Abstract
In coastal areas of Bangladesh, the problem of getting fresh drinking water is acute since the surface and groundwater of this area are affected by both seawater intrusion and anthropogenic activities. This study aims at assessing the hydrochemistry and quality of surface and groundwater of a south-western coastal area Rupsha Upazila of Bangladesh using geographical information system (GIS) technique. The hydrochemical facies revealed that the surface waters are mainly characterized by Na-Ca-HCO3-Cl and the groundwaters are characterized by Na-Cl-HCO3, indicating mixing composition of the natural water, while the Gibbs diagram indicates mixing processes of both the rock-water and evaporation-crystallization interactions of the surface and groundwater. The comparisons of the water quality parameters with World Health Organization (WHO) and Bangladesh (BD) standards show that surface water quality is better than groundwater in terms of total dissolved solids, chloride, iron, and arsenic concentrations. In surface water, arsenic concentration is within the WHO and BD standard but 40% of the groundwater samples exceeded the standard. Results also showed that 100% of the surface water samples exceeded the Escherichia coli and 62.85% of the groundwater samples exceeded the standard limit. Thus, the surface water of the study area can be a potential source to meet the future challenges for drinking water shortage problems as compared to aquifer water of the area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The consequences of global temperature change have significant effects on coastal zones. Due to temperature variation, coastal zones are highly vulnerable because of seawater intrusion which increases salinity level both in surface and groundwater that resulted in unsuitability for drinking water purposes (Minar et al. 2013). In Bangladesh, there are particular areas in the coastal zones where water from shallow and deep tube wells is not suitable for drinking due to high salinity. Moreover, the quality of surface water also varies because of the presence of different types of elements and dissolved salts. Since Bangladesh is an agricultural land, the quality of water plays a significant role for efficient agricultural production. Thus, the assessment of water quality can identify various elements, which are present in the water and can help to compare the values with the WHO (World Health Organization) guidelines and local standards (Babiker et al. 2007). The rapid growth of industrialization and population directly or indirectly create demanding pressure on the freshwater sources that resulted in decreased water quality (Singh et al. 2013). At present, different countries located at both northern and southern hemispheres are suffering from safe drinking water. Therefore, the water quality issue is now an emerging problem to most of the coastal areas especially located at delta. Bangladesh is situated in the largest delta of the world, and the country is dealing with poor water quality issue at an alarming rate. Many researchers in Bangladesh have been paid an utmost attention to the quality of drinking water at different regions in particularly south-western coastal areas. A comprehensive study of such kind is necessary to elucidate hydrochemical characteristics and quality of groundwater in south-western coastal area of Bangladesh. Datta and Ghosh (2015) reported that the municipal groundwater is significantly influenced by marine seawater and the drinking quality is also subjected to chemical vulnerability. This makes the groundwater unsafe for drinking purposes in coastal living area. In addition, Islam et al. (2017) revealed that the groundwater is slightly alkaline and brackish in the coastal area in southern part of Bangladesh. Seawater plays an important role in the geochemistry of the groundwater since salinity has strong relationship with alkalinity and other heavy metals contained in groundwater (Hasan 2016). The evidence also showed that mixing of seawater makes the water unsuitable for drinking (Lawson 2011). Previous study on investigating shallow groundwater’s hydrochemical characteristics in a south-western coastal area of Bangladesh indicated that salinity and total hardness problem produce unsafe water for domestic use (Bahar and Reza 2010).
The scarcity of freshwater and contamination of organic, inorganic, and biological substances in clean water demands development of water quality assessment methods to monitor the water quality effectively (Jähnig and Cai 2010). Through the assessment method, it is possible to change the human health situation by identifying the contamination and pollution scenario of water quality. For long-term monitoring of water quality, the physical, chemical, and biological method is necessary to make a positive impact on water quality assessment to identify pollutants (Liao et al. 2011). Typically, surface water assessment includes the analysis of temperature, dissolved oxygen, total dissolved solids, coliform bacteria, and organic and inorganic metals. On the other hand, groundwater assessment includes the aquifer characterization, the presence of toxic elements, and ions such as phosphate, sulfate, chromium, iron, copper, lead, zinc, and arsenic (Parparov et al. 2014).
