Abstract
Mercury (Hg) is a pollutant that poses a global threat, and it was listed as one of the ten leading ‘chemicals of concern’ by the World Health Organization in 2017. The review aims to summarize the sources of Hg, its combined effects on the ecosystem, and its remediation in the environment. The flow of Hg from coal to fly ash (FA), soil, and plants has become a serious concern. Hg chemically binds to sulphur-containing components in coal during coal formation. Coal combustion in thermal power plants is the major anthropogenic source of Hg in the environment. Hg is taken up by plant roots from contaminated soil and transferred to the stem and aerial parts. Through bioaccumulation in the plant system, Hg moves into the food chain, resulting in potential health and ecological risks. The world average Hg concentrations reported in coal and FA are 0.01–1 and 0.62 mg/kg, respectively. The mass of Hg accumulated globally in the soil is estimated to be 250–1000 Gg. Several techniques have been applied to remove or minimize elevated levels of Hg from FA, soil, and water (soil washing, selective catalytic reduction, wet flue gas desulphurization, stabilization, adsorption, thermal treatment, electro-remediation, and phytoremediation). Adsorbents such as activated carbon and carbon nanotubes have been used for Hg removal. The application of phytoremediation techniques has been proven as a promising approach in the removal of Hg from contaminated soil. Plant species such as Brassica juncea are potential candidates for Hg removal from soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg, hydrargyrum) is a potentially toxic element that has aroused global public health concern due to its toxicity (Alloway 2013; Raju et al. 2019). Hg in coal has been found in the form of cinnabar ore (HgS) and in association with pyrite (Hower et al. 2010). The melting point of Hg is − 38.8 °C, while the boiling point is 356.7 °C (Wang et al. 2012).
Once Hg is liberated into the atmosphere from ores, fossil fuels, and mineral deposits, it can be highly mobile and is deposited on surface soils, water bodies, and bottom sediments (Xu et al. 2015). The natural sources of Hg include degassing of the earth’s crust and evaporation from water bodies (oceans) (Rahman and Singh 2019), whereas emission from coal-burning thermal power plants (TPPs) is the main anthropogenic source. The most hazardous compound of Hg in the environment is methylmercury (MeHg) (Liang et al. 2009; Jagtap and Maher 2015; Rasulov et al. 2017), which is bioaccumulated and biomagnified in freshwater and saltwater fishes (Bradley et al. 2017). Due to its property of bioaccumulation in the food chain, methylmercury is considered the most concerning species of Hg (Cheng et al. 2013; Halbach et al. 2017; Bailon et al. 2018). A menacing disease called Minamata spread in Japan due to the consumption of fish contaminated with MeHg (Xinmin et al. 2006; Wang et al. 2015; Matsuyama et al. 2018). The harmful effect of Hg inhalation in humans is its neurotoxicity; i.e. Hg damages neural cells (Alloway 2013). Acute exposure to Hg may also cause psychological problems, such as anxiety, sleep disturbance, and depression (Gall et al. 2015).
The global anthropogenic emissions of Hg have been estimated at approximately 2000 Mg/year (1 Mg = 1 tonne = 106 g) (Pirrone et al. 2010; Streets et al. 2011), while emissions in India have been estimated at approximately 240 Mg/year (Pirrone et al. 2010). In China, the approximate anthropogenic Hg emissions in 2010 were reported to be 538 Mg (Zhang et al. 2015). According to the UNEP emission inventory, the highest Hg-emitting countries are China, India, Indonesia, Columbia, South Africa, Russia, Ghana, and the USA. The total emissions of these countries compose 56% (1095 Mg/year) of the total anthropogenic emissions to the atmosphere (UNEP 2013). Obrist et al. (2018) reported that the global mass of Hg accumulated in the soil is estimated to be in the range of 250–1000 Gg (1 Gg = 109 g).
Hg is remediated from soil and water through physical, chemical, and biological processes. Phytoremediation is the most effective and environmentally friendly technique for Hg removal from soil and water. Plant species like Brassica juncea is known to be the potential candidate for Hg removal from soil (Su et al. 2008).
The aim of the present study is to review the current knowledge on the major sources of Hg, as well as the effective Hg remediation techniques (physical separation and phytoremediation). The chemistry of Hg and assessment procedures thereof in various environmental media are explained in this review. The human health risks associated with Hg pollution are briefly discussed.
Chemistry of Hg
As Hg is a constituent element of the earth, it is bound with minerals in pyrite and sulphide form (Mukherjee et al. 2008). The metallic form of Hg is extracted from mercuric sulphide (HgS) ore (Donatello et al. 2012). It is also bound to other compounds as monovalent (Hg+) or divalent mercury (Hg2+) (Pudasainee et al. 2009) (Fig. 1). The simplest form of Hg is elemental mercury (Hg0), which itself is hazardous to humans and the environment. In natural conditions, Hg primarily forms complexes with OH−, Cl−, S2− and with the functional groups of organic ligands that contain sulphur (Dziok et al. 2015; Xu et al. 2015). Both oxidation and reduction processes are exhibited by Hg during the biotic process in the natural environment, and these processes result in changes in Hg speciation (Tan et al. 2004), which in turn affects the biological uptake of Hg (Xu et al. 2015). Hg is found in the form of organic and inorganic compounds (Renneberg and Dudas 2001; Xinmin et al. 2006).
Hg cycle in the environment (Alloway 2013)
The inorganic forms of Hg include mercuric chloride (HgCl2), mercuric sulphide (HgS), and mercuric oxide (HgO), while methylmercury (MeHg), dimethyl mercury ((CH3)2Hg), ethyl mercury (C2H5Hg), and phenylmercury (C6H5Hg) are the organic forms of Hg (Alloway 2013). The inorganic compounds of Hg are also called Hg salts, which are found in powder and crystal forms. Some organic forms of Hg, such as MeHg and phenyl mercury, exist as salts (methylmercuric chloride and phenylmercuric acetate) and are found as white crystalline solids in pure form (Alloway 2013). The biological behaviour of Hg depends upon the interconversion of its various forms. For example, inhaled Hg0 vapour is easily absorbed through mucous membranes and the lungs, where it rapidly oxidizes to other forms (Kabata-Pendias 2010). At room temperature and atmospheric pressure, Hg is found in a liquid state because the outermost orbital of Hg is filled with electrons, and it does not share its valence electrons easily. Hence, the bonding between Hg atoms is very weak and easily broken at normal temperature. In organic Hg, the carbon-Hg bond is chemically stable (Alloway 2013).
In water, the solubility of various compounds of Hg varies. Hg(I) chloride and HgS are less soluble, Hg(II) chloride is readily soluble, and Hg0 is insoluble in water. The inorganic form of Hg is methylated in water, which leads to the formation of very toxic MeHg in aquatic systems. The existence of bacterial species (Pseudomonas) in fish enables the methylation of Hg in water. Once MeHg is formed, it enters the food chain of the aquatic ecosystem (WHO 2004).
Methods of Hg determination in soil, coal, fly ash, and plant samples
The total Hg content (organic + inorganic) in soil, coal, fly ash (FA), and plant tissues can be determined by the process of acid digestion. (a) For Hg determination in soil, the samples are air-dried at room temperature and passed through a 2-mm stainless steel sieve to remove the large rock debris. Later, a portion of the homogenized soil sample (approximately 50 g) is further passed through a 0.149-mm stainless steel sieve (100 mesh) and oven-dried at 45 °C until a constant weight is achieved. Then, the total Hg can be determined by digesting the accurately weighed (0.5 g) samples with 50% aqua regia (HNO3:H2SO4; 1:3 v/v) on a hot plate for 30 min at 120 °C (USEPA 2007a; Park et al. 2013). (b) In coal and FA samples, the total Hg can be determined by mixing the samples with nitric acid (HNO3) and digestion on a hot plate at 70 °C for 90 min (ASTM D6414 2006; Park et al. 2013). (c). The total Hg can be determined in plant tissues by dissolving dried and sieved samples of plant tissue in an acid mixture of conc. HNO3 and conc. H2SO4 and heating on a hot plate at 95 °C for 30 min (Lomonte et al. 2008). As per the USEPA method ( 1991), 0.2–0.3 g of dried samples are digested with an acid mixture of conc. H2SO4 and conc. HNO3 (4:1) at 58 °C in a water bath until the plant tissues are dissolved (30–60 min).
