Abstract
The low ionic concentration meltwaters of the rivers originating from the Himalayan glaciers play a significant role in diluting the high solute load emanating from Ganga plain catchments. Hence, any change in the qualitative and quantitative characteristics of the Himalayan tributaries of River Ganga under the changing climatic scenario will impact the hydrochemical parameters of River Ganga as well. Hydrochemical investigations have been carried out in the River Alaknanda, a tributary of River Ganga during the period September 2016–May 2018 and revealed that TSS and COD values were observed above the prescribed criteria limit of 10 mg/L for drinking purpose for river as prescribed by CPCB. The anions for all sampling sites and seasons were observed to be in decreasing order of HCO3− > SO42− > Cl− > NO3− and cations Ca2+ > Mg2+ > Na+ > K+. The weathering of rock forming minerals of drainage basin is responsible for the chemical composition of river water. HCO3− being the dominant anion in the study area accounts for its presence due to carbonate and silicate weathering. Ion exchange process controls the major ion chemistry of the river water. The assessment and management of non-point sources (NPS) pollution are difficult by any deterministic method and require a vast amount of data to compensate for their extent of contamination, in the account of their prevailing nature in response to hydrological processes and land use patterns. In the present investigation, the application of a simple chemical mass balance approach based on law of conservation of mass/matter has been applied on River Alaknanda, a tributary of River Ganga for measuring the chemical mass loadings of some selected water quality constituents, viz., major cations (sodium, potassium, calcium, magnesium, and ammonium) and major anions (chloride, sulfate, nitrate, and phosphate) at upstream and downstream of different point source locations for examining the contribution made by non-point sources of pollution to the river. Time series analysis of various ion concentrations at point source sites and upstream/downstream sites inferred that the fluvial variations pertaining to ion concentration and flux are strongly dependent on the seasonal changes. More contribution (> 30–50%) for almost all constituents from uncharacterized sources was observed in the months of November to February, which may be attributed to intensified agricultural activities during the winter months particularly cereals and vegetables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The obtainability of freshwater resources is the root of socio-economic progress of a nation. Society is nevertheless dependent upon the river water for sustaining their immeasurable requirements, and thus, it becomes obligatory to uphold the quality of water within the prescribed limits to make sure an unremitting reserve of freshwater (Srinivas, 2019). The capricious nature of river water quality is inevitable of both spatial and temporal distribution (Seth et al., 2016). Various factors like atmospheric inputs and anthropogenic activities play a major role in governing the water chemistry and water quality of streams. Various physical and chemical parameters (dissolved oxygen, pH, river flow, turbidity) are accessible owing to the in situ probes. Whereas for the chemical concentration data, one has to rely on laboratory tests and the meltwater samples typically on weekly or monthly basis moreover even for post-monsoon or pre-monsoon (daily or storm data) for a predefined period (Bowes et al., 2015).
Rock weathering is the dominant factor in the overall hydrochemical characteristics. The major ion chemistry of river water is governed by weathering process in the drainage basin, minor contributions from cyclic sea salt, atmospheric provision (from terrestrial, marine, and anthropogenic sources) of chemical constituents and pollution. The relative concentration of cations and anions released in the rivers is contingent to the nature of parent rock and the proton source (Sarin et al., 1992; Singh & Hasnain, 1998).
Most of the studies in the Indian Himalaya were carried out either at the glacier portals or at the outlet of the tributaries. Ganga–Brahmaputra system accounts for ~ 3% of the total dissolved salts discharged into the world oceans via rivers, similar in magnitude to their contribution to the global water discharge (Sarin et al., 1989). The possible link between Himalayan uplift and Cenozoic climate change has resulted in natural weathering and continual geochemical processes (Chakrapani et al., 2009; Singh & Hasnain, 1998). In a glacial system, the discharge variation also has a direct implication on the sediment dynamics characteristics. This sediment dynamics characteristic is also associated with the development and progression of the subglacial zone as this zone is the main contributor of glacial sediments (Thayyen et al., 1999). The sediment outflow from the Gangotri glacier system plays an important role in solute acquisition during sediment–meltwater interaction and thereby in controlling the hydrochemical behavior of meltwater of the Gangotri glacier (Sharma et al., 2020).
Rivers across the world are extensively receiving pollution due to population growth, unplanned urbanization, and industrialization over the past few years through numerous point and non-point sources. The pollution from municipal garbage (from metropolitan or densely inhabited areas) and industrial wastewater loads (from a variety of industries) are easily identified and account for the point source (PS) pollution (Adu & Kumarasamy, 2018; Wu & Chen, 2013). Various pollutants such as underground leaching, atmospheric deposition, soil erosion, farmland drainage, surface runoff, and other approaches are responsible for causing soil, water, and air pollution and are referred to as non-point source (NPS) pollution. The aforementioned pollutants comprise of soil sediment particles, pesticides (phosphorus, nitrogen, and hazardous substances), drugs from aquaculture, solid waste (e.g., agricultural film, straw), waste from atmospheric particulates and/or rural areas, and fecal sewage of livestock and poultry (Brown & Froemke, 2012). The budding environmental issues for instance habitat destruction, reduction in biodiversity, and eutrophication are the consequences of NPS pollution. Wu et al. (2012) emphasized the two types of non-point source pollution, viz., agricultural/rural NPS and urban NPS particularly for water pollution.
As reported by Carpenter et al. (1998), water quality of many rivers, lakes, and coastal oceans has degraded, attributable to an increment of pollutant inputs into the rivers. Point sources can often be controlled by treatment at the source and are somewhat simple to measure and regulate. Non-point pollutants are comparatively less continuous, more intermittent and associated with recurrent agronomy or unbalanced processes, such as heavy rainfall or vital construction. Non-point inputs enter into the river system by various genres such as overland, underground, or through the atmosphere. Subsequently, non-point sources are difficult to measure and regulate. In order to control the non-point pollution, stress should be given on control of emission of pollutants to the environment, land management traditions, and changing the routine activities of mass population.
At present, non-point sources are accountable for beyond 50% of the problems relating to water quality (Jain et al., 2007). The enforcement of strict policies and superior engineering practices has notably reduced the point source pollution. Therefore, efforts have basically shifted to non-point source pollution, which comprise identifying priority management areas (PMAs) or critical source areas (CSAs) for an impeding watercourse. It is because of the non-point, mixed, and distributed nature of NPS pollution, PMAs are those discriminatory sections of watersheds producing great amount of NPS pollutants. The pollutant flux of river is dependent on chemical, physical and biological progressions within the complete upstream network and not only on the confined pollutant inputs. The futuristic management of NPS incorporates the reinforcement of multiple functional zones into PMAs (Shen et al., 2015). The identification of CSAs is still a challenging task for the hydrologists. Nowadays, numerous methods for its identification are available which includes both simple index-based methods as well as application of complex hydrological and water quality (HQW) models (Rudra et al., 2020). Yadav and Pandey (2017) studied the contribution of point sources and non-point sources to nutrient and carbon loads and their influence on the trophic status of the River Ganga at Varanasi, India, and reported the large differences in point and non-point sources of carbon and nutrient input into the River Ganga, although these variations were strongly influenced by the seasonality in surface runoff and river discharge.
