Abstract
Association of trace metal concentrations in water is problematic; however, its information is scarce and sometimes contradicted. This work presents variations in dissolved major constituents and trace element concentrations along the quaternary aquifers located in middle Upper Egypt (Minia and Assiut governorates). A total of 205 groundwater samples from these aquifers were collected. Auxiliary parameters (pH, alkalinity, and conductivity), major cations (Ca2+, Mg2+, Na+, and K+), dominant anions (HCO3−, SO42−, Cl−, and NO3−), and trace element (B, Fe, Cu, Mn, Ni, Pb, Cd, and Cr) concentrations were measured in all samples. Univariate (correlation coefficient and scatter matrix) analysis was employed combined with multivariate (principal coordinates analysis) analysis to identify the chemical characteristics of groundwater that are responsible for generating most of the variability within the dataset. Also, hierarchical cluster analysis was applied to classify the geochemical origin of the groundwater constituents. The results indicate that the groundwater pollution is mainly due to water–rock interactions, including aquifer matrix dissolution, redox reaction of trace metals, input from wastewater, and agricultural fertilizers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Less than 2.5% of the Earth’s water is classified as freshwater (Shiklomanov, 1993). Due to this limited freshwater resource, 1.6 billion people are facing water shortages and two-thirds of the world’s population faces water scarcity at least 1 month in a year (FAO, 2007; Mekonnen and Hoekstra, 2016). Freshwater is a restricted resource in arid and semiarid areas due to the limited and irregular distribution of precipitation throughout the year. There may also be a lack of freshwater supply in densely populated areas, and, with climate change and sea level rises projected, problems are expected to increase over the next decades (e.g. Oude Essink et al., 2010). Moreover, the limited freshwater resources are under threat from pollution, mainly caused by human activities. Human activities threaten the ecosystem by introducing large concentrations of chemicals into the water body. They are also responsible for point and non-point sources of pollutants such as trace metal contamination in aquatic systems. Widespread trace metal pollution is the main concern for ecological quality, particularly in aquatic systems because of the potential toxicity of several metals. Its occurrence in aquatic systems is often due to anthropogenic activities. Trace metals are insidious contaminants due to their nonbiodegradable nature (Needleman, 1990; Needleman et al., 1990; Goyer, 1993). Low concentrations can cause long-term toxicological effects. The accumulation, bioavailability, and mobility, which govern speciation of dissolved hazardous trace metals in the water system, are of critical interest due to their ecotoxicology and economic impact on social development and human health (Pape et al., 2012; Varol, 2013; Gurumurthy et al., 2014; Bu et al., 2015).
Aquatic systems are dynamic and often out of equilibrium. Water can contain a varied range of metal-complexing components. The resultant interactions dictate the transport, reactions, and spatial and temporal distribution of trace metals. Subsequently, the physicochemical speciation form in which a metal ion exists becomes the main issue because this determines its mobility, bioavailability, and toxicity in environmental compartments. Whereas the material morphology influences their transport behavior, their chemical composition modifies their reactivity. Many ligand complexing constituents existing in polluted waters are heterogeneous, multifunctional, and physicochemical complex molecules. Basically, trace metal dissolution in the contaminated medium is controlled by the redox conditions, which need to be better understood.
The dispersion of toxic metals in ecosystems increases with their mobility (Schröder et al., 2005). Heavy metals present under varying physicochemical forms of solubility and adsorption on mineral surfaces. Also, these metals form complexes with heterogeneous ligands (inorganic, via formation of organometallic complexes, with polysaccharides, microorganisms, iron and manganese oxides or oxyhydroxides, and clays) and are precipitated or entrapped in mineral phases in water systems that play important roles in altering metal speciation and transportation (Tessier et al. 1996; Santschi et al. 1997; Taillefert et al. 2000).
The changes in redox condition between oxic and anoxic water also affect the speciation and bioavailability of trace metals. The redox reactions of dissolved trace metal concentrations are also affected by such conditions as long-term natural and anthropogenic supply of metals, spatial distributions, seasonal fluctuations, water discharges, water temperature, pH, and dissolved oxygen (DO). Changes in the cycling and distribution of trace metals are a function of the internal hydrological variability conditions and external environment (Soto-Varela et al., 2014; Bu et al., 2015). For water system protection, as much information as possible is essential for the description of dissolved trace metal cycles (Weng and Wang, 2014).
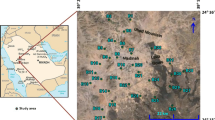
In our knowledge, it is the first work monitoring the trace elements contamination and quantified the spatial distribution of groundwater quality in this region. The goal of the present study is to survey dissolved trace metal concentrations in two middle Upper Egypt governorates, namely Minia in the north and Assiut in the south as shown in Fig. 1. This is intended to aid in monitoring, predicting sources, and understanding associated trace metals in groundwater. To assess these contaminant toxicities in the affected environment, deciphering the behavior and processes controlling the dissolved trace metals’ distribution is crucial.
