527.
Albuquerque, P. T. F., Frédou, T., Arruda, G. N., Silva Filho, C. A., Nascimento, A., Silva, M. J., & França, E. J. (2019). Tracking Hg historical inputs by 210Pb geochronology for the Itapessoca Estuarine Complex Pernambuco Brazil. Journal of Radioanalytical and Nuclear Chemistry, 321(3), 875–883. https://doi.org/10.1007/s10967-019-06665-9
Araújo, M. F., Valério, P., & Jouanneau, J.-M. (1998). Heavy metal assessment in sediments of the Ave river basin (Portugal) by energy-dispersive x-ray fluorescence spectrometry. X-Ray Spectrometry, 27(5), 305–312. https://doi.org/10.1002/(SICI)1097-4539(199809/10)27:5<305::AID-XRS275>3.0.CO;2-7
Araújo, M. F., Jouanneau, J. M., Valério, P., Barbosa, T., Gouveia, A., Weber, O., Oliveira, A., Rodrigues, A., & Dias, J. M. A. (2002). Geochemical tracers of northern Portuguese estuarine sediments on the shelf. Progress in Oceanography, 52(2–4), 277–297. https://doi.org/10.1016/s0079-6611(02)00011-3
Arz, H. W., Pätzold, J., & Wefer, G. (1998). Correlated millennial-scale changes in surface hydrography and terrigenous sediment yield inferred from last-glacial marine deposits off Northeastern Brazil. Quaternary Research, 50(02), 157–166. https://doi.org/10.1006/qres.1998.1992
Baby, S., Singh, N. A., Shrivastava, P., Nath, S. R., Kumar, S. S., Singh, D., & Vivek, K. (2008). Impact of dust emission on plant vegetation of vicinity of cement plant. Environmental Engineering and Management Journal, 7(1), 31–35. https://doi.org/10.1590/S1413-41522014019000000769
Balls, P. W., Hull, S., Miller, B. S., Pirie, J. M., & Proctor, W. (1997). Trace metal in Scottish estuarine and coastal sediments. Marine Pollution Bulletin, 34, 42–50.
Barbieri, M. (2016). The importance of enrichment factor (EF) and geoaccumulation index (Igeo) to evaluate the soil contamination. Journal of Geology & Geophysics, 5(1), 1–4. https://doi.org/10.4172/2381-8719.1000237
Barbieri, M., Sappa, G., Vitale, S., Parisse, B., & Battistel, M. (2014). Soil control of trace metals concentrations in landfill: A case study of the largest landfills in Europe Malagrotta Rome. Journal of Geochemical Exploration, 143, 146–154.
Barbosa, J. A., Souza, E. M., Lima Filho, M. F., & Neumann, V. H. (2003). A estratigrafia da Bacia Paraíba: Uma reconsideração. Estudos Geológicos, 13, 89–108.
Barbosa, J. A., Neumann, V. H., Lima Filho, M., Souza, E. D., & Moraes, M. D. (2007). Estratigrafia da faixa costeira Recife-Natal (Bacia da Paraíba e Plataforma de Natal) NE Brasil. Estudos Geológicos, 17(2), 3–30.
Barcellos, R. L., Figueira, R. C. L., França, E. J., Schettini, C. A., & Xavier, D. A. (2017). Changes of estuarine sedimentation patterns by urban expansion: The case of Middle Capibaribe Estuary Northeastern Brazil. International Journal of Geosciences, 8, 514–535. https://doi.org/10.4236/ijg.2017.84027
Barragán, J. M., & Andrés, M. (2015). Analysis and trends of the world’s coastal cities and agglomerations. Ocean & Coastal Management, 114, 11–20. https://doi.org/10.1016/j.ocecoaman.2015.06.004
Barreto, E. P., Silva, C. M. M., & Barbosa, A. G. (2011). Ordenamento territorial e conflitos socioambientais decorrentes da mineração em áreas urbanas: O caso da Região Metropolitana do Recife. Estudos Geológicos, 21(2), 133–148.
