Abstract
It is known that some persistent organic pollutants (POPs) are used worldwide, and these pollutants are dangerous for human health. However, there are still countries where measurements of these pollutants have not been adequately measured. Although many studies have been published for determining the concentrations of POPs in Turkey, there are limited studies in Latin American countries like Peru. For this reason, it is essential both to conduct a study in Peru and to compare the study with another country. This study is aimed at determining the atmospheric POPs such as polycyclic aromatic hydrocarbon (PAH), organochlorine pesticide (OCP), and polychlorinated biphenyl (PCB) concentrations using passive air samplers in Yurimaguas (Peru) and Bursa (Turkey). Molecular diagnosis ratios and ring distribution methods were used to determine the sources of PAHs. According to these methods, coal and biomass combustions were among the primary sources of PAHs in Peru, while petrogenic and petroleum were the primary sources of PAHs in Turkey. Then, α-HCH/γ-HCH and β-/(α+γ)-HCH ratios were used to determine the sources of OCPs. According to the α-HCH/γ-HCH ratios, the primary sources of OCPs in both countries were lindane. Similarly, according to β-/(α+γ)-HCH ratios, the HCHs have been historically used in Peru while they were recently utilized in Turkey. Finally, homologous group distributions were used to determine the sources of PCBs. Similar distributions of homologous groups were observed in the sampling sites in both countries. Also, the homologous group distributions obtained have been determined that industrial activities could be effective in the sampling areas in both countries. When the cancer risks that could occur via inhalation were evaluated, no significant cancer risk has been determined in both countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persistent organic pollutants (POPs) are characterized due to their persistence, bioaccumulative, long-range transport, and toxicity. Some polycyclic aromatic hydrocarbons (PAHs), organochlorine pesticides (OCPs), and polychlorinated biphenyls (PCBs) are among the well-known POPs (Qu et al. 2019; Yin et al. 2017). PAHs formed by the combination of two or more benzene rings and consisted of hundreds of different compounds are usually formed during incomplete combustion of organic substances such as wood, coal, gas, and tobacco. Among the PAH compounds, only 16 PAHs are listed as priority pollutants by the Environmental Protection Agency (EPA) (Andersson and Achten 2015). PCBs were widely produced in the industrial sector since 1929. Due to their toxic effects, PCB production and use were banned in many countries since the late 1970s (Dickerson et al. 2019). They were highly used in hydraulic fluids, capacitors, and transformers (Aydin et al. 2014). OCPs have been used as insecticides and herbicides for the protection of agriculture crops. Most of them were banned before the 1980s due to their toxicity and persistence characteristics (Cindoruk and Tasdemir 2007). Despite PCBs and OCPs were banned many decades ago, they can still be detected in the environment. For this reason, their measurements contribute to analyzing their current risk to human health and the environment.
Active and passive air samplers (PASs) are generally used to determine POP concentrations in the air. Although each of these two methods has advantages and disadvantages, the current concentrations of POPs in the air can be obtained directly with active air samplers (AASs). These systems have disadvantages such as high equipment costs, the need for a power supply, and their maintenance by experts (Mari et al. 2008; Tuduri et al. 2006). On the other hand, PASs are the most commonly used approach method due to they are cheap, noiseless, easy to use, and they do not need electricity and maintenance (Tromp et al. 2019). In addition, there are various studies in the literature in which POPs are commonly determined together with PASs (Hazrati and Harrad 2007; Zhang et al. 2013; Cetin et al. 2019; Meire et al. 2019; Bohlin-Nizzetto et al. 2020; Nguyen et al. 2020; Thang et al. 2020).
Many studies have evaluated the concentrations of POPs in different sites in Turkey (industrial, urban, semi-urban, and rural areas) (Cetin et al. 2007; Odabasi et al. 2008; Ozcan and Aydin 2009; Tasdemir and Esen 2008; Vardar et al. 2008; Yolsal et al. 2014). These investigations gave a great perspective of POPs in Turkey. It also supported previous papers about new POP sources and their risk to the environment. Although there are many studies that determined the atmospheric concentrations of POPs in developed and developing countries, there are still many regions such as Latin America, where the measurement of these pollutants is limited or absent. A similar situation exists in Peru. In addition to very few studies on POPs (i.e., PAHs, OCPs, and PCBs) in Peru, there is no study comparing concentration values with the Northern Hemisphere. The lack of studies makes difficulties in monitoring and reducing their presence in the environment and possible hazard effects on humans. This situation also restricts the study of industrial and urban sites of Peruvian cities in comparison with rural cities and other places around the world.
Although there are many studies that determine the atmospheric concentrations of POPs in passive air samplers in Turkey, there are no studies comparing the simultaneously measured concentration values with Peru. In this context, the main objectives of this study are to (i) measure and compare the concentrations of PAHs, OCPs, and PCBs between semi-urban points in Peru and a semi-urban point in Turkey; (ii) identify their possible sources, (iii) analyze the temporal changes of the pollutants; and (iv) determine health risk assessment via inhalation of POPs.
Materials and methods
Sampling points and programs
Yurimaguas (Peru) and Bursa (Turkey) cities have been chosen as sampling points. Yurimaguas is a semi-urban city located in the northeastern part of Peru. The main economic activities are related to agriculture, wood industry, and a connection point for the trade-off of products between the capital and northeastern cities. Bursa is in the northwestern part of Anatolia, and it is the fourth largest city in Turkey. The main economic activities belong to the textile, automotive, and food industries. In Yurimaguas, three different places were chosen for air samplings while in Bursa, only one. Since limited measurements were available in Peru, the chosen sampling site numbers were higher. All four sampling points represent the semi-urban point. Only the sampling point in Bursa is closer to the industry than the other sampling point. The sampling points are described in Table 1.
The sampling was carried out simultaneously for three months (June–August 2018). PASs with polyurethane foam (PUF) were used to collect the air samples, and a total of 12 samples were taken. The PUFs were changed every 30 days. They were wrapped in aluminum foil and taken to the laboratory in ice bags to avoid any contamination during the sampling. Samples contained in the sealed pouches were transported without contact with air and held at − 20 °C in a deep freeze until their analysis.
Sample analysis
The brand new PUFs were cleaned with distilled water for 24 h and then, with a soxhlet extraction including acetone (ACE) (twice) and hexane (HEX), and each step took 24 h. They were dried with a vacuum desiccator. Finally, they were wrapped with aluminum foil, put into a zip-lock bag, and stored in a deep freezer at − 20 °C until they were used to sampling.
The sample extraction procedure was made by soxhlet extraction with 250 mL of a solvent consisting of ACE:HEX (1:1, v:v). The obtained volume was reduced to 5 mL with a rotary evaporator (Laborota 4001-Heidolph, Germany). Then, 15 mL of HEX was added onto this solution, and it was reduced to 2 mL. PAHs, PCBs, and OCPs were fractionated with the use of a cleaning column, containing 3 g of deactivated silicic acid (3% of water), 2 g of deactivated alumina (6% of water), and 2 g of sodium sulphate (Na2SO4) (Esen and Kayikci 2018). The column was cleaned with 20 mL of dichloromethane (DCM) and 20 mL of petroleum ether (PE). PCBs were collected by adding 20 mL of PE to the cleaning column (fraction 1) (Cetin et al. 2007). Then, the PAH and OCP samples were gathered by pouring 20 mL of DCM (fraction 2) (Aydin et al. 2014; Tasdemir and Esen 2008). These two fractions were reduced to 5 mL using a rotary evaporator. Then, 10 mL of HEX was added to the 5 mL sample and reduced to 1 mL (Tasdemir and Esen 2007). For the PCB samples, the possible contamination of organic matter was removed by washing the samples with sulfuric acid (H2SO4). Finally, the PAH-OCP and PCB containing samples were stored in vials and kept at − 20 °C until instrumental analysis.
