Abstract
Iron is an essential mineral and one of the most abundant in soils, presenting itself in the environment as ferrous and ferric ions. As each oxidation state of iron has a different role in the environment, its speciation in environmental studies is important. The determination of ferrous iron received great attention from soil chemists because of its important role in agriculture, in redox processes, and as an electron acceptor in the catalysis of organic matter. Methodologies with the use of colorimetric reagents to determine ferrous iron are divergent and not very clear. In this study, we compared two colorimetric reagents (1,10-phenanthroline and ferrozine) to determine the total concentration of iron, ferrous and ferric ions in soil, using simple and low-cost methodologies. The determination of ferrous and total iron with 1,10-phenanthroline colorimetric reagent, following published instructions, did not correlate with ferrozine method, presenting an erroneous quantification. After neutralizing the extract of 1,10-phenanthroline with NaOH, both colorimetric methods allowed to quantify with precision and high yield the amount of ferrous and total iron extracted from the soil. The oxidation states of iron have a different contribution and importance to the environment. In this sense, the improvement of a widely used methodology is crucial for the better study of iron speciation in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As essential mineral, iron plays an important role in biological processes. Besides that, iron is the fourth most abundant element in soil in relation to other major elements (Coey 1988; Eskandari 2011; Tavakoli et al. 2014; Rout and Sahoo 2015), comprising around 5% of the lithosphere, being the most common range in soil from 0.5 to 5%. The global abundance of this element is approximately 45%, concentrations varying significantly accordingly to soil types and the presence of other sources (Kabata-Pendias and Pendias 2001).
Iron in the two oxidation states, Fe(II) (ferrous) and Fe(III) (ferric), are the most present in soil (Colombo et al. 2014). These oxidation states can easily change from one to another, being the redox equilibrium in function of pH, temperature, and additional components in a solution (Hoffmann 2005). Both ferrous and ferric iron are involved in the formation of organic and inorganic soluble complexes, colloids, and particulate phases (Achterberg et al. 2001; Hoffmann 2005). Ferric iron concentration in soil is frequently higher than other electron acceptors such as nitrate, sulfate, and oxygen. In this way, ferric iron serves as electron acceptor, releasing nutrients of organic matter mineralization (Lovley and Phillips 1986a).
The concentration of dissolved organic carbon in soils decreases in depth according to complexation and desorption between carbon and ferric iron (Lovley 1991). Moreover, low carbon availability is the result of high concentrations of iron at acidic pH in the presence of organic matter. These characteristics allow the formation of stable precipitates responsible to remove organic substances from soil (McKnight et al. 1992; Moore et al. 1992; Neu et al. 2017).
The change between ferric and ferrous iron is important to the cycle of nutrients and carbon in flooded soils. The labile organic matter is known to be an electron donor in the reaction of iron reduction mediated by microorganism (Emsens et al. 2016; Gabriel et al. 2020). This iron reduction is associated with methanogenesis suppression (Gabriel et al. 2020). The majority of iron in flooded soils is present in the ferrous form, characterizing the mobility. Under dry conditions, ferrous iron precipitates as ferric oxide, partially re-dissolving (Quesada et al. 2011).
Reactions of redox cycling and complexation of iron can influence several biogeochemical processes. Thus, the precise determination of iron is extremely important to understand these dynamics and to evaluate the potential interference of iron in the determination of other elements. Therefore the use of a fast methodology and low-cost reagents is relevant (Huang and Hall 2017). For these reasons, the speciation of an element or its oxidation state is crucial to biochemical and toxicological investigations (Kot and Namiesńik 2000). In this context, the determination of iron oxidation state and the accurate measurement of redox species are important (Pehkonen 1995; Xia et al. 2003).
It is known that plants may affect the abundance, the activity, and the diversity of microorganisms. Also, they may interfere in the iron availability and on the interactions between this essential micronutrient and microorganisms (Colombo et al. 2014). Therefore, is of extreme importance the investigation of the different forms of iron in soil in studies involving plants, microorganisms and nutrients, and also the mechanisms that regulates and associates these processes and its equilibrium.
