Abstract
Leaves of European larch, silver birch, and bilberry were sampled 5–7 times per growing season in 2010–2019 in a locality near the city of Litvínov in the Krušné Hory Mts. (Ore Mts.) near the Czech/German border. The locality is characterised by a large amount of plant-available Mn because of acidic soils in the study area. All three investigated plants at the studied site acquired manganese concentrations close to the definition of hyperaccumulation (ca. 10,000 mg kg−1). This paper presents the most detailed collection of plant material for the characterisation of seasonal dynamics of Mn concentrations in the foliage of the three studied plants under field conditions and compares this information with that in published studies. Time (day in the year or day in the growing season) and cumulative precipitation anomalies were major and minor variables, respectively, explaining Mn dynamics in leaves, while temperature and insolation anomalies were not significant. The three investigated species showed plant-specific Mn acquisition rates in the growing season and specific effects of precipitation. Seasonal dynamics must be considered if plant leaves are used for environmental monitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Manganese is an essential element in plants that contributes to the protein structure and phosphorylation of enzymes. Its deficiency causes damage to chloroplasts, affecting water photolysis in photosystem II, which supplies the electrons necessary for photosynthesis (Fernando & Lynch, 2015). Excessive concentrations of Mn in plant tissues may alter various processes, such as enzymatic activity, uptake, and redistribution and the use of other nutrients (Ca, Fe, Mg, N and P) (Lavres Junior et al., 2010). The toxicity limit of Mn, as well as tolerance to excess of this metal, is dependent on the plant species, in addition to its variety or genotype (Ducic & Polle, 2005; Kochian et al., 2004).
Seasonality is an important source of variability in many ecological systems (White, 2020). The seasonality of element concentrations in plants is not often addressed in current studies, perhaps because the collection of large datasets over several years is undoubtedly time-consuming (McMeans et al., 2015; Power et al., 2008; White, 2019, 2020). The available literature addressing seasonal changes in metal accumulation in plants is thus often limited to a single vegetation period or a single plant species (Březinová & Vymazal, 2015; Herndon et al., 2019; Kandziora-Ciupa et al., 2017; Kim & Kim, 2018; Vitória et al., 2015; Wislocka et al., 2006). Recognizing seasonal variation in metal accumulation is essential during biomonitoring programmes (Oliva et al., 2012). Viers et al. (2013) also observed considerable seasonal variability in element concentrations in plants, claiming that at the beginning (June) and at the end (September) of the season, biochemical processes probably did not start or end at the same time for all samples of larch they collected in 2006.
Manganese is the element of interest in this work. Viers et al. (2013) assigned Mn, together with Ca and Mg, to a group of accumulating elements based on its concentration pattern in the growing season. A 1-year observation of selected metals in bilberry leaves by Kandziora-Ciupa et al. (2013) indicated metal accumulation in the bilberry foliage increased with each subsequent month.
The amount of Mn in soils is highly variable; the concentration intervals reported are 50–3000 mg kg−1 (Bergmann, 1988; Marschner, 1995; Mengel & Kirkby, 2001). Manganese can become very mobile in the soil environment. Heal (2000) found that autumnal rains caused a Mn increase in surface soil solutions and runoff due to flushing from decomposing organic material. An opposite phenomenon was observed in summer, where a flux occurred from deeper soil horizons with less mobile Mn. According to Heal (2000), the stormier conditions and warmer temperatures occurring in temperate environments will cause autumn flushing of mobile Mn as a result of enhanced decomposition of organic material in upper soils with warmer summer temperatures.
The fate of Mn in soils is significantly affected by plants. Vegetation can accumulate Mn and mitigate its leaching from upper soil layers to deeper layers by reducing infiltration (Herndon et al., 2019). Manganese is subject to active uptake from lower soil horizons to the foliage, creating large Mn pools in the tree canopy (Herndon et al., 2019). The concentration of Mn in plant tissues depends on the plant species and growth conditions (Marschner, 1995), but in some cases, it can reach concentrations close to the estimated hyperaccumulation limit of 10,000 mg kg−1 for Mn (Baker et al., 1994; Boyd, 2004; Reeves & Baker, 2000; Verbruggen et al., 2009). Conifers can tolerate Mn concentrations in foliage up to 8,000 mg kg−1 (Bergmann, 1988). Similarly, high values of Mn can be found under suitable conditions in birch (Hrdlička & Kula, 1998, 2004). Bilberry leaves can also accumulate up to 11,000 mg kg−1 Mn (Kula et al., 2018). Mróz and Demczuk (2010) suggested that Vaccinium myrtillus is an accumulator of Mn, and such high concentrations of this element offer the possibility of using bilberry leaves for some beneficial purposes.
Manganese mobility and uptake by plants affect Mn vertical profiles in soils. The deposition of Mn-enriched foliage after the end of a growing season increases the concentration of Mn on the forest floor (Alriksson & Eriksson, 2001; Heinrichs & Mayer, 1980; Landre & Watmough, 2010; Navrátil et al., 2007). Mineral weathering caused by plants releasing organic acids into soil can result in high quantities of leached Mn (Berner et al., 2003; Bormann et al., 1998; Drever, 1994; Taylor et al., 2009). Deeper soil horizons are low in Mn, perhaps due to vegetation remobilizing labile Mn to the upper horizons or Mn leaching and being output from the catchment (Navrátil, et al., 2007).
The uptake of Mn increases at low concentrations of competing cations, including nutrients, such as Ca and Mg (Foy et al., 1969; Goss & Carvalho, 1992; Kogelmann & Sharpe, 2006; Juice et al., 2006; Gransee & Führs, 2013). Manganese is more available to plants at soil pH ca. 4 to 5 (Ducic & Polle, 2005; Lambers & Oliveira, 2019; Lambers et al., 2021) and reducing conditions (Heal, 2000) than under other conditions when insoluble Mn(III,IV) oxides are converted to mobile Mn2+ ions. In soils that are acidic, Mn can thus become toxic to plants (Fageria & Stone, 2008; Goss & Carvalho, 1992). According to Lu et al. (2010), an increase in available Mn in soils caused by anthropogenic activities is expected.