Instrumental analysis
In this study, 16 PAHs (Naphthalene (Nap), Acenaphthylene (Ace), Acenaphthene (Act), Fluorene (Fln), Phenanthrene (Phe), Anthracene (Ant), Fluoranthene (Fl), Pyrene (Py), Benz(a)anthracene (BaA), Chrysene (Chr), Benzo(b)fluoranthene (BbF), Benzo(k)fluoranthene (BkF), Benzo(a)pyrene (BaP), Indeno(1,2,3-cd)pyrene (IcdP), Dibenzo(a,h)anthracene (DahA), Benzo(g,h,i)perylene (BghiP)), 33 PCB congeners (PCB #8/5, 12/13, 16/32, 26, 44, 37/42, 41/64/71, 100, 74, 61/70, 66/95, 84, 99, 81/87, 85, 135/144, 118, 123, 153, 174, 180, 169, and 207), and 10 different OCPs (α-HCH, β-HCH, γ-HCH, δ-HCH, Heptachlor endo epoxide iso A, Endrin, Endosulfan-β, Endrin aldehyde, p,p′-DDT, and Methoxychlor) were analyzed.
The analysis of PAHs was carried out by employing an Agilent 7890 A gas chromatograph (GC) with a mass selective detector (Agilent 5975 C inert MSD) equipped with a 30 m HP5-MS column. The oven program for PAHs was 50 °C (1 min), then raised to 200 °C at a rate of 25 °C/min, and to 300 °C at a rate of 8 °C/min. The injector, ion source, and quadrupole temperatures were 295 °C, 300 °C, and 180 °C, respectively. Helium was used as a carrier gas at a flow of 1.4 mL/min. The surrogate standards were used to determine the efficiency values in the samples. The PCB and OCP analyses were achieved with a GC 7890A-μECD (Agilent, USA) (Micro-Electron Capture Detector) device. The HP-5MS capillary column was employed. The oven program for PCBs was 70 °C (2 min), to 150 °C at a rate of 25 °C/min, followed by 3 °C/min up to 200 °C, 8 °C/min up to 280 °C (8 min), and then 10 °C/min up to 300 °C (2 min). Similarly, the oven program for OCPs was 80 °C (1 min) and then 20 °C/min to 300 °C. The injector and detector inlet temperatures for both PCBs and OCPs were 250 °C and 320 °C, respectively. Helium was used as the carrier gas at a rate of 1.9 mL/min and high-purity nitrogen as make-up gas.
In this study, sampling rates for each POP compounds were calculated according to the model proposed by Herkert et al. (2016) (http://s-iihr41.iihr.uiowa.edu/pufpas_model/). Since the sampling rate (Rs) values were calculated for each sampling site based on the model, it is believed that atmospheric POP concentrations are accurately reflected. The sampling rates for the sampling points are given in Table S1 (Supplementary materials) for all compounds.
Quality assurance/quality control (QA/QC)
All equipment used during the sampling and laboratory procedures were washed with tap water, purified water, ACE, and PE, respectively. 1 mL surrogate standard was added to each sample and blank samples to determine the recovery efficiency before the extraction step. The surrogate standard contained naphthalene-d8, acenaphthene-d10, phenanthrene-d10, pyrene-d10, chrysene-d12, and perylene-d12 for PAHs; PCB#14, PCB#65, and PCB#166 for PCBs. However, external surrogate studies were done for OCPs (Cindoruk and Tasdemir 2014; Eker and Tasdemir 2018). The recovery efficiencies of all sampling points for POP compounds were found to be higher than 75%.
Blank samples were collected at least 10% of the total number of samples in order to determine any contamination during the transportation, storage, and laboratory analysis. Blank samples were subjected to the same laboratory procedure as the real samples. The instrument detection limits (IDLs), the lowest concentration of a calibration standard, were determined based on the Signal:Noise (S:N) ratio of 3:1. The IDL values for 1 μL injection were 0.1 ng for PAHs, 0.04 pg for OCPs, and 0.15 pg for PCBs. The method of detection limit (MDL) values were calculated for each measured compound as an average mass in blanks plus three times the standard deviation (average + 3 S.D.) (Tasdemir and Esen 2007). The MDL value was assumed to be equal to the IDL value when the analyte was not present in the blank sample. The MDL values for sampling points ranged from IDL to 30.48 ng for PAHs, from 0.24 to 4.83 pg for OCPs, and from 0.26 to 9.18 pg for PCBs. If any POP compounds had less than MDL values, then the concentration of that congener would have taken as half of the IDL values for statistical analysis.
The Pearson correlation coefficient and the coefficient of divergence
The Pearson correlation coefficient (PCC) is a widely used statistical approach to determine the temporal changes of pollutants (Liu et al. 2017). Temporal variations of pollutant concentrations are determined with the PCC values. The coefficient of divergence (COD) is employed to determine the differences between pollutant sources. The PCC and COD were calculated with the following equations:
where p is the number of individual POP compounds, j and k refer to different sampling points, i is the average concentration of ith POP compounds, and ā is the average POP concentration at sampling points (Bano et al. 2018; Chuang et al. 2019; Liu et al. 2017). If the PCC values are higher than 0.7, the POP concentrations among the sampling points do not change temporally (Sari et al. 2020a). The COD method is utilized to determine the similarities or differences between the POP concentrations measured in two sampling points (Bano et al. 2018; Shen et al. 2019). If COD values are higher than 0.2, it means that there are differences between sources of pollutants in the sampling points (Bano et al. 2018; Chuang et al. 2019; Liu et al. 2017; Shen et al. 2019).
Carcinogenic potentials of POPs via inhalation
Carcinogenic potentials of PAHs by inhalation are calculated using toxic BaPeq concentrations (Zhang et al. 2019). BaPeq concentration is calculated by Eq. 3.
where Ci is the individual PAH concentration (ng/m3), n is the number of the individual PAH compounds, and TEFi values are the toxicity equivalency factor of individual PAH compounds (Nap, Ace, Act, Fln, Phe, Fl, and Py for 0.001; Ant, Chr, DahA, and BghiP for 0.01; BaA and BbF for 0.1; BaP and IcdP for 1.0) (Mueller et al. 2019). In this study, the cancer risk calculation of PAHs by inhalation was calculated by the incremental lifetime cancer risk (ILCR) method developed by the U.S. Environmental Protection Agency (1991). ILCR values are calculated by Eq. 4.
where SF is the cancer slope factor (3.85 day kg/mg), EF is the exposure frequency (350 day/year for both adults and children) (Ali 2019), ED is the exposure duration (24 years for the adults, 6 years for the children), CF is conversion factor mg to ng (10−6), IR is the daily inhalation rate (20 m3/day for the adults, 10 m3/day for the children) (Ghanavati et al. 2019), BW is the body weight (70 kg for adults and 16.5 kg for children), and AT is the average lifespan for carcinogens (25,550 days for both adults and children) (Ranjbar Jafarabadi et al. 2019). Similarly, carcinogenic potentials of OCPs and PCBs by inhalation are calculated by Eq. 5 (Goel et al. 2016):
where CS is the total OCP or PCB concentration (pg/m3), ET is the daily exposure duration (24 hours/day), SF is the cancer slope factor (2 day kg/mg for ∑33PCBs, 1.8 day kg/mg for ∑HCHs, 9.1 day kg/mg for Heptachlor endo epoxide iso A, 0.34 day kg/mg for p,ṕ-DDT, and 1 day kg/mg was selected for other OCP congeners) (Fu et al. 2018; Qu et al. 2015), IR is the inhalation rate (0.83 m3/h for the adults, 0.42 m3/h for the children) (Ghanavati et al. 2019), CF is conversion factor mg to pg (10-9), and the other coefficients are the same as the 4th equation. According to the US-EPA, the risk of acceptable cancer is in the range from 1 × 10−6 to 1 × 10−4; higher cancer risk, if greater than 1 × 10−4; non-cancer risk, if less than 1 × 10−6 (Wang et al. 2019).