Acidic iron extraction is a classic and widely used methodology for the analysis of iron species in soil (Porsch and Kappler 2011). Two colorimetric reagents are commonly used in the analysis of ferric iron: 1,10-phenanthroline (PubChem CID: 1318, C12H8N2) (Harvey et al. 1955; Schilt 1969; Tamura et al. 1974; Luo et al. 1978; Freitas et al. 2015) and ferrozine (PubChem CID: 23662871, C20H13N4NaO6S2) (Sørensen 1982; Lovley and Phillips 1986a, b, 1987; Wallmann et al. 1993; Heron et al. 1994; Schnell et al. 1998). While ferrozine complexes with divalent iron in a broad range of pH (Stookey 1970), it is reported that 1,10-phenanthroline complex is very sensible to pH (Hummel and Willard 1938). Nonetheless, this issue has been neglected or not mentioned in the methodologies regarding iron determination in acidic extracts (Porsch and Kappler 2011).
Due to the importance of iron analysis in soil samples, the main objective of this study was to improve the iron speciation determination. Different methodologies were evaluated to quantify ferrous, ferric, and total iron, in order to establish a simple and adequate methodology for iron speciation analysis.
Materials and methods
Soil sample collection
Fifteen soil samples were collected in an agroforestry site located at a floodplain of the Tocantins River in the Amazon State, Brazil (2° 40′ 51″ S and 49° 39′ 05″ W), during the flooded period (May 2018). Samples were collected from 0 to 30 cm soil layer below the water column by using a hand core soil sampler with 1 to 3 m long × 5 cm diameter with a beveled leading edge, retention valve, and extrusion apparatus in sections of the soil profile. Samples were transported on ice and stored at 4 °C until extraction.
Iron extraction
Iron was extracted under oxic conditions according to Porsch and Kappler (2011). Wet soil samples (0.5 g) were mixed with 25 mL of 1 M HCl which has been bubbled with nitrogen gas (N2) for 1 h, in closed 50 mL centrifuge tubes. Thereafter, the suspensions were bubbled with nitrogen gas during 2 min. The tubes closed and incubated at 70 °C in a water bath in the dark for 1 h, avoiding major air contact (Porsch and Kappler 2011). The suspensions were vacuum filtered in a 0.45 μm pore size cellulose ester membrane filters (MF-Millipore™). Extraction samples were maintained in the dark at 4 °C in penicillin-type flasks closed with gas proof butyl rubber stoppers (Bellco Glass 2048) until analysis. This prevents reduction of Fe(III) and oxidation of Fe(II).
Analytical standard curves
Fe(II) stock solution was prepared from ferrous ammonium sulfate hexahydrate (PubChem CID: 15942308, Fe(NH4)2(SO4)2∙6H2O) in 0.1 M H2SO4 (PubChem CID: 1118). Permanganometry was used to determine the title of the stock solution. Standard solutions were diluted in 1 M HCl (PubChem CID: 313) and were also used as spiking solutions (9:1 sample/standard; v:v) in order to assess the performance of the colorimetric reactions and their photometric determination in each plate. Standard solutions were prepared in the moment of the analysis.
Determination of total iron and Fe(II)
Total iron and Fe(II) concentrations were determined by UV-Vis absorption spectrometry in a microplate reader (HTX Multi-Mode Microplate Reader, Synergy™) by using 96 well optical microplates. Reactions were performed in three technical replicates. Fe(III) concentrations were determined by the difference between total and ferrous iron. In addition to colorimetric analysis, total iron was determined by microwave plasma atomic emission spectroscopy (MP-AES 4200, Agilent Technologies) as reference method (emission wavelength 371.993 nm).
Determination of total iron and Fe(II) with 1,10-phenanthroline
The methodology with 1,10-phenanthroline (Sigma-Aldrich®) was based on Tarafder and Thakur (2013) and Freitas et al. (2015). We performed both methodologies previously presented by the authors and the modified method. The modified method consists of the neutralization of soil extract pH with NaOH (PubChem CID: 14798) in the moment of the analysis. This improves the colored complex formation and stability (Online Resource).