This paper is a follow-up on our preceding study (Kula et al., 2018) on extreme Mn concentrations in bilberry leaves at a Czech mountainous site impacted by acidification due to industrial activities, in particular coal mining and exploitation in neighbouring areas in the Czech Republic, Germany, and Poland. Our work now additionally includes the results of the Mn monitoring obtained for two tree species common in the study area, European larch and silver birch, which also accumulate a considerable amount of Mn. Kula et al. (2018) found considerable Mn seasonal dynamics in bilberry foliage; however, the impacts of time, precipitation, and other seasonal parameters were not determined. The considerable unexplained variability in seasonal data (Kula et al., 2018) is thus newly addressed using a Mn concentration dataset gathered in the decade of 2010–2019. Our aim in this work was to decipher individual seasonal dynamics of the Mn concentrations in these three plant species and to assess variation among seasons by correlating results with precipitation, temperature, and insolation. The study is based on long-term monitoring in actual landscapes and not on short-term laboratory experiments that are mostly reported in the research literature. The seasonal dynamics of Mn in the studied plants can also be of relevance for understanding the nutrient uptake strategies of plants (Albornoz et al., 2021; Lambers et al., 2015) as well as element cycles in acid rain-impacted or contaminated soils (Herndon et al., 2019).
Materials and methods
Site characteristics
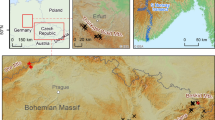
The permanent research area is situated at the foot (SW slope) of the Krušné Hory Mts. at 480–495 m a.s.l. near the city of Litvínov (Czech Republic, 50°37′03.80″N; 13°37′25.17″E). The area is heavily affected by air pollution due to nearby opencast brown coal mining and related activities (power and heating plants and a refinery) (Fig. 1). Of eleven monitored tree species growing there, Betula pendula Roth occupies 65%, and Larix decidua Mill. occupies 3% of the forest stand. Bilberry covers smaller patches on the forest floor and is more represented at higher altitudes in the Krušné Hory Mts. The geological structure of the study area is predominantly granite porphyry and partly orthogneiss. Generally, these rocks, similar to other rocks of the Krušné Hory Mts., are poor in nutrients (Forest management plan, 2010).
The prevailing soil type in the studied forest stands is Dystric Cambisol. Three soil pits were excavated on the site where the total concentrations of the selected elements were established by a handheld X-ray fluorescence analyser (Olympus Innov-X Delta, Waltham, MA, USA). Handheld XRF is a device that is used as a very quick in situ method for analysing various elements in soils. As a source of radiation, a 4 W, 200 µA (max) X-ray tube was used.
The mean pH values were determined at an accredited laboratory in 2010 (Laboratory-Morava, 2010) and published in Kula et al. (2018). The measured pH and mean values (from all soil pits) of the selected element concentrations in the topsoil horizon with accumulated organic matter (Ah) and in the subsoil (Bv), where mineral layers were present, are shown in Table 1.
The mean active pH in both observed soil horizons can be defined as very strongly acidic (3.59–4.38), which is similar to that at other sites in the Krušné Hory Mts. The concentration of calcium in the topsoil is very high. The topsoil contents of iron, potassium, aluminium, phosphorus, and zinc are very high to excessive, and the topsoil contents of Mn are also very high (893–1,379 mg kg−1). The same situation is observed in the subsoil horizon (Mn 499–4,135 mg kg−1), except for calcium, the concentration of which in the subsoil is considered high (Fiala et al., 2013).
Meteorological datasets and their processing
Data from the continuous monitoring of air temperature and cumulative rainfall at the permanent research plot (May 2010 to December 2019) were obtained from the MeteoUNI (Amet Velké Bílovice) climatic station that recorded the climatic conditions of the area. Daily precipitation, temperature, and insolation values were processed to obtain temperature, precipitation, and insolation anomalies as potential variables for Mn concentrations.
The average air temperatures in the growing season (April to October) were not substantially different during the period from 2010 to 2017 (13.4–14.1 °C). In addition, 2018 was exceptionally warm and dry, when the average air temperature in the growing season exceeded 16 °C and precipitation was the lowest in comparison with that in the other years (249 mm) (Table 2). Except for 2018, the total precipitation levels for the studied season ranged from 287 to 461 mm, with 2011 being the year with the highest value (Kula et al., 2018). According to Kula et al. (2018), the manganese flow into forest stands in the study area from the atmosphere was not significant.
Fieldwork and laboratory analyses
Sampling of larch and birch leaves at the permanent research site took place monthly during the growing seasons (April to October) over a period of 10 years (2010–2019). The growing season begins when the average air temperature is over 5 °C for a period of 24 h (Sobíšek et al., 1993). Samples were collected from randomly localized trees in the permanent research plot. Dried larch and birch leaves were placed separately in pots made of tungsten carbide and ground in a RETSCH MM 400 vibrating mill (Haan, North Rhine-Westphalia, Germany) (grinding time 3 min, intensity 30 Hz, milling fineness < 0.5 mm). The ground samples were stored in PE powder bottles. Acid digestion of the samples was performed in CEM MW MARS 5 (Microwave Solvent Extraction Labstation, Matthews, North Carolina, USA) with a mixture of 5 ml of HNO3 and 5 ml of deionized water. The sample weight was 0.252 g. After slowly heating the sample, a mineralization temperature of 210 °C was reached and maintained for 20 min. After cooling to 60 °C, deionized water was added to the digested sample to reach a total volume of 25 ml. Determination of Mn contents in the digested samples of the selected plant species was performed by inductively coupled plasma optical emission spectroscopy (ICP-OES) (Perkin Elmer Optima 8000, Waltham, Massachusetts, USA) at a wavelength of 257.6 nm.
Results
Mn concentration growth in the respective years
Tables 3, 4, and 5 show the statistically significant linear increase in Mn in the leaves of the studied species for the respective growing seasons in the 10 years of monitoring (all individual figures depicting Mn accumulation in leaves of the studied species in all growing seasons are given in Online Resource 1). The fit was not possible to determine for bilberry in the exceptionally dry year of 2015, which caused the shedding of bilberry leaves in September followed by new sprouting from which leaves were collected in autumn, with Mn concentrations similarly as low as those at the beginning of the growing season. The time of leaf collection was expressed as a day of the growing season. The beginning of the growing season was specific to each year; it is defined as the first date when the temperature does not drop below 5 °C for 24 h. The resulting determination indices show the statistically significant temporal effect on Mn concentrations in birch, larch, and bilberry leaves towards the end of the growing season. The last columns in Tables 3, 4, and 5 show extrapolated concentrations of manganese at the end of the growing season as a result of the regression function calculated for day 250 of the growing season. All these extrapolated values are extremely high, particularly for larch.