Rupsha Upazila, the selected area for this study, is a south-western coastal area of Bangladesh. This area was selected because of degradation of aquifer led by poor management of water resources. In Rupsha Upazila, most of the sources of water for residents come from the surface water bodies such as ponds or lakes which are now facing severe environmental pollution and the groundwater is significantly affected by saltwater intrusion. Some of the deep tube well water in this area has an unbearable odor which brings the groundwater unsuitable for drinking. Therefore, evaluation of water quality in that area is inevitable for providing safe drinking water to residents and to identify the major sources of pollution in surface and groundwater.
The main purpose of this study was to assess hydrochemical characteristics and quality of surface and groundwater in Rupsha Upazila of Bangladesh. The specific objectives of this study are (a) to identify the surface and groundwater quality parameters for drinking purposes, (b) to evaluate the hydrochemical facies and its mechanisms controlling the groundwater and surface water using Piper and Gibbs diagram, and (c) to develop drinking water quality mapping using geographical information system (GIS) technique.
Materials and methods
Study area
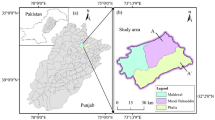
This study was conducted in Rupsha Upazila which is located in south-western coastal area of Bangladesh and between 22° 43′ and 22° 52′ north latitudes and 89° 33′ and 89° 52′ east longitudes. Rupsha Upazila named after Rupsha River covers 120.15 km2 which is bounded by Terokhada Upazila on the north, Batiaghata and Fakirhat Upazilas on the south, Fakirhat and Mollahat Upazilas on the east, and Khulna Sadar and Dighalia Upazilas on the west. Rupsha Upazila consists of 5 unions (local government unit), 64 mouzas (local administrative district), and 78 villages with a total population of 180,000 (Statistics 2011).
However, the study area is located in the Ganges tidal floodplain of the Ganges-Brahmaputra-Meghna (GBM) deltaic region of Bangladesh and, geologically, in the Faridpur Trough with a flat topography (JICA 2011) having an average elevation of about 3.32 m (Bahar and Reza 2010) and sloping gently towards the Bay of Bengal (JICA 2011). Considering the depositional history of the GBM delta, the study area consists of Late Holocene to Recent alluvium of Quaternary geological time (Bahar and Reza 2010), which is crisscrossed by a large number of rivers. Geomorphologically, the study area is shaped by fluvial processes and, thus, fluvio-tidal origin in nature, while the area consists of a complex network of tidal rivers associated with natural levees, bars, back swamps, floodplains, deltaic plains, and abandoned river channels as notable geomorphic units (JICA 2011). Moreover, the area is divided into seven lithostratigraphic units, which are characterized by various proportions of sand, silt, and clay with a limited coarser sand fraction (JICA 2011). Such various proportions of the alluvium of the study area demonstrate that the alluvium comprises coarse to very fine sand, silt, and silty clay commonly found from the surface to a depth of about 300 m, whereas the soils are commonly peaty in nature, while both calcareous and non-calcareous soils at the surface are well noted (Bahar and Reza 2010). However, considering heterogeneous mixture of the alluvium of the study area, the aquifers are classified as upper (50–100 m from the surface), main (250–350 m from the surface), and deep aquifers (below 250–350 m) (Datta and Ghosh 2015) and it is estimated that the transmissivity, storage coefficient, and specific yield of this area are 3900 m2/day, 2 × 103, and 16.6%, respectively (Bahar and Reza 2010).
Water sampling and analysis
The water samples were collected during monsoon period (June to August) from 50 sampling stations located in the study area as shown in Fig. 1. The information regarding well depths were collected from the record taken by the well owners and local government offices. There are two types of tube wells used in this study area for water supply such as (1) shallow wells (1–150 m depth) and (2) deep wells (160–300 m depth). The sources of surface water in the area are mainly ponds and lakes. All water samples were collected from the study area based on its availability. The groundwater and surface water sampling stations are enlisted in Tables 1 and 2.