All samples are digested until a clear transparent solution is obtained. The digested solution is filtered through Whatman filter paper (Whatman #42, pore size 2.5 μm), and the filtrate is diluted to 100 mL with de-ionized water. The total Hg content in diluted solution is determined by cold vapour atomic absorption spectrometry (CVAAS) (Dołęgowska and Michalik 2019). The cold vapour technique is applied for Hg determination due to the volatile nature of Hg; in this process, a suitable reducing agent (SnCl4 or NaBH4) is used to generate a cold vapour of Hg, and the absorbance is measured at 253.7 nm (USEPA 1991; Sanchez-Rodas et al. 2010; Csuros and Csuros 2016).
The sequential extraction procedure (SEP) or fractionation of Hg in soil samples and FA can be carried out by using two popular methods. The first method (a) is the Kingston method (Han et al. 2003; Reis et al. 2010; Fernández-Martínez et al. 2005). In this method, the mobile fraction of Hg (MHg), semi-mobile fraction of Hg (SMHg) and non-mobile fraction of Hg (NMHg) can be extracted from a sample.
(b) The BCR (Community Bureau of Reference) fractionation technique has also been used by several researchers to extract various fractions (exchangeable fraction, amorphous Fe-Mn oxides, organic-crystalline iron oxides, and residual fraction) of Hg in soils (Li et al. 2009; Subirés-Muñoz et al. 2011), as given in Table 1.
Sources of mercury in the environment
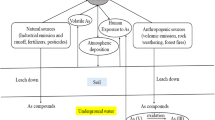
Mercury enters environmental media through natural and anthropogenic sources (Fig. 2). Environmental media are the major categories of the physical environment (abiotic components of the environment) and consist of soil, air, and water (UN. 1997). The release of contaminants such as Hg from point and diffuse sources leads to their further deposition into the physical environment, through which these contaminants move into and become bioaccumulated in living organisms. The two major sources of Hg in the physical environment are as follows:
- (a)
Natural sources: In nature, Hg exists as Hg0 or as HgS and is found in the earth’s crust (Kostova et al. 2013). Atmospheric releases of Hg occur through volcanic activity (Martín and Nanos 2016) or from outgassing from rocks. The other natural source of Hg emission into the environment is geothermal activity (Gustin 2003).
- (b)
Anthropogenic sources: The anthropogenic sources of Hg are categorized into point sources and diffuse sources. The point sources include coal-fired TPPs and incinerators, while the diffuse sources include landfills, sewage sludge-amended fields, and mine wastes (Alloway 2013). The total Hg emissions in 2010 from the combustion of fossil fuels such as coal are estimated at approximately 475 metric tonnes and the value varies according to the Hg content in fossil fuels (Streets et al. 2018). The mining and smelting of ores also contribute Hg to the environment and are considered important sources of anthropogenic air pollution (Zhang et al. 2014). The incineration of Hg-containing waste, such as batteries and various industrial wastes (scrubber sludge), also contributes to Hg concentrations in all environmental media (Gosar et al. 2006). The use of Hg-containing pesticides and fungicides in the agricultural field is hazardous and may cause a severe health hazard to humans through the consumption of Hg-contaminated food. The use of Hg in thermal sensing instruments, pharmaceuticals, electrical switches, and preparations of dental amalgams are also chief anthropogenic contributors of Hg in the environment (Chen et al. 2010). The most common sources of Hg are explained in detail in the following paragraphs:
Coal
Coal is formed from prehistoric vegetation remains that accumulated in swamps and peat bogs 360 years ago during the Carboniferous period (Alloway 2013). The coal quality depends upon the organic maturity of the coal, i.e. the temperature, pressure, and length of time during coal formation. Initially, brown coal (lignite) is formed from peat with low organic maturity. The Hg content in coal depends on the geological distribution of coal on the earth, and Hg is an undesirable component of various types of coals (lignite and sub-bituminous). The highest concentration of Hg in coal is attributed to hydrothermal fluid circulation in coal (Yudovich and Ketris 2005a, b) (Table 2).
Coal is the main source of energy in India and other developing countries, such as China, Australia, and Russia (Mukherjee et al. 2008). Industrial development is highly dependent on energy production from coal (Hossain et al. 2015; Özkul 2016), which is the main anthropogenic source of Hg emissions to the environment (Li et al. 2017). In China, the energy supply from coal constitutes 70% of the total energy supply, and this value is higher than the global average of 28% of the total energy supply from coal (Li et al. 2017).
It has been reported that the Hg content ranges from 0.01 to 1.1, 0.2 to 2, 0.03 to 1.3, and 0.01 to 2 mg/kg in Indian, Belgian, Canadian, and American coals, respectively, while the maximum Hg content in Russian coal has been reported to reach 20 mg/kg (Agrawal et al. 2008). In one of the earliest studies on Hg content in Indian coal, Ghosh et al. (1994) reported that the Hg content varied from 0.01 to 1.5 mg/kg. The study reported that Hg content in lignite coal ranged from 0.01 to 0.21 mg/kg, with an average value of 0.06 mg/kg. The Hg content in coals from various regions of India was also reported, as follows: in tertiary coal from Assam, Hg (average) = 0.16 mg/kg; in coals from Jammu Kashmir, Hg (average) = 0.8 mg/kg; in coals from Damodar valley coalfields, Hg (average) = 0.52 mg/kg; and in coals from Son Mahanadi coalfields, Hg (average) = 0.56 mg/kg. Ghosh et al. (1994) also reported that most of the Hg in Indian coal is present in the specific gravity fractions 1.6–1.7 and that fractions above 1.7 indicate inorganically associated Hg in coal.
Park et al. (2008) reported that anthracite coal in Korea contained 0.43 mg/kg Hg, while various studies on Chinese coal revealed that the Hg content ranged from 0.01 to 0.5 mg/kg (Tang and Huang 2004) and 0.003 to 10.5 mg/kg (Ren et al. 2006). Luo et al. (2013) conducted a study on the occurrence of Hg in coal, and their research was based on five coal samples. Their study focused on the removal techniques of various forms of Hg present in coal before its utilization. It was observed that the pyrite-bound Hg in coal could be removed through the process of pyrolysis, i.e. heating coal at high temperature (400–600 °C). Extraction with dilute HNO3, followed by pyrolysis, was also one of the methods for the removal of Hg from pyrite-bound coal (Luo et al. 2013). In an assessment study on 51 types of coal used in Japanese TPPs, the Hg contents in coal varied from 0.0068 to 0.243 mg/kg (Ohki et al. 2014). Toole-O'Neil et al. (1999) reported that the average Hg concentration in coal was approximately 0.2 mg/kg, and the data were collected from the United States Geological Survey’s COALQUAL database. Dabrowski et al. (2008) reported that the Hg content in coals from South Africa was 0.2 mg/kg.
Fly ash
Coal-fired TPPs have been installed for electricity generation and are operated in countries such as India and China. Coal combustion in TPPs leads to the production of glossy particles, popularly known as FA (Mahajan et al. 2012), ranging in size from 0.01 to 100 μm (Ansari et al. 2011). FA is also a part of solid waste that contains a significant amount of toxic metals, such as Hg, and causes environmental problems. China is considered the largest emitter of global anthropogenic Hg, and the major source of Hg emissions is ash released from the combustion of coal (Wang and Luo 2017). A study by Wang and Luo (2017) concluded that the combustion of domestic coal, steam coal, and coal gangue contributes approximately 50% of the total anthropogenic emissions of Hg in China. Their study presented data on the emission rate of Hg due to coal combustion in TPPs, and their results showed that the Hg emission rate from TPPs was 50.21%, while 83.61% of Hg emissions were due to the combustion of coal in domestic coal stoves. It was also estimated that coal-fired TPPs release 26% of the global anthropogenic Hg emissions into the environment (Zhao et al. 2017). In one study, it was reported that the contribution of Hg0 (38.95%) released from the stacks of coal-fired TPPs was higher than that of oxidized Hg (Hg2+). The emission of Hg0 was 13 times higher than Hg2+ emission, while Hg occupied the smallest ratio, 0.07% in bottom ash (Zhao et al. 2017).