For total iron analysis, were added into each well of the microplate 60 μL of the extract sample pierced from the flasks with a syringe and 40 μL of the reductant agent, which is hydroxylamine hydrochloride (PubChem CID: 443297, Sigma-Aldrich®) 10% (w/v) in 1 M HCl. This mixture was kept during 5 min in room temperature (~ 24 °C) for iron reduction. Then, 40 μL of 2.5 M NaOH (to neutralize the pH of the solution) and 60 μL of 1,10-phenanthroline 0.3% (w/v) in 0.1 M sodium acetate/acetic acid buffer pH 4.5 were added. The absorbance at 510 nm was measured after 15 min of reaction.
For Fe(II) analysis, 100 μL of the extract sample pierced from flasks with a syringe, 40 μL of 2.5 M NaOH (to neutralize the pH of the solution), and 60 μL of 1,10-phenanthroline 0.3% (w/v) in 0.1 M sodium acetate/acetic acid buffer pH 4.5 were added into each well of the microplate. The absorbance at 510 nm was measured after 15 min of reaction.
Determination of total iron and Fe(II) with ferrozine
The methodology with ferrozine (3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4′′-disulfonic acid sodium salt) (Sigma-Aldrich®) was based on Schnell et al. (1998) and Lovley and Phillips (1986b).
For total iron analysis, 100 μL of the extract sample pierced from the flasks with a syringe was mixed in assay tubes with 1 mL of the reductant agent, which is hydroxylamine hydrochloride 10% (w/v) in 1 M HCl. This mixture was kept 5 min in room temperature (~ 24 °C) for iron reduction. Thereafter, 100 μL of this mixture was mixed with 1 mL of ferrozine 0.1% (w/v) in 50 mM HEPES (Sigma-Aldrich®) buffer pH 7.0. After 30 min of reaction, 200 μL was transferred into each well of the microplate, and then the absorbance at 562 nm was measured.
For Fe(II) analysis, 100 μL of the extract sample pierced from the flasks syringe was mixed with 1 mL of ferrozine 0.1% (w/v) in 50 mM HEPES buffer pH 7.0 in assay tubes. After 15 min of reaction, 200 μL was transferred into each well of the microplate, and the absorbance at 562 nm was measured.
Determination of total iron by MP-AES
The same extract samples used in the colorimetric methods were subjected to the microwave plasma atomic emission spectrometry (MP-AES 4200, Agilent Technologies) determination as reference method for total iron. The analytical standard curve used was the same as to the colorimetric analysis, varying from 8 to 100 mg L−1, depending on each experiment and samples iron concentration. The emission wavelength used was 371.993 nm (Fe). The results were therefore compared against the ones from the 1,10-phenanthroline and ferrozine methods.
Iron speciation with visual MINTEQ
We used the Visual MINTEQ 3.1 software to evaluate the speciation of Fe(II) and Fe(III) ions in different pH conditions. This analysis allows the understanding of the ionic species available in solution under different pH conditions (Gustafsson 2019).
Statistical analysis
Standard calibration curves were fitted with linear regressions for all methods. Data were subjected to descriptive analyses. Methods were compared by using linear regressions. Limits of detection and quantification were determined according to Hage and Carr (2012) (Table 1).
Results and discussion
Soil chemical characteristics are one of the most important properties (USDA Soil Taxonomy 1999). Determining the total concentration of an element and its oxidation state is the key to know its availability for plants and microorganisms (Ure 1990).
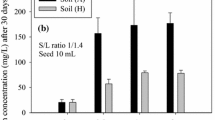
Iron extraction from soil sample in acidic conditions (with weak HCl, 1 M) inhibits Fe(II) oxidation and is an important soil pre-treatment to iron analysis when redox speciation is necessary (Huang and Hall 2017). The determination of total iron with colorimetric reagents was slightly overestimated when compared to the reference method (MP-AES). This determination ranged from 5 to 25% (\( \overline{x} \) = 14%) based on ferrozine and from 6 to 23% (\( \overline{x} \) = 15%) based on 1,10-phenanthroline after pH neutralization of the extract solution with NaOH before complexation. However, the difference between both methods was not significant (Fig. 1, Table 1). An overestimation of iron concentrations was already observed (Braunschweig et al. 2012; Huang and Hall 2017), and this may be due to the high amount of organic matter dissolved in the samples (Huang and Hall 2017). Samples spiked with standard solutions at the ratio of 10% resulted in values 1% lower than the expected based on the sample quantification.