Modelling non-point pollution is of utmost importance and declared as a foremost challenge for the researchers and decision makers regardless of the fact that influence of point and non-point sources of pollution individually is uncompromising. The assessment and management of NPS pollutants is difficult by any deterministic method and requires vast amount of data to compensate their extent of contamination, in account of their prevailing nature in response to hydrological processes and land use patterns. The wide-ranging inorganic farming practices and excessive irrigation is responsible for majority of NPS pollution across many river basins of the world. The non-point sources should be evaluated on the basis of spatial and temporal changes and furthermore it is necessary to prototype the pathway/direction of runoff (Srinivas, 2019). To assess the non-point source pollution, various water quality models have been developed and widely used to determine the rate at which rivers disperse pollutants and to simplify the complicated natural processes of generation and relocation of NPS pollutants (Adu & Kumarasamy, 2018). Generally, in literature two ways have been mentioned to assess NPS pollution: traditional models and mechanistic models (Liu et al., 2015). Studies on assessment of non-point source pollution have prominently flourished in literature during the recent years through various NPS models such as Integrated Watershed Management Model (IWMM) and Water Quality Analysis Simulation Program (WASP) model (Lai et al., 2011), Soil and Water Assessment Tool (SWAT) (Shen et al., 2008; Wu & Chen, 2013; Zhai et al., 2012), WATFLOOD (Leon et al., 2001), load apportionment model (LAM) (Chen et al., 2015), nutrient balance model (Wang et al., 2019), and semi-distributed land use-based runoff process model (SLURP) (Chen et al., 2013) which require time series of water quality data along with all sorts of data, such as land use, population, soil, pesticide, livestock breeding, and fertilizer use for such modeling. These models may be based on any of the following techniques—quadratic programming, linear programming, nonlinear programming, stochastic programming, or dynamic programming (Archibald & Marshall, 2018). The hydrologic community throughout the world suffers from the issue of model complexity and the concerns become more adamant when the subject shifted to the models of NPS pollution. Although the reliability of these models is in no doubt yet rigorous efforts need to be devoted in developing sensitivity analysis, uncertainty analysis and optimization (Rudra et al., 2020). Table 1 discusses the work of different researchers on various NPS models with their merits and demerits.
Further, to study the sedimentary processes and the reactions occurring at water course, the simple and effortless chemical mass balance approach has also been widely used and reported in literature during the past years (Berndtsson, 1990; Ismail et al., 2005; Jain, 1996, 2000; Jain et al., 1998, 2007; Kelley & Nater, 2000; Mosley et al., 2012). The present study involves the application of a simple chemical mass balance approach drew on law of conservation of mass/matter on River Alaknanda, a tributary of River Ganga for measuring the chemical mass loadings of some selected water quality constituents, viz., major cations (sodium, potassium, calcium, magnesium, and ammonium) and major anions (chloride, sulfate, nitrate, and phosphate) at upstream and downstream locations and point source locations for examining the contribution made by non-point sources of pollution to the river. The study also features the hydrochemical characteristics of River Alaknanda and its tributaries, Pinder and Mandakini, thus developing a baseline data on its status.
Materials and methods
Study area
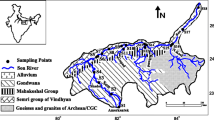
The study is focused on the mighty River Alaknanda, a major tributary of River Ganga. The Satopanth twin glaciers and the Bhagirath Kharak mark the origin of the River Alaknanda at an elevation of 3800 m. The total catchment area of the river is 10,237 km2. The River Alaknanda is joined by the Saraswati, Dhauliganga, Garunganga, Patalganga, Birehiganga, Nandakini, and Pindar streams on the left bank and Mandakini on the right bank. The river traverses for a distance of 240 km from its source to Devprayag. The river flows through narrow and deep gorges in the upper course and then enters at an elevation of 900 m in its lower course, 25 km downstream of Joshimath (Fig. 1). The River Alaknanda basin experiences strong monthly variations in the stream flows and is gifted with both sedimentary and highly metamorphosed gneissic rocks. Among the major tributaries of Alaknanda, Pinder, and Mandakini rivers in the study area are considered as a source of point source pollution. Pinder river originates from the Pindari Glacier which is located in Bageshwar district of Kumaon region in Uttarakhand and is situated at an altitude of 3820 m. Pinder river mouth is located at Karanprayag where it ends by its confluence with River Alaknanda. The river flows from east to west having a total length of 124 km and drainage area is 1688 km2. River Mandakini originates near Kedarnath in Uttarakhand from the Chorabari Glacier and joins Alaknanda at Rudraprayag. It runs for a length of 80 km with an average slope of 42 m per km up to the confluence at Rudraprayag.
Water sample collection and analysis
Water samples from six locations along River Alaknanda [Joshimath, Karanprayag (U/S), Karanprayag (D/S), Rudraprayag (U/S), Rudraprayag (D/S), and Devprayag] and its tributaries (Pinder and Mandakini) were collected on monthly basis over a time span of 2 years (September 2016–May 2018) by dip/grab sampling method using a standard water sampler (Hydro Bios, Germany). To obtain a homogeneous sample for laboratory analysis, water samples from the two tributaries (Pinder and Mandakini) and upstream/downstream sites of the river were collected from three different points via one third, one half, and two thirds across the width of the river. The approximated chosen depth from the surface level of the river to collect the river water samples was 0.15 m. This was done to avoid any contribution from the ineffectual floating material which could hamper the homogeneity of the sample and provide encumbrance in analysis. Polyethylene bottles were pre-cleaned at the laboratory level for the storage of river water samples.
The various water quality parameters monitored include pH, electrical conductivity (EC), total suspended solids (TSS), alkalinity, hardness, major cations (sodium, potassium, calcium, magnesium), major anions (bicarbonate, chloride, sulfate, nitrate), minor ions (fluoride, phosphate, ammonium), and demand parameters (DO, BOD, COD). Water samples were preserved using appropriate reagents (conc. H2SO4 for COD analysis and alkaline MnSO4 and alkali azide for determination of DO). Water samples were filtered using 0.45 µm membrane Whatman filter paper, and filtered river water samples were brought to the laboratory for analysis and kept in sampling kits whose temperature was sustained at 4 °C. Ion Chromatograph with Auto Titrator (Metrohm, Switzerland) was used to perform hydrochemical analysis of filtered river water samples collected from River Alaknanda. A portable conductivity meter and pH meter were used on field to measure EC and pH, respectively. Details of the analysis are given in Sharma et al. (2019) and maintained analytical precision for all the analytes (anions and cations) < 5% and accuracy < 5%.
Chemical mass balance
The following equation demonstrates the indirect measurements of the sum of sources in receiving water and can be pertained to acquire information on the contributions made from individual sources:
where QD and QU represent flows at the downstream and upstream sites, CD and CU represent the concentrations of ions in river at downstream and upstream sites, and \(\sum \mathrm{Li}\) (i = 1,…,n) is the consecutive individual loading to the river, neglecting losses and/or generation within the river system.