Materials and methods
Water samples collection and analysis
Groundwater samples were collected from Minia and Assiut governorates. Electrical conductivity (EC) and pH values were measured in situ. EC values were determined using a WPA CM 35 Conductivity Meter. pH values were determined according to ASTM D-1293 using an Eu-tech cyberscan-pH 310 meter with a combined glass electrode. The samples were stored in 500-ml transparent glass bottles in a refrigerator at about 20 °C to prevent any change in volume due to evaporation and transported to the laboratory. The analyses were carried out in the Central Health Laboratories, Abdein, Cairo, Egypt. The elements (B, Fe, Cu, Mn, Ni, Pb, Cd, and Cr) were detected using an atomic absorption spectrometer (GBC, GF 3000, USA). Other parameters including alkaline species (CO3, OH, and HCO3) were measured according to ASTM D-3875 calculations done using Alkalinity Calculator Ver. 2.10 (USGS). Minor constituents such as nitrate (NO3−) were tested using the methods of the APHA (1998). Anions (HCO3−, SO42−, and Cl−) and cations (Ca2+, Mg2+, Na+, and K+) were determined according to ASTM D-4327 using ion chromatography. The instrument used was a Dionex 600 equipped with high capacity columns (AS9 and CS12) for anion and cations, respectively. Trace metals (Fe and Cu) were estimated very precisely using the inductively coupled plasma (ICP) emission technique according to ASTM D-1976.
Study area
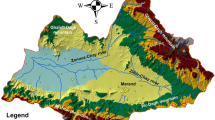
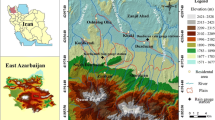
Minia Governorate in Upper Egypt is located in the vicinity of coordinates 28° 06′ 35.57″ N and 30° 45′ 1.08″ E. It is about 245 km south of Cairo. The following cities are in this governorate from north to south: El Idwa, Maghagha, Beni Mazar, Matai, Samalut, Minia (old name of the city is Men'at Khufu), Abu Qurqas, Mallawi, and Deir Mawas. The area has a hot climate with arid to semiarid conditions rainless in summer, with mild to rare precipitation in winter. Minia Governorate is essentially covered by Middle Eocene to Recent sedimentary rocks. The stratigraphic sequence built up from base to top is as follows: Middle Eocene limestone; Pliocene clays; Plio-Pleistocene sand and gravel; Pleistocene sand and gravel; and Holocene silt and clay (Zaki et al. 2001). The Pleistocene semi-confined aquifer represents the main water-bearing formation in Minia Governorate, consisting of gravels and sands. This aquifer directly rests on the Pliocene clay, which acts as the base of the aquifer and the fissured Eocene limestone as shown in Fig. 2a. The thickness of this aquifer decreases to the west of the area (towards an Eocene plateau), and it is hydraulically connected with the underlined Eocene aquifer through many faults (Sanad 2010). The surface water (particularly the irrigation canals) is the main source of aquifer recharge. The depth of water in the Pleistocene aquifer varies with locality, from 0.9 to 8 m, while the water level ranges between 29.4 and 43 m. The groundwater level declines in a south to north direction due to the hydraulic gradient due to the surface water in the irrigation and drainage system (Abou Heleika and Niesner 2009; Kamel 2012). The flow of groundwater is directed generally towards the River Nile (northeast).
Assiut Governorate is bounded by a limestone plateau and is located in the vicinity of 27° 10′ 51.46″ N and 31° 11′ 1.25″ E. Assiut includes 11 towns extending from Dirout in the North to El-Badary in the south. Assiut Governorate stretches for about 120 km along the Nile banks, and its distance from Minia Governorate is about 112 km. It is bounded in the east and the west by agricultural lands and limestone plateau. The sedimentary rock origin is Lower Eocene to Quaternary (Fig. 2b). The Lower Eocene carbonate rocks are represented by the plateaux bordering the Nile Valley. The Quaternary sediments represent a water-bearing formation. The Quaternary deposits are composed of sand and gravel plains (Pleistocene) and are generally capped by silty clay layers as alluvial deposits (Holocene) and are unconformably resting on the Eocene carbonate (Waleed et al., 2009; El-Aassar et al., 2016).
Statistical analysis
The PAST statistical program (Hammer et al., 2001) was used to recognize principal coordinate analysis (PCoA) on the results from the groundwaters and to examine the relations between the variables. SPSS version 25 statistical program was used to perform cluster analysis (CA). The Origin Pro 8 statistical program was used to determine the scatter matrix and the correlation between each groundwater variable.