For water sampling, 50 plastic bottles (each of 1 L volume) were collected and washed by 4 to 5 times using de-ionized water. Immediately after collection of each water sample, pH, electrical conductivity (EC), total dissolved solids (TDS), and temperature were measured in the field by a portable pH and an EC/TDS meter (HI-9635, portable waterproof Multirange Conductivity/TDS meter, HANNA). The collected water samples were sealed on site using polythene paper and tapes to avoid any external air intrusion into the samples. After that, the samples were brought to the laboratory and stored below 4 °C temperature for further water quality analysis. In the laboratory, sodium and potassium ions of the water samples were determined by using the flame photometer (Flame photometer-models PEP 7 and PEP 7/C), while calcium and magnesium ions were determined by EDTA titrimetric method. Bicarbonates, fluorides, and bromides were determined by potentiometric method, while chloride ions were determined using standard silver nitrate solution by ion-selective electrode method (Cole-Parmer iodine electrode, model no. 27502-13). In the case of nitrate, sulphate, and phosphate ions, the spectrophotometric method (Thermo spectronic, UV-visible Spectrophotometers, Helios 9499230 45811) was used to determine their amounts. The iron contents were determined by titrimetric method and the arsenic contents were determined by the Econo Quick test kit (Industrial Test Systems, Rock Hill, SC).
The microbial analyses of the water samples were performed to assess the potential health risk to human and evaluate the drinking water quality. A total of 50 water samples were collected from the entire study area. A 500-mL plastic bottle was used for microbiological water sampling. Before sampling, the bottles were sterilized in an autoclave at 121 °C for 20 min. Before sterilizing the bottles, four or five drops of aqueous sodium thiosulfate solution were used to inactivate residual chlorine but did not affect the microorganisms in the water sample. Samples were transported to the laboratory under colder temperature by using icebox and analyzed within 6 h of their collection.
For surface water sampling, sterilized bottles were held by the lower part and submerged it to a depth of about 20 cm below the water surface with the mouth faced slightly upwards. After filling the surface water into the bottle, the stopper was placed on the bottle mouth. After sampling, the samples were transported to the laboratory in a dark and cold place at about 4 °C and processed within 6 h of their collection.
The membrane filtration technique (American Public Health Association 1998) was used to estimate the counts of coliforms in the surface water samples. This technique was used because of having relatively large number of water samples and the method gives a direct count of total coliforms, fecal coliforms, and Escherichia coli present in a water sample.
Total dissolved solids measurement
The major ions that constitute and contribute to most of the TDS in natural water involve hydrogen, calcium, magnesium, sodium, potassium, carbon, nitrogen, oxygen, silicon, phosphorus, sulfur, chlorine, and iron. The analysis of such parameters is fundamental in assessing groundwater (Ramesh and Anbu 1996). Therefore, this study has analyzed the major ions of the groundwater that are used for drinking and other purposes. The analyses of physio-chemical properties of water samples were conducted in the laboratory of Environmental Science Discipline at Khulna University, Khulna, Bangladesh, and Department of Public Health and Engineering, Khulna, Bangladesh (DPHE). The concentrations of major ions listed above were determined in the laboratory by using standard methods (Ramesh and Anbu 1996). The analyses of water temperature, pH, salinity (Microprocessor pH meter), EC, and turbidity (Microprocessor pH meter) were performed on-site at the station.
Microbiological method
For the E. coli and fecal coliform (FC) count, 100 mL sample of water was filtered by a 0.45-μm pore-size membrane filter (Millipore Corp., Bedford, MA, USA), and after that, filters were being placed on top of the membrane of fecal coliform (mFC) and then mTEC agar plates, respectively (American Public Health Association 1998). To quantify FC, the mFC plates were incubated at a temperature of 44 °C for 18 to 24 h. The plates of mFC agar plates were also incubated at temperature of 35 ± 0.5 °C for 2 h and then the incubation temperature was raised to 44.5 ± 0.2 °C and kept for 22 to 24 h to quantify the E. coli. Color changes and the blue colonies were counted as FC. Yellow-green, yellow-brown, and yellow-pale colonies were numbered as E. coli and expressed by the colony-forming unit (CFU) per 100 mL.