The data processing showed several differences between individual plants. The main differences are shown in Fig. 2 with regression lines constructed for all years. The intercepts in the linear regressions for bilberry were nonsignificant for most years if time was expressed as a day in the growing season, while mean temporal Mn acquisition rates (slopes in regression lines) were largest for this plant (Table 5, Fig. 2). Birch showed the largest intercept and the smallest acquisition rates (Table 3, Fig. 2). Larch showed a moderate intercept when time was expressed as a day in the growing season (Table 4, Fig. 2). The intercept for larch was nonsignificant with time expressed as a day in the year (Table 6). The mean Mn acquisition rates for birch were lower than those for larch and bilberry.
The Mn concentration increase over time in bilberry was most remarkably different from the line; i.e. the Mn concentration increased the fastest at the beginning of growth, and this phase was followed by saturation before the end of the growing season, i.e. a fast decrease in the mean daily Mn acquisition rate before the end of the growing season (Fig. 3). The different shapes of Mn versus time for bilberry make the comparison of acquisition rates based on linear regression only approximate, but they underpin the variable behaviour of individual plant species. Theoretically, there could be a connection with different transpiration and exudate chemistries of the species, but determining that connection would require further research. The sampled bilberries were growing near or beneath birch trees, so an interaction between Mn cycling, including joint soil chemistry and transpiration, in those plants can be expected. Additionally, subsurface Mn translocations by precipitation or evaporation extremes could play a role, but this was not the subject of our current study.
Meteorological variables affecting Mn concentration growth
We processed the entire dataset of individual plant species jointly for all years to test the influence of further possible variables, i.e. to test whether further variables could explain the observed interannual variability (unprocessed data are given in Online Resource 2). While r2 values were typically > 0.9 for the respective plants and years (Tables 3, 4, and 5), the fits were worse for birch and bilberry when all years were processed jointly. Combinations of several variables were thus tested: day in the growing season or day in the year as temporal variables and precipitation, temperature, or insolation anomalies as further variables. The results of the search for significant variables are summarized in Table 6. If they were not statistically significant, then the intercepts or independent variables were omitted (their t values were < 1.5). Temperature, precipitation, and insolation anomalies were calculated as described in the Materials and methods.
Cumulative precipitation amounts (CPs) were fitted versus day in the growing season (G) or year (Y) by linear regression for all studied years. Then, daily cumulative precipitation anomalies (CPAs) were calculated as the difference between actual cumulative precipitation amounts and the joint fitted line, and the resulting differences were averaged for the time period between two successive samplings.
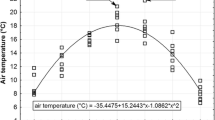
Mean temperatures in the growing seasons were fitted against day in the growing season for all studied years using parabolic regression (second-order polynomial). Temperature anomalies in the growing seasons (TAG) for the interval between two successive samplings were calculated as the mean difference between the actual daily temperature and the fitted parabolic trend.
Insolation anomalies were obtained by fitting daily insolation (W m−2) values for all years using the Lorentz function. Isolation anomalies in growing seasons (IAG) were then calculated as daily differences between actual and fitted values and averaged for each interval between successive samplings.
Multilinear regression analyses were performed with day in the growing season or in year of the date between two successive samplings, mean precipitation, temperature, and insolation anomalies for the interval between successive samplings as independent variables and Mn concentrations in the latter sampling as dependent variables. This scheme was used because we assumed that the Mn concentration on the actual sampling date should reflect the entire period between two successive samplings.
Two temporal variables were compared in this analysis: day in the middle of the time interval between successive samplings counted from the beginning of the year (DY) or from the beginning of the growing season (DG). DY produced better fits for larch than for birch and bilberry, if that tree had a longer vegetation period than the season defined in our work. DG produced better fits for birch and bilberry than for larch, as inferred from the regression coefficients (Table 6). Precipitation amounts were expressed as cumulative precipitation anomalies from the beginning of the growing season (CPAG) or from the beginning of the running year (CPAY), all calculated from daily data. Neither precipitation amounts nor cumulative precipitation values improved the fits. CPAY improved fits for birch (with a negative slope), while CPAG improved fits for bilberry and larch (with a positive slope). The positive CPAY (more annual precipitation) thus decreased the Mn concentrations in birch leaves, indicating a possible loss of Mn by washing in the soil profiles. The positive CPAG increased the Mn concentrations in larch and bilberry leaves, indicating a possible greater uptake of Mn in more humid periods. Temperature and insolation anomalies were not statistically significant, as indicated in Table 6.
Particular attention was given to the influence of the precipitation rate on the Mn concentration in the birch leaves because the negative influence of CPAY on the Mn concentration seemed surprising. There was an interrelation between the Mn concentration at the end of the growing season, extrapolated to the 250th day (the end) of the growing season in the preceding year, and the initial Mn concentration in the following year, inferred from the intercept of the corresponding linear concentrations (Fig. 4). This figure shows that the Mn concentration in the leaf litter could be the source of Mn at the start of the subsequent season. Interestingly, the difference between those two concentrations was proportional to the CPAY at the beginning of the growing season (Fig. 5), as more precipitation in winter leached more Mn from the litter and postponed its uptake by birch during the growing season. No such relations were found for larch and bilberry.
Discussion
Manganese cycle in the environment
All three studied plants showed similar annual patterns of Mn concentrations over time (Fig. 2). The continuous increase in Mn concentrations during the growing season was in accordance with the results of past studies but showed unprecedented details and temporal extent. Most preceding studies were based on two or three successive samplings, while more detailed (more frequent) sampling was rare. A seasonal increase in Mn concentrations in foliage was found for larch by Navrátil et al. (2007) and Kandziora-Ciupa et al. (2013) and for bilberry by Kula et al. (2018) (Table 7). Surprisingly, the hyperaccumulation of Mn was observed in mycorrhizal species, including the studied plants, and mycorrhizal hyphae are supposed to intercept metals, including Mn (Lambers et al., 2015). To determine a connection with the mycorrhizal environment, more detailed element (P, Fe, Ca, and Mg) analyses of leaves will be conducted in future research.