Results and discussions
Groundwater characteristics
All groundwater samples’ physicochemical parameters for the area under investigation are summarized in Table 1 with minimum, maximum, mean, and standard deviation values. The groundwater samples fluctuate from acidic to alkaline conditions with pH values ranging from 2.82 to 9.7 with a mean value of 7.82 ± 0.60 (mostly alkaline pHs). The electrical conductivity (EC) and alkalinity values vary significantly as shown in Fig. 3, with ranges of 2100–320 μS cm−1 and 695–102 mg L−1, with a mean value of 829.53 ± 345.87 μS cm−1 and 299.05 ± 89.01 mg L−1, respectively. Groundwater conductivities indicated that most of the samples had a high ionic strength. The concentrations of the cations Ca2+, Mg2+, Na+, and K+ ranged from 345 to 2 mg L−1, 180–3 mg L−1, 197.57–12.56 mg L−1, and 25–0.8 mg L−1 with mean values of 75.70 ± 41.15 mg L−1, 39.50 ± 25.11 mg L−1, 53.87 ± 31.78 mg L−1, and 4.25 ± 5.28 mg L−1, respectively. The concentration of dissolved anions such as HCO3−, SO42−, Cl−, and NO3− varied from 730 to 90 mg L−1, 751 to 6.5 mg L−1, 505 to 14 mg L−1, and 0.80 to 0 mg L−1 with the mean concentrations of 271.65 ± 106.07 mg L−1, 123.79 ± 125.01 mg L−1, 82.80 ± 71.37 mg L−1, and 0.46 ± 6.10 mg L−1, respectively. The dominant major cations in the groundwater samples are in the order of Ca2+ > Na+ > Mg2+ > K+, while the dominant anions are HCO3− > Cl− > SO42− > NO3−. In line with Tariq et al. (2008) and Ledesma-Ruiz et al. (2015), the higher standard deviations than the mean displayed by K+, SO42−, Cl−, and NO3− indicated their randomly fluctuating concentration levels in the groundwater. This revealed that the geochemistry of the investigated area is not homogenous. The elevated concentrations of HCO3− and Ca2+ ions indicated that the study area might be affected by carbonate mineral dissolution. Holland (1978) mentioned that 74 ± 10% of Ca2+ and 40 ± 20% of Mg2+ in groundwater results from carbonate mineral dissolution.
The trace elements B, Fe, Cu, Mn, Ni, Pb, Cd, and Cr concentrations ranged from 1.5 to 0.008 mg L−1, 3.5–0 mg L−1, 1.85–0 mg L−1, 9–0 mg L−1, 0.40–0 mg L−1, 0.34–0 mg L−1, 0.45–0 mg L−1, and 0.04–0 mg L−1, respectively. The mean concentration of these elements follows the descending order: Mn > Fe > B > Cu > Cd > Pb > Ni > Cr. The mean values of Mn (0.514 ± 0.713 mg L−1), Fe (0.381 ± 0.526 mg L−1), Cd (0.035 ± 0.075 mg L−1), and Pb (0.026 ± 0.056 mg L−1) are higher than the water quality standards set by Egyptian Standard Methods Decision (Decree of Health Ministry No. 458/2007) and international organization (WHO, 2011). This suggests the possibility of infiltration of these metals from anthropogenic sources.
Groundwater pollution sources and quality controlling factors
The groundwater data subjected to the statistical method principal coordinates analysis (PCoA) retains most of the data variation while reducing the dimensionality, to elucidate the relationship of group variables. This reduction proceeds by identifying principal vectors in which the variation in the data is maximal and expressed in the patterns of correlation between variables. Applying PCoA to identify the main variability characters of dissolved elements in the groundwater helps to distinguish patterns dominating the element content (Mora et al., 2017). According to Liu et al. (2003), they involve the use of only factors with eigenvalues exceeding one. Only three important components are significant for groundwater datasets based on eigenvalues more than 1 as revealed in Table 2.
Figure 4 a reveals four groups originated from the effect of both component 1 with an eigenvalue of 2.14, which represents 26.20%, and component 2 with an eigenvalue of 1.17, which represents 14.24%. The first group contains EC, Na+, Mg2+, Cl−, B, Ca2+, and SO42−, these elements resulting from different possible sources in groundwater: coming naturally from the aquifer matrix through water–rock interactions and anthropogenically as a result of pollution plumes. Hem (1985) and Battistel et al. (2016) indicate that Cl−, B, and SO42− often relate to sedimentary rocks. However, Srivastava and Ramanathan (2008), Chetelat and Gaillardet (2005), and Wu et al. (2014) stated that the elevated concentrations of Cl−, B, and SO42− in groundwater are frequently considered an indicator of contamination phenomena. Likewise, Nigro et al. (2017), Li et al. (2019), and Wu et al. (2019) stated that Na+, Mg2+, and Ca2+ are a natural constituent of the groundwater because these cations may derive from the leaching of rocks, but they can also derive from landfill leachate. In line with Bodrud-Doza et al. (2016) and references therein, EC indicates geogenic hydrogeochemical evolution of groundwater by geological weathering conditions due to high ionic concentrations and probably caused by high anthropogenic activities with ion exchange. Consequently, such input from cations and anions would be the greatest contributing influence on the electrical conductance. The second group contains HCO3−, NO3−, alkalinity, pH, and K+, which suggests weathering of carbonates and contamination by fertilizer usage, especially nitrogenous fertilizer (KNO3). Alkaline pH and HCO3− reflect intensive weathering of carbonate environments, and alkaline nature of groundwater. The third group contains two heavy metals, Cd and Pb, that may relate to anthropogenic sources such as wastewater, municipal (electric) waste, and sewage drains. The fourth group contains the rest of the heavy metals, Ni, Fe, Mn, Cu, and Cr, that are expected to leach from weathering of rocks, which is the primary source of these metals. Possible anthropogenic sources of these metals are wastes and sewage sludges through leaching from topsoil and entry into groundwater.