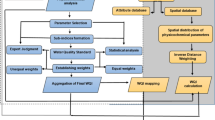
Water quality mapping technique
ArcGIS 10.3.1 was used for the preparation of surface and groundwater quality map of the study area. The thematic maps for the parameters were prepared using inverse distance weighting (IDW), a spatial analyst tool. IDW is a method of interpolation that estimates cell values by averaging the values of sample data points in the neighborhood of each processing cell. The closer a point is to the center of the cell being estimated, the more influence, or weight, it has in the averaging process (ESRI 2011).
Results and discussions
Analytical accuracy of water quality parameter analysis
Analytical accuracy is essential for identifying the chemistry of natural water. The analytical accuracy of water quality samples was confirmed by determining electro neutrality (EN) of each analyzed water sample (Datta and Ghosh 2015). The calculated EN values for the surface water, deep groundwater, and shallow groundwater samples were less than 10%. The relationship between total cations and total anions for the surface water and groundwater were found to be linear (regression coefficient of 0.99). Therefore, the analyses performed for calculating EN values and developing ionic relatationship for both the surface and groundwater indicated that the analytical accuracy of the water sample analyses is well accepted and the total cations and the total anions of the surface and groundwater are strongly correlated.
Hydrochemical facies of the surface and groundwater
Hydrochemical facies is necessary to identify the chemical background of the water and these facies can be explained by plotting Piper diagram (Piper and Roth 1953). In aquifers, the process of chemical interactions can be determined by hydrochemical facies (Sivasubramanian et al. 2013). Piper diagram is generally used to illustrate the major ionic composition of the surface water and groundwater samples (Ophori and Toth 1989). Using Piper diagram, it can be identified that which materials are dissolved in the water and what is the hydrochemical problem in that particular area. The Piper diagram of the surface and groundwater samples is shown in Fig. 2. The following hydrochemical facies were identified for the surface water: (a) Ca-Na-HCO3-Cl (13.3%); (b) Ca-Na-Cl-HCO3-Cl (6.67%); (c) Na-Ca-HCO3-Cl (40%); (d) Na-Ca-Cl-HCO3 (26.7%); (e) Na-Cl (6.67%); and (f) Ca-Na-HCO3 (6.67%). Results suggested that most of the surface waters are characterized by Na-Ca-HCO3-Cl facies. On the other hand, the following hydrochemical facies are identified for the groundwater: (a) Na-Cl-HCO3 (37.14%); (b) Na-HCO3-Cl (2.85%); (c) Na-Ca-Cl-HCO3 (8.57%); (d) Na-Ca-Mg-HCO3-Cl (8.57%); (e) Na-Cl (2.85%); (f) Ca-Na-HCO3-Cl (2.85%); (g) Na-Ca-HCO3-Cl (2.85%); (h) Na-HCO3 (2.85%); (i) Ca-Mg-Na-HCO3 (5.74); (j) Na-Cl-HCO3 (2.85%); (k) Ca-Na-HCO3 (2.85%); (l) Ca-Mg-HCO3-Cl (5.74%); (m) Ca-Mg-HCO3 (5.74%); (n) Na-Ca-Mg-Cl-HCO3 (2.85%); (o) Na-Ca-Cl-HCO3 (2.85%); and (p) Ca-Na-HCO3-Cl (2.85%). It was observed that most of the groundwaters are characterized by Na-Cl-HCO3 facies, indicating mixing composition of the groundwater.
Mechanisms controlling water quality
The Gibbs diagram was used for the evaluation of hydrochemistry of water in the study area. Mechanisms controlling water are a meaningful reaction between the water and minerals of aquifers, which is important to understand the nature of water quality. The Gibbs diagram has been widely used to find which factor among rain, rock-water interaction, and evaporation dominantly influences the water composition (Gibbs 1970). The Gibbs diagram was prepared by plotting [Na / (Na + Ca)] ratio against the respective TDS concentration. The Gibbs diagram explaining the mechanisms controlling RupshaUpazila’s water quality is illustrated in Fig. 3. The Gibbs diagram of the surface water shows that all samples are fallen in the rock-water interaction dominance field, indicating that the chemistry of surface water of Rupsha Upazila is dominated by the interaction between water and rock material. The cation exchange and silicate weathering can be dominant processes controlling the chemical composition of the surface water of Rupsha Upazila (Koffi et al. 2017). Since surface water samples were collected during the monsoon (rainy) period (June to August), it may be expected that the samples should have fallen under precipitation zone due to dilution effect of rainwater. However, in this study, surface water samples do not show any precipitation dominance because the hydrochemical interaction processes of the surface water with the basinal lithostratigraphic column have acquired dissolved loads to the surface water, and thus, surface water samples are situated under rock dominance zone. The groundwater ionic composition of Rupsha Upazila found to be dominated by the chemical interaction of water and rock as well as seawater intrusion. In addition, a small portion of groundwater samples were represented by evaporation-crystallization process.