Beurlen, K. (1967). Estratigrafia da faixa sedimentar costeira Recife-João Pessoa. Boletim Da Sociedade Brasileira De Geologia, 16(1), 43–55.
Biscaye, P. E. (1965). Mineralogy and sedimentation of recent deep-sea clay in the Atlantic Ocean and adjacent seas and oceans. Geological Society of America Bulletin, 76(7), 803–832.
Blanchet, C. L., Thouveny, N., Vidal, L., Leduc, G., Tachikawa, K., Bard, E., & Beaufort, L. (2007). Terrigenous input response to glacial/interglacial climatic variations over southern Baja California: A rock magnetic approach. Quaternary Science Reviews, 26, 3118–3133. https://doi.org/10.1016/j.quascirev.2007.07.008
Blomqvist, S., Larsson, U., & Borg, H. (1992). Heavy metal decrease in the sediments of a Baltic bay following tertiary sewage treatment. Marine Pollution Bulletin, 24, 258–266.
Brazil. (1951). Decreto nº 30.015, de 27 de setembro de 1951. Autoriza Itapessoca Agroindustrial Limitada, a lavrar calcário no município de Goiana, Estado de Pernambuco. Diário Oficial da União: seção 1, Brasília, DF, p.14.585. https://www2.camara.leg.br/legin/fed/decret/1950-1959/decreto-30015-27-setembro-1951-339407-publicacaooriginal-1-pe.html
Canadian Council of Ministers of the Environment. (2001). Canadian sediment quality guidelines for the protection of aquatic life: Summary tables. Updated. In Canadian Environmental Quality Guidelines. Canada, Winnipeg: Canadian Council of Ministers of the Environment.
CPRH. (2003). Diagnóstico Socioambiental do Litoral Norte de Pernambuco. Recife: Companhia Pernambucana do Meio Ambiente.
Coleman, M. L., Raiswell, R., Brown, A., Curtis, C. D., Aplin, A. C., Ortoleva, P. J., Gruszczynski, M., Lyons, T., Lovley, D. R., & Eglinton, G. (1993). Microbial mineralization of organic matter—Mechanisms of self organization and inferred rates of precipitation of diagenetic minerals. Philosophical Transaction the Royal Society London, A344, 69–87. https://doi.org/10.1098/rsta.1993.0076
Curtis, C. D. (1987). Mineralogical consequence of organic matter degradation in sediments: Inorganic/organic diagenesis. In J. K. Legget & G. G. Zuffa (Eds.), Marine clastic sedimentology—Concepts and case studies (pp. 108–123). Norwell: Graham and Troman Inc. https://doi.org/10.1007/978-94-009-3241-8_6.
Davis, A., Jr., Welty, A. T., Borrego, J., Morales, J. A., Pendon, J. G., & Ryan, J. G. (2000). Rio Tinto estuary (Spain): 5000 years of pollution. Environmental Geology, 39(10), 1107–1116. https://doi.org/10.1007/s002549900096
Delgado, J., Nieto, J. M., & Boski, T. (2010). Analysis of the spatial variation of heavy metals in the Guadiana Estuary sediments (SW Iberian Peninsula) based on GIS-mapping techniques. Estuarine Coastal and Shelf Science, 88(1), 71–83. https://doi.org/10.1016/j.ecss.2010.03.011
Deysel, K. (2007). Leucoxene study: A mineral liberation analysis (MLA) investigation. In The 6th International Heavy Minerals Conference ‘Back to Basics’. The Southern African Institute of Mining and Metallurgy.
Emmerson, R. H. C., O’Reilly-Wiese, S. B., Macleod, C. L., & Lester, J. N. (1997). A multivariate assessment of metal distribution in intertidal sediments of the Blackwater Estuary UK. Marine Pollution Bulletin, 34, 960–968.