Figure 4 b reveals seven groups originated from the effect of both component 3 with an eigenvalue of 1.02, which represents 12.50%, and component 2 with an eigenvalue of 1.17, which represents 14.24%. The first group contains EC, Na+, Mg2+, Cl−, and NO3−, as in the previous section (component 1 vs. component 2). These elements may occur naturally in groundwater through water–rock interactions and anthropogenically as a result of agricultural activity. The second group contains HCO3−, pH, and alkalinity that reveals intensive weathering of carbonate deposits and alkaline pH of groundwater. The third group matches with the results found in component 1 vs. component 2 and contains Cd and Pb, which strengthens the suggestion that these metals originated from the same anthropogenic sources. The fourth group contains Ni, Cu, and Fe that suggests that these metals have entered the groundwater as a result of human activities. The fifth group contains K and Mn suggesting that leaching from weathering of rocks is the primary source of these metals. The sixth group contains Cr and B indicates that these metals may originate from wastes and sewage. The seventh group contains Ca2+ and SO42− implying that these species may derive from calcium sulfate fertilizers. Fertilizers, especially nitrogen (Ca (NO3)2 and KNO3) and sulfur (CaSO4 and MgSO4) fertilizers, are presumably the most common aquifer contaminants.
Comparison and grouping of groundwater constituents
Cluster analysis (CA) revealed three main clusters for datasets of analyzing parameters. CA was applied to detect physicochemical groupings in the groundwater datasets, as shown in Fig. 5. This analysis indicates a complex hydrochemistry in the area with ion exchange between weathering minerals in the aquifers, domestic sewage effects, leaching of materials, and the infiltration of agricultural fertilizers. Parameters belonging to the same cluster were likely derived from the same source or conditions.
In agreement with Omo-Irabor et al. (2008) and Li et al. (2018), Cluster 1 included fertilizers and nutrients that contain K+ and NO3−, which might be explained by leaching of agricultural fertilizers from the soil horizon. Also, K+ may originate from biogenic sources due to leaching from decomposed vegetation and resulting from rock weathering of silicates and K-feldspars from the aquifer medium causing groundwater contamination. Cluster 2 consists of redox-sensitive elements such as Pb, Cd, Mn, Cu, Cr, Fe, and Ni, and reflects the influence of domestic and agricultural pollution deriving from the distal part of domestic sewage and drainage channels that connect to the groundwater. Changes in pH and redox conditions play a significant role in the variability and/or cyclicity of dissolved trace metals because the pH-dependent redox process influences how trace elements are scavenged in aquatic environments. Whereas the oxidation process may be affected by increased pH, trace metals can combine into the particulate phase and coprecipitate (Mora et al. 2017). Cluster 3 includes elements resulting from water–rock interactions that consist of EC, Na+, Cl−, Ca2+, SO42−, Mg2+, B, alkalinity, HCO3−, and pH from the dissolution of minerals under alkaline conditions due to the weathering of aquifer matrix, especially carbonates. The concentration variations of these elements and parameters are controlled by dilution of mineralized groundwater by non-mineralized superficial waters during the aquifer recharge process.
Correlation matrix analysis
Pearson’s correlation (PC) coefficient was used to identify the interrelationship and association pattern among diverse geochemical constituents (Fig. 6). The correlation coefficient values of the analyzed water quality parameters are presented in Table 3. The correlation matrix showed inter-parameter relationships, which agreed with the results obtained from PCoA and CA, as well as new associations between the parameters that were not adequately identified in the previous sections. Strong (p < 0.01) and significant correlations (p < 0.05) were observed in the groundwater dataset. The dataset was examined based on the linear correlation between every pair of groundwater constituent in terms of significant positive correlation coefficient, r ≥ 0.248, at p < 0.01. Table 3 reveals that pH positively correlated with HCO3− (r = 0.337) with a 99% confidence level (Fig. 7). This indicated that the alkaline pH was obtained from carbonate-rich sediments such as limestone and dolomite (indicating a geogenic source of groundwater contamination). As stated by Ledesma-Ruiz et al. (2015), Li et al. (2018), Li et al. (2019), and Wu et al. (2019), the existing carbonates in the aquifer rock might have been dissolved and added to the groundwater during movement, infiltration, and irrigation. Mollema and Antonellini (2016) emphasize that groundwater rich in dissolved elements typically meets more alkaline water. Also, Taillefert et al. (2002) indicated that many parameters such as pH, reduced sulfur, sorption by inorganic materials, precipitation of carbonate, and the presence of Mn2+ and Fe2+ control the release of trace metals. With changes in pH and alkalinity, trace elements may undergo deposition or go again into the