Results and discussion
Atmospheric PAH concentrations and possible sources
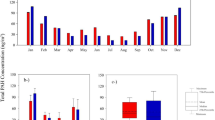
Monthly air samples were taken from semi-urban sampling points to determine the total PAH concentration values in Peru (LR, LB, and CR) and Turkey (BUU) in the summer of 2018, respectively. The average concentration of the total 16 PAH (∑16PAH) compounds sampled with PASs is presented in Fig. 1.
The ∑16PAH concentrations in the LR, LB, CR, and BUU sampling points were 17 ± 2, 14 ± 2, 32 ± 3, and 8 ± 4 ng/m3, respectively. The highest PAH concentration levels were observed in CR, while the lowest PAH concentration levels were observed in BUU. This is probably because the CR sampling point represents a region near the main road and open-air cooking while the BUU sampling point reflects a region with less PAH sources than other regions. Also, the BUU sampling point is located in a region where many pine trees are present. The resins existed in the leaves of pine trees around the BUU sampling point work as absorbent surfaces for PAHs in the gas-phase (Chun 2011; Sari et al. 2020b). The concentration levels obtained from this study were comparable to various studies in the literature. For example, two different studies carried out in the cities of Chile (Concepcion and Temuco) reported PAH concentrations between 50 and 230 ng/m3 (∑15PAH) for industrial areas and 14-74 ng/m3 (∑13PAH) for urban areas (Pozo et al. 2012, 2015). The concentration levels obtained in this study were similar to the previous studies conducted in the same regions. For instance, PAH concentration levels obtained in the studies carried out by Evci et al. (2016) and Vardar et al. (2008) showed that the atmospheric Σ15PAH concentrations for the summer seasons were 7.0 ± 0.6 ng/m3 and 13.5 ± 4.2 ng/m3, respectively.
Molecular diagnostic ratios (MDRs) are one of the most common and most straightforward methods used to identify PAH sources (Konstantinova et al. 2019). Ant/(Ant+Phe), Fl/(Fl+Py), BaA/(BaA+Chr), and BaP/BghiP ratios were used as MDRs in this study. These ratios helped to identify the sources of PAHs in the sampling sites. The Ant/(Ant+Phe) ratio is generally used in the determination of combustion and oil sources. The Fl/(Fl+Py) and BaA/(BaA+Chr) ratios are mostly utilized in the decision of biomass combustion. Finally, Bap/BghiP ratio is employed to determine the traffic emission (Choi 2014; Tobiszewski and Namieśnik 2012; Yunker et al. 2002). The analysis of PAH ring distribution is also important in the evaluation of source identification. PAHs are divided into three groups according to the number of benzene rings: 2–3 rings, 4 rings, and 5–6 rings (Jiao et al. 2017). The MDR and distribution of the number of the benzene ring profile obtained from this study are shown in Fig. 2a, b.
According to the MDRs, the coal burning was effective at all sampling points in Peru (Fig. 2a). Although the use of coal and wood for heating did not occur in Yurimaguas, the use of these fuels for cooking was high and continuous throughout the year. In the BUU sampling point, the petrogenic and petroleum-derived PAHs were generally influential. According to the BaP/BghiP ratios, the traffic emissions did not dominate at all sampling points.
The predominance of 2-, 3-, and 4-ring PAHs is explained by their high volatility in comparison with 5- and 6-ring PAHs. It was reported that 2- and 3-ring PAHs were originated by the combustion of biomass and fuels (Lai et al. 2017), while 4-ring PAHs, such as CHR, were used to be detected emissions from incomplete combustion of carbon-rich fuels (Eccleshare et al. 2017). As a result, biomass and coal combustions were the primary sources of PAHs at the sampling points according to both MDRs and ring distributions (Fig. 2b).
Atmospheric OCP concentrations and possible sources
The average concentration of the total 10 OCP (∑10OCP) compounds sampled with the PASs is presented in Fig. 3. The ∑10OCP concentrations in the LR, LB, CR, and BUU sampling points were 415 ± 53, 260 ± 34, 282 ± 72, and 628 ± 385 pg/m3, respectively. The highest OCP concentrations were observed in the BUU sampling point, while the lowest OCP concentration levels were observed in the LB sampling point. Since the LR and BUU sampling points are close to the agricultural areas, high OCP concentrations were detected at these two sampling points.
In a study conducted in urban and semi-rural areas in Mexico, the ∑10OCP concentration values were reported as 200 and 269 pg/m3, respectively (Bohlin et al. 2008). A complete study carried out in 7 different regions of Mexico reported DDTs, lindane, and HCHs, and endosulfan concentrations in a range of 15–1975, 8–104, and 26–3730 pg/m3, respectively (Wong et al. 2009). The presence of more agricultural fields and the use of illegal OCPs may cause a high concentration in comparison with our study. The regional atmospheric transport from neighbor Latin American countries, where the use of these pesticides was extended, could also be an important source of OCPs to Peru (Estellano et al. 2012; Taiwo 2019). Wong et al. (2009), in their study about OCPs in Mexico, found that wind trajectories carried OCPs from the agricultural sites.
The sum of the total HCH concentrations (∑HCH (sum of α-, β-, γ-, and δ-HCHs)) in the LR, LB, CR, and BUU sampling points were 220 ± 51, 138 ± 33, 174 ± 56, and 438 ± 375 pg/m3, respectively. In general, technical HCHs (α-HCH 55-80%, β-HCH 5-14%, γ-HCH 8-15%, and δ-HCH 2-16%) have been used extensively in agriculture from the 1950s to the early 1980s (Da et al. 2014). From these isomers, only γ-HCH has specific insecticidal properties; for instance, lindane contains more than 90% of γ-HCH (Vijgen et al. 2019). The HCH isomers (α-, β-, γ-, and δ-) have different physicochemical properties. For example, α-HCH is more likely to enter the atmosphere and be transported over long distances, while β-HCH is more resistant to hydrolysis and environmental degradation (Da et al. 2014). α-HCH/γ-HCH ratios are among the most common methods employed to determine the sources and transport of HCHs in the atmosphere (Hao et al. 2019). If the α-HCH/γ-HCH ratio has a value ranging from 4 to 7, technical HCHs prevail; if the α-HCH/γ-HCH ratio is lower than 4, the lindane is dominant (Botwe et al. 2017). Moreover, the β-/(α+γ)-HCH ratio is another approach used to determine the usage history of technical HCH and lindane (Liu et al. 2012). If this ratio is lower than 0.5, it indicates that HCH and/or lindane are used recently, and if this ratio is higher than 0.5, it was utilized in the past. In this study, the β-/(α+γ)-HCH versus α-HCH/γ-HCH ratios obtained for the sampling points are shown in Fig. 4.