The impact of extract neutralization for determining Fe(II) was as relevant as for total iron. Samples subjected to 1,10-phenanthroline method without neutralization with NaOH did not correlate with ferrozine method. On the other hand, same samples subjected to NaOH addition improved ferrous iron determination. Thereat, ferrozine and 1,10-phenanthroline methods presented similar performance on Fe(II) determination in a broad range of concentrations. This result was based on the significant correlation and equation coefficients (Fig. 2, Table 1).
Pioneering studies reported the complex of 1,10-phenanthroline with Fe(II) in the range of pH 2 to 9 (Fortune and Mellon 1938). However, ferrous complex is not stable under strong acidic conditions (Hummel and Willard 1938). This information has been neglected or not mentioned in the methodologies regarding iron determination in acidic soil extracts, characteristic that can be clearly proved in this study. Despite the inclusion of a buffer solution in the original assay, it was not enough to promote the best conditions for the chemical reaction when working with 1 M HCl extraction solution.
In relation to ferrozine, the formation of the complex with Fe(II) occurs in the range of pH 4 to 9. Below this range, the lower the pH, the lower is the complex stability. However, in this case, the relationship between color intensity and concentration is still linear (Stookey 1970). Therefore, iron quantitative analysis could be performed below pH 4, unlike as for the 1,10-phenanthroline method (Online Resource).
The analysis of the distribution of iron species in different pH ranges using visual MINTEQ software allowed to observe the high availability of Fe(II) and Fe(III) ions at low pH values. These results agreed with what was observed by the different quantification methodologies, at pH 2 to microwave plasma atomic emission spectroscopy and at pH 4.5 to colorimetry with 1,10-phenanthroline. The use of ferrozine as colorimetric reagent at pH 7.0 provided less availability of Fe(III) ions compared to other methodologies. Consecutively, there is an increase in the availability of hydroxide ions in solution (Fig. 3).
The classes of soils most present in the world are oxisols, aridisols, alfisols, and entisols. The characteristics of these soils are the dominance of minerals with high amount of iron (USDA Soil Taxonomy 1999). In well-drained soil, the pedogenic forms of crystalline iron are the most abundant mineral present. This element is considered an essential micronutrient for plants and microorganisms, contributing to its growth. In this way, the amount of iron available to these organisms is crucial to study soil characteristics and life-sustaining processes, since the deficiency of this element is a problem for many crops (USEPA 2003; Colombo et al. 2014). In addition, the geochemistry of iron in soil is associated with other processes, such as chemical, physical, and biological, which can influence the micronutrient availability when factors that change it affect the different forms of iron (Colombo et al. 2014).
Ferrous iron is the form absorbed by plants as a micronutrient, and Fe(III) is the unavailable insoluble form (USEPA 2003). In this way, the analysis of Fe(II) concentration in agricultural soil is important, since iron deficiency may cause diverse problems to the health and growth of plants (USEPA 2003). In addition, Fe(II) and Fe(III) interaction with microorganisms and organic molecules is important to understand the properties of the metal oxides (Colombo et al. 2014). Therefore, a detailed methodology for analyzing iron redox species in soil is extremely important.
We demonstrated that without neutralizing the pH with 1,10-phenanthroline reagent, even using a buffer, the total quantification of iron and Fe(II) in acidic soil samples is erroneous and underestimated (Figs. 1 and 2). Another study has already warned that the final pH of the reagents and sample mixture can lead to inefficient iron quantification. This is due to the competitive complexation of the 1,10-phenanthroline with other ions, which leads to a non-linear relationship between iron concentration and color intensity (Jeitner 2014, Online Resource). However, this characteristic is not always taken into account when developing protocols for analyzing ferrous or total iron in environmental samples by colorimetry (Luo et al. 1978; Tarafder and Thakur 2013; Freitas et al. 2015).