It has been observed that approximately 30–80% of the total Hg content in coal is found in FA after the combustion process (Wang et al. 2012; Deng et al. 2014). The total Hg content in coal-FA depends on the chemistry of the feed coal and its quality (Hower et al. 2017). In India and China, coal-fired TPPs are the largest emission source of Hg into the environment (Mukherjee et al. 2008; Hu and Cheng 2016). In China, industrial boilers and TPPs together emit approximately 250 tonnes of Hg every year (Hu and Cheng 2016). The results of a study by Hower et al. (2017) showed that the Hg content in FA depends on the coal source and indicated that a low Hg content in the feed coal resulted in a low concentration of Hg in FA. The Hg concentration in FA collected from a TPP in Spain was 0.1 mg/kg (Font et al. 2012), while in the Philippines, the Hg content in FA samples collected from three TPPs (Sual coal power plant, Mauban coal power plant, and Masinloc coal power plant) was 1.2, 1.9, and 1.2 mg/kg, respectively (Brigden and Santillo 2002) (Table 3). In an experimental study conducted by Dabrowski et al. (2008), the average Hg emissions from a coal-fired TPP in South Africa were 9.8 tonnes/year, while the calculated emission factor fell within the range of 0.02 to 0.16 gm Hg/tonnes coal burned. Goodarzi (2006) conducted a study on the assessment of the total Hg content in coal-FA samples collected from the electrostatic precipitator (ESP) and bag house of seven coal-fired TPPs in Canada. FA samples were collected from TPPs that use sub-bituminous coal, low-sulphur bituminous coal, and high-sulphur bituminous feed coal, and the average Hg contents were 0.049, 0.855, and 1.25 mg/kg, respectively. The study found that the Hg content in the FA collected from the baghouse of the power plant was higher than that of the FA collected from the ESP, which means that Hg was less enriched in the ESP than the baghouse of the power plant. A study by Li et al. (2017) concluded that the Hg concentration in the soils surrounding TPPs will increase if the power plants continue to operate for a long period. Zhao et al. (2017) reported that the FA collected from the ESP of a TPP contained more Hg than bottom ash collected from the same power plant, and the Hg concentration in the FA (0.134 mg/kg) was 45 times higher than the Hg content in the bottom ash (0.003 mg/kg).
Industrial release
Industrial processes, cement production, the incineration of medical devices the use of dental amalgam causes health risks amongst dental personnel due to Hg exposure) (Nagpal et al. 2017), alkali and metal processing (discharges from the Hg-cell chlor-alkali industry are the largest anthropogenic source of Hg emission into water bodies), and chemical and municipal wastes are also major sources of Hg in the environment (Fthenakis et al. 1995). Liquid metallic Hg is used in gold extraction and hence contaminates rivers via discharge (Kabata-Pendias 2010; Mbanga et al. 2019).
Mercury pollution in soil
Anthropogenic Hg deposition to soil occurs through atmospheric deposition. Soil plays a pivotal role in the biogeochemical cycle of Hg because it acts as one of the most important pools of Hg (Martín and Nanos 2016), and Hg has a long retention time in soil. In soil, the behaviour of Hg is very complicated and is controlled by adsorption and desorption processes (Montoya et al. 2019). The deposition of atmospheric Hg0 to soil occurs over a large spatial and temporal scale (Lin et al. 2010; O'Connor et al. 2019), while atmospheric Hg2+ is quickly deposited into soil through dry or wet deposition. Hg that has been deposited in the soil is trapped by organic matter in the soil (Wang et al. 2012), and oxidized Hg (Hg2+) forms inorganic mercuric salts and minerals (O'Connor et al. 2019). Trees (especially deciduous trees) are also considered a Hg sink during the growing season. Therefore, in addition to the deposition of Hg to soil from FA, another form of Hg deposition to soil is the dropping of Hg-laden plant leaves on the ground (Alloway 2013). The concentration of Hg in soil mainly depends on edaphogenetic parameters and the composition of the parent material (Gil et al. 2010). It has been reported in some studies that the volatility of Hg increases with increases in the moisture content of soil (Steinnes 1995; Kabata-Pendias and Mukherjee 2007). In recent years, the Hg concentration in soil has increased by a factor of 3 to 10, which is mainly due to the long-range transport of Hg (Xu et al. 2015). Generally, the global average background concentrations of Hg in different types of soil range between 0.58 and 1.18 mg/kg, and the mean value is estimated at 1.1 mg/kg (Kabata-Pendias 2010).
In one study, Dragović et al. (2013) found that the Hg concentration in soils collected from a depth of 0–10 cm in the area surrounding the Nikola Tesla TPP (Belgrade, Serbia) was 2.1 mg/kg, while in a similar type of study carried out by Pastrana-Corral et al. (2017), the Hg concentration in 32 soil samples collected from a depth of 0–5 cm in the vicinity of the Playas de Rosarito TPP (Mexico) was 0.05 mg/kg. The Hg concentrations in soils collected from cropland and forest areas near the Jinsha TPP (China) were 0.70 and 0.30 mg Hg/kg, respectively (Huang et al. 2017). Another study was conducted on Hg pollution in soil, in which 57 samples of surface soil and 108 samples of deeper soil were collected from 5 different industrial areas. The results showed that the average Hg content in surface soils was higher than the background Hg concentration in the areas surrounding the city (Beijing, China). The Hg concentration in 48% of the total analysed soil samples exceeded the critical value of 1.0 mg Hg/kg. The study also reported that the average Hg concentrations in 108 soil samples (27 from each of four different depths) collected from depths of 20, 80, 180, and 400 cm were 3.64, 3.65, 0.98, and 1.11 mg/kg, respectively. The Hg content in the soil samples collected from depths of 0–80 cm was high, which was due to the deposition of Hg-containing waste products released from various industries (Luo et al. 2009). Martín and Nanos (2016) assessed Hg contents in soil and reported that the Hg content in Spanish soil fell within the range of 0.001 to 7.564 mg/kg, with an average concentration of 0.0672 mg Hg/kg. The results showed that the Hg concentration in 50% of the soil samples fell below the level of 0.037 mg/kg, while 66% of the soil samples exceeded a Hg level of 0.025 mg/kg. The study also demonstrated that an increased Hg content was found in topsoils, which was due to the deposition of Hg-laden FA in the areas near coal-fired TPPs. Arbestain et al. (2009) reported that the Hg content in soil was high (> 6500 mg/kg) in the proximity of an old Hg fulminate production plant. The maximum Hg content (10197 mg Hg/kg) was found in soils in the immediate vicinity of an abandoned Hg mine waste in Spain, while the concentration decreased with increasing distance from the mining site (Fernández-Martínez et al. 2005).
Gil et al. (2010) found that 40% of Mediterranean calcareous soil samples exceeded the established reference value of 0.025 mg Hg/kg. In their research, 53 soil samples were collected for the determination of Hg, and the results showed that the Hg content in these samples varied from 0.0094 to 1.585 mg/kg. The symptoms of Hg toxicity in soil can be observed at a level of 0.3 mg Hg/kg, which is also known as the threshold value (Martín and Nanos 2016). Müller et al. (2001) investigated the long-term effect of Hg pollution on the soil microbial community and observed a reduction in the size of bacterial and protozoan populations in Hg-contaminated soil. It was also observed that the bacterial population growing in Hg-contaminated soil media was structurally less diverse. The study of Palmieri et al. (2006) found the continuous deposition of Hg into soil through erosion in cinnabar mines. Therefore, soil samples collected from areas near cinnabar mines in Brazil had a very high Hg content. Soares et al. (2015) concluded that Hg0 accumulates in tropical soils. The oxidation of gaseous Hg0 occurs after the retention of gaseous Hg by soils. The retention capacity of Hg by soils varies with the type of soil. It was also observed from the results of the study that the A-horizon (top layer of the soil) of soil adsorbed more Hg than the B-horizon (subsoil) of the same type of soil. Organic matter plays an important role in the adsorption of gaseous Hg in the soil, and therefore, the A-horizon (high organic matter) of soil contains more Hg. The adsorption of Hg onto soil also depends upon the pH of the soil, and it was observed in an experimental study by Yin et al. (1996) that the adsorption of Hg decreases significantly above soil pH values. The formation of Hg complexes with organic carbon has also been observed at a pH value of 5 due to the availability of large amounts of dissolved soil organic carbon.