groundwater; that is, trace element solubility is pH dependent. The range of pH values observed in samples is 2.82–9.7. Averyt et al. (2004) noted the pH effects on the stability of trace element complexes with ligands in water. Also, Table 3 reveals the general observed trend of trace metals in conditions of increased ionic strength and alkaline pH, as well as the increased electrolyte concentration and decreased oxidation rate, which would decrease the activity of the reacting species. B showed a positive correlation with EC (r = 0.304), as did Mg+ (r = 0.296), with a weak positive correlation for Na+ (r = 0.261) and a positive correlation with SO42− (r = 0.319). In line with Battistel et al. (2016), these correlations may be due to anion–cation exchange and indicate that the source of B in groundwater is natural release from carbonates (Fig. 8). Also, these conditions slow and decrease complexants-colloids formation that scavenge or complex with trace elements. Three groups of complexants-colloids formation may be differentiated. The first is an Fe-colloids-based group that may adsorb and/or complex with Cu (r = 0.421), Mn (r = 0.248), Ni (r = 0.284), and Cr (r = 0.359). The second group is a Cu-colloids-based group that may adsorb and/or complex with Cr (r = 0.485). The third group is a Mn-colloids-based group that may adsorb and/or complex with Pb (r = 0.326), Cd (r = 0.251), and Cr (r = 0.407), as shown in Fig. 9. The correlations in Table 3 suggest that the dissolved trace metals in the groundwater are not strongly affected by colloidal destabilization. In line with Pullin and Cabaniss (2003), the complete complexation of the metals resulting from the effect of a lower metal to larger ligand ratio indicates that the positively charged species must be reacting with negatively charged inorganic ligands (or vice-versa). Pb had a strong positive correlation with Cd (r = 0.627) as shown in Fig. 10. Most probably, these metals concentrate in the clay fraction and are released by sediments during redox conditions throughout the aquifer profile and/or are released from the same sources of pollution. Pb and Cd concentrations in groundwater generally increased with the ionic strength and groundwater salinity. Zhang et al. (1992) and Emmenegger et al. (1998) noted that metals and cations show increasing sorption at increasing pH (i.e. sorption increases with alkalinity). In agreement with Pinheiro et al. (1996), the trace element (B, Fe, Cu, Mn, Ni, Pb, Cd, and Cr) heterogeneity might be pH-dependent because of elevated concentrations and a variety of water contents that act as inorganic ligands (HCO3−, SO42−, Cl− and NO3−) with different ranges and binding sites being available. Generally, metals are sorbed and complexed with inorganic ligands most strongly at elevated pH (> 5). The various metals remain more heterogeneous in groundwater with higher pH values that range from 5 to the highest detected value of 9.7. Whereas reductive dissolution occurs at low pH ranging from < 5 to the lowest detected value of 2.82 in the suboxic zone. Thus, metal complexes are split at pH < 5. Mollema et al. (2015a, b) recognized that redox reactions in the aquifer control water chemistry and consequently concentrations of trace metals in the groundwater.
Table 3 reveals that EC had a positive correlation with alkalinity (r = 0.307), Ca2+ (r = 0.314), Mg+ (r = 0.354), HCO3− (r = 0.344), and SO42− (r = 0.268) and a strong positive correlation with Na+ (r = 0.776) and Cl− (r = 0.502). The salinity load in groundwater is controlled first by Na+ and then by Cl− and HCO3−. Generally, the groundwater is high hard water and has greater alkalinities (up to 695 mg L−1) indicating that the carbonate from aquifer sediments was the essential source of the water alkalinity. Alkalinity is positively correlated with Mg2+ (r = 0.295) and had a strong positive correlation with HCO3− (r = 0.603) as shown in Figs. 11 and 12. The relatively low acidity and high alkalinity in most samples was probably caused by leaching of carbonates. In line with Mollema et al. (2015a, b), high HCO3− concentrations (up to 730 mg L−1) will be caused by CaCO3 dissolution and redox reactions. As HCO3− is generated during the weathering of carbonates, pH values increase in the groundwater.
As revealed in Table 3 and shown in Fig. 13, there are strong positive correlations among the main constituents of groundwater such as Ca+ with Mg+ (r = 0.723), Ca2+ with SO42− (r = 0.769), Ca2+ with Cl− (r = 0.378), Mg2+ with HCO3− (r = 0.488), Mg2+ with SO42− (r = 0.642), Mg2+ with Cl− (r = 0.323), Na+ with K+ (r = 0.318), Na+ with HCO3− (r = 0.288), Na+ with Cl− (r = 0.475), and K+ with HCO3− (r = 0.297). These correlation results indicate mixed origin of these parameters as a result of the lithologic influence of aquifer matrix and water interactions (i.e. geogenic derived from aquifer materials through water–rock interactions by dissolution processes). In addition, infiltration from drains, human wastewater (septic tanks), stagnant water, and fertilizers and secondary salts from infiltration of agriculture water affect the water table in these regions. All these factors are responsible for the ionic alteration of groundwater in the study area.