Our results show that, although the methods are widely used and referenced in the literature, there is a problem regarding the neutralization of the solution to iron determination after acidic extraction when using the 1,10-phenanthroline reagent. Thus, this step is essential for speciation of iron in soil samples for environmental purposes and requires better attention when choosing reagents and procedures.
Conclusions
Simple and low-cost methodologies for determining iron in different oxidation states are extremely important. The difficulty of finding an effective methodology, with details in literature, may lead to inconsistent results. We present an optimized colorimetric method that allows equivalent analyses of multiple soil extractions. We demonstrated that both ferrozine and 1,10-phenanthroline reagents can be used to determine iron in acidic extracts with good precision and high-throughput. Our results also highlight the importance of adjusting the final pH of the reaction mixture with NaOH when using 1,10-phenanthroline reagent. This factor has received little attention in previous studies, but it can substantially influence the quantification results. In addition, using a microplate, we can save reagent and generate less chemical waste, once the final volume is reduced than by using a cuvette. Therefore, the analyst might take into consideration other issues than precise determination of iron in soil when choosing the best methodology. For example, ferrozine is non-toxic, while 1,10-phenanthroline is dangerous to the environment and human health. On the other hand, 1,10-phenanthroline might have lower acquisition cost than ferrozine, which could be an important issue depending on budget availability and regional market. Once the oxidation states of iron have different contribution and importance to the environment, the improvement of a widely used methodology demonstrates the significance of well-detailed and well-described procedures. This allows obtaining accurate results in the analysis of ferrous and total iron present in soil.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Achterberg, E. P., Holland, T. W., Bowie, A. R., Mantoura, R. F. C., & Worsfold, P. J. (2001). Determination of iron in seawater. Analytica Chimica Acta, 442(1), 1–14.
Braunschweig, J., Bosch, J., Heister, K., Kuebeck, C., & Meckenstock, R. U. (2012). Reevaluation of colorimetric iron determination methods commonly used in geomicrobiology. Journal of Microbiological Methods, 89(1), 41–48.
Coey, J. M. D. (1988). Magnetic properties of iron in soil iron oxides and clay minerals. In J. W. Stucki, B. A. Goodman, & U. Schwertmann (Eds.), Iron in soils and clay minerals (pp. 397–466). Dordrecht: Springer.
Colombo, C., Palumbo, G., He, J., Pinton, R., & Cesco, S. (2014). Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. Journal of Soils and Sediments, 14, 538–548.
Emsens, W., Aggenbach, C. J. S., Schoutens, K., Smolders, A. J. P., Zak, D., & van Diggelen, R. (2016). Soil iron content as a predictor of carbon and nutrient mobilization in rewetted fens. PLoS One, 11, e0153166.
Eskandari, H. (2011). The importance of iron (Fe) in plant products and mechanism of its uptake by plants. Journal of Applied Environmental and Biological Sciences, 1(10), 448–452.
Freitas, P. C., Carvalho, R. M., Silva, J. S. A., Prado, T., Duarte, E. R., Frescura, V. L. A., & Chaves, E. S. (2015). Extração assistida por ultrassom para determinação colorimétrica de ferro em solo: uma comparação com espectrometria de massa com plasma indutivamente acoplado. Química Nova, 38(4), 570–574.
Fortune, W. B., & Mellon, M. G. (1938). Determination of iron with o-phenanthroline: A spectrophotometric study. Industrial and Engineering Chemistry, Analytical Edition, 10(2), 60–64.
Gabriel, G. V. M., Oliveira, L. C., Barros, D. J., Bento, M. S., Neu, V., Toppa, R. H., Carmo, J. B., & Navarrete, A. A. (2020). Methane emission suppression in flooded soil from Amazonia. Chemosphere, 250, 126263.