All three species at the studied sites reached the limit of hyperaccumulation (10,000 mg kg−1) for Mn (Baker et al., 1994; Boyd, 2004; Reeves & Baker, 2000; Verbruggen et al., 2009), highly exceeding the expected toxicity limits for plants at 400–1000 mg kg−1 (Kabata-Pendias, 2011; Kandziora-Ciupa et al., 2013). However, symptoms of Mn phytotoxicity, such as leaf chlorosis in older leaves or growth limitations (Bergmann, 1988; Kitao et al., 1997; Marschner, 1995; Wissemeier & Horst, 1992; Wu, 1994), were not observed in this study. The Mn concentrations reported for the same species by other authors (Table 7) were usually substantially lower. Our study site is obviously extraordinary regarding soil chemistry. We believe that the soil chemistry may be affected by geogenic anomalies common to the Krušné Hory Mts. in combination with acidification due to the proximity of the chemical factory (Unipetrol) (Fig. 1). The extreme accumulation reported at the study site is, however, similar to that at other sites on the ridges of the Krušné Hory Mts., as reported previously by Kula et al. (2012, 2018). In comparison to at our site, in the Karkonosze-Izera Block (Sudetes Mts., SW Poland), which is contaminated by metals and uranium, much lower Mn concentrations were found both in soils (62–883 mg kg−1) and in birch leaves (284–1790 mg kg−1) (Wislocka et al., 2006). Alternatively, in the Krakow-Częstochowa Uplands (Poland), which are affected by air pollutants from various sources, metallurgical emissions and pulp mills, Hrdlička and Kula (2010) measured “only” 2,692 mg kg−1 of Mn in birch leaves (Table 7). Hrdlička and Kula (2010) found Mn concentrations in larch needles to be 12,542 mg kg−1 (October 8th, 2010). Such large concentrations affect the cycling of biogeochemical elements in forest ecosystems, as described in the Introduction. These concentrations should cause persistent transfer of Mn from deeper soil strata to topsoil via Mn-enriched litter, followed by leaching of Mn during litter leaching by winter precipitation and snow melt. This mechanism was named “plant pump” by Reimann et al. (2018) after Goldschmidt (1937) described the process in his study. Goldschmidt (1937) also discussed the consequences of mobile elements leaching back to deeper soil strata after leaves are shed; i.e. plants can generally enhance the vertical transfer of some elements in soil profiles. These transfers and their consequences on topsoil/subsoil ratios were also highlighted in some preceding studies (Alriksson & Eriksson, 2001; Berner et al., 2003; Bormann et al., 1998; Drever, 1994; Heinrichs & Mayer, 1980; Landre & Watmough, 2010; Navrátil et al., 2007; Taylor et al., 2009). The main point of the study by Reimann et al. (2018) was that element allocation to plant organs depends on metabolism, physiology, and structure linked to biological functions and cannot be attributed exclusively to the substrate and environmental background. The results of Reimannn et al. (2018) study stressed the importance of understanding the biological mechanisms of plant–soil interactions to correctly quantify anthropogenic impacts on soil and plant geochemistry. This is particularly relevant to the hyperaccumulating plants prevailing in the studied areas of the Krušné Hory Mts. because the presence of accumulators such as birch and bilberry may not be coincidental on acid soils in the study area.
Manganese dynamics in leaves
The Mn concentration in the leaves of the three examined species from a single locality apparently followed a simple pattern of persistent accumulation over time as a major source of variability, as documented by regression coefficients in Tables 3, 4, 5, and 6. Precipitation acted as a minor but in some cases statistically significant source of variability at the studied site and area, as documented by the slight increase in r2 after using yearly or seasonal precipitation anomalies in the fits in Table 6. Lambers et al. (2021) observed increasing leaf Mn concentrations with increasing mean annual precipitation and decreasing leaf Mn with increasing mean annual temperature. We did not observe temperature effects of the Mn concentration dynamics. Lei (2007) found that the humid climate population of Populus cathayana was more sensitive to Mn stress than the dry climate population. A more significant impact of precipitation and temperature at our study site would be expected if these parameters were critical for plant growth, as occurred with the extreme event impacts on bilberry in 2015: this year was excluded from data processing.
The results of our study show that Mn concentrations in leaves are so dynamic that they could not be discussed without information on the date of sampling and precipitation patterns. This conclusion is relevant for environmental monitoring studies based on sampling plant materials, as with bilberry, but without specifying the date of the sampling and precipitation distribution in the season (Reimann et al., 2001, 2018; Salemaa et al., 2004; Brekken & Steinnes, 2004; Białonska et al., 2007). Because the impact of precipitation is so specific to plant species, even foliar Mn concentrations in plants collected on the same date in the same locality could be seasonally and interannually variable.
Toxic Mn concentration in leaves
The optimal concentrations of Mn or even phytotoxicity levels for some plants given in the literature range from 400–1000 mg kg−1 (Kabata-Pendias, 2011; Kandziora-Ciupa et al., 2013; Reeves, 2006), and these concentrations were exceeded several times in the plants investigated in our study. The Mn concentrations in larch, birch, and bilberry accumulated several thousand mg kg−1 Mn without symptoms of Mn phytotoxicity, as described by Bergmann (1988), Kitao et al. (1997), Marschner (1995), Wissemeier and Horst (1992), and Wu (1994). Our results were much closer to the limit of Mn hyperaccumulation in plants (10,000 mg kg−1) (Baker et al., 1994; Boyd, 2004; Reeves & Baker, 2000; Verbruggen et al., 2009). Baker and Brooks (1989) listed 8 species as hyperaccumulators based on the hyperaccumulation limit. The list is continuously expanding; thus, Fernando et al. (2013) reported 22 hyperaccumulators, but their list does not include larch, birch, or bilberry. In fact, the Mn concentrations found for the three studied plants in the study area substantially exceed the values reported for those species in the literature (Table 7).