According to the α-HCH/γ-HCH ratios, lindane was dominant in all sampling points during the measurement period. This pesticide is known for its toxic properties and stability and often causes water and soil pollution (Kartalovic et al. 2015). The α-HCH/γ-HCH ratios obtained in this study are similar to several studies in the literature. For example, in a study conducted by Wu et al. (2020) in the northwest Pacific Ocean, they reported an average value for α-HCH/γ-HCH ratio as 0.61 ± 0.24. On the other hand, a mean value for the α-HCH/γ-HCH ratio from urban areas in Turkey was 2.26 (Kurt-Karakus et al. 2018). Odabasi et al. (2008) affirmed that low α-HCH/γ-HCH ratios could be attributed to the effect of regional sources supporting our previous assumption. Similarly, according to β-/(α+γ)-HCH ratios, the HCHs could recently be used in the BUU and CR sampling points. Pokhrel et al. (2018) found ratios between 0.1 and 0.5; these ratios were similar to the values in our study (0.05-0.78), confirming the use of lindane in our sampling points.
Atmospheric PCB concentrations and possible sources
The concentration of the total 33 PCB (∑33PCB) compounds sampled with the PASs is presented in Fig. 5. The ∑33PCB concentrations in the LR, LB, CR, and BUU were 351 ± 22, 352 ± 16, 316 ± 15, and 603 ± 83 pg/m3, respectively. The highest average PCB concentration level was observed in the BUU, while the lowest PCB concentration levels were measured in the CR yet not different from the other Peru sites.
The sampling points from Peru showed low variation during the three months of sampling, and statistically significant differences among the samples were not observed (p > 0.05). However, the average PCB value measured in the BUU was higher than the values reported in the Peru sites. Jaward et al. (2004) reported ∑29PCB concentrations between 20 and 1700 pg/m3 across 22 European countries. The same author, in another study, showed that ∑29PCB concentrations in four Asian countries (China, Singapore, Japan, and South Korea) were between 5 and 336 pg/m3 (Jaward et al. 2005). It was expected to measure lower concentrations in our studies because European countries have used a massive amount of PCBs in comparison with other parts of the world (United Nations Environment Programme 2002). However, industrial sites in Asia may contain higher PCB concentrations than in Europe. Xing et al. (2009) reported the average ∑37PCB concentration of 415 ± 355 ng/m3 in a recycling center of China, which is about 200 times higher than the highest PCB concentration declared by Jaward et al. (2004). Pozo et al. (2012) and Tombesi et al. (2014) have reported ∑48PCB concentrations of 200 ± 13 pg/m3 and 100–350 pg/m3 in Buena Bahia (Argentina) and Chile (Concepcion), respectively. The Health and Environmental Ministry of Peru reported ∑7PCB concentrations lower than 0.016 pg/filter in 6 important cities of Peru in 2017 (Health Ministry of Peru 2017). These results are difficult to compare with other values due to the low number of PCB congeners analyzed and the different units used to present the results.
In a previous study in the BUU by Cindoruk and Tasdemir (2008), they reported ∑37PCB concentrations in the gas-phase as 434 ± 11 pg/m3. However, our study demonstrated an increase in PCB concentrations. The newly open depot for e-waste, 50 m from the sampling point, could explain this increase in PCB levels. Moreover, PCB concentrations in more industrialized cities in Turkey, such as Aliaga, Kocaeli, and Izmir, showed atmospheric PCB concentrations between 13 and 388 times higher than our results (Aydin et al. 2014; Cetin et al. 2007, 2017). In general, industrial sites presented higher concentrations than the urban and rural sites.
Homolog groups of PCBs can be used as a standard method for determining the possible sources, environmental transport, and behavior of PCBs in the environment (Habibullah-Al-Mamun et al. 2019). The percentage distribution of monthly homolog group each sampling point is shown in Fig. 6.
Homolog group distributions were examined, and similar results were obtained in all sampling points. The predominant homolog groups were Tri- (19–29%), Tetra- (19–25%), and Penta- (12–22%) CBs. Various studies around the world show that Tri- and Tetra-CB homologs are dominant in industrial and urban areas (Birgül et al. 2017; Pozo et al. 2012; Tasdemir et al. 2004). In general, higher molecular weight PCBs are in the particle phase. Gas-phase pollutants are mostly sampled in PAS. This situation is thought to be one of the reasons for the low contribution of high chlorinated homologs in this study (Birgül et al. 2017). Hepta- and Nona-CBs covered 4–8% and 0–5% of total homologs, respectively. Also, considering the historical background of PCB production, low molecular weight homolog groups were mostly produced (Arinaitwe et al. 2018; Breivik et al. 2002). Therefore, it is likely to measure low-chlorinated PCBs in the atmosphere.
Temporal changes and differences among pollutant sources of POPs
The PCC and COD results obtained for PAHs, OCPs, and PCBs in the sampling points are shown in Fig. 7 and Fig. 8, respectively.
Except for the BUU in June (PCC < 0.7), PAH concentrations within four sampling points did not change temporally. The analysis for the identification of PAH sources indicated differences between Peru and Turkey. When the PCC values for OCPs were examined, generally low correlation levels were observed. The PCC values for OCPs in the BUU and CR sampling points were higher than 0.74 in all months. Since pesticides have been recently used in these two sampling points, no differences were expected between them (Fig. 4). Differences between temporal changes in the LR and LB sampling points in June and July were thought to be due to the fact that the LR sampling point was located closer to agricultural activities than the LB. The high PCC values observed in August were explained by the start of pesticide usage from this month (Rodrigues et al. 2018). For this reason, low PCC values were observed in June–July and high PCC values in August in both sampling points. The calculated PCC values for PCBs were found to be higher than 0.7 in all sampling points and months.
The COD values were higher than 0.2 among almost all paired sites. These results indicated the differences in source types (Liu et al. 2017). The COD distributions of PAHs in the LR-LB sampling points were close to 0.2; the LR-BUU, the LB-BUU, and the CR-BUU samples yielded typically a result higher than 0.5. This means there were differences between PAH sources in both countries. The CR sampling point represents a region near to the main road and anthropogenic sources, while the BUU sampling point represents a region with less PAH sources than other points. In Peru, wood and coal are widely used for heating and cooking, while natural gas is generally consumed in Turkey. High COD rates were observed due to these differences between the two countries. According to COD distributions in OCP samples, the CR-BUU sampling points were found to be less than 0.2. Also, in June, the LB-LR sampling point was also close to 0.2. The COD values in the LR-BUU and LB-BUU sampling points were always higher than 0.2. It appeared that similar sources of OCPs were found in the CR-BUU sampling points. When the β-/(α+γ)-HCH ratios of these two sampling points were analyzed, it was determined that pesticides had been used recently. COD distributions in PCBs are similar to those of OCPs. COD values in the LR-LB sampling point in July and in the CR-BUU sampling point in August were less than 0.2. When the distributions of both the PCC and COD values were examined, similarities were observed between the temporal distribution and sources of pollutants in the CR-BUU sampling points in June-July for OCPs and in August for PCBs.