Gustafsson, J. P. (2019). Visual MINTEQ, v. 3.1. Stockholm: Royal Institute of Technology, Div. of Land and Water Resources Engineering Available at: https://vminteq.lwr.kth.se.
Hage, D. S., & Carr, J. D. (2012). Química analítica e análise quantitativa (pp. 101–103). São Paulo: Pearson Prentice Hall.
Harvey, A. E., Smart, J. A., & Amis, E. S. (1955). Simultaneous spectrophotometric determination of iron(II) and total iron with 1,l0-phenanthroline. Analytical Chemistry, 27(1), 26–29.
Heron, G., Crouzet, C., Bourg, A. C. M., & Christensent, T. H. (1994). Speciation of Fe(II) and Fe(III) in contaminated aquifer sediments using chemical extraction techniques. Environmental Science & Technology, 28(9), 1698–1705.
Hoffmann, P. (2005). Speciation of iron. In R. Cornelis, J. Caruso, H. Crews, & K. Heumann (Eds.), Handbook of elemental speciation II - Species in the environment, food, medicine and occupational health (pp. 200–217). Hoboken: John Wiley & Sons.
Huang, W., & Hall, S. J. (2017). Optimized high-throughput methods for quantifying iron biogeochemical dynamics in soil. Geoderma, 306(15), 67–72.
Hummel, F. C., & Willard, H. H. (1938). Determination of iron in biological materials. Industrial and Engineering Chemistry, Analytical Edition, 10(1), 13–15.
Jeitner, T. M. (2014). Optimized ferrozine-based assay for dissolved iron. Analytical Biochemistry, 454(1), 36–37.
Kabata-Pendias, A., & Pendias, H. (2001). Trace elements in soils and plants (pp. 310–313). Boca Raton: CRC Press LLC.
Kot, A., & Namiesńik, J. (2000). The role of speciation in analytical chemistry. Trends in Analytical Chemistry, 19(2–3), 69–79.
Lovley, D. R., & Phillips, E. J. P. (1986a). Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Applied and Environmental Microbiology, 51(4), 683–689.
Lovley, D. R., & Phillips, E. J. P. (1986b). Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac river. Applied and Environmental Microbiology, 52(4), 751–757.
Lovley, D. R., & Phillips, E. J. P. (1987). Rapid assay for microbially reducible ferric iron in aquatic sediments. Applied and Environmental Microbiology, 53(7), 1536–1540.
Lovley, D. R. (1991). Dissimilatory Fe(III) and Mn(IV) reduction. Microbiological Reviews, 55(2), 259–287.
Luo, C., Hwang, J., Puno, T., & Tsai, H. (1978). Determination of trace iron(II) in the presence of iron(III). Journal of the Chinese Chemical Society, 25(3), 119–123.
McKnight, D. M., Bencala, K. E., Zellweger, G. W., Aiken, G. R., Feder, G. L., & Thorn, K. A. (1992). Sorption of dissolved organic-carbon by hydrous aluminum and iron-oxides occurring at the confluence of deer creek with the Snake River, Summit County, Colorado. Environmental Science & Technology, 26(7), 1388–1396.
Moore, T. R., Souza, W., & Koprivnjak, J. F. (1992). Controls on the sorption of dissolved organic carbon by soils. Soil Science, 154(2), 120–129.
Neu, V., Krusche, A. V., & Ferraz, J. (2017). Dinâmica do carbono orgânico dissolvido na solução do solo sob diferentes coberturas vegetais na Amazônia Central. In S. S. Vasconcelos, M. L. P. Ruivo, & A. M. M. Lima (Eds.), Amazônia em tempo: Impactos do uso da terra em diferentes escalas (pp. 1–504). Belém: Universidade Federal do Pará: Museu Paraense Emílio Goeldi: Embrapa Amazônia Oriental.
Pehkonen, S. (1995). Determination of the oxidation states of iron in natural waters. Analyst, 120(11), 2655–2663.
Porsch, K., & Kappler, A. (2011). FeII oxidation by molecular O2 during HCl extraction. Environmental Chemistry, 8, 190–197.