The probability of occurrence of inaccuracy is very high while calculating the total contribution made by the point and non-point sources of pollution by adding the individual loadings. Such calculations involving large amount of data is always accompanied by analytical and/or systematic errors. The simple equation above mentioned is a mass budget and can be utilized to devise a hypothesis that will aim at noticeably more accurate estimation of \(\sum Li\).
The chemical mass balance modelling procedure requires the following: (i) daily discharge data of river applicable over the period of sampling, (ii) identification of point sources of pollution, upstream and downstream sites throughout the river stream, and (iii) estimation of water quality data (of chemical species) or the nutrient data assessed in the laboratory.
The primary significance of water quality engineering come about in ascertaining mass loading of input, namely, the rate of total mass of a substance discharged into an explicit water body. For defined sources with incessant flow, the following equation gives the input load:
where C(t) represents the input concentration (Ml−3), Q(t) represents the input flow (l3 T−1), and L(t) represents the mass rate (load) of input [MT−1 (kilogram per second)], all quantities occurring concurrently at time t.
A variety of NPS models have been accomplished which requires vast amount of data to estimate loading from non-point sources. Such models are however not suitable for Indian conditions due to restrictions in obtainability of data and thus the alternate indirect approach using upstream/downstream river constituents’ data assist to characterize the non-point sources. The chemical mass balance approach is similar to tracer approach in which a particular type of nutrient is identified and its contribution is quantified (Vega et al., 2000). This approach used by many professionals is a quantitative approach in which evaluation of input load, output load and uncharacterized/retention load of chemical constituents in the river is accomplished (Silveira et al., 2011). The analysis of large quantity of effluent and water samples demands for an immense utilization of resources and monetary inputs. This approach has an added advantage over such concerns. Another application of this approach heads toward the determination of chemical load to the rivers (Berndtsson, 1990; Bukaveckas et al., 2005; Dolan & El-Shaarawi, 1989; Jain, 1996; Jain et al., 2007).
Results
Hydrochemical characteristics of River Alaknanda
The water quality, hydrochemical characteristics, and their governing factors provide significant information for water resource management in any aquatic system. Water samples collected from the study area were analyzed for hydro-chemical parameters. The hydro-chemical data of River Alaknanda and tributaries Pinder and Mandakini is presented in Table 2. Longitudinal variations of different water quality constituents for River Alaknanda are given in Fig. 2.
The pH of River Alaknanda ranged from 6.8 to 8.3 with minimum value observed at Devprayag while the maximum value was reported at Joshimath and Karanprayag (U/S). Alaknanda showed a minimum value 62.6 µS/cm for EC at Rudraprayag (U/S) while the maximum value 245 µS/cm was observed at Joshimath, which may be attributed to tourist activity in the area. TDS and TSS showed remarkable temporal fluctuations. TDS of the river water varied from 40.06 to 156.8 mg/L while TSS varied from 0.08 to 1954 mg/L. The alkalinity in natural streams is generally due to the presence of carbonates, bicarbonates and hydroxides. The alkalinity of River Alaknanda varied from 26.5 to 83.6 mg/L, and maximum value was observed at Devprayag. Total hardness of river water varied from 9.6 to 110.7 mg/L. A sudden change in hardness was observed at Rudraprayag (U/S) in monsoon and winter season during the study period.
The dissolved oxygen (DO) concentration is the primary indicator for the quality of river water while both biochemical oxygen demand (BOD) and chemical oxygen demand (COD) are studied to determine the degree of pollution in rivers. The DO levels at all sites of River Alaknanda were above the minimum criteria limit of 4 mg/L for river for drinking purpose prescribed by CPCB and ranged from 8.0 to 12.9 mg/L. It was implied from the data that DO generally shoots up during winter season and recedes in summer and monsoon. Maximum DO was observed at Devprayag in June 2017. In this study, the BOD and COD range was observed between 0.3 to 3.4 mg/L and 2.08 to 41.1 mg/L, respectively. Both these parameters showed abnormalities during the study period at almost all sites having BOD less than the maximum criteria limit of 3 mg/L for the river for drinking purpose prescribed by CPCB and COD higher than maximum criteria limit of 10 mg/L for the river for drinking purpose prescribed by CPCB. Minimum concentration of BOD was noticed in winter season during the study period, and maximum BOD concentration was observed at Joshimath and Rudraprayag (D/S) in the month of July 2017 and March 2018, respectively, which may be attributed to tourist activity in the area. The maximum concentration of COD was observed at Rudraprayag (U/S) in the month of January 2017, i.e., in winter season and gradually decreases in summer followed by monsoon.
The concentration of major anions, viz., chloride, fluoride, sulfate, nitrate, and phosphate ranged from 0.115 to 6.33, 0.048 to 0.716, 3.7 to 41.2, 0.03 to 2.5, and 0.0001 to 1.63 mg/L, respectively. The anions for all sampling sites and seasons were observed to be in decreasing order of HCO3− > SO42− > Cl− > NO3−. Similar trend was reported by Singh et al. (2014) and Sharma et al. (2019). After HCO3−, SO42− is the next dominant anion in River Alaknanda. All anions SO42−, HCO3−, Cl−, and NO3− show decreasing concentrations in monsoon due to dilution. Similar trend was observed by Chakrapani (2005). Bicarbonate was the dominant anion on average accounting for 72.25% during the study period. On an average, SO42− accounted for 24.70%, followed by Cl− 1.95% and NO3− 1.09% of total anions. The concentration of major cations, viz., sodium, potassium, calcium, magnesium, and ammonium ranged from 0.8 to 7.4, 1.4 to 4.1, 9.8 to 31.32, 1.1 to 8.32, and 0.0 to 3.2 mg/L, respectively. The order of concentration of cations in the waters of River Alaknanda varied as Ca2+ > Mg2+ > Na+ > K+. A similar trend was also observed in the Ganga headwaters by Sarin et al. (1992) and Sharma et al. (2019). The major cations Na+, K+, Ca2+, and Mg2+ constitute more than 40% of the total dissolved solids (TDS). Among cations (Ca2+ + Mg2+) dominance accounted for 89.76% of total cations. Calcium was the dominant cation on average accounting for 65.03%, during the study period. On an average, Mg2+ accounted for 24.73% followed by Na+ 6.79% and K+ 3.44% of total cations.
Hydrochemical characteristics of tributaries Pinder and Mandakini
The longitudinal variations of water quality constituents for tributaries Pinder and Mandakini are given in Fig. 3. The water of the two tributaries was neutral to alkaline (7.4–8.3 and 6.8–8.0 for Pinder and Mandakini, respectively) in nature just like other Himalayan streams. EC varied from 95 to 225 μS/cm at Pinder and 65.3 to 169 μS/cm at Mandakini. The TDS of Pinder and Mandakini were in the range 60.5 to 144 mg/L and 41.8 to 108 mg/L, respectively. TSS values for both the tributaries displayed an extensive temporal variation. Poorly permeable soils and high erosion in Pinder leads to higher TSS concentration as compared to Mandakini. However, both tributaries have TSS values above the prescribed criteria limit of 10 mg/L for drinking purpose for river as prescribed by CPCB. The alkalinity of Pinder and Mandakini water ranged from 10 to 92 mg/L and 23.7 to 60.3 mg/L, respectively. Total hardness ranged from 36 to 105 mg/L in the water of tributary Pinder and 29.4 to 78.6 mg/L in the water of tributary Mandakini.