Elliot, M., & Quintino, V. (2007). The Estuarine Quality Paradox environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Marine Pollution Bulletin, 54(6), 640–645.
Fernández, Z. H., dos Santos Júnior, J. A., dos Santos Amaral, R., et al. (2017). EDXRF as an alternative method for multielement analysis of tropical soils and sediments. Environmental Monitoring and Assessment, 189, 447. https://doi.org/10.1007/s10661-017-6162-5
Field, A. (2009). Discovering statistics using SPSS (3rd ed.). Sage Publications Ltd.
Freitas, S. S., & Nóbrega, C. C. (2014). The benefits of co-processing wasted tires for the cement industry. Engenharia Sanitária e Ambiental, 19(3), 293–300. https://doi.org/10.1590/S1413-41522014019000000769
Frenz, M., Wynn, R. B., Georgiopoulou, A., Bender, V. B., Hough, G., Masson, D. G., Talling, P. J., & Cronin, B. T. (2009). Provenance and pathways of late Quaternary turbidites in the deep-sea Agadir Basin northwest African margin. International Journal of Earth Sciences, 98, 721–733. https://doi.org/10.1007/s00531-008-0313-4
Garcia-Ordiales, E., Loredo, J., Esbrí, J. M., Lominchar, M. A., Millan, R., & Higueras, P. (2014). Stream bottom sediments as a means to assess metal contamination in the historic mining district of Almadén (Spain). International Journal of Mining Reclamation and Environment, 28(6), 357–376. https://doi.org/10.1080/17480930.2014.967917
Godoy, J. M., Moreira, I., Wanderley, C., Simões Filho, F. F., & Mozeto, A. A. (1998). An alternative method for the determination of excess 210Pb in sediments. Radiation Protection Dosimetry, 1(4), 111–115.
Hair, J. F., Black, W. C., Babin, B. J., Anderson, R. E., & Tatham, R. L. (2006). Multivariate data analysis (6th ed.). Pearson Prentice Hall.
Holban, E., Diacu, E., & Daescu, V. (2015). Soil quality variation in a cement plant in Romania. Scientific Bulletin, 77(2), 73–80.
IBGE. (2011). Atlas geográfico das zonas costeiras e oceânicas do Brasil. Instituto Brasileiro de Geografia e Estatística.
IPEA. (2011). Caderno de Diagnóstico: Resíduos Sólidos da Atividade de mineração. Brasília: Instituto de Pesquisa Econômica Aplicada.
Jacobsen, S. B., & Kaufman, A. J. (1999). The Sr C and O isotopic evolution of Neoproterozoic seawater. Chemical Geology, 161, 37–57.
Joshi, L., & Ku, T. L. (1979). Measurement of 2l0Pb from a sediment core off the coast of California. Journal of Radioanalytical and Nuclear Chemistry, 52(2), 329–334.
Kaiser, H. F. (1958). The varimax criterion for analytic rotation in factor analysis. Psychometrika, 23(3), 187–200. https://doi.org/10.1007/BF02289233
Kaiser, H. F. (1960). The application of electronic computers to factor analysis. Education and Psychological Measurement, 20, 141–151.
Kleiven, H. F., Kissel, C., Laj, C., Ninnemann, U. S., Richter, T. O., & Cortijo, E. (2007). Reduced North Atlantic deep water coeval with the glacial Lake Agassiz fresh water outburst. Science, 319, 60–64. https://doi.org/10.1126/science.1148924
Knoll, G. F. (2010). Radiation detection and measurement (4th ed., p. 754). Chichester Wiley.