Spatial distribution maps
The contamination sources’ diversity and the variety of anthropogenic contamination make this study difficult. Figure 14 a–s depict the most contaminated groundwater of the investigated aquifers. Samples located along the studied aquifers show different trends. High pH levels were found in Assiut and north Minia aquifers (Fig. 14a). High levels of B were dispersed along different sites in both Assiut and Minia aquifers (Fig. 14b). High levels of Fe were found in Assiut aquifer (Fig. 14c), and high levels of Cu were found in south Minia and largely in south Assiut (Fig. 14d). Elevated Mn levels were found in south Minia and all the Assiut aquifer (Fig. 14e). High levels of Ni were dispersed along different sites in both Assiut and Minia aquifers (Fig. 14f). High Pb levels were dispersed among different sites in Assiut aquifer and at one site in north Minia (Fig. 14g). Elevated Cd levels were dispersed among different sites in the Assiut aquifer (Fig. 14h). High levels of Cr were dispersed along different sites in Minia especially in the north of the aquifer and in a small area in Assiut (Fig. 14i). Elevated EC levels were found among different sites in the aquifers especially north Minia (Fig. 14j). An increase in the alkalinity level was found among different sites in the aquifers especially north Minia and middle Assiut aquifers (Fig. 14k). Increased Ca levels were concentrated at north Minia and middle Assiut aquifers (Fig. 14l), and increased Mg levels found in all Assiut aquifers and north Minia aquifer (Fig. 14m). High levels of Na were dispersed along different sites in both Assiut and Minia aquifers (Fig. 14n), and high levels of K dispersed along different sites especially at south Assiut aquifer (Fig. 14o). High levels of HCO3 were dispersed in south Minia and Assiut aquifer (Fig. 14p), and increased SO4 levels dispersed along different sites in both Assiut and Minia aquifers (Fig. 14q). Elevated Cl levels were found among different sites in both Assiut and Minia aquifers (Fig. 14r), and high levels of NO3 in south Assiut aquifer (Fig. 14s). As mentioned above, the PCoA and CA results indicated that the interference of both natural processes (increase concentrations of elements resulting from water–rock interactions) and the impacts of human activities (increase concentrations of fertilizers, nutrients and redox-sensitive elements) are the principal factors affecting groundwater quality. Also, these multivariate statistical results reveal that the major ions in groundwater are controlled by water–rock interactions such as the dissolution of carbonates, silicates, and soil leaching. Whereas, negative impacts of trace elements in the groundwater mainly originated from anthropogenic input such as sewage effluents and agricultural activities in the study area.
Conclusion
The present paper addresses groundwater pollution in middle Upper Egypt. Groundwater is usually considered to be less directly contaminated by various toxic substances compared with surface water. However, groundwater quality is a growing concern due to contamination by a variety of pollutants. Based on the results obtained in this study, the following findings can be summarized:
-
•Groundwater of the study area (Minia and Assiut governorates) is characterized by high concentrations of trace elements such as Fe, Mn, Cd, and Pb.
-
•The results of linear correlations, PCoA, and CA were used to investigate the sources of groundwater contamination and indicate that the geochemical characteristics of the dissolved groundwater constituents are strongly influenced by the impact of weathering, dissolution of deposits (especially carbonate-rich sediments), ion exchange, and water–rock interaction.
-
•Also, statistical results indicated that pollution, which might be attributed to domestic sewage and effluent discharge from agricultural drains, has an adverse impact on the aquifers. Meanwhile, this study suggests that agricultural effluent and release of domestic sewage should be reduced.
References
Abou Heleika, M. M., & Niesner, E. (2009). Configuration of the limestone aquifers in the central part of Egypt using electrical measurements. Hydrogeology Journal, 17, 433–446.
APHA. (1998). Standard methods for the examination of water and wastewater (20th ed.). New York: American public health association (APHA), American Water Works Association (AWWA), and water pollution control federation (WPCF).
Averyt, K. B., Kim, J. P., & Hunter, K. A. (2004). Effect of pH on measurement of strong copper binding ligands in lakes. Limnology and Oceanography, 49, 20–27.
Battistel, M., Hurwitz, S., Evans, W. C., & Barbieri, M. (2016). The chemistry and isotopic composition of waters in the lowenthalpy geothermal system of Cimino-Vico Volcanic District, Italy. Journal of Volcanology and Geothermal Research, 328, 222–229.
Bodrud-Doza, M. d., Islam, T., Ahmed, F., Das, S., Saha, N., & Rahman, M. S. (2016). Characterization of groundwater quality using water evaluation indices, multivariate statistics and geostatistics in Central Bangladesh. Water Science, 30, 19–40.
Bu, H., Wang, W., Song, X., & Zhang, Q. (2015). Characteristics and source apportionment of dissolved trace elements in the Jinshui River of the South Qinling Mts., China. Environmental Science and Pollution Research, 22, 14248–14257.
Chetelat, B., & Gaillardet, J. (2005). Boron isotopes in the Seine River, France: A probe of anthropogenic contamination. Environmental Science and Technology, 39, 2486–2493.
Dawoud, M. A., El Arabi, N. E., Khater, A. R., & Wonderen, J. v. (2006). Impact of rehabilitation of Assiut barrage, Nile River, on groundwater rise in urban areas. Journal of African Earth Sciences, 45, 395–407.
Decree of Health Ministry (No.458), (2007). Egyptian standards for drinking water and domestic uses (in Arabic Language).
El-Aassar, M., Hussien, R. A., & Ghoubachi, S. Y. (2016). Groundwater quality and vulnerability assessment in the new reclamation areas, Assuit governorate, West Nile River, Egypt. Journal of American Science, 12(11).