Health risk assessment of POPs via inhalation
The carcinogenic risk via inhalation of POPs for adults and children is given in Table 2. According to the results of cancer risk by inhalation for PAHs, OCPs, and PCBs, it was determined that there was not any risk for both adults and children in two countries. However, the risk of cancer that may arise as a result of exposure to PAHs in Peru is higher than in Turkey. This was probably related to the effect of pollutant sources and their effectiveness. For example, since the BUU sampling point represented an area away from PAH sources than other sampling points, less cancer risk was calculated for both adults and children. On the other hand, according to the β-/(a+γ)-HCH ratios in the BUU sampling point, there was an indication of recent pesticide usage. Due to pesticide use, higher results were obtained at the BUU sampling point than other sampling points for both adults and children. Finally, due to the open waste storage for electronic waste near the BUU sampling point, the inhalational exposure at this sampling point yielded higher PCB risk values for both adults and children compared with other sampling points. The use of coal and wood for cooking was high and continuous throughout the year in Yurimaguas, Peru. Therefore, the CR sampling point, although within limits, poses a higher risk for PAH inhalation compared with other regions. According to the β-/(a+γ)-HCH ratios in the sampling points in Peru, there was a historical use of pesticides in general. At the same time, low PCB concentrations have been observed because the sampling points in Peru are far from industries. As a result, there is no risk of cancer in both age groups that may occur via inhalation.
Conclusion
In this study, the measurements of atmospheric PAHs, OCPs, and PCBs concentrations in Yurimaguas (Peru) and Bursa (Turkey) were carried out during the summer season (June–August) in 2018. The highest PAH concentrations and the lowest OCP and PCB concentrations were measured in Peru. Molecular diagnostic ratio (MDR) and ring profile results indicated that anthropogenic sources like open-air cooking, coal burning, and traffic were much more effective in Peru. Considering the OCPs, the usage of lindane was dominant in both countries. The reason for the high PCB concentrations at the BUU sampling point was due to the commissioning of the electronic waste storage area near the sampling area. The Pearson correlation coefficient (PCC) method was used to correlate the results from both sampling points. In addition, the coefficient of divergence (COD) method was employed to investigate the differences between the pollutant sources in both sampling points. According to these methods, the concentration levels were similar for PAH and PCBs; yet, the sources of pollutants were different. Finally, the risk of cancer that can occur in both adults and children via inhalation was evaluated. There was no risk for POPs in both countries during the sampling period. This paper represents the first study to compare Turkey with a Latin American country. In this context, further studies must be carried out in Latin American countries and the evaluation with more industrialized countries as Turkey, as well.
References
Ali, N. (2019). Polycyclic aromatic hydrocarbons (PAHs) in indoor air and dust samples of different Saudi microenvironments; health and carcinogenic risk assessment for the general population. Science of the Total Environment, 696, 133995. https://doi.org/10.1016/j.scitotenv.2019.133995.
Andersson, J. T., & Achten, C. (2015). Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycyclic Aromatic Compounds, 35, 330–354. https://doi.org/10.1080/10406638.2014.991042.
Arinaitwe, K., Muir, D. C. G., Kiremire, B. T., Fellin, P., Li, H., Teixeira, C., & Mubiru, D. N. (2018). Prevalence and sources of polychlorinated biphenyls in the atmospheric environment of Lake Victoria, East Africa. Chemosphere, 193, 343–350. https://doi.org/10.1016/j.chemosphere.2017.11.041.
Aydin, Y. M., Kara, M., Dumanoglu, Y., Odabasi, M., & Elbir, T. (2014). Source apportionment of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in ambient air of an industrial region in Turkey. Atmospheric Environment, 97, 271–285. https://doi.org/10.1016/j.atmosenv.2014.08.032.
Bano, S., Pervez, S., Chow, J. C., Matawle, J. L., Watson, J. G., Sahu, R. K., Srivastava, A., Tiwari, S., Pervez, Y. F., & Deb, M. K. (2018). Coarse particle (PM10–2.5) source profiles for emissions from domestic cooking and industrial process in Central India. Science of the Total Environment, 627, 1137–1145. https://doi.org/10.1016/j.scitotenv.2018.01.289.
Birgül, A., Kurt-Karakus, P. B., Alegria, H., Gungormus, E., Celik, H., Cicek, T., & Güven, E. C. (2017). Polyurethane foam (PUF) disk passive samplers derived polychlorinated biphenyls (PCBs) concentrations in the ambient air of Bursa-Turkey: spatial and temporal variations and health risk assessment. Chemosphere, 168, 1345–1355. https://doi.org/10.1016/j.chemosphere.2016.11.124.
Bohlin, P., Jones, K. C., Tovalin, H., & Strandberg, B. (2008). Observations on persistent organic pollutants in indoor and outdoor air using passive polyurethane foam samplers. Atmospheric Environment, 42, 7234–7241. https://doi.org/10.1016/j.atmosenv.2008.07.012.
Bohlin-Nizzetto, P., Melymuk, L., White, K. B., Kalina, J., Madadi, V. O., Adu-Kumi, S., et al. (2020). Field- and model-based calibration of polyurethane foam passive air samplers in different climate regions highlights differences in sampler uptake performance. Atmospheric Environment, 238(July). https://doi.org/10.1016/j.atmosenv.2020.117742.
Botwe, B. O., Kelderman, P., Nyarko, E., & Lens, P. N. L. (2017). Assessment of DDT, HCH and PAH contamination and associated ecotoxicological risks in surface sediments of coastal Tema Harbour (Ghana). Marine Pollution Bulletin, 115, 480–488. https://doi.org/10.1016/j.marpolbul.2016.11.054.
Breivik, K., Sweetman, A., Pacyna, J. M., & Jones, K. C. (2002). Towards a global historical emission inventory for selected PCB congeners - a mass balance approach: 2. Emissions. Science of the Total Environment, 290, 199–224. https://doi.org/10.1016/S0048-9697(01)01076-2.
Cetin, B., Yatkin, S., Bayram, A., & Odabasi, M. (2007). Ambient concentrations and source apportionment of PCBs and trace elements around an industrial area in Izmir, Turkey. Chemosphere, 69, 1267–1277. https://doi.org/10.1016/j.chemosphere.2007.05.064.
Cetin, B., Yurdakul, S., Keles, M., Celik, I., Ozturk, F., & Dogan, C. (2017). Atmospheric concentrations, distributions and air-soil exchange tendencies of PAHs and PCBs in a heavily industrialized area in Kocaeli, Turkey. Chemosphere, 183, 69–79. https://doi.org/10.1016/j.chemosphere.2017.05.103.
Cetin, B., Yurdakul, S., & Odabasi, M. (2019). Spatio-temporal variations of atmospheric and soil polybrominated diphenyl ethers (PBDEs) in highly industrialized region of Dilovasi. Science of the Total Environment, 646, 1164–1171. https://doi.org/10.1016/j.scitotenv.2018.07.299.
Choi, S. D. (2014). Time trends in the levels and patterns of polycyclic aromatic hydrocarbons (PAHs) in pine bark, litter, and soil after a forest fire. Science of the Total Environment, 470–471, 1441–1449. https://doi.org/10.1016/j.scitotenv.2013.07.100.
Chuang, H. C., Sun, J., Ni, H., Tian, J., Lui, K. H., Han, Y., Cao, J., Huang, R. J., Shen, Z., & Ho, K. F. (2019). Characterization of the chemical components and bioreactivity of fine particulate matter produced during crop-residue burning in China. Environmental Pollution, 245, 226–234. https://doi.org/10.1016/j.envpol.2018.10.119.