Total Hg concentrations in surface and mineral soil of the Norwegian Arctic region were determined by Halbach et al. (2017) (Table 4), and Svalbard was selected as the study area for investigating the long-range transport of Hg to the Arctic region. In the 57 total surface and mineral soil samples, the Hg concentrations ranged from 0.041 to 0.254 and 0.004 to 0.060 mg/kg, respectively. The results clearly showed that the deposition of Hg from the atmosphere is directly associated with Hg accumulation in the surface soil in the study area. The study also showed that the major pathway by which Hg enters the terrestrial ecosystems of the Arctic is atmospheric Hg deposition. Hu et al. (2017) assessed greenhouse soil that was sampled from three intensive greenhouse vegetable production systems along the coast of the Yellow Sea (China) for Hg determination and reported that the surface soils contained 0.05 mg Hg/kg.
Mercury pollution in vegetables and tree leaves
Vegetables growing in Hg-contaminated soil become contaminated due to the uptake of Hg in their roots and edible parts. Several tree species growing on Hg-contaminated soil are also affected by the deposition of Hg-laden FA and mine dust and the uptake of Hg from the soil. Li et al. (2017) reported Hg concentrations in vegetables growing near a coal-fired TPP region and found that the vegetable species Solanum lycopersicum, Cucumis sativus, and Lactuca sativa contained 0.0718, 0.0384, and 0.039 mg Hg/kg, respectively (Table 5). Patel et al. (2015) reported Hg concentrations in various tree leaves and found that the Hg contents in Mangifera indica, Butea monosperma, Tectona grandis, and Azadirachta indica were 3.3, 1.6, 1.8, and 16.4 mg/kg, respectively. It was reported that up to 18 mg/kg Hg occurred in green cabbages (Brassica oleracea) grown in soils contaminated with Hg in the Lanmuchuang Hg mining area of China (Qiu et al. 2006). A study was carried out by Ostos et al. (2015) to assess wild edible mushrooms for Hg content. The study reported that the total Hg content in a wild species of mushroom (Boletus aereus) was 8 mg/kg. Hu et al. (2017) measured Hg contents in leafy, rootstalk, and fruit vegetables and found that the concentrations in the three types of vegetables ranged from 0.0003 to 0.004, 0.0001 to 0.002, and 0.0001 to 0.001 mg/kg, respectively. The potential health risk of Hg was also calculated for the consumption of all three types of vegetables in the study area, and the calculations revealed that the highest Hg content was observed in leafy vegetables. Cheng et al. (2013) measured the concentration of MeHg in foodstuffs and reported that the MeHg content in the edible part of carrots ranged from 0.02 to 0.91 ng/g, while in cucumbers, the concentration ranged from 0.01 to 0.02 ng/g. The study also measured average MeHg contents of 30.63 and 1.77 ng/g in fish and rice, respectively.
Effects of mercury pollution on human health and the environment
Hg toxicity affects all organisms and their ecosystem processes (Wang et al. 2012; Spahić et al. 2018, 2019) (Fig. 3). The major health hazard of Hg in humans is related to exposure to MeHg through food (Pavlish et al. 2003). MeHg exposure primarily occurs through the ingestion of aquatic organisms, mainly fish (El Mahmoud-Hamed et al. 2019), and MeHg is distributed through human tissues by complete absorption in the bloodstream (Ha et al. 2016). In humans, the brain (central nervous system), kidneys, and liver are the main organs where MeHg accumulates (WHO 2003; Cheng et al. 2013; Genthe et al. 2018). The other effects of high-dose exposure to Hg are hearing defects, visual changes, loss of speech, and death in critical conditions. MeHg poisoning leads to the destruction of neuronal cells. It is also transferred to the placenta and efficiently contributes to the poor development of the child’s brain. So, it can be said that the prenatal life is more sensitive than an adult due to the exposure of MeHg (Alloway 2013). The toxic effects of Hg depend upon the dose and rate of exposure of various forms of Hg, and primarily brain is the target organ for inhaled Hg vapour (Beckers and Rinklebe 2017). In humans, Hg exposure is estimated by the determination of Hg content in blood, hair, and urine. The guidelines for daily intake of Hg in adult have been given in Table 6, while the standard guidelines for Hg exposure in environmental media are presented in Table 7.
The fertility of soil decreases with an increased concentration of Hg in the soil (Kabata-Pendias 2010; Alloway 2013). The most common symptoms of Hg toxicity in plants are growth inhibition, poor root development, the failure of metabolic processes such as the production of chlorophyll pigment and the photosynthesis respiration rate and poor yield. The accumulation of Hg in root tissue inhibits the uptake of K+ ions by plants (Kabata-Pendias 2010). Shiyab et al. (2009) reported that Hg could induce oxidative stress in plant cells and enhance the process of lipid peroxidation (Wang et al. 2012).
Mechanism of Hg toxicity
The possible mechanism of Hg toxicity at the cellular level demonstrates that Hg can damage cells by blocking important molecules (e.g. enzymes and polynucleotides). The transport of ions and disruption of cell membranes are also affected by Hg binding on the outer membrane (Azevedo and Rodriguez 2012). Hg has a high affinity for reaction with sulphydryl (–SH) and phosphate groups (Patra et al. 2004). Antioxidant characteristics are also affected by Hg via interference with non-enzymatic antioxidants (glutathione and non-protein thiols) and enzymatic antioxidants (superoxide dismutase, ascorbate peroxidase, and glutathione reductase) (Fig. 4) (Azevedo and Rodriguez 2012). In the case of seed germination, Hg reduces seed viability, while the interaction of Hg with –SH groups (tissues rich in –SH ligands) leads to the formation of S–Hg–S bridges, resulting in the disruption of group stability and restraining seed germination (Alloway 2013).
Mechanism of Hg toxicity in cell (Kabata-Pendias 2010)
Genotoxic effects of Hg are very rare, but some studies demonstrated that Hg could cause lethal errors in the genetic materials of plants and animals (Patra et al. 2004; Alloway 2013). Hg ions bind with the reactive sites present in DNA (deoxyribonucleic acid) molecules and form covalent bonds inside cells. The binding of Hg with DNA molecules results in damage to chromosomes in humans, while in plant cells, Hg binding can provoke spindle alterations and chromosomal aberrations. The sequences of amino acids also become imbalanced due to the interaction of Hg ions with the sulphur atoms of amino acids and nucleosides (Zalups 2000).
Remediation of mercury from environmental media
Physical techniques of Hg remediation
Soil washing
Soil washing is a physical separation technique of Hg from soil in which water is used to minimize the concentration of Hg in soil (Dermont et al. 2008; Xu et al. 2015). The principle of the soil washing process is based on the concept that most toxic substances bind with the fine particles (clay and slit) in soil and that these contaminants are removed along with the particles by washing with water (USEPA 2007). When chemicals are applied to remove Hg from soil, the process of removal is called chemical extraction, and this method may be used with physical separation. Ghosh et al. (1994) suggested that the beneficiation of coal may also be carried out prior to its use in various industries to reduce Hg levels in coal. The main advantage of this technology is the reduced volume of soil that needs to be further disposed or treated. However, this process is not feasible when there is strong bonding between Hg and soil particles (Xu et al. 2015).
Selective catalytic reduction and wet flue gas desulphurization
Selective catalytic reduction (SCR) and wet flue gas desulphurization (WFGD) are two important techniques used for the removal of Hg from coal-FA. The SCR technique is useful in the conversion of Hg0 to Hg2+. The oxidation of Hg0 in SCR methods plays a crucial role in the removal of Hg0. WFGD is used for the removal of sulphur dioxide (SO2) from flue gas in coal-fired TPPs. In this process, sulphur can be removed by mixing the coal or coal-FA with lime slurry. WFGD treatment has good effects on the removal of Hg2+ from flue gas (emission from coal combustion), and it can be very useful in capturing gaseous Hg, i.e. particulate Hg (Hgp) (Zhao et al. 2017). However, in some cases, re-emission occurs during the removal of Hg2+. The availability of excess oxygen in the flue gas that is in contact with the desulphurization solution may decrease the reduction of Hg2+ to Hg0 (Omine et al. 2012).
Thermal treatment
Thermal treatment is another important technique that may be applied for the efficient removal of Hg in soil (Ma et al. 2015). In thermal treatment, high temperature (320–700 °C) is applied to remove Hg from soil through the process of volatilization (USEPA 2007). In addition to high temperature, reduced pressure is applied to volatize Hg, and the process of volatilization is followed by condensation, which converts Hg vapour into liquid Hg0. In the thermal treatment process, the efficiency of Hg removal from the soil matrix is 41.99% (USEPA 2007; Xu et al. 2015), and high-temperature and low-pressure treatment can remove high concentrations of Hg (up to 34,000 mg/kg) from the soil. However, the removal of Hg through this technique has some disadvantages: a high capital cost and the interruption of the heat transfer process due to the presence of larger particles (Xu et al. 2015).