The DO levels of both tributaries were reportedly higher during winter season, followed by a gradual decrease to its lowest value during monsoon season. The concentration of DO ranged from 7.4 to 11.6 mg/L in the water of tributary Pinder and 7.8 to 11.5 mg/L in the water of tributary Mandakini. It was observed that for both Pinder and Mandakini, DO concentrations were above the minimum criteria limit of 5 mg/L for river for drinking purpose prescribed by CPCB. In this study, the BOD concentration at Pinder ranged from 0.5 to 3.6 mg/L, and at Mandakini, it ranged from 0.7 to 3.2 mg/L, while the COD concentration at Pinder varied from 2.1 to 27.4 mg/L, and at Mandakini, it was in the range of 2.1 to 32 mg/L. From our analysis, it was concluded that in both the tributaries the BOD concentrations were less than the maximum criteria limit of 3 mg/L for river for drinking purpose prescribed by CPCB while the COD concentrations were higher than maximum criteria limit of 10 mg/L for drinking purpose prescribed by CPCB.
The anions for all sampling seasons and sites were observed to be in decreasing order of HCO3− > SO42− > Cl− > NO3−. A similar trend was reported by Singh et al. (2014). Bicarbonate was the dominant anion on average accounting for 78.89% during the study period for the tributaries of River Alaknanda. On an average, SO42− accounted for 17.32% followed by chloride 2.46% and nitrate 1.32% of total anions. The order of concentration of cations varied as Ca2+ > Mg2+ > Na+ > K+. Among the cations, (Ca2+ + Mg2+) dominance accounted for 91.47% of total cations in tributaries of River Alaknanda. Calcium was the dominant cation on average accounting for 50.64%, during the study period for the tributaries of River Alaknanda. On an average, Mg2+ accounted for 40.79% followed by Na+ 5.54% and K+ 2.99% of total cations.
Hydrogeochemical characteristics of River Alaknanda
Geochemical conditions have a marked influence on the surface water quality. Hydrogeochemical studies explain the relationship of water chemistry to the aquifer lithology for water quality. Such relationship would help not only to explain the origin and distribution of dissolved constituents but also to illuminate the factors controlling the surface water chemistry. Gibbs (1970) proposed a speculation to illuminate the major natural mechanisms controlling the composition of dissolved salts of the world waters. Three mechanisms—atmospheric precipitation, rock dominance, and the evaporation–crystallization process—are the major factors controlling the composition of dissolved salts of the world waters. Other second-order factors, such as relief, vegetation and composition of material in the basin dictate only minor deviations within the zones dominated by the three prime factors. Gibbs plot is a diagrammatic representation of the mechanisms responsible for controlling the chemical composition of various water bodies on the surface of the earth. Almost all collected surface water samples from River Alaknanda fall in rock dominance zone (Fig. 4) suggesting precipitation induced chemical weathering along with dissolution of rock forming minerals. Few samples are away from this zone reflecting the contribution of anthropogenic activity responsible for chemical composition of surface water of the study area.
Chemical mass balance approach
The chemical mass balance approach mentioned above has been productively employed in the current work to evaluate the contribution built up by non-point sources of pollution to the River Alaknanda. The water quality parameters taken into consideration were sodium, potassium, calcium, magnesium, ammonium, chloride, sulfate, nitrate, and phosphate. Bicarbonate ion (HCO3−) having highest concentration in river water has not been considered for mass balance as its dynamics can be regulated by gaseous exchange (Bouchez et al., 2017). The important water quality characteristics (flow/discharge of river and ion concentration) of water samples collected monthly from upstream and downstream sites of River Alaknanda besides two tributaries, Pinder and Mandakini entering the river are given in Table 3.
Discussion
The aquatic life is substantially affected by a change in temperature. The temperature of river water plays an important role and induces changes in biodegradation rate, dissolved oxygen, solubility of salts, and other physicochemical parameters (Rao & Nageswararao, 2010), and thus, the water temperature was recorded on site. The spatial and temporal alterations in the levels of DO, BOD, and COD during the study period may also be attributed to the changing temperature of river water apart from the fundamental reason of anthropogenic activities. It has been seen in our study that DO concentrations depict seasonal alterations with maximum values reported in winter. High polluted water is an indication of low DO values and vice versa. The area under study has reported high concentrations in DO levels inferring a good status of the water quality. Moreover, in the time span of study, the maximum concentrations were reported in winter season pertaining to increased O2 retaining capacity of water (Khanna et al., 2011). COD is high at lower reaches of River Alaknanda, which is indicative of the fact that anthropogenic pollution is responsible for high concentration of COD. Moreover, occurrence of this result is also due to the effluent discharge from various small industrial installations located in the area.
The weathering of rock forming minerals of drainage basin is responsible for the chemical composition of glacial meltwater (Raiswell, 1984; Singh & Hasnain, 1998). Major ion chemistry of the Ganga source waters—the Bhagirathi, Alaknanda, and its tributaries—have been attempted to assess the chemical weathering processes and concluded the domination of the weathering of carbonate rocks by carbonic and sulfuric acids in the high altitude Himalaya (Sarin et al., 1992). Meltwater chemistry is found to be governed by the coupled reaction involving sulfide oxidation and carbonate dissolution in Ganga headwaters (Hasnain & Thayyen, 1999; Iqbal, 2001; Sharma et al., 2019; Singh & Hasnain, 2002; Singh et al., 2012, 2014; Stachnik et al., 2016). HCO3− being the dominant anion in the study area accounts for its presence due to carbonate and silicate weathering. The next position was attained by SO42− ion accounting to the phenomena of pyrite oxidation and together they accounted for > 95% of global river water composition. The two important reactions involving dissolution of atmospheric CO2 in water and the oxidation of sulfides generates H+ into the stream which chemically weather carbonates, silicates, and alumino-silicates in the drainage basin (Berner & Berner, 2012; Chakrapani et al., 2009; Mackenzie & Garrels, 1971; Raiswell, 1984; Singh & Hasnain, 1998). The sulfate present in the river water results mainly from the oxidation of pyrites or dissolution of gypsum. Chloride which is the next abundant ion in the study area has remarkably less contribution to the total anions present. The presence of Cl− affects the hardness of water (Khan et al., 2020) and the corresponding data of total hardness provides a justification to the lower concentrations of chloride. Both hardness and chloride concentrations were below the acceptable limit of 200 mg/L and 250 mg/L respectively for drinking purpose (BIS, 2012). The mainstream River Alaknanda shows low concentration of chloride ion and its input into the stream accounts for atmospheric deposition (Chakrapani et al., 2009; Singh & Hasnain, 1998). The other anions, F−, NO3−, and PO43− were present in very low concentration in the mainstream of Alaknanda and its tributaries. The effect of agricultural practices on the river bank inputs pesticide residues such as DDT, endosulfan, and dieldrin having the main constituent as NO3− (Jain, 2002; Semwal & Akolkar, 2006). The use of phosphate fertilizers is the main cause of phosphate ion in the streams. Other factors governing the entry of phosphate include sewage and domestic waste water discharge, breakdown of mineral rock, soil structure, and increased weathering (Jain, 2002; Kotnala et al., 2016). As the level of these ions is very low, it can be said that River Alaknanda is far away from anthropogenic activities and agricultural practices.