Chun, M. Y. (2011). Relationship between PAHs concentrations in ambient air and deposited on pine needles. Environmental Health and Toxicology, 26, e2011004. https://doi.org/10.5620/eht.2011.26.e2011004.
Cindoruk, S. S., & Tasdemir, Y. (2007). Characterization of gas/particle concentrations and partitioning of polychlorinated biphenyls (PCBs) measured in an urban site of Turkey. Environmental Pollution, 148, 325–333. https://doi.org/10.1016/j.envpol.2006.10.018.
Cindoruk, S. S., & Tasdemir, Y. (2008). Atmospheric gas and particle phase concentrations of polychlorinated biphenyls (PCBs) in a suburban site of Bursa, Turkey. Environmental Forensics, 9, 153–165. https://doi.org/10.1080/15275920801888442.
Cindoruk, S. S., & Tasdemir, Y. (2014). The investigation of atmospheric deposition distribution of organochlorine pesticides (OCPs) in Turkey. Atmospheric Environment, 87, 207–217. https://doi.org/10.1016/j.atmosenv.2014.01.008.
Da, C., Liu, G., & Yuan, Z. (2014). Analysis of HCHs and DDTs in a sediment core from the Old Yellow River Estuary, China. Ecotoxicology and Environmental Safety, 100, 171–177. https://doi.org/10.1016/j.ecoenv.2013.10.034.
Dickerson, A. S., Ransome, Y., & Karlsson, O. (2019). Human prenatal exposure to polychlorinated biphenyls (PCBs) and risk behaviors in adolescence. Environment International, 129, 247–255. https://doi.org/10.1016/j.envint.2019.04.051.
Eccleshare, L., Selzer, S., & Woodward, S. (2017). An efficient synthesis of substituted chrysenes. Tetrahedron Letters, 58, 393–395. https://doi.org/10.1016/j.tetlet.(2016).12.004.
Eker, G., & Tasdemir, Y. (2018). Atmospheric deposition of organochlorine pesticides (OCPs): species, levels, diurnal and seasonal fluctuations, transfer velocities. Archives of Environmental Contamination and Toxicology, 75(4), 625–633. https://doi.org/10.1007/s00244-018-0560-8.
Esen, F., & Kayikci, G. (2018). Polycyclic aromatic hydrocarbons in indoor and outdoor air in Turkey: estimations of sources and exposure. Environmental Forensics, 19, 39–49. https://doi.org/10.1080/15275922.2017.1408162.
Estellano, V. H., Pozo, K., Harner, T., Corsolini, S., & Focardi, S. (2012). Using PUF disk passive samplers to simultaneously measure air concentrations of persistent organic pollutants (POPs) across the Tuscany region, Italy. Atmospheric Pollution Research, 3, 88–94. https://doi.org/10.5094/APR.2012.008.
Evci, Y. M., Esen, F., & Taşdemir, Y. (2016). Monitoring of long-term outdoor concentrations of PAHs with passive air samplers and comparison with meteorological data. Archives of Environmental Contamination and Toxicology, 71, 246–256. https://doi.org/10.1007/s00244-016-0292-6.
Fu, L., Lu, X., Tan, J., Zhang, H., Zhang, Y., Wang, S., & Chen, J. (2018). Bioaccumulation and human health risks of OCPs and PCBs in freshwater products of Northeast China. Environmental Pollution, 242, 1527–1534. https://doi.org/10.1016/j.envpol.2018.08.046.
Ghanavati, N., Nazarpour, A., & Watts, M. J. (2019). Status, source, ecological and health risk assessment of toxic metals and polycyclic aromatic hydrocarbons (PAHs) in street dust of Abadan, Iran. Catena, 177, 246–259. https://doi.org/10.1016/j.catena.2019.02.022.
Goel, A., Upadhyay, K., & Chakraborty, M. (2016). Investigation of levels in ambient air near sources of Polychlorinated Biphenyls (PCBs) in Kanpur, India, and risk assessment due to inhalation. Environmental Monitoring and Assessment, 188. https://doi.org/10.1007/s10661-016-5280-9.
Habibullah-Al-Mamun, M., Kawser Ahmed, M., Saiful Islam, M., Tokumura, M., & Masunaga, S. (2019). Occurrence, distribution and possible sources of polychlorinated biphenyls (PCBs) in the surface water from the Bay of Bengal coast of Bangladesh. Ecotoxicology and Environmental Safety, 167, 450–458. https://doi.org/10.1016/j.ecoenv.2018.10.052.
Hao, Y., Li, Y., Han, X., Wang, T., Yang, R., Wang, P., Xiao, K., Li, W., Lu, H., Fu, J., Wang, Y., Shi, J., Zhang, Q., & Jiang, G. (2019). Air monitoring of polychlorinated biphenyls, polybrominated diphenyl ethers and organochlorine pesticides in West Antarctica during 2011–2017: Concentrations, temporal trends and potential sources. Environmental Pollution, 249, 381–389. https://doi.org/10.1016/j.envpol.2019.03.039.
Hazrati, S., & Harrad, S. (2007). Calibration of polyurethane foam (PUF) disk passive air samplers for quantitative measurement of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs): factors influencing sampling rates. Chemosphere, 67(3), 448–455. https://doi.org/10.1016/j.chemosphere.2006.09.091.
Health Ministry of Peru. (2017). Monitoreo y Análisis de Bifenilos Policlorados en el Aire en ciudades priorizadas del Perú. https://minpetel.com/publicaciones-y-videos-para-gestion-de-pcb. Accessed 2 Apr 2020.
Herkert, N. J., Martinez, A., & Hornbuckle, K. C. (2016). A model using local weather data to determine the effective sampling volume for PCB congeners collected on passive air samplers. Environmental Science and Technology, 50, 6690–6697. https://doi.org/10.1021/acs.est.6b00319.
Jaward, F. M., Farrar, N. J., Harner, T., Sweetman, A. J., & Jones, K. C. (2004). Passive air sampling of PCBs, PBDEs, and organochlorine pesticides across Europe. Environmental Science and Technology, 38, 34–41. https://doi.org/10.1021/es034705n.
Jaward, F. M., Zhang, G., Nam, J. J., Sweetman, A. J., Obbard, J. P., Kobara, Y., & Jones, K. C. (2005). Passive air sampling of polychlorinated biphenyls, organochlorine compounds, and polybrominated diphenyl ethers across Asia. Environmental Science and Technology, 39, 8638–8645. https://doi.org/10.1021/es051382h.
Jiao, H., Wang, Q., Zhao, N., Jin, B., Zhuang, X., & Bai, Z. (2017). Distributions and sources of polycyclic aromatic hydrocarbons (PAHs) in soils around a chemical plant in Shanxi, China. International Journal of Environmental Research and Public Health, 14. https://doi.org/10.3390/ijerph14101198.
Kartalovic, B., Babic, J., Prica, N., Zivkov-Balos, M., Jaksic, S., Mihaljev, Z., & Cirkovic, M. (2015). The presence of lindane in different types of honey in the Pannonian region. Bulgarian Journal of Agricultural Science, 21, 208–212.