Thus, with respect to the broad range of reported Mn concentrations in plant leaves in Table 7, the question arises as to whether the plants reported in our paper should be called hyperaccumulators or simply plants growing on acidic soils, with mechanisms for ensuring even large amounts of Mn (and perhaps also other elements mobilized in acidic soils) are nontoxic. Larch, birch, and bilberry are among the most common plant species in the mountain forests of the Czech Republic. It is important to determine whether the observed accumulation is a consequence of natural factors, such as felsic, crystalline, chemically resistant bedrock producing acid soils that have low to very low Mg2 + and Ca2 + in the research area (Hrdlička & Kula, 2004), or acid rain from coal combustion or the chemical industry in areas surrounding the Krušné Hory Mts. from the east, north, and west (Fig. 1). The determination could be based on a comparison of the Mn concentrations in the plant leaves in the study area and in areas with the same bedrock and clean air level during the highest atmospheric pollution period in the twentieth century. In both those cases, the high Mn content in leaves provides huge amounts of plant-available Mn for plants in the subsequent season (from litter), and thus, less Mn-tolerant plants cannot grow in the litter of those leaves.
The extremely high Mn levels in the assimilatory organs of plants can have negative effects on insect feeding. A recent study by Martínek et al. (2020) concluded that the high Mn concentrations in birch leaves increase mortality and time until pupation of the Cabera pusaria caterpillars. Energy requirements for excreting Mn have caused massive mortality among individuals with 3,000 mg kg−1 or more Mn in their diet. The problem of excess Mn in leaves applies to additional insect species. The high manganese concentration in the food of Lymantria dispar caterpillars increased mortality of the caterpillar’s first instar, and the development of caterpillars was prolonged, possibly due to the energy requirements for manganese allocation. Diets with a high Mn concentration increased the mortality rate while decreasing the food consumption and fertility of Melolontha hippocastani (Martínek, 2018). Insects may develop mechanisms that can eliminate the negative impacts of high doses of manganese, e.g. translocation of this element into frass and exuviae or its secretion in excrements. The sensitivity of insects to developmental stages is differentiated and associated with mortality; the elimination of Mn is energy-consuming (Kula et al., 2014; Martínek et al., 2017). Mn accumulation in the studied plants can thus have consequences for whole forest ecosystems that contain those plants.
The mechanism behind the accumulation of Mn in the leaves of the studied plants via soil acidification can be a result of the change in the proportions among plant-available cations. Mn uptake by plants can be facilitated by the combined action of Mn mobilization and decreased concentrations of Ca2+ and Mg2+ (Joslin et al., 1992; Ohki, 1984; Schlegel et al, 1992; Terry et al., 1975; Thornton et al., 1989; Kochenderfer & Helvey, 1989). An explanation for why the studied plants in the Krušné Hory Mts. accumulate such large concentrations of Mn could be the low concentrations of Mg because the two elements behave as antagonists in plant uptake (Heenan et al., 1981; Goss & Carvalho, 1992; Gransee & Führs, 2013). This interrelation between the proportions of the three mentioned cations, Ca, Mg, and Fe and Mn uptake, will be addressed in our further research.
Conclusions
The tree species birch and larch and bilberry, a common plant in central European mountain forests, can accumulate ca. 1% Mn in the dry weight of leaves during the growing season. Mn uptake proceeds during the whole growing season generally linearly with time, and in the case of bilberry, the rate declines by the end of the growing season. Mn uptake by larch and bilberry is enhanced by greater precipitation during the growing season, while the Mn concentration in birch leaves decreases with more precipitation before the growing season, probably due to leaching from the leaf litter. Litter decay in winter could retain Mn ions in biogeochemical cycling in mountain forest soils, causing element uptake in the following growing season. Extreme Mn concentrations are a consequence of low soil pH, to which natural (geogenic) and anthropogenic (acid rains) contributions are not known. More attention should be given to the concentration ratios of Ca, Mg, and Mn in soils because it is known that Mg deficiency in soils results in an elevated uptake of Ca and Mn.
Availability of data and material
The datasets generated and/or analysed during the current study are available in Supplementary information or from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Albornoz, F. E., et al. (2021). Revisiting mycorrhizal dogmas: Are mycorrhizas really functioning as they are widely believed to do? Applied Soil Ecology, 3(1), 73–82.
Alriksson, A., & Eriksson, H. M. (2001). Distribution of Cd, Cu, Pb and Zn in soil and vegetation compartments in stands of five boreal tree species in N.E, Sweden. Water, Air, and Soil Pollution, 1, 461–475.
Baker, A. J. M., Reeves, R. D., & Hajar, A. S. M. (1994). Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & Presl (Brassicaceae). New Phytologist, 127, 61–68. https://doi.org/10.1111/j.1469-8137.1994.tb04259.x
Baker, A. J. M., & Brooks, R. R. (1989). Terrestrial higher plants which hyperaccumulate metallic elements – A review of their distribution, ecology and phytochemistry. Biorecovery, 1, 81–126.
Bergmann, W. (1988). Ernährungsstörungen bei Kulturpflanzen. (Entstehung, visuelle und analytische Diagnose). Second ed., Jena: VEB Gustav Fischer Verlag.
Berner, E., Berner, R., & Moulton, K. (2003). Plants and mineral weathering: Present and past. Treatise on Geochemistry, 5, 169–188.
Białońska, D., Zobel, A., Kuraś, M., Tykarska, T., & Sawicka-Kapusta, K. (2007). Phenolic compounds and cell structure in bilberry leaves affected by emissions from a Zn–Pb smelter. Water, Air, and Soil Pollution, 181, 123–133.
Bormann, B. T., Wang, D., Snyder, M. C., Bormann, F. H., Benoit, G., & April, R. (1998). Rapid, plant-induced weathering in an aggrading experimental ecosystem. Biogeochemistry, 43, 129–155.
Boyd, R. S. (2004). Ecology of metal hyperaccumulation. New Phytologist, 162, 563–567.
Brekken, A., & Steinnes, E. (2004). Seasonal concentrations of cadmium and zinc in native pasture plants: Consequences for grazing animals. Science of the Total Environment, 326, 181–195.
Březinová, T., & Vymazal, J. (2015). Evaluation of heavy metals seasonal accumulation in Phalaris arundinacea in constructed treatment wetland. Ecological Engineering, 79, 94–99.
Creighton, L. G., & Spiers, J. M. (1992). Inheritance of tolerance to mineral element-induced chlorosis in rabbiteye blueberry. International Journal of Horticultural Science, 27, 148–151.