Quesada, C. A., Lloyd, J., Anderson, L. O., Fyllas, N. M., Schwarz, M., & Czimczik, C. I. (2011). Soils of Amazonia with particular reference to the RAINFOR sites. Biogeosciences, 8, 1415–1440.
Rout, G. R., & Sahoo, S. (2015). Role of iron in plant growth and metabolism. Reviews in Agricultural Science, 3, 1–24.
Schilt, A. A. (1969). Analytical applications of 1,10-phenanthroline and related compounds (pp. 1–9). London: Pergamon.
Schnell, S., Ratering, S., & Jansen, K. (1998). Simultaneous determination of iron(III), iron(II), and manganese(II) in environmental samples by ion chromatography. Environmental Science & Technology, 32(10), 1530–1537.
Sørensen, J. (1982). Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Applied and Environmental Microbiology, 43(2), 319–324.
Stookey, L. L. (1970). Ferrozine - A new spectrophotometric reagent for iron. Analytical Chemistry, 42(7), 779–781.
Tamura, H., Goto, K., Yotsuyanagi, T., & Nagayama, M. (1974). Spectrophotometric determination of iron(II) with 1,10-phenanthroline in the presence of large amounts of iron(III). Talanta, 21(4), 314–318.
Tarafder, P. K., & Thakur, R. (2013). An optimised 1,10-phenanthroline method for the determination of ferrous and ferric oxides in silicate rocks, soils and minerals. Geostandards and Geoanalytical Research, 37(2), 155–168.
Tavakoli, M. T., Chenari, A. I., Rezaie, M., Tavakoli, A., Shahsavari, M., & Mousavi, S. R. (2014). The importance of micronutrients in agricultural production. Advances in Environmental Biology, 8(10), 31–35.
USDA Soil Taxonomy. (1999). A basic system of soil classification for making and interpreting soil surveys. Agricultural Handbook (Vol. 436, 2nd ed.). Washington, DC: United States Department of Agriculture, Natural Resources Conservation Service, Soil Taxonomy.
USEPA. (2003). United State Environmental Protection Agency. Ecological soil screening level for iron, Interim final. OSWER Directive 9285 (pp. 7–69). Washington, DC: U.S. Environmental Protection Agency.
Ure, A. M. (1990). Trace elements in soil: Their determination and speciation. Fresenius' Journal of Analytical Chemistry, 337, 577–581.
Xia, L., Wu, Y., Jiang, Z., Li, S., & Hu, B. (2003). Speciation of Fe(III) and Fe(II) in water samples by liquid–liquid extraction combined with low-temperature electrothermal vaporization (ETV) ICP-AES. International Journal of Environmental Analytical Chemistry, 83(11), 953–962.
Wallmann, K., Hennies, K., König, I., Petersen, W., & Knauth, H. (1993). New procedure for determining reactive Fe(III) and Fe(II) minerals in sediments. Limnology and Oceanography, 38(8), 1803–1812.
Code availability
Not applicable.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 (process no. 88882.306808/2018-01) and pro-equipment project (proposal no. 189683) CAPES no. 11/2014 and was supported by grants #2013/50940-0, #2016/10796-5, and #2016/16687-3, São Paulo Research Foundation (FAPESP). AAN was supported by grant #2017/03575–5, FAPESP.
Author information
Authors and Affiliations
Contributions
GVMG performed the experiments and wrote the manuscript. LMP contributed in the performance of the experiments, performed the statistics, participated on the discussion, and reviewed and edited the manuscript. LMTR performed the Visual MINTEQ analysis and discussion and prepared Fig. 3. AAN collected the soil samples and reviewed and edited the manuscript. WGB reviewed and edited the manuscript. JBC and LCO reviewed and edited the manuscript and contributed with the resources. All authors approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 142 kb)
Rights and permissions
About this article
Cite this article
de Mello Gabriel, G.V., Pitombo, L.M., Rosa, L.M.T. et al. The environmental importance of iron speciation in soils: evaluation of classic methodologies. Environ Monit Assess 193, 63 (2021). https://doi.org/10.1007/s10661-021-08874-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-08874-w