Konstantinova, E., Minkina, T., Sushkova, S., Antonenko, E., & Konstantinov, A. (2019). Levels, sources, and toxicity assessment of polycyclic aromatic hydrocarbons in urban topsoils of an intensively developing Western Siberian city. Environmental Geochemistry and Health, 9. https://doi.org/10.1007/s10653-019-00357-9.
Kurt-Karakus, P. B., Ugranli-Cicek, T., Sofuoglu, S. C., Celik, H., Gungormus, E., Gedik, K., et al. (2018). The first countrywide monitoring of selected POPs: polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs) and selected organochlorine pesticides (OCPs) in the atmosphere of Turkey. Atmospheric Environment, 177(January), 154–165. https://doi.org/10.1016/j.atmosenv.2018.01.021.
Lai, Y. C., Tsai, C. H., Chen, Y. L., & Chang-Chien, G. P. (2017). Distribution and sources of atmospheric polycyclic aromatic hydrocarbons at an industrial region in Kaohsiung, Taiwan. Aerosol and Air Quality Research, 17, 776–787. https://doi.org/10.4209/aaqr.2016.11.0482.
Liu, W.-X., He, W., Qin, N., Kong, X.-Z., He, Q.-S., Ouyang, H.-L., Yang, B., Wang, Q.-M., Yang, C., Jiang, Y.-J., Wu, W.-J., & Xu, F.-L. (2012). Residues, distributions, sources, and ecological risks of OCPs in the water from Lake Chaohu, China. The Scientific World Journal, 2012, 1–16. https://doi.org/10.1100/2012/897697.
Liu, Y., Yan, C., Ding, X., Wang, X., Fu, Q., Zhao, Q., Zhang, Y., Duan, Y., Qiu, X., & Zheng, M. (2017). Sources and spatial distribution of particulate polycyclic aromatic hydrocarbons in Shanghai, China. Science of the Total Environment, 584–585, 307–317. https://doi.org/10.1016/j.scitotenv.2016.12.134.
Mari, M., Schuhmacher, M., Feliubadaló, J., & Domingo, J. L. (2008). Air concentrations of PCDD/Fs, PCBs and PCNs using active and passive air samplers. Chemosphere, 70, 1637–1643. https://doi.org/10.1016/j.chemosphere.2007.07.076.
Meire, R. O., Khairy, M., Aldeman, D., Galvão, P. M. A., Torres, J. P. M., Malm, O., & Lohmann, R. (2019). Passive sampler-derived concentrations of PAHs in air and water along Brazilian mountain transects. Atmospheric Pollution Research, 10(2), 635–641. https://doi.org/10.1016/j.apr.2018.10.012.
Mueller, A., Ulrich, N., Hollmann, J., Zapata Sanchez, C. E., Rolle-Kampczyk, U. E., & von Bergen, M. (2019). Characterization of a multianalyte GC-MS/MS procedure for detecting and quantifying polycyclic aromatic hydrocarbons (PAHs) and PAH derivatives from air particulate matter for an improved risk assessment. Environmental Pollution, 255, 112967. https://doi.org/10.1016/j.envpol.2019.112967.
Nguyen, T. N. T., Kwon, H. O., Lammel, G., Jung, K. S., Lee, S. J., & Choi, S. D. (2020). Spatially high-resolved monitoring and risk assessment of polycyclic aromatic hydrocarbons in an industrial city. Journal of Hazardous Materials, 393(February), 122409. https://doi.org/10.1016/j.jhazmat.2020.122409.
Odabasi, M., Cetin, B., Demircioglu, E., & Sofuoglu, A. (2008). Air-water exchange of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) at a coastal site in Izmir Bay, Turkey. Marine Chemistry, 109, 115–129. https://doi.org/10.1016/j.marchem.2008.01.001.
Ozcan, S., & Aydin, M. E. (2009). Polycyclic aromatic hydrocarbons, polychlorinated biphenyls and organochlorine pesticides in urban air of Konya, Turkey. Atmospheric Research, 93, 715–722. https://doi.org/10.1016/j.atmosres.2009.02.012.
Pokhrel, B., Gong, P., Wang, X., Chen, M., Wang, C., & Gao, S. (2018). Distribution, sources, and air–soil exchange of OCPs, PCBs and PAHs in urban soils of Nepal. Chemosphere, 200, 532–541. https://doi.org/10.1016/j.chemosphere.2018.01.119.
Pozo, K., Harner, T., Rudolph, A., Oyola, G., Estellano, V. H., Ahumada-Rudolph, R., Garrido, M., Pozo, K., Mabilia, R., & Focardi, S. (2012). Survey of persistent organic pollutants (POPs) and polycyclic aromatic hydrocarbons (PAHs) in the atmosphere of rural, urban and industrial areas of Concepciόn, Chile, using passive air samplers. Atmospheric Pollution Research, 3, 426–434. https://doi.org/10.5094/APR.2012.049.
Pozo, K., Estellano, V. H., Harner, T., Diaz-Robles, L., Cereceda-Balic, F., Etcharren, P., Pozo, K., Vidal, V., Guerrero, F., & Vergara-Fernández, A. (2015). Assessing polycyclic aromatic hydrocarbons (PAHs) using passive air sampling in the atmosphere of one of the most wood-smoke-polluted cities in Chile: the case study of Temuco. Chemosphere, 134, 475–481. https://doi.org/10.1016/j.chemosphere.2015.04.077.
Qu, C., Qi, S., Yang, D., Huang, H., Zhang, J., Chena, W., Yohannes, H. K., Sandy, E. H., Yang, J., & Xing, X. (2015). Risk assessment and influence factors of organochlorine pesticides (OCPs) in agricultural soils of the hill region: a case study from Ningde, southeast China. Journal of Geochemical Exploration, 149, 43–51. https://doi.org/10.1016/j.gexplo.2014.11.002.
Qu, C., Albanese, S., Lima, A., Hope, D., Pond, P., Fortelli, A., et al. (2019). The occurrence of OCPs, PCBs, and PAHs in the soil, air, and bulk deposition of the Naples metropolitan area, southern Italy: implications for sources and environmental processes. Environment International, 124(October 2018), 89–97. https://doi.org/10.1016/j.envint.2018.12.031.
Ranjbar Jafarabadi, A., Riyahi Bakhtiari, A., Mitra, S., Maisano, M., Cappello, T., & Jadot, C. (2019). First polychlorinated biphenyls (PCBs) monitoring in seawater, surface sediments and marine fish communities of the Persian Gulf: distribution, levels, congener profile and health risk assessment. Environmental Pollution, 253, 78–88. https://doi.org/10.1016/j.envpol.2019.07.023.
Rodrigues, E. T., Alpendurada, M. F., Ramos, F., & Pardal, M. Â. (2018). Environmental and human health risk indicators for agricultural pesticides in estuaries. Ecotoxicology and Environmental Safety, 150, 224–231. https://doi.org/10.1016/j.ecoenv.2017.12.047.
Sari, M. F., Esen, F., & Tasdemir, Y. (2020a). Biomonitoring and source identification of polycyclic aromatic hydrocarbons (PAHs) using pine tree components from three different sites in Bursa, Turkey. Archives of Environmental Contamination and Toxicology, 78, 646–657. https://doi.org/10.1007/s00244-020-00722-1.
Sari, M. F., Gurkan Ayyildiz, E., & Esen, F. (2020b). Determination of polychlorinated biphenyls in honeybee, pollen, and honey samples from urban and semi-urban areas in Turkey. Environmental Science and Pollution Research, 27, 4414–4422. https://doi.org/10.1007/s11356-019-07013-w.