Emmenegger, L., King, D. W., Sigg, L., & Sulzberger, B. (1998). Oxidation kinetics of Fe (II) in a eutrophic Swiss lake. Environmental Science and Technology, 32, 2990–2996.
FAO. (2007). Coping with water scarcity: Challenge of the twenty-first century. UN-Water.
Goyer, R. A. (1993). Lead toxicity: Current concerns. Environmental Health Perspectives, 100, 177–187.
Gurumurthy, G. P., Balakrishna, K., Tripti, M., Audry, S., Riotte, J., Braun, J. J., & Shankar, H. N. U. (2014). Geochemical behaviour of dissolved trace elements in a monsoon-dominated tropical river basin, southwestern India. Environmental Science and Pollution Research, 21, 5098–5120.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 9pp.
Hem, J. D. (1985). Study and interpretation of the chemical characteristics of natural water. U.S. Geological Survey Water-Supply paper, 1473, 19–32.
Holland, H. D. (1978). The chemistry of the atmosphere and oceans (p. 351). New York: John Wiley and Sons.
Kamel, A. K. (2012). Hydrochemical evaluation of the water sources in Minia district, Egypt. M.Sc. of Geology Minia, Faculty of Science, Minia University, Egypt, 120 p.
Ledesma-Ruiz, R., Pastén-Zapata, E., Parra, R., Harter, T., & Mahlknecht, J. (2015). Investigation of the geochemical evolution of groundwater under agricultural land: A case study in northeastern Mexico. Journal of Hydrology, 521, 410–423.
Li, P., He, S., Yang, N., & Xiang, G. (2018). Groundwater quality assessment for domestic and agricultural purposes in Yan′an City, northwest China: Implications to sustainable groundwater quality management on the Loess Plateau. Environmental Earth Sciences, 77, 775.
Li, P., He, X., & Guo, W. (2019). Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan′an City on the Loess Plateau of northwest China. Human and Ecological Risk Assessment, 25(1–2), 11–31. https://doi.org/10.1080/10807039.2018.1553612.
Liu, C. W., Lin, K. H., & Kuo, Y. M. (2003). Application of factor analysis in the assessment of groundwater quality in a Blackfoot disease area in Taiwan. Science of the Total Environment, 313, 77–89.
Mekonnen, M. M., & Hoekstra, A. Y. (2016). Four billion people facing severe water scarcity. Science Advances, 2(2), e1500323.
Mollema, P. N., & Antonellini, M. (2016). Water and (bio)chemical cycling in gravel pit lakes: A review and outlook. Earth-Science Reviews, 159, 247–270.
Mollema, P. N., Antonellini, M., Dinelli, E., Greggio, N., & Stuyfzand, P. J. (2015a). The influence of flow-through saline gravel pit lakes on the hydrologic budget and hydrochemistry of a Mediterranean drainage basin. Limnology and Oceanography, 60, 2009–2025.
Mollema, P. N., Stuyfzand, P. J., Juhasz-Holterman, M. H. A., Van Diepenbeek, P. M. J. A., & Antonellini, M. (2015b). Metal accumulation in an artificially recharged gravel pit lake used for drinking water supply. Journal of Geochemical Exploration, 150, 35–51.
Mora, A., Mahlknecht, J., Baquero, J. C., Laraque, A., Alfonso, J. A., Pisapia, D., & Balza, L. (2017). Dynamics of dissolved major (Na, K, Ca, Mg, and Si) and trace (Al, Fe, Mn, Zn, Cu, and Cr) elements along the lower Orinoco River. Hydrological Processes, 31, 597–611.
Needleman, H. L. (1990). The future challenge of lead toxicity. Environmental Health Perspectives, 89, 85–89.
Needleman, H. L., Schell, A., Bellinger, D., Leviton, A., & Allred, E. N. (1990). The long-term effects of exposure to low-doses of lead in childhood—An 11-year follow-up report. The New England Journal of Medicine, 322, 83–88.
Nigro, A., Sappa, G., & Barbieri, M. (2017). Application of boron and tritium isotope for tracing landfill contamination in groundwater. Journal of Geochemical Exploration, 172, 101–108.
Omo-Irabor, O. O., Olobaniyi, S. B., Oduyemi, K., & Akunna, J. (2008). Surface and groundwater water quality assessment using multivariate analytical methods: A case study of the Western Niger Delta, Nigeria. Physics and Chemistry of the Earth, Parts A/B/C, 33, 666–673.
Oude Essink, G. H. P., van Baaren, E. S., & de Louw, P. G. B. (2010). Effects of climate change on coastal groundwater systems: A modeling study in the Netherlands. Water Resources Research, 46, W00F04.
Pape, P. L., Ayrault, S., & Quantin, C. (2012). Trace element behavior and partition versus urbanization gradient in an urban river (Orge River, France). Journal of Hydrology, 472(473), 99–110.
Pinheiro, J. P., Mota, A. M., Simões Gonçalves, M. L. S., van der Weijde, M., & van Leeuwen, H. P. (1996). Comparison between polarographic and potentiometric speciation for cadmium/humic acid systems. Journal of Electroanalytical Chemistry, 410, 61–68.