Stabilization
Stabilization is a physical-chemical separation technique in which Hg is converted into chemical forms that are stable and highly insoluble in various ranges of soil pH (Alloway 2013; Xu et al. 2015). The availability of Hg for uptake in plants is decreased through stabilization (USEPA 2007). The process involves mixing soil with chemical binders (thiol-functionalized zeolite and powdered activated carbon) to make a paste or slurry and then conversion into a solid form (Wang et al. 2012). Stabilization includes immobilization, in which the solubility and mobility of Hg are reduced by the addition of stabilizing agents to the contaminated soil. Since Hg is a Lewis acid, it forms complexes with Lewis bases. The addition of reduced sulphur to Hg-contaminated soil leads to the formation of a precipitate (HgS), which in turn results in the immobilization of Hg in the soil. Piao and Bishop (2006) reported that Hg could be reduced to 2300 mg/kg by using sulphide in Hg-contaminated soil. Iron chips (Wang et al. 2012) and tire rubber (Meng et al. 1998) have also been used for the stabilization of Hg in contaminated soil. In a study by Sierra et al. (2010), a physical separation technique was used for the remediation of Hg-containing pyrite ash. The results of the study showed that the physical separation method is effective when the grain size is below 125 μm. The limitation of the process is that the leachability of the metal increases with decreasing pH (Xu et al. 2015).
Electro-remediation
Electro-remediation is the controlled application of direct current between electrodes through the soil. The entire system comprises three compartments (two electrode compartments and a third (soil) compartment, which is placed between the two electrodes). During the treatment of Hg-contaminated soil, ions move through an ion exchange membrane from the soil to the electrodes (Hansen et al. 1997; Pazos et al. 2010). Hansen et al. (1997) investigated the electro-dialytic remediation of soil and found that the addition of oxidizing agents and chloride to the soil would mobilize Hg, hence increasing the Hg removal rate from soil. The results showed that the addition of chelates to the soil increased Hg solubility and enhanced the efficiency of the electro-remediation process. The efficiency of this process is very much affected by the soil pH, the solubility of Hg in soil, and soil organic matter. The major disadvantage of this technique is the requirement of acidic conditions for treatment (Virkutyte et al. 2002).
Adsorption
The removal of Hg through the adsorption process is one of the most important and feasible methods to remove Hg from solid surfaces (Yu et al. 2016). Several adsorbents, such as activated carbon (Lu et al. 2014), mesoporous carbon (Anbia and Dehghan 2014), carbon nanotubes (Habuda-Stanić and Nujić 2015), and iron oxides (Figueira et al. 2011), have been used for the efficient removal of Hg. The physical characteristics (surface area, pore size, pore volume, and pore number) of the adsorbent may affect the adsorption capacity, which can lead to an increased or decreased Hg removal efficiency. For example, the sulphurization of activated carbon reduces the number of micropores, which slows the Hg removal efficiency (Yu et al. 2016). Ojea-Jiménez et al. (2012) used colloidal nanoparticles as adsorbents for Hg removal from aqueous solutions. Low-cost adsorbents such as copper chloride, copper oxide, and artificial zeolite have also been used for the removal of Hg0 (Yu et al. 2016). The bacterial species Paecilomyces catenlannulatus was also used for Hg removal at the optimum pH of 7 and exhibited a maximum adsorption capacity of 140.85 mg/g (Li et al. 2013). The specific properties of the adsorbent, such as the pore size distribution, surface area, and polarity, may affect the efficiency of adsorption (Yu et al. 2016).
Biological technique of Hg remediation: phytoremediation
Amongst various technologies to remediate Hg-contaminated sites, phytoremediation is an alternative green technique to expensive remediation technology (Lim et al. 2004). This technology is used to remove or reduce the level of contaminants in soil through the process of translocation, in which the contaminants are translocated from the soil to plant tissue (Salt et al. 1995). Typically, plants such as Jatropha curcas and Indian mustard (Brassica juncea) are used for the phytoremediation of Hg from soils (Su et al. 2008; Marrugo-Negrete et al. 2015). In contrast, high concentrations of Hg in soil affect biomass, inhibit the growth of plants, and produce long-term effects on soil fertility (Sahi et al. 2006). Photosynthesis activity is reduced and nutrient and mineral absorption in the plant system is inhibited (Patra et al. 2004) due to the phytotoxic effects of Hg (Alloway 2013).
Phytoremediation technology is also used for the restoration of mining-affected areas. Moreover, the knowledge and behaviour of Hg in plant systems are also essential to apply this technology in the field (Millán et al. 2014). A study conducted by Millán et al. (2014) summarized that plants might be used to sense the degree of contamination and the long-term effects of Hg toxicity in environmental media. The study also reported that a high Hg content in soil shows a higher capability of Hg accumulation in the aerial parts of the plant. Another study on the phytoremediation of Hg by Su et al. (2008) reported that beard grass (Polypogon monspeliensis) accumulated a limited amount of Hg in its shoots, i.e. less than 65 mg/kg, while the accumulation of Hg in the roots was higher. The study reported that Indian mustard (Brassica juncea) plants growing on freshly spiked soil accumulated a modest amount of Hg in the shoots and found that the mustard plants exhibited several stress symptoms, such as a loss of water content and chlorosis, during growth. Su et al. (2008) investigated Chinese brake fern (Pteris vittata) and found that Hg accumulation in the plant was higher (1469 mg Hg/kg in shoots in freshly spiked soil) than that in P. monspeliensis and B. juncea and fewer stress symptoms were exhibited. Cassina et al. (2012) used two plant species (B. juncea and Helianthus annuus) for the effective remediation of Hg and observed that B. juncea was more effective in Hg uptake, while the sunflower plant gave a better response regarding plant biomass production. The study also found that the phytoremediation capacity of plants was improved by the use of plant hormones (cytokinin) and thioligands. Hussein et al. (2007) investigated transgenic tobacco plants to study the phytoremediation of Hg through the uptake of various forms of Hg into roots and shoots of tobacco plants that had been genetically engineered with bacterial merA and merB genes through the chloroplast genome.
Phytoremediation was also used for Hg removal from water through Typha domingensis in a constructed wetland, and the results showed that the aquatic macrophytes (T. domingensis) had great potential for Hg removal from contaminated water due to their high Hg accumulation capacity (273.3515 ± 0.7234 mg/kg) (Gomes et al. 2014).
Phytoremediation through algae and aquatic plants
One study reported that macroalgae such as Ulva lactuca (Chlorophyta), Gracilaria gracilis (Rhodophyta), and Fucus vesiculosus (Phaeophyta) could also be used for Hg removal from saline water. The results of the study showed that massive amounts of Hg (up to 209 mg of Hg per gram of macroalgae d.w.) were accumulated in all three seaweed species and found that 99% of Hg could be removed from seawater through macroalgae. U. lactuca was the fastest Hg accumulator amongst the three species of macroalgae (Henriques et al. 2015).
Lafabrie et al. (2011) investigated the bioaccumulation of Hg in Vallisneria neotropicalis (submerged aquatic plant species) and observed that Vallisneria species played an important role in the bioaccumulation of Hg and could be used as a warning signal of Hg contamination in sediments. Isaksson et al. (2007) studied Hg accumulation in Lemna minor (aquatic plant) and found that L. minor sequestered Hg in its biomass. The study demonstrated that Hg concentration in plant tissues and water was positively correlated and that therefore, L. minor is a good candidate for the phytoremediation of Hg from water. the aquatic macrophytes Pistia stratiotes and Azolla pinnata were also used as potential candidates for Hg removal from open-cast coal mine effluent. The study noted that Hg was removed from the coal mine effluent by the process of rhizofiltration and subsequent Hg accumulation. The maximum removal of Hg in both plant species was observed at a retention time of 21 days, and the removal capacity of the plants decreased moderately. The study also demonstrated that the maximum Hg accumulation occurred in the plant’s root. The study determined that P. stratiotes had a higher Hg removal efficiency than A. pinnata and there was a minimal effect of Hg toxicity on P. stratiotes (Mishra et al. 2009).