Shen, R., Liu, Z., Chen, X., Wang, Y., Wang, L., Liu, Y., & Li, X. (2019). Atmospheric levels, variations, sources and health risk of PM2.5-bound polycyclic aromatic hydrocarbons during winter over the North China Plain. Science of the Total Environment, 655, 581–590. https://doi.org/10.1016/j.scitotenv.2018.11.220.
Taiwo, A. M. (2019). A review of environmental and health effects of organochlorine pesticide residues in Africa. Chemosphere, 220, 1126–1140. https://doi.org/10.1016/j.chemosphere.2019.01.001.
Tasdemir, Y., & Esen, F. (2007). Urban air PAHs: Concentrations, temporal changes and gas/particle partitioning at a traffic site in Turkey. Atmospheric Research, 84(1), 1–12. https://doi.org/10.1016/j.atmosres.2006.04.003.
Tasdemir, Y., & Esen, F. (2008). Deposition of polycyclic aromatic hydrocarbons (PAHs) and their mass transfer coefficients determined at a trafficked site. Archives of Environmental Contamination and Toxicology, 55, 191–198. https://doi.org/10.1007/s00244-007-9096-z.
Tasdemir, Y., Vardar, N., Odabasi, M., & Holsen, T. M. (2004). Concentrations and gas/particle partitioning of PCBs in Chicago. Environmental Pollution, 131, 35–44. https://doi.org/10.1016/j.envpol.2004.02.031.
Thang, P. Q., Kim, S. J., Lee, S. J., Kim, C. H., Lim, H. J., Lee, S. B., et al. (2020). Monitoring of polycyclic aromatic hydrocarbons using passive air samplers in Seoul, South Korea: spatial distribution, seasonal variation, and source identification. Atmospheric Environment, 229(November 2019), 117460. https://doi.org/10.1016/j.atmosenv.2020.117460.
Tobiszewski, M., & Namieśnik, J. (2012). PAH diagnostic ratios for the identification of pollution emission sources. Environmental Pollution, 162, 110–119. https://doi.org/10.1016/j.envpol.2011.10.025.
Tombesi, N., Pozo, K., & Harner, T. (2014). Persistent organic pollutants (POPs) in the atmosphere of agricultural and urban areas in the Province of Buenos Aires in Argentina using PUF disk passive air samplers. Atmospheric Pollution Research, 5, 170–178. https://doi.org/10.5094/APR.2014.021.
Tromp, P. C., Beeltje, H., Okeme, J. O., Vermeulen, R., Pronk, A., & Diamond, M. L. (2019). Calibration of polydimethylsiloxane and polyurethane foam passive air samplers for measuring semi volatile organic compounds using a novel exposure chamber design. Chemosphere, 227, 435–443. https://doi.org/10.1016/j.chemosphere.2019.04.043.
Tuduri, L., Harner, T., & Hung, H. (2006). Polyurethane foam (PUF) disks passive air samplers: wind effect on sampling rates. Environmental Pollution, 144, 377–383. https://doi.org/10.1016/j.envpol.2005.12.047.
U.S. Environmental Protection Agency, 1991. Risk assessment guidance for superfund. Volume I Human health evaluation manual (Part B, Development of Risk-based preliminary remediation goals) I, 66. EPA/540/1-89/002
United Nations Environment Programme (2002). Eastern and Western South America. Switzerland.
Vardar, N., Esen, F., & Tasdemir, Y. (2008). Seasonal concentrations and partitioning of PAHs in a suburban site of Bursa, Turkey. Environmental Pollution, 155, 298–307. https://doi.org/10.1016/j.envpol.2007.11.026.
Vijgen, J., de Borst, B., Weber, R., Stobiecki, T., & Forter, M. (2019). HCH and lindane contaminated sites: European and global need for a permanent solution for a long-time neglected issue. Environmental Pollution, 248, 696–705. https://doi.org/10.1016/j.envpol.2019.02.029.
Wang, X., Celander, M. C., Yin, X., Zhang, Z., Chen, Y., Xu, H., Yu, X., Xu, K., Zhang, X., & Kanchanopas-Barnette, P. (2019). PAHs and PCBs residues and consumption risk assessment in farmed yellow croaker (Larimichthys crocea) from the East China Sea, China. Marine Pollution Bulletin, 140, 294–300. https://doi.org/10.1016/j.marpolbul.2019.01.062.
Wong, F., Alegria, H. A., Bidleman, T. F., Alvarado, V., Angeles, F., Galarza, A. A., Bandala, E. R., De La Hinojosa, I. C., Estrada, I. G., Reyes, G. G., Gold-Bouchot, G., Zamora, J. V. M. Í., Murguía-GONZÁLEZ, J., & Espinoza, E. R. (2009). Passive air sampling of organochlorine pesticides in Mexico. Environmental Science and Technology, 43, 704–710. https://doi.org/10.1021/es802385j.
Wu, Z., Lin, T., Hu, L., Guo, T., & Guo, Z. (2020). Atmospheric legacy organochlorine pesticides and their recent exchange dynamics in the Northwest Pacific Ocean. Science of the Total Environment, 727, 138408. https://doi.org/10.1016/j.scitotenv.2020.138408.
Xing, G. H., Chan, J. K. Y., Leung, A. O. W., Wu, S. C., & Wong, M. H. (2009). Environmental impact and human exposure to PCBs in Guiyu, an electronic waste recycling site in China. Environment International, 35, 76–82. https://doi.org/10.1016/j.envint.2008.07.025.
Yin, G., Zhou, Y., Strid, A., Zheng, Z., Bignert, A., Ma, T., et al. (2017). Spatial distribution and bioaccumulation of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in snails (Bellamya aeruginosa) and sediments from Taihu Lake area, China. Environmental Science and Pollution Research, 24(8), 7740–7751. https://doi.org/10.1007/s11356-017-8467-x.
Yolsal, D., Salihoglu, G., & Tasdemir, Y. (2014). Air-soil exchange of PCBs: Levels and temporal variations at two sites in Turkey. Environmental Science and Pollution Research, 21, 3920–3935. https://doi.org/10.1007/s11356-013-2353-y.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33, 489–515. https://doi.org/10.1016/S0146-6380(02)00002-5.
Zhang, L., Dong, L., Yang, W., Zhou, L., Shi, S., Zhang, X., et al. (2013). Passive air sampling of organochlorine pesticides and polychlorinated biphenyls in the Yangtze River Delta, China: concentrations, distributions, and cancer risk assessment. Environmental Pollution, 181, 159–166. https://doi.org/10.1016/j.envpol.2013.06.033.
Zhang, J., Yang, L., Ledoux, F., Courcot, D., Mellouki, A., Gao, Y., Jiang, P., Li, Y., & Wang, W. (2019). PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) and nitrated PAHs (NPAHs) in rural and suburban areas in Shandong and Henan Provinces during the 2016 Chinese New Year’s holiday. Environmental Pollution, 250, 782–791. https://doi.org/10.1016/j.envpol.2019.04.040.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Sari, M.F., Córdova Del Águila, D.A., Tasdemir, Y. et al. Atmospheric concentration, source identification, and health risk assessment of persistent organic pollutants (POPs) in two countries: Peru and Turkey. Environ Monit Assess 192, 655 (2020). https://doi.org/10.1007/s10661-020-08604-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-08604-8