Konfirst, M. A., Kuhn, G., Monien, D., & Scherer, R. P. (2011). Correlation of Early Pliocene diatomite to low amplitude Milankovitch cycles in the ANDRILL AND-1B drill core. Marine Micropaleontology, 80, 114–124. https://doi.org/10.1016/j.marmicro.2011.06.005
Kouassi, N. L. B., Yao, K. M., Sangare, N., Trokourey, A., & Metongo, B. S. (2018). The mobility of the trace metals copper zinc lead cobalt and nickel in tropical estuarine sediments Ebrie Lagoon Côte d’Ivoire. Journal of Soils and Sediments, 19(2), 929–944. https://doi.org/10.1007/s11368-018-2062-8
Lacerda, L. D., Maia, L. P., Monteiro, L. H. U., Souza, G. M., Bezerra, L. J. C., & Menezes, M. O. T. (2006). Manguezais do Nordeste e mudanças ambientais. Ciência Hoje, 39, 24–49.
Lee, C. L., Fang, M. D., & Hsieh, M. T. (1998). Characterization and distribution of metals in surficial sediments in Southwestern Taiwan. Marine Pollution Bulletin, 36, 464–471.
Liu, W., Li, X., Shen, Z., Wang, D., Wai, O. W., & Li, Y. (2003). Multivariate statistical study of heavy metal enrichment in sediments of the Pearl River Estuary. Environmental Pollution, 121(3), 377–388. https://doi.org/10.1016/s0269-7491(02)00234-8
Lopez, P., Navarro, E., Marce, R., Ordonez, J., Caputo, L., & Armengol, J. (2006). Elemental ratios in sediments as indicators of ecological processes in Spanish reservoirs. Limnetica, 25(1–2), 499–512. https://doi.org/10.23818/limn.25.34
Loring, D. H. (1991). Normalization of heavy-metal data from estuarine and coastal sediments. ICES Journal of Marine Science, 48, 101–115. https://doi.org/10.1093/icesjms/48.1.101
Mabesoone, J. M. (1967). Sedimentologia da faixa costeira de Recife-João Pessoa. Boletim Da Sociedade Brasileira De Geologia, 16(1), 57–72.
MacLeod, K. G., Huber, B. T., Pletsch, T., Röhl, U., & Kucera, M. (2001). Maastrichtian foraminiferal and paleoceanographic changes on Milankovitch time scales. Paleoceanography, 16, 133–154. https://doi.org/10.1029/2000PA000514
Manso, V. A. V., Coutinho, P. N., Pedrosa, F. J., Macedo, R. J., Silva, A. C., Gois, L. A., Barcellos, R. L., Arruda, S. D. D., Soares Junior, C. F. A., Madruga Filho, J. D., Arrais, M. M. C., & Madruga, M. M. D. (2018). Pernambuco. In M.M.A. Dieter Muehe (Ed.), Panorama da Erosão Costeira no Brasil Pernambuco (1st ed., pp. 345–380). Brasília: Biblioteca do Ministério do Meio Ambiente.
Marguí, E., Queralt, I., Carvalho, M. L., & Hidalgo, M. (2005). Comparison of EDXRF and ICP-OES after microwave digestion for element determination in plant specimens from an abandoned mining area. Analytica Chimica Acta, 549, 197–204.
Marsh, R., Mills, R. A., Green, D. R. H., Salter, I., & Taylor, S. (2007). Controls on sediment geochemistry in the Crozet region. Deep Sea Research Part II: Topical Studies in Oceanography, 54, 2260–2274. https://doi.org/10.1016/j.dsr2.2007.06.004
Medeiros, C., Kjerfve, B., Araújo, M., & Neumann-Leitão, S. (2001). The Itamaracá Estuarine Ecosystem, Brazil. In U. Seeliger, & Kjerfve, B. (Eds.), Coastal marine ecosystems of Latin America Ecological studies 144 (pp. 71–81). Berlin: Springer-Verlag.
Ministério do Meio Ambiente. (1993). Lei nº 8.723, de 28 de Outubro de 1993. Dispõe sobre a redução de emissão de poluentes por veículos automotores e dá outras providências. D.O.U, seção 1: p. 16.313.https://www.planalto.gov.br/CCivil_03/leis/L8723.html
Moragas, W. M., & Schneider, M. O. (2003). Biocidas: Suas Propriedades e seu Histórico no Brasil. Caminhos De Geografia, 3(10), 26–403.