Drever, J. I. (1994). The effect of land plants on weathering rates of silicate minerals. Geochimica Et Cosmochimica Acta, 58, 2325–2332.
Ducic, T., & Polle, A. (2005). Transport and detoxification of manganese and copper in plants. Brazilian Journal of Plant Physiology, 17, 103–112.
EC-UN/ECE, Stefan. K., Fürst, A., Hacker, R., Bartels, U. (1995). Forest foliar condition in Europe - Results of large-scale foliar chemistry surveys. EC, UN/ECE.
Fageria, N. K., Stone, L. F. (2008). Micronutrient deficiency problems in South America. In: Alloway, B. J. (ed.), Micronutrient deficiencies in global crop production. Springer, Dordrecht.
Fernando, D. R., et al. (2013). Microbeam methodologies as powerful tools in manganese hyperaccumulation research: Present status and future directions. Frontiers of Plant Science, 4.
Fernando, D. R., & Lynch, J. P. (2015). Manganese phytotoxicity: New light on an old problem. Annals of Botany, 116, 313–319.
Fiala, P., Reininger, D., Samek, T., Němec, P., & Sušil, A. (2013). A survey of nutrition forest in the Czech Republic, 1996–2011. Brno: Central Institute for Supervising and Testing in Agriculture.
Forest management plan. (2010). Forest management plan: Litvínov city forests 2010–2019. Text part. Ekoles–Projket s.r.o., Jablonec nad Nisou.
Foy, C. D., Fleming, A. L., & Armlger, W. H. (1969). Differential tolerance of cotton varieties to excess manganese. Agronomy Journal, 61, 690–694.
Goldschmidt, V. M. (1937). The principles of distribution of chemical elements in minerals and rocks. Journal of the Chemical Society, 655–673.
Goss, M. J., & Carvalho, M. J. (1992). Manganese toxicity: The significance of magnesium for the sensitivity of wheat plants. Plant and Soil, 139, 91–98.
Gransee, A., & Führs, H. (2013). Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant and Soil, 368, 5–21.
Heal, K. V. (2000). Manganese and land-use in upland catchments in Scotland. Science of the Total Environment, 265, 169–179.
Heenan, D., & Campbell, L. (1981). Influence of potassium and manganese on growth and uptake of magnesium by soybeans (Glycine max (L.) Merr. Cv. Bragg). Plant and Soil, 61, 447–456.
Heinrichs, H., & Mayer, R. (1980). The role of forest vegetation in the biogeochemical cycle of heavy metals. Journal of Environmental Quality, 9, 111–118.
Herndon, E., Yarger, B., Frederick, H., & Singer, D. (2019). Iron and manganese biochemistry in forested coal mine spoil. Soil Systems, 3, 13. https://doi.org/10.3390/soilsystems3010013
Hrdlička P., Kula E. (1998). Element content in leaves of birch (Betula verrucosa Ehrh.) in an air polluted area. Trees, 13 (2), 68–73.
Hrdlička, P., & Kula, E. (2004). Changes in the chemical content of birch (Betula pendula Roth) leaves in the air polluted Krušné hory mountains. Trees, 18, 237–244.
Hrdlička, P., & Kula, E. (2010). Changes in element content of birch leaves (Betula pendula Roth) in polluted air. Polish J. of Environ. Stud., 20(3), 661–667.
Joslin, J. D., Kelly, J. M., & Van Mlegroet, H. (1992). Soil chemistry and nutrition of North American spruce-fir stands: Evidence for recent change. Journal of Environmental Quality, 21, 1250.
Juice, S. M., Fahey, T. J., Siccama, T. G., Driscoll, C. T., Denny, E. G., & Eagar, C. (2006). Response of sugar maple to calcium addition to Northern Hardwood Forest. Ecology, 87, 1267–1280.
Kabata-Pendias, A. (2011). Trace elements in soil and plants (Fourth ed.). Boca Raton: CRC Press 520 pp.
Kandziora-Ciupa, M., Ciepał, R., Nadgórska-Socha, A., et al. (2013). A comparative study of heavy metal accumulation and antioxidant responses in Vaccinium myrtillus L. leaves in polluted and non-polluted areas. Environmental Science and Pollution Research, 20, 4920–4932.
Kandziora-Ciupa, M., Nadgórska-Socha, A., Barczyk, G., et al. (2017). Bioaccumulation of heavy metals and ecophysiological responses to heavy metal stress in selected populations of Vaccinium myrtillus L. and Vaccinium vitis-idaea L. Ecotoxicology, 26, 966–980.
Kim, H. T., & Kim, J. G. (2018). Seasonal variations of metal (Cd, Pb, Mn, Cu, Zn,) accumulation in a voluntary species, Salix subfragilis, in unpolluted wetlands. Science of the Total Environment, 610–611, 1210–1221. https://doi.org/10.1016/j.scitotenv.2017.08.137
Kitao, M., Lei, T. L., & Koike, T. (1997). Effects of manganese toxicity on photosynthesis of white birch (Betula platyphylla var. japonica) seedlings. Physiologia Plantarum, 101, 249–256.
Kogelmann, W. J., & Sharpe, W. E. (2006). Soil acidity and manganese in declining and non-declining sugar maple stands in Pennsylvania. Journal of Environmental Quality, 35, 433–441.
Kochenderfer, J. N., Helvey, J. D. (1989). Hydrologic impacts of mechanized site preparation in the central Appalachians. In: Kochendetfer, J. N., Crews, J. T., Smith, H. C. Effects of fertilization on the growth and development of a Japanese larch plantation in West Virginia: The 7th central hardwood forest conference: 1995 March 5–8; Carbondale, IL. Res. Pap. NE- 700. U. S. Department of Agriculture, Forest Service.
Kochian, L. V., Hoekenga, O. A., & Pineros, M. A. (2004). How do crop plants tolerate acid soils? Mechanisms of aluminium tolerance and phosphorus efficiency. Annual Review of Plant Biology, 55, 459–493.
Kozanecka, T., Chojnicki, J., & Kwasowski, W. (2002). Content of heavy metals in plant from pollution-free regions. Polish Journal of Environmental Studies, 11(4), 395-400.
Kula, E., Hrdlička, P., Hedbávný, J., & Švec, P. (2012). Various content of manganese in selected forest tree species and plants in the undergrowth. Beskydy, 5(1), 19–26.