Phytoremediation through herbaceous plants
The wild plant Cyrtomium macrophyllum (perennial herbaceous plant widely distributed in China) has high phytoremediation potential for Hg. In a pot experiment, it was found that C. macrophyllum species could grow in soils with high Hg levels (500 mg/kg), and no toxic effects were observed during the plant’s growth at a high Hg concentration. The experimental work found that C. macrophyllum had excellent Hg translocation and accumulation abilities, and the species was therefore considered to be a good candidate for the remediation of Hg from highly polluted soil (Xun et al. 2017). One study also reported that rice plants growing on mine soil accumulate Hg in their tissues (Meng et al. 2014) and high amounts of MeHg can be accumulated in the aerial part of the rice plant. Sorkhoh et al. (2010) studied the bioremediation of Hg from soils contaminated with crude oil through rhizosphere technology and found that rhizobacteria played a valuable role in the phytoremediation of Hg. Several plant species, such as Hordeum vulgare, Lupinus albus, Lens esculenta, and Cicer aretinum (commonly grown in Spain), are also able to absorb and accumulate Hg in their plant tissue (Rodriguez et al. 2007). The plant species Impatiens walleriana showed higher accumulation of Hg in its leaves than in the flower and stems (Pant et al. 2010).
Liu et al. (2017) used five different plant species (Opuntia stricta, Aloe vera, Setcreasea purpurea, Chlorophytum comosum, and Oxalis corniculata) to test the Hg accumulation capacities at various concentrations. The results showed that Hg accumulation in the roots of all plant species was higher than that in the shoots. The results indicated that the rate of Hg accumulation in O. corniculata was maximum amongst all plant species, and it was recommended that O. corniculata had high potential for phytoremediation of Hg from soils with a concentration level of less than 500 μg/L.
Conclusions
The natural environment is becoming polluted due to natural and anthropogenic contributions of Hg to environmental media. The combustion of coal-FA is the major anthropogenic source of Hg, while the natural Hg content in soil depends on the soil geochemistry of the parent material.
Once released into the atmosphere, Hg (especially Hg0) may undergo long-range transportation and accumulate on the top surface of the soil, on plant leaves, and in aquatic environments, where it is converted into MeHg (the most toxic form of Hg). MeHg becomes biomagnified at the trophic level and imbalances the food chain, which results in the disturbance of the natural ecosystem.
Several remediation techniques have been considered to reduce the level of Hg contamination from the environment to avoid health hazards. Processes such as selective catalytic reduction and wet flue gas desulphurization have been applied to reduce Hg levels in coal-FA, whereas coal washing is used to remove Hg from coal prior to the coal combustion process. Moreover, physical separation methods, such as soil washing, thermal treatment stabilization, and electro-remediation, are also used for Hg removal from soil.
Phytoremediation is one of the most effective, economically viable, and environmentally friendly methods used for the remediation of Hg. Physical separation methods affect soil properties, but phytoremediation techniques improve the quality (physical, chemical, and biological) of soil. Therefore, plant-based treatment methods have been widely accepted for Hg removal. Several plant species, such as Brassica juncea, Jatropha curcas, and Polypogon monspeliensis, are suitable for Hg remediation. This review enriches our knowledge on the sources of Hg, its toxic effects, and various remediation techniques.
References
Agrawal, P., Mittal, A., Kumar, M., & Tripathi, S. K. (2008). Mercury exposure in Indian environment due to coal fired thermal power plants and existing legislations. International Journal of Forensic Medicine and Pathology, 1, 41.
Ali, M. H., & Al-Qahtani, K. M. (2012). Assessment of some heavy metals in vegetables, cereals and fruits in Saudi Arabian markets. The Egyptian Journal of Aquatic Research, 38, 31–37.
Alloway, B. J. (2013). Heavy metals in soils: trace metals and metalloids in soils and their bioavailability, vol 22. Dordrecht: Springer.
Anbia, M., & Dehghan, R. (2014). Functionalized CMK-3 mesoporous carbon with 2-amino-5-mercapto-1, 3, 4-thiadiazole for Hg (II) removal from aqueous media. Journal of Environmental Sciences, 26, 1541–1548.
Ansari, F. A., Gupta, A. K., & Yunus, M. (2011). Fly-ash from coal-fed thermal power plants: Bulk utilization in horticulture–a long-term risk management option. International Journal of Environmental Research, 5, 101–108.
Arbestain, M. C., Rodriguez-Lado, L., Bao, M., & Macias, F. (2009). Assessment of mercury-polluted soils adjacent to an old mercury-fulminate production plant. Applied and Environmental Soil Science. https://doi.org/10.1155/2009/387419.
ASTM (2006). ASTM D6414: standard test methods for total mercury in coal and coal combustion residues by acid extraction or wet oxidation/cold vapour atomic absorption.
ATSDR. (1999). Toxicological Profile for mercury. Atlanta: US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry.
Azevedo, R., & Rodriguez, E. (2012). Phytotoxicity of mercury in plants: a review. Journal of Botany, 2012, 1–6.
Bai, X., Li, W., Wang, Y., & Ding, H. (2017). The distribution and occurrence of mercury in Chinese coals. International Journal of Coal Science & Technology, 4, 172–182.
Bailey, E. A., Gray, J. E., & Theodorakos, P. M. (2002). Mercury in vegetation and soils at abandoned mercury mines in southwestern Alaska, USA. Geochemistry: Exploration, Environment, Analysis, 2, 275–285.
Bailon, M. X., David, A. S., Park, Y., Kim, E., & Hong, Y. (2018). Total mercury, methyl mercury, and heavy metal concentrations in Hyeongsan River and its tributaries in Pohang city, South Korea. Environmental Monitoring and Assessment, 190, 274.
Beckers, F., & Rinklebe, J. (2017). Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Critical Reviews in Environmental Science and Technology, 47, 693–794.
Belkin, H. E., Tewalt, S. J., Hower, J. C., Stucker, J. D., & O'Keefe, J. M. K. (2009). Geochemistry and petrology of selected coal samples from Sumatra, Kalimantan, Sulawesi, and Papua, Indonesia. International Journal of Coal Geology, 77, 260–268.
Biester, H., Müller, G., & Schöler, H. F. (2002). Binding and mobility of mercury in soils contaminated by emissions from chlor-alkali plants. Science of the Total Environment, 284, 191–203.
Boszke, L., Kowalski, A., Astel, A., Barański, A., Gworek, B., & Siepak, J. (2008). Mercury mobility and bioavailability in soil from contaminated area. Environmental Geology, 55, 1075–1087.
Bradley, M., Barst, B., & Basu, N. (2017). A review of mercury bioavailability in humans and fish. International Journal of Environmental Research and Public Health, 14, 169.
Brigden, K., & Santillo, D. (2002). Heavy metal and metalloid content of fly ash collected from the Sual, Mauban and Masinloc coal-fired power plants in the Philippines, 2002. Greenpeace Araştırma Laboratuarı Teknik Notu, 7, 2002.
Burmistrz, P., Kogut, K., Marczak, M., & Zwoździak, J. (2016). Lignites and subbituminous coals combustion in Polish power plants as a source of anthropogenic mercury emission. Fuel Processing Technology, 152, 250–258.
Cassina, L., Tassi, E., Pedron, F., Petruzzelli, G., Ambrosini, P., & Barbafieri, M. (2012). Using a plant hormone and a thioligand to improve phytoremediation of Hg-contaminated soil from a petrochemical plant. Journal of Hazardous Materials, 231, 36–42.
Chen, X., Xia, X., Wu, S., Wang, F., & Guo, X. (2010). Mercury in urban soils with various types of land use in Beijing, China. Environmental Pollution, 158, 48–54.
Cheng, Z., Wang, H. S., Du, J., Sthiannopkao, S., Xing, G. H., Kim, K. W., Yasin, M. S. M., Hashim, J. H., & Wong, M. H. (2013). Dietary exposure and risk assessment of mercury via total diet study in Cambodia. Chemosphere, 92, 143–149.
Csuros, M., & Csuros, C. (2016). Environmental sampling and analysis for metals. CRC Press. https://doi.org/10.1201/9781420032345.
Dabrowski, J. M., Ashton, P. J., Murray, K., Leaner, J. J., & Mason, R. P. (2008). Anthropogenic mercury emissions in South Africa: Coal combustion in power plants. Atmospheric Environment, 42, 6620–6626.
Dahl, O., Pöykiö, R., & Nurmesniemi, H. (2008). Concentrations of heavy metals in fly ash from a coal-fired power plant with respect to the new Finnish limit values. Journal of Material Cycles and Waste Management, 10, 87–92.