The presence of the major cation Ca2+ into the streams is mainly because of igneous rock minerals of which calcium is an essential constituent. Mg2+ can be derived from carbonate rocks containing calcite (CaCO3) and dolomite CaMg(CO3)2 and chiefly biotite, as well as dolomite (Chakrapani et al., 2009). The other major ions Na+ and K+ are released into the river water from aluminosilicates through silicate weathering (sodium feldspar and potassium feldspar). The existence of these minerals in the Alaknanda basin has been supported by Singh and Hasnain (1998).
The source of major ions in River Alaknanda may further be evaluated by scatter plots of different ions. The scatter plot of (Ca + Mg) v/s TZ+ shows that all the points fall above 1:1 equiline (Fig. 5). The relatively high contribution of (Ca + Mg) to the total cations (TZ+) and high (Ca + Mg)/(Na + K) ratio indicate that carbonate weathering of granites is a major source of dissolved ions in the surface water of the study area. Similar ratio was also reported in Gangotri glacier in Garhwal Himalaya (Kumar et al., 2009). The scatter plot of (Na + K) v/s TZ+ shows that all the points fall above 1:1 equiline with a low ratio indicating a relatively low contribution of dissolved ions from silicate weathering (Fig. 5). Na+, K+, and dissolved silica in the drainage basin are mainly derived from the weathering of silicate minerals, with clay minerals as by-products. These types of contribution from silicate weathering are also reported by other researchers (Iqbal, 2001).
The plot of (Ca + Mg) v/s HCO3− for most of the samples in the study area indicates an excess of Ca + Mg over HCO3− suggesting an extra source of Ca and Mg. This requires that a portion of the (Ca + Mg) has to be balanced by other anions like SO42− and/or Cl− (Paudyal et al., 2016). The plot of (Ca + Mg) v/s HCO3 + SO4 is a major indicator to identify the ion exchange process activated in the study area. Plot of (Ca + Mg) v/s HCO3 + SO4 shows that most of the plotted points clusters around the 1:1 equiline and some fall in HCO3 + SO4 indicating the ion exchange process which may be due to the excess of HCO3 + SO4 (Fig. 5). The scatter plots between Ca + Mg and HCO3 + SO4 show good correlation, wherein all points are on the equiline. This attributes that a portion of HCO3 + SO4 is balanced by cations derived from silicate rock weathering (Singh et al., 2014). The plot of Na+ v/s Cl− shows that Na+ values are much higher than Cl−. It indicates that most of the points lie below the 1:1 equiline reflecting contribution of silicate weathering through the release of Na+ (Fig. 5). Low ratio of Na+ to Cl− indicates low contribution from atmospheric precipitation and evaporates dissolution and negates possible impact of atmospheric pollution on the river water (Kumar et al., 2009).
Concentration–flow–flux relationship
The analysis on water quality time series affords a principle means of exploring the dynamics relating river ion source and is used extensively in hydrology. Chemical constituent concentration-flow relationships turned out to be successful in recent years to support the relative chemical contributions to the river from regular and inputs pertaining to rain (Bowes et al., 2015). From the relationship between ion concentrations and river flow at different sites, it can be accomplished that all the constituents exhibited a dilution of concentration with the increasing flow of the river (Fig. 6).
The chemical loading or flux (F in kg/day) from a watershed is given by discharge/flow (termed Q, m3/sec) times the solute concentration (referred as C, mg/L) (Bouchez et al., 2017).
This equation representing the relationship between the three factors, concentration, discharge and solute export also known as C-Q relationship has been emphasized for characterization in hydrology. Godsey et al. (2009) conducted their study on small basins in US and suggested best functional fit of C-Q relationship through power law:
where a and b represent the fit parameters. The power law relationship between C and Q mentioned above can be very well seen in C v/s Q plots. The exponent, b in the above equation has a physical interpretation. If the value of b is equal to 0, or a slope of zero would indicate that a river stream would behave chemostatically, i.e., there is a limited variability between concentration and discharge. On the other hand, a slope of − 1 indicates that solute concentrations vary inversely with the flow of river. Dilution process is the governing factor controlling concentrations (Godsey et al., 2009). This section demonstrates a brief description about concentration–discharge–flux relationship across River Alaknanda and its tributaries for a time period of one year (April 2017–March 2018) with minimal human impacts. From the relationship between ion concentrations and river flow at different sites the relative variability of concentration and discharge can be accomplished and further it can be inferred that all the constituents exhibited a dilution of concentration with the increasing flow of the river (Fig. 6). As seen from the figure, more or less the concentration of all the chemical constituents exhibited a best fit logarithmic relationship with the flow having a negative slope.
Climate impacts an influential effect on fluxes (Hooper et al., 2001). Quantifying fluxes precisely is important mainly from a mass- balance perception as it has major repercussions to ecological conditions of downstream receiving waters (Zhang et al., 2019). The daily discharge at upstream and downstream sites of River Alaknanda and two tributaries for predefined study period were studied against the fluxes of water quality constituents and ascertaining that there is a strong correlation between discharge and fluxes as seen from the plots given in Fig. 7. Flux is strongly controlled by stream discharge such that flux variations are as easy to characterize empirically as discharge variations. The r2 value of nearly all constituents depicts a perfect linear relationship between the two and justifies Eq. 3. It can be seen from the r2 values at upstream sites that Ca, K, and NO3 have values ≈ 1 which shows that solute export has a strong correlation with daily discharge. The ions having least correlation were NH4 and SO4. Similarly, for both Pinder and Mandakini, the best r2 values were depicted by SO4 (0.95 and 0.80, respectively) and K (0.97 and 0.99, respectively). For the downstream sites the best correlation was observed for Ca (0.99) and SO4 (0.80) while the least correlation was observed by NH4 (0.01) and Cl (0.09). It can be inferred from the above discussion that the decrease of concentration pertaining to increase in flow can be attributed to dilution phenomena; however, direct proportionality occurs with constituent fluxes.
Point source loadings
The daily discharge data was collected from Central Water Commission (CWC, New Delhi) for River Alaknanda, and the values ranged from 18.44 to 2230.66 m3/s over the time span of twelve months (April 2017 to March 2018) with highest value reported in monsoon season (July 2017) while the minimum value was in March 2018. The contribution of Pinder:Mandakini ratios were evaluated for different water quality constituents to compare the contributions made by point source sites. The ratio of loadings from these two sources (Pinder:Mandakini) for Na+, K+, Ca2+, Mg2+, Cl−, SO42−, NO3−, PO43−, and NH4+ were 19:8, 61:15, 565:101, 33:4, 13:5, 121: 28, 27:8, 0.298:0.124, and 1:0.3, respectively. The loadings of various constituents from two major input sites on the course of River Alaknanda, viz., Pinder and Mandakini during April 2017 to March 2018 that join the river system gives a major interpretation that the major contribution was observed for Pinder more specifically during the monsoon season for almost all constituents (Fig. 8). Although, Pinder is the tributary of Alaknanda, yet, it is larger contributor of flows at the confluence of Pinder with Alaknanda. Even, up to Devprayag, more than 50% contribution of flow of Alaknanda supplemented from Pinder stream.