Kula, E., Martinek, P., Chromcová, L., & Hedbávný, J. (2014). Development of gypsy moth (Lymantria dispar L.) affected by manganese in food. Environmental Science and Pollution Research, 21, 11987–11997.
Kula, E., Wildová, E., Hrdlička, P. (2018). Accumulation and dynamics of manganese content in bilberry (Vaccinium myrtillus L.). Environmental Monitoring and Assessment, 190–224. https://doi.org/10.1007/s10661-018-6604-8
Laboratory-Morava. (2010). Standardized internal laboratory methods for chemical analyses soil and plant (p. 30). Studénka: Laboratory Morava, Inc., accredited laboratory.
Lambers, H., et al. (2015). Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends in Plant Science, 20, 83–90.
Lambers, H., Oliveira, R. S. (2019). Plant physiological ecology, 3rd ed. Springer, Cham. https://doi.org/10.1007/97830-302-9639-1
Lambers, H., Wright, I. J., Guilherme Pereira, C., et al. (2021). Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant and Soil, 461, 43–62. https://doi.org/10.1007/s11104-020-04690-2
Landre, A. L., & Watmough, S. A. (2010). Metal pools, fluxes, and budgets in an acidified forested catchment on the precambrian shield, Central Ontario, Canada. Water, Air, and Soil Pollution, 209, 209–228.
Lavres Junior, J., Reis, A. R., Rossi, M. L., Cabral, C. P., Nogueira, N. L., & Malavolta, E. (2010). Changes in the ultrastructure of soybean cultivars in response to manganese supply in solution culture. Science in Agriculture, 67, 287–294.
Lei, Y., Korpelainen, H., & Li, C. (2007). Physiological and biochemical responses to high Mn concentrations in two contrasting Populus cathayana populations. Chemosphere, 65, 686–694.
Lu, X. K., Mo, J. M., Gilliam, F. S., Zhou, G. Y., & Fang, Y. T. (2010). Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Global Change Biology, 16(10), 2688–2700.
Marschner, H. (1995). Mineral nutrition of higher plants (2nd ed.). Academic Press.
Martinek, P., Kula, E., & Hedbavný, J. (2017). Reaction of leaf weevil Phyllobius arborator (Coleoptera, Curculionidae) to manganese content in diet Environmental. Environmental Entomology, 46(1), 131–136.
Martinek, P., Kula, E., & Hedbavný, J. (2018). Reaction of Melolontha hippocastani adults to high manganese content in food. Ecotoxicology and Environmental Safety, 148, 37–43.
Martínek, P., Hedvábný, J., Kudláček, T., Šťasta, M., & Kula, E. (2020). Adverse responses on Cabera pusaria caterpillars to high dietary manganese concentration. Entomol. Exp. Et Appl., 168, 635–643.
McMeans, B. C., McCann, K. S., Humphries, M., Rooney, N., & Fisk, A. T. (2015). Food web structure in temporally-forced ecosystems. Trends in Ecology & Evolution, 30(October), 662–672.
Mengel, K., & Kirkby, E. A. (2001). Principles of plant nutrition (5th ed.). Kluwer Academic Publishers.
Migeon, A., Richaud, P., Guinet, F., Chalot, M., & Blaudez, D. (2009). Metal accumulation by woody species on contaminated sites in the north of France. Water, Air, and Soil Pollution, 204, 89–101.
Mróz, L., Demczuk, M. (2010). Contents of phenolics and chemical elements in bilberry (Vaccinium myrtillus L.) leaves from copper smelter area. Polish Journal of Ecology, 58(3), 475–486.
Navrátil, T., Shanley, J. B., Skřivan, P., Krám, P., Mihaljevič, M., & Drahota, P. (2007). Manganese biogeochemistry in a central Czech Republic catchment. Water, Air, and Soil Pollution, 186, 149–165.
Ohki, K. (1984). Manganese deficiency and toxicity effects on growth, development, and nutrient composition in wheat. Agronomy Journal, 76, 213–218.
Oliva, M., José Vicente, J., & Gravato, C., Guilhermino, L., Dolores Galindo- Riaño, M. (2012). Oxidative stress biomarkers in Senegal sole, Solea senegalensis, to assess the impact of heavy metal pollution in a Huelva estuary (SW Spain): Seasonal and spatial variation. Ecotoxicology and Environmental Safety, 75(1), 151–162.
Pickens, C. J., Sharpe, W. E., & Edwards, P. J. (1995). The effects of doubling annual N and S deposition on foliage and soil chemistry and growth of Japanese larch (Larix leptolepis Sieb. and Zucc.) in north central West Virginia. In: Gottschalk, Kurt W.; Fosbroke, Sandra LC, ed. Proceedings, 10th Central Hardwood Forest Conference; 1995 March 5-8; Morgantown, WV.: Gen. Tech. Rep. NE-197. Radnor, PA: US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. 131-140(Vol. 197).
Power, M. E., Parker, M. S., & Dietrich, W. E. (2008). Seasonal reassembly of a river food web: Floods, droughts, and impacts of fish. Ecological Monographs, 78(2), 263–282.
Reeves, R. D. (2006). Hyperaccumulation of trace elements by plants. In J.-L. Morel, G. Echevarria, & N. Goncharova (Eds.), Phytoremediation of metal-contaminated soils. NATO Science, 68, 25–52.
Reeves, R. D., & Baker, A. J. M. (2000). Metal – Accumulating plants. In I. Raskin & B. D. Ensley (Eds.), Phytoremediation of toxic metals: Using plants to clean-up the environment (pp. 193–230). John Wiley and Sons.
Reimann, C., Fabian, K., Flem, B., Andersson, M., Filzmoser, P., & Englmaier, P. (2018). Geosphere-biosphere circulation of chemical elements in soil and plant systems from a 100 km transect from southern central Norway. Science of the Total Environment, 639, 129–145.
Reimann, C., Koller, F., Frengstad, B., Kashulina, G., Niskavaara, H., & Englmaier, P. (2001). Comparison of the element composition in several plant species and their substrate from a 1500000-km2 area in Northern Europe. Science of the Total Environment, 278, 87–112.
Reimann, C., Arnoldussen, A., Finne, T., Koller, R., & Englmaier, P. (2007a). Element contents in leaves of four plant species (birch, mountain ash, fern and spruce) along anthropogenic and geogenic concentration gradients. Science of the Total Environment, 377, 416–433.