Drinking water quality mapping
For assessing the drinking water quality of the surface and groundwater in Rupsha Upazila, the water sample parameters were compared with the World Health Organization (WHO) standards and Bangladesh drinking water quality standards. The statistics of water quality parameter for surface and groundwater sample is described in Table 3. Table 4 represents the comparisons of the water quality parameters with the WHO and Bangladesh (BD) standards. To display the condition of contamination of surface water and groundwater with different ions and microbial contamination, water quality mapping for TDS, EC, chloride, arsenic, iron, and E. coli was developed and presented in Figs. 4, 5, and 6 to understand the accurate condition of the water in study area. Table 4 indicates that pH values for both the surface and groundwater are within the range of WHO and BD standards.
TDS of water is an important parameter in assessing its potability (Islam et al. 2017). The analytical data of the surface and groundwater samples showed that 6.6% and 65.71% of the samples had TDS concentration above the WHO and BD standards (Table 4). According to past study (Davis and Dewiest 1966), the classifications of the groundwater (GW) and surface water (SW) based on TDS are shown in Table 5 and Fig. 4. Results indicated that 66.7% of surface water is desirable for drinking while groundwater is unsuitable for drinking purposes (Table 5). The analytical data of EC showed that 57.13% groundwater exceeded the EC standard of WHO and BD. According to previous study (Ducci 1999), the classification of the groundwater and surface water quality based on EC is shown in Table 5 and Fig. 4. It clearly indicated that surface water is desirable for drinking water purposes based on TDS concentration, and EC in surface water is also under optimum conditions and vice versa for groundwater sample.
Presence of chloride in water is considered as one of the major causes of salinity (Islam et al. 2017; Mogheir et al. 2013). Chloride ion in drinking water mostly provides palatability when present between low to medium concentration. However, high concentration of chloride ion in drinking water can make unpleasant to drink. Based on the water quality analysis of chloride ion (Cl−), all surface water samples were within the WHO and BD standards, but 11.42% groundwater samples exceeded the WHO and BD standards. According to Ducci (1999), the classification of the groundwater and surface water quality based on chloride concentration is shown in Table 5 and Fig. 5. Results suggested that groundwater quality is poor based on chloride ion concentration and will affect the taste of drinking water.
Arsenic contamination is one of the most important issues regarding public health and safety in Bangladesh which is the most arsenic affected country in the world (Islam et al. 2017). Arsenic has long-term effect and it can cause skin cancer and damage to the kidney. It can also affect several skin diseases like pigmentation and thickening (Kapaj et al. 2006). However, due to the massive groundwater arsenic contamination, the government of Bangladesh has determined the arsenic standard of 0.05 mg/L. Arsenic in the surface water samples collected in this study were within the BD standard but 40% of the groundwater samples exceeded the BD standard (Table 4). According to the BD water quality standard, the classification of the surface and groundwater quality based on arsenic concentration is shown in Table 6 and Fig. 5. Results suggested that surface water is suitable for drinking than groundwater.
Based on study results of iron (Fe) concentration in water, it was observed that 6.6% surface water and 2.85% groundwater exceeded the limit of WHO and BD standards. Thus, both surface water and groundwater are suitable for drinking purposes in terms of iron concentration (Tables 4 and 6, Fig. 6).