Nagarajan, R., Sial, A., Armstrong-Altrin, J., Madhavaraju, J., & Nagendra, R. (2008). Carbon and oxygen isotope geochemistry of Neoproterozoic limestones of the Shahabad Formation Bhimabasin Karnataka Southern India. Revista Mexicana Ciencias Geológicas, 25(2), 225–235.
Nascimento-Silva, M. V., Sial, A. N., Ferreria, V. P., Neumann, V. H., Barbosa, J. A., Pimentel, M. M., & Lacerda, L. D. (2011). Cretaceous-Paleogene transition at the Paraíba Basin Northeastern Brazil: Carbon-isotope and mercury subsurface stratigraphies. Journal of South American Earth Sciences, 32, 379–392.
Nizou, J., Hanebuth, T. J. J., Heslop, D., Schwenk, T., Palamenghi, L., Stuut, J. B., & Henrich, R. (2010). The Senegal River mud belt: A high-resolution archive of paleoclimatic change and coastal evolution. Marine Geology, 278, 150–164. https://doi.org/10.1016/j.margeo.2010.10.002
Oliveira, J. A. R. (2017). Zoneamento Ambiental e Ocupação Territorial Geográfica do Distrito de Pontas de Pedra no Município de Goiana Litoral Norte de Pernambuco – Brasil. OBSERVATORIUM: Revista Eletrônica de Geografia, 8(21), 31–52.
Oliveira, L. E. E., Santos, L. D., Flores-Montes, M. J., & Barcellos, R. L. (2017). Influência da maré na variabilidade sedimentar da barra de Catuama Ilha de Itamaracá Pernambuco-Brasil. Estudos Geológicos (UFPE), 27, 110–122.
Oliveira, P. E. (1940). História da pesquisa do Petróleo no Brasil Serviço Público Agrícola (15th ed., pp. 1–208). Rio de Janeiro: Ministério da Agricultura.
Olsen, C. R., Simpson, H. J., Bopp, R. F., William, S. C., Peng, T. H., & Deck, B. L. (1978). A geochemical analysis of the sediments and sedimentation in the Hudson Estuary. SEPM Journal of Sedimentary Research, 48(2), 401–418. https://doi.org/10.1306/212f7496-2b24-11d7-8648000102c1865d
Pélage, L., Domalain, G., Lira, A., Travassos, P. E. P., & Frédou, T. (2019). Coastal land use in northeast Brazil: Mangrove coverage evolution over three decades. Tropical Conservation Science, 12, 1–15. https://doi.org/10.1177/1940082918822411
Sampaio, J. A., Almeida, S. L. M. (2005). Calcário e Dolomito. In A.B. Luz, & A.F. Lins (Eds.), Rochas & Minerais Industriais: usos e aplicações. Rio de Janeiro: Centro de Tecnologia Mineral – CETEM.
Santos, J. L., & Oliveira, A. L. N. (2015). Archaeology and urban history: Research notes on the island of Itamaracá Colonial. História Unicap, 2(3), 45–53.
Schwartz, S. B. (2005). A commonwealth within itself the early Brazilian sugar industry 1550–1670. Revista De Indias, 60(233), 79–116.
Shimadzu Corporation. (2006). EDX software Ver.1.00 Rel.017 – Additional manual. Shimadzu: Instrument Division.
Sinex, S. A., & Wright, D. A. (1988). Distribution of trace metals in the sediments and biota of Chesapake Bay. Marine Pollution Bulletin, 19, 425–431.
Silva, J. B., Galvíncio, J. D., de Barros Corrêa, A. C., da Silva, D. G., & Machado, C. C. C. (2011). Classificação Geomorfológica dos Estuários do Estado de Pernambuco (Brasil) com Base em Imagens do LANDSAT 5/TM (Geomorphologic classification of estuaries of the state of Pernambuco (Brazil) based on Landsat 5 TM images). Revista Brasileira De Geografia Física, 4(1), 118–133.