Reimann, C., Arnoldussen, A., Englmaier, P., Filzmoser, P., Finne, T. E., Garret, R. G., et al. (2007). Element concentrations and variations along 120-km transect in southern Norway – Anthropogenic vs. geogenic vs. biogenic element sources and cycles. Applied Geochemistry, 22, 851–871.
Salemaa, M., Derome, J., Helmisaari, H. S., Nieminen, T., & Vanha-Majamaa, I. (2004). Element accumulation in boreal bryophytes, lichens and vascular plants exposed to heavy metal and sulphur deposition in Finland. Science of the Total Environment, 324, 141–160.
Sanderson, K., Jordan, C., & Fillmore, S. (2008). Leaf nutrient ranges for wild blueberries in Prince Edward Island. International Journal of Fruit Science, 8, 63–68.
Sembratowicz, I., Rusinek, E., Ognik, K., Truchlinsky, J. (2009). Concentrations of trace elements and heavy metals at selected medicinal plants harvested in two vegetation periods. Department of Biochemistry and Toxicology. University of Life Sciences, 55 (1), 22–28.
Schlegel, H., Amundson, R. G., & Huttermann, A. (1992). Element distribution in red spruce (Pica rubens) fine roots; evidence for aluminium toxicity at Whiteface Mountain. Canadian Journal of Forest Research, 22, 1132–1138.
Schweitzer, C. J., Sharpe, W. E., Edwards, P. J. (1999). The effect of soil manganese on Japanese Larch (Larix leptolepis Sieb. And Zucc.) Seedlings in the greenhouse. In: Stringer, Jeffrey W.; Loftis, David L., eds. 1999. Proceedings, 12th central hardwood forest conference; 1999 February 28-March 1–2; Lexington, KY. Gen. Tech. Rep. SRS-24. Asheville, NC: U. Department of Agriculture, Forest Service, Southern Research Station.
Skřivan, P., Navrátil, T., Vach, M., Sequens, J., Kurian, M., & Kvidova, O. (2002). Biochemical cycles of metals in the environment: Factors controlling their content in the tissues of selected forest tree species. Scientia Agriculturae Bohemica, 2, 71–78.
Sobíšek, B., Munzar, J., Krška, K., et al. (1993). Meteorological Dictionary Interpretation & Terminology. Academia.
Taylor, L. L., Leake, J. R., Quirk, J., Hardy, K., Banwart, S. A., & Beerling, D. J. (2009). Biological weathering and the long-term carbon cycle: Integrating mycorrhizal evolution and function into the current paradigm. Geobiology, 7, 171–191.
Terry, N., Evans, P. S., & Thomas, D. E. (1975). Manganese toxic effects on leaf cell multiplication and expansion and on dry matter yield of sugar beets. Crop Science, 15, 205–208.
Thornton, F. C., Schaedle, M., & Raynal, D. J. (1989). Tolerance of red oak and American and European beech seedlings to aluminium. Journal of Environmental Quality, 18, 541–545.
Verbruggen, N., & Herma ns, C., Schat, H. . (2009). Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist, 181, 759–776.
Viers, J., Prokushkin, A. S., Pokrovsky, O. S., et al. (2013). Seasonal and spatial variability of elemental concentrations in boreal forest larch foliage of Central Siberia on continuous permafrost. Biogeochemistry, 113, 435–449. https://doi.org/10.1007/s10533-012-9770-8
Vitória, A. P., da Silva Santos, J. L., Barros Salomao, M. S. M., de Oliveira Vieira, T., Da Cunha, M., Pireda, S. F., & Rabelo, G. R. (2015). Influence of ecologic type, seasonality, and origin of macrophyte in metal accumulation, anatomy and ecophysiology of Eichhornia crassipes and Eichhornia azurea. Aquatic Botany, 125, 9–16.
White, E. R. (2020). Seasonality in ecology: Progress and prospects in theory. Ecological Complexity, 44, 100867.
White, E. R. (2019). Minimum time required to detect population trends: The need for long-term monitoring programs. BioScience, 69(1), 40–46.
Wislocka, M., Krawczyk, J., Klink, A., & Morrison, L. (2006). Bioaccumulation of heavy metals by selected plants species from uranium mining dumps in the society MTS Poland. Polish Journal of Environmental Studies, 15(5), 811–818.
Wissemeier, A. H., & Horst, W. J. (1992). Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata L. Walp.). Plant and Soil, 143, 299–309.
Wu, S. (1994). Effect of manganese excess on the soybean plant cultivated under various growth conditions. Journal of Plant Nutrition, 17, 993–1003.
Acknowledgements
The authors thank T. Matys Grygar (Faculty of Environment, J. E. Purkyně University in Ústí nad Labem, and Institute of Inorganic Chemistry CAS, Řež) for discussions and help with manuscript preparation. The authors also thank a student Gabriela Bilkova for her help within filedworks and samples preparation.
Funding
The presented paper was supported by grant UJEP-SGS-2020–44-005–3 provided as part of a student grant competition at Jan Evangelista Purkyně University in Ústí nad Labem (Czech Republic) and by grant UJEP-IGA-TC-2019–44-02–2 provided by the Internal Grant Agency of the same institution. The authors acknowledge the assistance provided by the Research Infrastructure NanoEnviCz (project No. LM2015073) and by project INVUST (CZ.02.1.01/0.0/0.0/16_017/0002678), supported by the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to the conception and design of the manuscript, acquisition of data, analysis and interpretation of the data, revisions, and final approval of the version to be submitted for publication.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Bilberry, larch, and birch are Mn-accumulating species.
• Seasonality is essential for determining element intake in plants.
• Environmental monitoring should include several samplings per season.
• Leaves on Mn-tolerant species accumulate more Mn under acidic soil conditions.
• Precipitation affects manganese uptake by plants.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wildová, E., Elznicová, J. & Kula, E. Seasonal dynamics of manganese accumulation in European larch (Larix decidua Mill.), silver birch (Betula pendula Roth), and bilberry (Vaccinium myrtillus L.) over 10 years of monitoring. Environ Monit Assess 193, 612 (2021). https://doi.org/10.1007/s10661-021-09415-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09415-1