Other cations (sodium, potassium, calcium, magnesium) and anions (PO43−, NO3−, and SO42−) in water sample were investigated for this study. Sodium ion (Na+) is essential ion for human health because a higher Na+ intake may cause hypertension, congenial heart diseases, nervous disorder, and kidney diseases. In addition, high salt contents in drinking water decrease palatability of water and causes intestinal irritation and laxative effects in humans (Islam et al. 2017; WHO 2003). Based on water quality analysis, it was observed that 13.3% surface water and 65.71% groundwater samples exceeded the WHO and BD standards indicating that groundwater has high sodium content. Potassium ion (K+) is also a critical function for the humans but higher potassium intake causes kidney failure and type 1 diabetes. For K+ concentration, 13.3% surface water and 17.14% groundwater samples were above the WHO and BD standards, respectively. Both calcium ion and magnesium ions are responsible for hardness of the water. For Ca2+, all surface water samples were within the WHO and BD standards and 45.71% of groundwater exceeded the limit of WHO and BD standards. For Mg2+, no surface water samples exceeded the WHO and BD standards, but 37.14% groundwater exceeded the WHO and BD standards. Moreover, comparing by the hardness classification (Pennak 1971), the surface water (146 mg/L as CaCO3) and groundwater (338 mg/L as CaCO3) in the study area were slightly hard and very hard, respectively. The analytical data of PO43−, NO3−, and SO42− concentrations for both surface and groundwater were within the allowable limit of WHO and BD standards.
In terms of microbiological contamination in drinking water, all surface water and 97.14% groundwater exceeded the TC limit of WHO and BD standards. For FC, all samples of the surface water and 80% of the groundwater exceeded the WHO and BD standards. In addition, for E. coli, 100% surface water samples and 62.85% groundwater samples exceeded the WHO and BD standards. According to WHO (2003), human risk due to E. coli-contaminated water is categorized into five types and described in Table 7 and Fig. 6 for surface and groundwater investigated in this study. The surface water samples are not risk free from drinking aspect, while 37.14% of the groundwater samples are risk free. This suggested that most of the water sources in Rupsha Upazila are in danger due to waterborne diseases.
Based on water quality parameters studied in this study, it is recommended that source of surface water in Rupsha Upazila would be better to use for drinking water purposes compared to groundwater. However, surface water did not meet the standard of E. coli which is a major threat for public health. Therefore, surface water must be treated in water treatment plant before using for drinking.
Conclusions
In developed countries, surface water is the major source of drinking water because of the availability of advanced water treatment processes. However, in developing countries such as Bangladesh, groundwater is the most extractable source compared to surface water because of the economic feasibility to avoid costly water treatment processes. The environmental assessment in Rupsha Upazila of Bangladesh indicated that surface water quality is better as compared to groundwater in terms of physico-chemical perspectives, and hence, the development of the management strategies for surface water utilization for drinking purposes is necessary.
References
American Public Health Association, A. (1998). Standard methods for the examination of water and wastewater.
Babiker, I. S., Mohamed, M. A., & Hiyama, T. (2007). Assessing groundwater quality using GIS. Water Resources Management, 21(4), 699–715.
Bahar, M. M., & Reza, M. S. (2010). Hydrochemical characteristics and quality assessment of shallow groundwater in a coastal area of Southwest Bangladesh. Environmental Earth Sciences, 61(5), 1065–1073.
Datta, D. K., Ghosh, P. K. (2015). Groundwater of the municipalities of southwestern coastal Bangladesh. Surface and sub-surface water in Asia: issues and perspectives. IOS Press BV, The Netherlands, 120-145.
Davis, S., & Dewiest, R. (1966). Hydrogeology. New York: Wiley.
Ducci, D. (1999). GIS techniques for mapping groundwater contamination risk. Natural Hazards, 20(2–3), 279–294.
ESRI, R. (2011). ArcGIS desktop: release 10. Environmental Systems Research Institute, CA.
Gibbs, R. (1970). Mechanisms controlling world water chemistry. Science, 170(3962), 1088–1090.
Hasan, M. (2016). Correlation among TOC and heavy metals in groundwater used for irrigation in Bangladesh. In: Civil engineering, Vol. MSc Bangladesh University of Engineering and Technology. Dhaka, Bangladesh.