Szefer, P., Glasby, G. P., Kusak, A., Szefer, K., Jankowska, H., Wolowicz, M., & Ali, A. A. P. (1998). Evaluation of the anthropogenic influx of metallic pollutants into Puck Bay Southern Baltic. Applied Geochemistry, 13, 293–304.
Tam, N. F. Y., & Wong, Y. S. (1993). Retention of nutrients and heavy metals in mangrove sediment receiving wastewater of different strengths. Environmental Technology, 14, 719–729.
Thomson, J., Higgs, N. C., Croudace, I. W., Colley, S., & Hydes, D. J. (1993). Redox zonation of elements at an oxic post-oxic boundary in deep-sea sediments. Geochimica Et Cosmochimica Acta, 57, 579–595.
Thurstone, L. L. (1947). Mutiple factor analysis. University of Chicago Press.
Topan, J. G. O., & Lima Filho, M. (2017). Estudo Aeromagnetométrico e Morfotectônico da Sub-Bacia Olinda Bacia Paraíba e do Embasamento Adjacente. Anuário Do Instituto De Geociências, 40(3), 281–293.
Turekian, K. K., & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the Earth’s crust. Geological Society of America Bulletin, 72, 175–192.
Valença, L. M. M., & Souza, N. G. A. D. (2017). Geologia e recursos minerais da folha Itamaracá SB. 25-YC-VI: estados de Pernambuco e Paraíba. CPRM.
Wanderley, C. V. A., Godoy, J. M., Godoy, M. L. D. P., Rezende, C. E., & Lacerda, L. D. (2014). Evaluating sedimentation rates in the estuary and shelf region of the Paraíba do Sul River southeastern Brazil. Journal of Brazilian Chemistry Society, 25, 50–64.
Westphal, H. (2006). Limestone–marl alternations as environmental archives and the role of early diagenesis: A critical review. International Journal of Earth Sciences, 95(6), 947–961. https://doi.org/10.1007/s00531-006-0084-8
Whitfield, M., & Elliott, M. (2011). Ecosystem and biotic classifications of estuaries and coasts. In E. Wolanski & D. S. McLusky (Eds.), Treatise on estuarine and coastal science (1st ed., pp. 99–124). Academic Press.
Xavier, D. A., Barcellos, R. L., Figueira, R. C. L., & Schettini, C. A. F. (2016). Evolução sedimentar do estuário do Rio Capibaribe (Recife-PE) nos últimos 200 anos e suas relações com a atividade antrópica e processo de urbanização in Portuguese. Tropical Oceanography, 44, 74–88.
Yadav, D. V., Jain, R., & Rai, R. K. (2009). Impact of heavy metals on sugarcane. Soil Heavy Metals, 19, 339–367. https://doi.org/10.1007/978-3-642-02436-8_16
Zhang, J., & Liu, C. L. (2002). Riverine composition and estuarine geochemistry of particulate metals in China—Weathering features anthropogenic impact and chemical fluxes. Estuarine Coastal and Shelf Science, 54, 1051–1070.
Acknowledgements
The English text of this paper has been revised by Sidney Pratt, Canadian, MAT (The Johns Hopkins University), RSAdip—TESL (Cambridge University).
Funding
The Fundação Capes, Fundação Rondônia de Amparo ao Desenvolvimento das Ações Científicas e Tecnológicas e à Pesquisa (FAPERO), Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) provided financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Albuquerque, P.T.F., Fredou, T., Barcellos, R.L. et al. Anthropogenic influences on sedimentary geochemistry of Itapessoca Estuarine Complex, Pernambuco, Brazil. Environ Monit Assess 194, 43 (2022). https://doi.org/10.1007/s10661-021-09699-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09699-3