Differential loadings
For evaluating the alterations in the load and/or differential concentration to the river in the monitored period, the upstream–downstream approach is incredibly beneficial by providing a comparison between upstream and downstream loadings with the differential loading (Dolan & El-Shaarawi, 1989). The difference between the total point source load to the river and consequent losses by virtue of volatilization, adsorption, sedimentation, degradation, settling, biological, chemical or physical phenomena, and evaporation account for the uncharacterized non-point contribution (Fig. 9). On evaluating the estimated differential loadings for the various water quality constituents and point source loadings of analogous constituent, it was found out that both the factors are comparable, and thus, it became obligatory to neglect the contributions from the point sources and their occurrence does not govern the uncharacterized non-point sources of pollution.
Chemical mass balance
The chemical mass balance approach is successfully utilized to examine the proposition that various factors (agricultural runoff, groundwater interactions, or sediment contributions) results in the attenuation of nutrient concentrations at downstream sites. This approach works on the simple mathematical mechanism of adding the contributions made by the upstream, tributaries, and point sources to get the net effect of contributions from uncharacterized sources by simple subtraction from the load data at the downstream sites. The input fluxes from tributaries and point sources and output fluxes are usually drawn from rigorous measurements of discharge and intermittent sampling in the selected locations (Fig. 10). Calculations from the mass balance revealed that contribution of sodium, potassium and nitrate from uncharacterized sources is minimum in July 2017 and maximum in February 2018 which corresponds to 3.80 and 62.70%, 0.00 and 56.11%, and 0.17 and 69.80%, respectively. Calcium contribution from uncharacterized sources is minimum in June 2017 (0.86%) and maximum in February 2018 (53.35%). Magnesium contribution from uncharacterized sources is minimum in April 2017 (1.40%) and maximum in December 2018 (53.05%). Ammonium contribution from uncharacterized sources is minimum in July 2017 (0.45%) and maximum in April 2017 (79.61%). Chloride contribution from uncharacterized sources is minimum in June 2017 (23.48%) and maximum in April 2017 (82.11%). Sulfate contribution from uncharacterized sources is minimum in July 2017 (2.32%) and maximum in December 2017 (67.72%). Phosphate contribution from uncharacterized sources is minimum in January 2018 (3.11%) and maximum in December 2017 (95.69%) (Table 4).
More contribution (> 30–50%) for almost all constituents from uncharacterized sources was observed in the months of November to February, which may be attributed to intense agricultural activities during the winter months particularly cereals and vegetables along with the runoff due to winter rains/snowmelt coming from the landscape. Knowing the concentration of water quality constituents at the upstream and downstream sites, mass balance approach can be applied to infer the uncharacterized inputs into the river system. The appearance of uncharacterized inputs as inferred from the study represents that there ought to be some small point sources of pollution which remains unmarked in the course of study. The additional inputs needed to balance the chemical mass approach are explained by the factors such as non-point sources of pollution due to agricultural practices, groundwater interference, remobilization of contaminated underneath sediments, or a blend of these resources.
The NPS contribution mainly from agricultural activities may be considered as N-P-K contribution. In our study area, average contribution of NO3, NH4, PO4, and K was observed to be 31.94%, 28.86%, 51.92%, and 20.64%, respectively, which may be attributed to the wash off from the agricultural activities on terrace farming in the hilly terrain. Although this contribution is quite low but significant from NPS pollution point of view and cannot be ignored while implementing any water quality management plan.
Conclusion
Rock weathering is the dominant factor in the overall hydrochemical characteristics. The major ion chemistry of river water is governed by weathering process in the drainage basin, minor contributions from cyclic sea salt, atmospheric provision (from terrestrial, marine, and anthropogenic sources) of chemical constituents and pollution. In the River Alaknanda, bicarbonate is the dominant anion followed by sulfate and calcium is the dominant cation followed by magnesium. Lack of resources and adequate data hinders the assessment and modelling of non-point sources of pollution and thus has a severe impact on the environment. Considering all such obstructions, simple chemical mass balance approach has been applied in this study for the Alaknanda River for estimating the non-point sources of pollution. The investigations on time series of discharge/flow at two tributaries, upstream and downstream sites indicated that there is a strong dependence of ion concentration with the seasonal changes. Another significant inference drawn from the study is that nutrient concentration varies inversely with the flow of the river and somewhat shows a logarithmic trend and flux increases with flow. The contributions of uncharacterized non-point sources of pollution possibly may be attributed from the variance in estimating point source loadings and differential loading due to underground water contribution, cultivation practices, sediment water interactions and some unnoticed point sources of pollution. This approach provides a supplementary benefit of considerable deduction in the expenditure in analysis of a large number of samples.
In the Upper Ganga Basin, there are very few gauge and discharge sites maintained by Central Water Commission, Ministry of Jal Shakti, Govt. of India. Other vast amount of historical time series data, viz., water quality data, hydrological data, soil type data, land use pattern data, is required for any deterministic modeling. There is a need to strengthen to data repository by establishing the monitoring stations at different locations in the basin to understand the complete hydrological processes in the low temperature environment.
Data availability
The analysis of major cations and anions was carried on Ion Chromatograph (Metrohm, Switzerland). Details of the analysis are given in Sharma et al. (2019) cited in the reference list and maintained analytical precision for all the analytes (anions and cations) < 5% and accuracy < 5%. The corresponding author confirms that he had full access to all the data used in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Discharge data used in the study was provided by Central Water Commission, Ministry of Jal Shakti, Govt. of India, New Delhi, and is thankfully acknowledged. The analytical data that support the findings of this study will be available from the corresponding author upon reasonable request.
References
Adu, J. T., & Kumarasamy, M. V. (2018). Assessing non-point source pollution models: A review. Polish Journal of Environmental Studies, 27(5).
Amin, M. M., Veith, T. L., Collick, A. S., Karsten, H. D., & Buda, A. R. (2017). Simulating hydrological and nonpoint source pollution processes in a karst watershed: A variable source area hydrology model evaluation. Agricultural Water Management, 180, 212–223.
Archibald, T. W., & Marshall, S. E. (2018). Review of mathematical programming applications in water resource management under uncertainty. Environmental Modelling & Assessment, 23, 753–777. https://doi.org/10.1007/s10666-018-9628-0
Berndtsson, R. (1990). Transport and sedimentation of pollutants in a river reach: A chemical mass balance approach. Water Resources Research, 26(7), 1549–1558.
Berner, E. K., & Berner, R. A. (2012). Global environment: Water, air, and geochemical cycles. Princeton University Press.
BIS, (2012). Bureau of Indian Standards, New Delhi. 2–3.
Bouchez, J., Moquet, J. S., Espinoza, J. C., Martinez, J. M., Guyot, J. L., Lagane, C., Filizola, N., Noriega, L., Sanchez, L. H., & Pombosa, R. (2017). River mixing in the Amazon as a driver of concentration-discharge relationships. Water Resources Research, 53(11), 8660–8685.