Dai, S., Ren, D., Chou, C. L., Finkelman, R. B., Seredin, V. V., & Zhou, Y. (2012). Geochemistry of trace elements in Chinese coals: a review of abundances, genetic types, impacts on human health, and industrial utilization. International Journal of Coal Geology, 94, 3–21.
Deng, S., Zhang, C., Liu, Y., Cao, Q., Xu, Y. Y., Wang, H. L., & Zhang, F. (2014). A full-scale field study on chlorine emission of pulverized coal-fired power plants in China. Research of Environmental Sciences, 27, 127–133.
Dermont, G., Bergeron, M., Mercier, G., & Richer-Laflèche, M. (2008). Soil washing for metal removal: a review of physical/chemical technologies and field applications. Journal of Hazardous Materials, 152, 1–31.
Dołęgowska, S., & Michalik, A. (2019). The use of a geostatistical model supported by multivariate analysis to assess the spatial distribution of mercury in soils from historical mining areas: Karczówka Mt., Miedzianka Mt., and Rudki (south-central Poland). Environmental Monitoring and Assessment, 191, 302.
Donatello, S., Fernández-Jiménez, A., & Palomo, A. (2012). An assessment of Mercury immobilisation in alkali activated fly ash (AAFA) cements. Journal of Hazardous Materials, 213, 207–215.
Dragović, S., Ćujić, M., Slavković-Beškoski, L., Gajić, B., Bajat, B., Kilibarda, M., & Onjia, A. (2013). Trace element distribution in surface soils from a coal burning power production area: A case study from the largest power plant site in Serbia. Catena, 104, 288–296.
Dziok, T., Strugała, A., Rozwadowski, A., & Macherzyński, M. (2015). Studies of the correlation between mercury content and the content of various forms of sulfur in Polish hard coals. Fuel, 159, 206–213.
EA. (2009). Contaminants in soil: updated collation of toxicological data and intake values for humans. Mercury. Science Report SC050021/SR TOX7. Bristol: Environment Agency.
El Mahmoud-Hamed, M. S., Montesdeoca-Esponda, S., Santana-Del Pino, A., Zamel, M. L., Brahim, M., T’feil, H., Santana-Rodiguez, J. J., Sidoumou, Z., & Sidi’Ahmed-Kankou, M. (2019). Distribution and health risk assessment of cadmium, lead, and mercury in freshwater fish from the right bank of Senegal River in Mauritania. Environmental Monitoring and Assessment, 191, 493.
Fernández-Martínez, R., Loredo, J., Ordóñez, A., & Rucandio, M. I. (2005). Distribution and mobility of mercury in soils from an old mining area in Mieres, Asturias (Spain). Science of The Total Environment, 346, 200–212.
Fernández-Martínez, R., Larios, R., Gómez-Pinilla, I., Gómez-Mancebo, B., López-Andrés, S., Loredo, J., Ordóñez, A., & Rucandio, I. (2015). Mercury accumulation and speciation in plants and soils from abandoned cinnabar mines. Geoderma, 253, 30–38.
Figueira, P., Lopes, C. B., Daniel-da-Silva, A. L., Pereira, E., Duarte, A. C., & Trindade, T. (2011). Removal of mercury (II) by dithiocarbamate surface functionalized magnetite particles: application to synthetic and natural spiked waters. Water Research, 45, 5773–5784.
Font, O., Córdoba, P., Leiva, C., Romeo, L. M., Bolea, I., Guedea, I., Moreno, N., Querol, X., Fernandez, C., & Díez, L. I. (2012). Fate and abatement of mercury and other trace elements in a coal fluidised bed oxy combustion pilot plant. Fuel, 95, 272–281.
Fthenakis, V. M., Lipfert, F. W., Moskowitz, P. D., & Saroff, L. (1995). An assessment of mercury emissions and health risks from a coal-fired power plant. Journal of Hazardous Materials, 44, 267–283.
Gall, J. E., Boyd, R. S., & Rajakaruna, N. (2015). Transfer of heavy metals through terrestrial food webs: a review. Environmental Monitoring and Assessment, 187, 201.
Genthe, B., Kapwata, T., Le Roux, W., Chamier, J., & Wright, C. Y. (2018). The reach of human health risks associated with metals/metalloids in water and vegetables along a contaminated river catchment: South Africa and Mozambique. Chemosphere, 199, 1–9.
Ghosh, S. B., Das, M. C., Ghosh, B., Roy, R. R. P., & Banerjee, N. N. (1994). Mercury in Indian coals. Journal of Chemical Technology, 1, 237-240.
Gil, C., Ramos-Miras, J., Roca-Pérez, L., & Boluda, R. (2010). Determination and assessment of mercury content in calcareous soils. Chemosphere, 78, 409–415.
Gnamuš, A., Byrne, A. R., & Horvat, M. (2000). Mercury in the soil-plant-deer-predator food chain of a temperate forest in Slovenia. Environmental Science & Technology, 34, 3337–3345.
Gomes, M. V. T., de Souza, R. R., Teles, V. S., & Mendes, É. A. (2014). Phytoremediation of water contaminated with mercury using Typha domingensis in constructed wetland. Chemosphere, 103, 228–233.
Goodarzi, F. (2006). Characteristics and composition of fly ash from Canadian coal-fired power plants. Fuel, 85, 1418–1427.
Gosar, M., Šajn, R., & Biester, H. (2006). Binding of mercury in soils and attic dust in the Idrija mercury mine area (Slovenia). Science of The Total Environment, 369, 150–162.
Gustin, M. S. (2003). Are mercury emissions from geologic sources significant? A status report. Science of the Total Environment, 304, 153–167.
Ha, E., Basu, N., Bose-O’Reilly, S., Dorea, J. G., McSorley, E., Sakamoto, M., & Chan, H. M. (2016). Current progress on understanding the impact of mercury on human health. Environmental Research, 152, 419–433.
Habuda-Stanić, M., & Nujić, M. (2015). Arsenic removal by nanoparticles: a review. Environmental Science and Pollution Research, 22, 8094–8123.
Halbach, K., Mikkelsen, Ø., Berg, T., & Steinnes, E. (2017). The presence of mercury and other trace metals in surface soils in the Norwegian Arctic. Chemosphere, 188, 567–574.
Han, Y., Kingston, H. M., Boylan, H. M., Rahman, G. M., Shah, S., Richter, R. C., et al. (2003). Speciation of mercury in soil and sediment by selective solvent and acid extraction. Analytical and Bioanalytical Chemistry, 375, 428–436.
Hansen, H. K., Ottosen, L. M., Kliem, B. K., & Villumsen, A. (1997). Electrodialytic remediation of soils polluted with Cu, Cr, Hg, Pb and Zn. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental and Clean Technology, 70, 67–73.
Henriques, B., Rocha, L. S., Lopes, C. B., Figueira, P., Monteiro, R. J., Duarte, A. C., Pardal, M. A., & Pereira, E. (2015). Study on bioaccumulation and biosorption of mercury by living marine macroalgae: prospecting for a new remediation biotechnology applied to saline waters. Chemical Engineering Journal, 281, 759–770.
Hossain, M. N., Paul, S. K., & Hasan, M. M. (2015). Environmental impacts of coal mine and thermal power plant to the surroundings of Barapukuria, Dinajpur, Bangladesh. Environmental Monitoring and Assessment, 187, 202.
Hower, J. C., Senior, C. L., Suuberg, E. M., Hurt, R. H., Wilcox, J. L., & Olson, E. S. (2010). Mercury capture by native fly ash carbons in coal-fired power plants. Progress in Energy and Combustion Science, 36, 510–529.
Hower, J. C., Clack, H. L., Hood, M. M., Hopps, S. G., & Thomas, G. H. (2017). Impact of coal source changes on mercury content in fly ash: Examples from a Kentucky power plant. International Journal of Coal Geology, 170, 2–6.
Hu, Y., & Cheng, H. (2016). Control of mercury emissions from stationary coal combustion sources in China: Current status and recommendations. Environmental Pollution, 218, 1209–1221.
Hu, W., Huang, B., Tian, K., Holm, P. E., & Zhang, Y. (2017). Heavy metals in intensive greenhouse vegetable production systems along Yellow Sea of China: Levels, transfer and health risk. Chemosphere, 167, 82–90.