Islam, S. D.-U., Majumder, R. K., Uddin, M. J., Khalil, M. I., & Alam, M. F. (2017). Hydrochemical characteristics and quality assessment of groundwater in Patuakhali district, southern coastal region of Bangladesh. Exposure and Health, 9(1), 43–60.
Jähnig, S., & Cai, C. (2010). River water quality assessment in selected Yangtze tributaries: background and method development. Journal of Earth Science, 21, 876–881.
JICA. (2011). Feasibility study for Khulna Water Supply Improvement Project in Bangladesh. Japan International Cooperation Agency, Final report, Tokyo, Japan.
Kapaj, S., Peterson, H., Liber, K., & Bhattacharya, P. (2006). Human health effects from chronic arsenic poisoning—a review. Journal of Environmental Science and Health, Part A, 41(10), 2399–2428.
Koffi, K. V., Obuobie, E., Banning, A., & Wohnlich, S. (2017). Hydrochemical characteristics of groundwater and surface water for domestic and irrigation purposes in Vea catchment, Northern Ghana. Environmental Earth Sciences, 76(4), 185.
Lawson, E. (2011). Physico-chemical parameters and heavy metal contents of water from the Mangrove Swamps of Lagos Lagoon, Lagos, Nigeria. Advances in Biological Research, 5(1), 8–21.
Liao, Y., Xu, J., & Wang, W. (2011). A method of water quality assessment based on biomonitoring and multiclass support vector machine. Procedia Environmental Sciences, 10, 451–457.
Minar, M., Hossain, M. B., & Shamsuddin, M. (2013). Climate change and coastal zone of Bangladesh: vulnerability, resilience and adaptability. Middle-East Journal of Scientific Research, 13(1), 114–120.
Mogheir, Y., Foul, A. A., Abuhabib, A., & Mohammad, A. W. (2013). Large-scale brackish water desalination plants in Gaza Strip: assessments and improvements. Journal of Water Reuse and Desalination, 3(3), 315–324.
Ophori, D., & Toth, J. (1989). Patterns of ground-water chemistry, Ross Creek Basin, Alberta, Canada. Groundwater, 27(1), 20–26.
Parparov, A. D., Berman, T., & Hambright, K. D. (2014). Water quality assessment. Aquatic Ecology, 6, 607–615.
Pennak, R. W. (1971). Toward a classification of lotic habitats. Hydrobiologia, 38(2), 321–334.
Piper, W. W., & Roth, W. (1953). Perfect crystals of zinc sulfide. Physical Review, 92(2), 503.
Ramesh, R., Anbu, M. (1996). Chemical methods for environmental analysis: water and sediment. Macmillan India.
Singh, P. K., Tiwari, A., Panigarhy, B., & Mahato, M. (2013). Water quality indices used for water resources vulnerability assessment using GIS technique: a review. International Journal of Earth Science Engineering, 6(6–1), 1594–1600.
Sivasubramanian, P., Balasubramanian, N., Soundranayagam, J. P., & Chandrasekar, N. (2013). Hydrochemical characteristics of coastal aquifers of Kadaladi, Ramanathapuram District, Tamilnadu, India. Applied Water Science, 3(3), 603–612.
Statistics, B.B. (2011). Statistical yearbook of Bangladesh. Statistics Division, Ministry of Planning, Dhaka, Government of the People’s Republic of Bangladesh.
WHO. (2004). Guidelines for drinking-water quality. World Health Organization.
WHO. (2003). Guidelines for safe recreational water environments: coastal and fresh waters. World Health Organization.
Acknowledgments
The authors acknowledge the laboratory support provided by Mr. Shankar Kumar Sarker and Ms. Morzina Begum, Section Officer (Lab), Environmental Science Discipline, Khulna University, during the water sample analyses. We would like to give thanks to the officials of Department of Public Health and Engineering, Khulna, Bangladesh (DPHE), for assistance in analyzing the water samples.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, A., Ghosh, P.K., Hasan, M. et al. Surface and groundwater quality assessment and identification of hydrochemical characteristics of a south-western coastal area of Bangladesh. Environ Monit Assess 192, 258 (2020). https://doi.org/10.1007/s10661-020-8227-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-8227-0