Bowes, M. J., Jarvie, H. P., Halliday, S. J., Skeffington, R. A., Wade, A. J., Loewenthal, M., Gozzard, E., Newman, J. R., & Palmer-Felgate, E. J. (2015). Characterising phosphorus and nitrate inputs to a rural river using high-frequency concentration–flow relationships. Science of the Total Environment, 511, 608–620.
Brown, T. C., & Froemke, P. (2012). Nationwide assessment of nonpoint source threats to water quality. BioScience, 62(2), 136–146.
Bukaveckas, P. A., Guelda, D. L., Jack, J., Koch, R., Sellers, T., & Shostell, J. (2005). Effects of point source loadings, sub-basin inputs and longitudinal variation in material retention on C, N and P delivery from the Ohio River Basin. Ecosystems, 8, 825–840.
Carpenter, S. R., Caraco, N. F., Correll, D. L., Howarth, R. W., Sharpley, A. N., & Smith, V. H. (1998). Non point pollution of surface waters with phosphorus and nitrogen. Ecological Applications, 8(3), 559–568.
Chakrapani, G. J. (2005). Major and trace element geochemistry in upper Ganga River in the Himalayas, India. Environmental Geology, 48(2), 189–201.
Chakrapani, G. J., Saini, R. K., & Yadav, S. K. (2009). Chemical weathering rates in the Alaknanda-Bhagirathi river basins in Himalayas, India. Journal of Asian Earth Sciences, 34(3), 347–362.
Chen, D., Hu, M., Guo, Y., & Dahlgren, R. A. (2015). Reconstructing historical changes in phosphorus inputs to rivers from point and nonpoint sources in a rapidly developing watershed in eastern China. Science of the Total Environment, 533(15), 196–204.
Chen, H., Teng, Y., & Wang, J. (2013). Load estimation and source apportionment of nonpoint source nitrogen and phosphorus based on integrated application of SLURP model, ECM, and RUSLE: A case study in the Jinjiang River, China. Environmental Monitoring and Assessment, 185(2), 2009–2021.
Dolan, D. M., & El-Shaarawi, A. H. (1989). Inferences about point source loadings from upstream/downstream river monitoring data. Environmental and Monitoring Assessment, 12, 343–357.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 170(3962), 1088–1090.
Godsey, S. E., Kirchner, J. W., & Clow, D. W. (2009). Concentration–discharge relationships reflect chemostatic characteristics of US catchments. Hydrological Processes: An International Journal, 23(13), 1844–1864.
Hasnain, S. I., & Thayyen, R. J. (1999). Controls on the major-ion chemistry of the Dokriani glacier meltwaters, Ganga basin, Garhwal Himalaya, India. J. Glaciol., 45(149), 87–92.
Hooper, R. P., Aulenbach, B. T., & Kelly, V. J. (2001). The National Stream Quality Accounting Network: A flux-based approach to monitoring the water quality of large rivers. Hydrological Processes, 15(7), 1089–1106.
Iqbal, A. S. H. S. (2001). Dudu Glacier, an alpine meltwater stream in Ganga headwater, Garhwal Himalaya. Journal of China University of Geosciences, 12(1), 75–83.
Ismail, W. R., Siow, A. Y., & Ali, A. (2005). Water quality and chemical mass balance of tropical freshwater wetland, Beriah Swamp, Perak. Jurnal Teknologi (f), 43, 65–84.
Jain, C. K. (1996). Application of chemical mass balance approach to upstream/downstream river monitoring data. Journal of Hydrology, 182, 105–115.
Jain, C. K., Bhatia, K. K. S., & Seth, S. M. (1998). Assessment of point and non-point sources of pollution using a chemical mass balance approach. Hydrological Sciences, 43(3).
Jain, C. K. (2000). Application of chemical mass balance approach to determine nutrient loading. Hydrological Sciences Journal, 45(4), 577–588.
Jain, C. K. (2002). A hydro-chemical study of a mountainous watershed: The Ganga, India. Water Research, 36(5), 1262–1274.
Jain, C. K., Singhal, D. C., & Sharma, M. K. (2007). Estimating nutrient loadings using chemical mass balance approach. Environmental Monitoring and Assessment, 134, 385–396.
Kelley, D. W., & Nater, E. A. (2000). Source apportionment of lake bed sediments to watersheds in an Upper Mississippi basin using a chemical mass balance method. CATENA, 41(4), 277–292.
Khan, M. Y. A., Hu, H., Tian, F., & Wen, J. (2020). Monitoring the spatio-temporal impact of small tributaries on the hydrochemical characteristics of Ramganga River, Ganges Basin, India. International Journal of River Basin Management, 18(2), 231–241.
Khanna, D. R., Bhutiani, R., Matta, G., Singh, V., & Bhadauriya, G. (2011). Physico-chemical property of River Ganga flatfoot hills of Garhwal Himalayas. Environment Conservation Journal, 12(3), 163–168.
Kotnala, G., Dobhal, S., & Chauhan, J. S. (2016). Monitoring the self-purification capacity of the River Alaknanda stretch at Srinagar, Uttarakhand, India. International Journal of River Basin Management, 14(4), 491–498.
Kumar, K., Miral, M. S., Joshi, S., Pant, N., Joshi, V., & Joshi, L. M. (2009). Solute dynamics of meltwater of Gangotri glacier, Garhwal Himalaya, India. Environmental Geology, 58(6), 1151–1159.
Lai, Y. C., Yang, C. P., Hsieh, C. Y., Wu, C. Y., & Kao, C. M. (2011). Evaluation of non-point source pollution and river water quality using a multimedia two-model system. Journal of Hydrology, 409(3–4), 583–595.
Leon, L. F., Soulis, E. D., Kouwen, N., & Farquhar, G. J. (2001). Nonpoint source pollution: A distributed water quality modeling approach. Water Research, 35(4), 997–1007.
Liu, X., Li, D., Zhang, H., Cai, S., Li, X., & Ao, T. (2015). Research on nonpoint source pollution assessment method in data sparse regions: a case study of Xichong River Basin, China. Advances in Meteorology, 2015.
Mackenzie, F. T., & Garrels, R. M. (1971). Evolution of sedimentary rocks. Norton.
Mosley, L. M., Zammit, B., Leyden, E., Heneker, T. M., Hipsey, M. R., Skinner, D., & Aldridge, K. T. (2012). The impact of extreme low flows on the water quality of the Lower Murray River and Lakes (South Australia). Water Resources Management, 26, 3923–3946.
Paudyal, R., Kang, S., Sharma, C. M., Tripathee, L., Huang, J., Rupakheti, D., & Sillanpää, M. (2016). Major ions and trace elements of two selected rivers near Everest region, southern Himalayas, Nepal. Environmental Earth Sciences, 75(1), 46.
Raiswell, R. (1984). Chemical models of solute acquisition in glacial melt waters. Journal of Glaciology, 30(104), 49–57.
Rao, G. S., & Nageswararao, G. (2010). Study of groundwater quality in Greater Visakhapatnam City, Andhra Pradesh (India). Journal of Environmental Science & Engineering, 52(2), 137–146.