Pullin, M. J., & Cabaniss, S. E. (2003). The effects of pH, ionic strength, and iron–fulvic acid interactions on the kinetics of nonphotochemical iron transformations. I. Iron (II) oxidation and iron(III) colloid formation. Geochimica et Cosmochimica Acta, 67, 4067–4077.
RIGW (Research Institute of Groundwater). (1992). Hydrogeological map of Egypt, scale 1:100,000 (2nd ed.). Map Sheet of: El-Minia.
Sanad, E.Y. (2010). Geophysical and hydrogeological studies for evaluation the groundwater potentiality in the reclaimed area, West Minia District, Egypt, department. Of geology, Faculty of Science, Minia University., Egypt, 178 p.
Santschi, P. H., Lenhart, J. J., & Honeyman, B. D. (1997). Heterogeneous processes affecting trace contaminant distribution in estuaries: The role of natural organic matter. Marine Chemistry, 58, 99–125.
Schröder, T. J., Hiemstra, T., Vink, J. P., & van der Zee, S. E. (2005). Modeling of the solid-solution partitioning of heavy metals and arsenic in embanked flood plain soils of the rivers Rhine and Meuse. Environmental Science and Technology, 39, 7176–7184.
Shiklomanov, I. (1993). World freshwater resources. In P. H. Gleick (Ed.), Water in crisis: A guide to the World's fresh water resources. New York: Oxford University press.
Soto-Varela, F., Rodríguez-Blanco, M. L., Taboada-Castro, M. M., & Taboada-Castro, M. T. (2014). Identifying environmental and geochemical variables governing metal concentrations in a stream draining headwaters in NW Spain. Applied Geochemistry, 44, 61–68.
Srivastava, S. K., & Ramanathan, A. L. (2008). Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environmental Geology, 53, 1509–1528.
Taillefert, M., Lienemann, C. P., Gaillard, J. F., & Perret, D. (2000). Speciation, reactivity, and cycling of Fe and Pb in a meromictic lake. Geochimica et Cosmochimica Acta, 64, 169–183.
Taillefert, M., Macgregor, B. J., Gaillard, J.-F., Lienemann, C.-P., Perret, D., & Stahl, D. A. (2002). Evidence for a dynamic cycle between Mn and Co in the water column of a stratified lake. Environmental Science and Technology, 36, 468–476.
Tariq, S. R., Shah, M. H., Shaheen, N., Jaffar, M., & Khalique, A. (2008). Statistical source identification of metals in groundwater exposed to industrial contamination. Environmental Monitoring and Assessment, 138, 159–165.
Tessier, A., Fortin, D., Belzile, N., DeVitre, R. R., & Leppard, G. G. (1996). Metal sorption to diagenetic iron and manganese oxyhydroxides and associated organic matter: Narrowing the gap between field and laboratory measurements. Geochimica et Cosmochimica Acta, 60, 387–404.
Varol, M. (2013). Dissolved heavy metal concentrations of the Kralkızı, Dicle and Batman dam reservoirs in the Tigris River basin, Turkey. Chemosphere, 93, 954–962.
Waleed, S. S., Abd El-Monaim, A. E., Mansour, M. M., & El-Karamany, M. F. (2009). Evaluation of groundwater aquifer in the area between El-Qusiya and Manfalut using vertical electric soundings (Ves) technique. Journal of Engineering Sciences, Assiut University, 37, 1193–1207.
Weng, N., & Wang, W.-X. (2014). Variations of trace metals in two estuarine environments with contrasting pollution histories. Science of the Total Environment, 485–486, 604–614.
WHO (2011). World Health Organization: Guidelines for Drinking-Water Quality, 4th ed.
Wu, J., Li, P., Qian, H., Duan, Z., & Zhang, X. (2014). Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: A case study in Laoheba phosphorite mine in Sichuan, China. Arabian Journal of Geosciences, 7, 3973–3982.
Wu, J., Li, P., Wang, D., Ren, X., & Wei, M. (2019). Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Human and Ecological Risk Assessment. https://doi.org/10.1080/10807039.2019.1594156.
Zaki, R., El Bakry, A., El Shemi, A., & Fanous, F. A. (2001). Petrography and geochemistry of the Eocene limestone units, east El Minia district, north Upper Egypt. In 5 th international conference on geochemistry, Alexandria University, Egypt, II (pp. 113–148).
Zhang, Y., Charlet, L., & Schindler, P. W. (1992). Adsorption of protons, Fe(II) and Al(III) on lepidocrocite (γ-FeOOH). Colloids and Surfaces, 63, 259–268.
Acknowledgment
Many thanks are due to Dr. Julian O’Dea Director (retired), Chemical Review, Australian Department of Health for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 863 kb)
Rights and permissions
About this article
Cite this article
Snousy, M.G., Morsi, M.S., Elewa, A.M. et al. Groundwater vulnerability and trace element dispersion in the Quaternary aquifers along middle Upper Egypt. Environ Monit Assess 192, 174 (2020). https://doi.org/10.1007/s10661-020-8109-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-8109-5