Rudra, R. P., Mekonnen, B. A., Shukla, R., Shrestha, N. K., Goel, P. K., Daggupati, P., & Biswas, A. (2020). Currents status, challenges, and future directions in identifying critical source areas for non-point source pollution in Canadian conditions. Agriculture, 10(10), 468.
Sarin, M. M., Krishnaswami, S., Dilli, K., Somayajulu, B. L. K., & Moore, W. S. (1989). Major ion chemistry of the Ganga-Brahmaputra river system: Weathering processes and fluxes to the Bay of Bengal. Geochimica Cosmochimica Acta, 53, 997–1009.
Sarin, M. M., Krishnaswami, S., Trivedi, J. R., & Sharma, K. K. (1992). Major ion chemistry of the Ganga source waters: Weathering in the high altitude Himalaya. Proceedings – Earth and Planetary Sciences, 101(1), 89–98.
Semwal, N., & Akolkar, P. (2006). Water quality assessment of sacred Himalayan rivers of Uttaranchal. Current Science, 91(4), 486–496.
Seth, R., Mohan, M., Singh, P., Singh, R., Dobhal, R., Singh, K. P., & Gupta, S. (2016). Water quality evaluation of Himalayan rivers of Kumaun region, Uttarakhand, India. Applied Water Science, 6(2), 137–147.
Sharma, M. K., Thayyen, R. J., Jain, C. K., Arora, M., & Lal, S. (2019). Assessment of system characteristics of Gangotri glacier headwater stream. Science of the Total Environment, 662, 842–851.
Sharma, M. K., Thayyen, R. J., & Lal, S. (2020). Role of sediment in solute acquisition in the Himalayan Glacier meltwater stream‐Gangotri Glacier, Uttarakhand, India. Hydrological Processes, https://doi.org/10.1002/hyp.14018
Shen, Z., Hong, Q., Yu, H., & Liu, R. (2008). Parameter uncertainty analysis of the non-point source pollution in The Daning River watershed of the Three Gorges Reservoir Region, China. Science of the Total Environment, 405, 195–205.
Shen, Z., Zhong, Y., Huang, Q., & Chen, L. (2015). Identifying non-point source priority management areas in watersheds with multiple functional zones. Water Research, 68(1), 563–571.
Silveira, R. P., Rodregues, A. P. C., Santelli, R. E., Cordeiro, R. C., & Bidone, E. D. (2011). Mass balance in the monitoring of pollutants in tidal rivers of the Guanabara Bay, Rio de Janeiro, Brazil. Environmental Monitoring and Assessment, 181, 165–173.
Singh, A. K., & Hasnain, S. I. (1998). Major ion chemistry and weathering control in a high altitude basin: Alaknanda River, Garhwal Himalaya, India. Hydrological Sciences, 43(6), 825–843.
Singh, A. K., & Hasnain, S. I. (2002). Aspects of weathering and solute acquisition processes controlling chemistry of sub-Alpine proglacial streams of Garhwal Himalaya, India. Hydrological Processes, 16(4), 835–849.
Singh, A. P., Dhadse, K., & Ahalawat, J. (2019). Managing water quality of a river using an integrated geographically weighted regression technique with fuzzy decision-making model. Environmental Monitoring and Assessment, 191(6), 1–17.
Singh, V. B., Ramanathan, A. L., Pottakkal, J. G., Sharma, P., Linda, A., Azam, M. F., & Chatterjee, C. (2012). Chemical characterisation of meltwater draining from Gangotri Glacier, Garhwal Himalaya, India. Journal of Earth System Science, 121(3), 625–636.
Singh, V. B., Ramanathan, A. L., Pottakkal, J. G., & Kumar, M. (2014). Seasonal variation of the solute and suspended sediment load in Gangotri glacier meltwater, Central Himalaya, India. Journal of Asian Earth Science, 79, 224–234.
Srinivas, R., Singh, A. P., Dhadse, K., & Garg, C. (2019). An evidence based integrated watershed modelling system to assess the impact of non-point source pollution in the riverine ecosystem. Journal of Cleaner Production, 246, 118963.
Stachnik, Ł, Yde, J. C., Kondracka, M., Ignatiuk, D., & Grzesik, M. (2016). Glacier naled evolution and relation to the subglacial drainage system based on water chemistry and GPR surveys (Werenskioldbreen, SW Svalbard). Annals of Glaciology, 57(72), 19–30.
Thayyen, R. J., Gergan, J. T., & Dobhal, D. P. (1999). Particle size characteristics of suspended sediments and subglacial hydrology of Dokriani Glacier, Garhwal Himalaya, India. Hydrological Sciences Journal, 44(1), 47–61.
Vega, E., Mugica, V., Carmona, R., & Valencia, E. (2000). Hydrocarbon source apportionment in Mexico City using the chemical mass balance receptor model. Atmospheric Environment, 34(24), 4121–4129.
Wang, G., Li, J., Sun, W., Xue, B., Yinglan, A., & Liu, T. (2019). Non-point source pollution risks in a drinking water protection zone based on remote sensing data embedded within a nutrient budget model. Water Research, 157, 238–246.
Wu, L., Long, T., & Cooper, W. J. (2012). Temporal and Spatial Simulation of Adsorbed Nitrogen and Phosphorus Nonpoint Source Pollution Load in Xiaojiang Watershed of Three Gorges Reservoir Area. China. Environmental Engineering Science, 29(4), 238–247.
Wu, Y., & Chen, J. (2013). Investigating the effects of point source and nonpoint source pollution on the water quality of the East River (Dongjiang) in South China. Ecological Indicators, 32, 294–304.
Yadav, A., & Pandey, J. (2017). Contribution of point sources and non-point sources to nutrient and carbon loads and their influence on the trophic status of the Ganga River at Varanasi, India. Environmental Monitoring and Assessment, 189(9), 1–19.
Zhai, X., Zhang, Y., Wang, X., Xia, J., & Liang, T. (2012). Non-point source pollution modelling using Soil and Water Assessment Tool and its parameter sensitivity analysis in Xin’anjiang catchment, China. Hydrological Processes, 28(4), 1627–1640.
Zhang, Q., Blomquist, J. D., Moyer, D. L., & Chanat, J. G. (2019). Estimation bias in water-quality constituent concentrations and fluxes: A synthesis for chesapeake bay rivers and streams. Frontiers in Ecology and Evolution, 7, 109.
Acknowledgements
This research work is a part of NMSHE project titled “Integrated Hydrological Studies for Upper Ganga Basin up to Rishikesh” sponsored by Department of Science & Technology, Govt. of India, New Delhi vide DST Sanction No. DST/SPLICE/CCP/NMSHE/TF-4/NIH/2015-G and hereby acknowledged. The authors are thankful to the Director, National Institute of Hydrology, Roorkee, for providing analytical facilities and financial support for carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, M.K., Kumar, P., Bhanot, K. et al. Assessment of non-point source of pollution using chemical mass balance approach: a case study of River Alaknanda, a tributary of River Ganga, India. Environ Monit Assess 193, 424 (2021). https://doi.org/10.1007/s10661-021-09203-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09203-x