Huang, X., Hu, J., Qin, F., Quan, W., Cao, R., Fan, M., & Wu, X. (2017). Heavy metal pollution and ecological assessment around the Jinsha Coal-Fired Power Plant (China). International Journal of Environmental Research and Public Health, 14, 1589.
Hussein, H. S., Ruiz, O. N., Terry, N., & Daniell, H. (2007). Phytoremediation of mercury and organomercurials in chloroplast transgenic plants: enhanced root uptake, translocation to shoots, and volatilization. Environmental Science & Technology, 41, 8439–8446.
Isaksson, R., Balogh, S. J., & Farris, M. A. (2007). Accumulation of mercury by the aquatic plant Lemna minor. International Journal of Environmental Studies, 64, 189–194.
Jagtap, R., & Maher, W. (2015). Measurement of mercury species in sediments and soils by HPLC–ICPMS. Microchemical Journal, 121, 65–98.
Kabata-Pendias, A. (2010). Trace elements in soils and plants. Washington DC: CRC Press.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. Springer Science & Business Media. https://doi.org/10.1007/978-3-540-32714-1.
Kolker, A., Panov, B. S., Panov, Y. B., Landa, E. R., Conko, K. M., Korchemagin, V. A., Shendrik, T., & McCord, J. D. (2009). Mercury and trace element contents of Donbas coals and associated mine water in the vicinity of Donetsk, Ukraine. International Journal of Coal Geology, 79, 83–91.
Kostova, I., Vassileva, C., Dai, S., Hower, J. C., & Apostolova, D. (2013). Influence of surface area properties on mercury capture behaviour of coal fly ashes from some Bulgarian power plants. International Journal of Coal Geology, 116, 227–235.
Lafabrie, C., Major, K. M., Major, C. S., & Cebrián, J. (2011). Arsenic and mercury bioaccumulation in the aquatic plant, Vallisneria neotropicalis. Chemosphere, 82, 1393–1400.
Li, Y., Yang, L., Ji, Y., Sun, H., & Wang, W. (2009). Quantification and fractionation of mercury in soils from the Chatian mercury mining deposit, southwestern China. Environmental Geochemistry and Health, 31, 617–628.
Li, Z., Wu, L., Liu, H., Lan, H., & Qu, J. (2013). Improvement of aqueous mercury adsorption on activated coke by thiol-functionalization. Chemical Engineering Journal, 228, 925–934.
Li, R., Wu, H., Ding, J., Fu, W., Gan, L., & Li, Y. (2017). Mercury pollution in vegetables, grains and soils from areas surrounding coal-fired power plants. Scientific Reports, 7, 46545.
Liang, Y., Dongxing, Y. U. A. N., Min, L. U., Zhenbin, G. O. N. G., Xiyao, L. I. U., & Zhang, Z. (2009). Distribution characteristics of total mercury and methylmercury in the topsoil and dust of Xiamen, China. Journal of Environmental Sciences, 21, 1400–1408.
Lim, J. M., Salido, A. L., & Butcher, D. J. (2004). Phytoremediation of lead using Indian mustard (Brassica juncea) with EDTA and electrodics. Microchemical Journal, 76, 3–9.
Lin, C. J., Gustin, M. S., Singhasuk, P., Eckley, C., & Miller, M. (2010). Empirical models for estimating mercury flux from soils. Environmental Science & Technology, 44, 8522–8528.
Liu, Z., Wang, L. A., Xu, J., Ding, S., Feng, X., & Xiao, H. (2017). Effects of different concentrations of mercury on accumulation of mercury by five plant species. Ecological Engineering, 106, 273–278.
Lomonte, C., Gregory, D., Baker, A. J., & Kolev, S. D. (2008). Comparative study of hotplate wet digestion methods for the determination of mercury in biosolids. Chemosphere, 72, 1420–1424.
Lu, X., Jiang, J., Sun, K., Wang, J., & Zhang, Y. (2014). Influence of the pore structure and surface chemical properties of activated carbon on the adsorption of mercury from aqueous solutions. Marine Pollution Bulletin, 78, 69–76.
Luo, W., Lu, Y., Wang, B., Tong, X., Wang, G., Shi, Y., Wang, T., & Giesy, J. P. (2009). Distribution and sources of mercury in soils from former industrialized urban areas of Beijing, China. Environmental Monitoring and Assessment, 158, 507–517.
Luo, G., Ma, J., Han, J., Yao, H., Xu, M., Zhang, C., Chen, G., Gupta, R., & Xu, Z. (2013). Hg occurrence in coal and its removal before coal utilization. Fuel, 104, 70–76.
Luo, Y., Duan, L., Wang, L., Xu, G., Wang, S., & Hao, J. (2014). Mercury concentrations in forest soils and stream waters in northeast and south China. Science of the Total Environment, 496, 714–720.
Lusilao-Makiese, J., Tessier, E., Amouroux, D., Tutu, H., Chimuka, L., & Cukrowska, E. M. (2012). Speciation of mercury in South African coals. Toxicological and Environmental Chemistry, 94, 688–706.
Ma, F., Peng, C., Hou, D., Wu, B., Zhang, Q., Li, F., & Gu, Q. (2015). Citric acid facilitated thermal treatment: an innovative method for the remediation of mercury contaminated soil. Journal of Hazardous Materials, 300, 546–552.
Mahajan, V. E., Yadav, R. R., Dakshinkar, N. P., Dhoot, V. M., Bhojane, G. R., Naik, M. K., Shrivastava, P., Naoghare, P. K., & Krishnamurthi, K. (2012). Influence of mercury from fly ash on cattle reared nearby thermal power plant. Environmental Monitoring and Assessment, 184, 7365–7372.
Marrugo-Negrete, J., Durango-Hernández, J., Pinedo-Hernández, J., Olivero-Verbel, J., & Díez, S. (2015). Phytoremediation of mercury-contaminated soils by Jatropha curcas. Chemosphere, 127, 58–63.
Martín, J. A. R., & Nanos, N. (2016). Soil as an archive of coal-fired power plant mercury deposition. Journal of Hazardous Materials, 308, 131-138.
Matsuyama, A., Yano, S., Taninaka, T., Kindaichi, M., Sonoda, I., Tada, A., & Akagi, H. (2018). Chemical characteristics of dissolved mercury in the pore water of Minamata Bay sediments. Marine Oollution Bulletin, 129, 503–511.
Mbanga, O., Ncube, S., Tutu, H., Chimuka, L., & Cukrowska, E. (2019). Mercury accumulation and biotransportation in wetland biota affected by gold mining. Environmental Monitoring and Assessment, 191, 186.
Meng, X., Hua, Z., Dermatas, D., Wang, W., & Kuo, H. Y. (1998). Immobilization of mercury (II) in contaminated soil with used tire rubber. Journal of Hazardous Materials, 57, 231–241.
Meng, M., Li, B., Shao, J. J., Wang, T., He, B., Shi, J. B., Ye, Z. H., & Jiang, G. B. (2014). Accumulation of total mercury and methylmercury in rice plants collected from different mining areas in China. Environmental Pollution, 184, 179–186.
Millán, R., Lominchar, M. A., Rodríguez-Alonso, J., Schmid, T., & Sierra, M. J. (2014). Riparian vegetation role in mercury uptake (Valdeazogues River, Almadén, Spain). Journal of Geochemical Exploration, 140, 104–110.
Mishra, V. K., Tripathi, B. D., & Kim, K. H. (2009). Removal and accumulation of mercury by aquatic macrophytes from an open cast coal mine effluent. Journal of Hazardous Materials, 172, 749–754.
Montoya, A. J., Lena, J. C., & Windmöller, C. C. (2019). Adsorption of gaseous elemental mercury on soils: Influence of chemical and/or mineralogical characteristics. Ecotoxicology and Environmental Safety, 170, 98–106.
Mukherjee, A. B., Zevenhoven, R., Bhattacharya, P., Sajwan, K. S., & Kikuchi, R. (2008). Mercury flow via coal and coal utilization by-products: a global perspective. Resources, Conservation and Recycling, 52, 571–591.
Müller, A. K., Westergaard, K., Christensen, S., & Sørensen, S. J. (2001). The effect of long-term mercury pollution on the soil microbial community. FEMS Microbiology Ecology, 36, 11–19.
Nagpal, N., Bettiol, S. S., Isham, A., Hoang, H., & Crocombe, L. A. (2017). A review of mercury exposure and health of dental personnel. Safety and Health at Work, 8, 1–10.
Noda, N., & Ito, S. (2018). Mercury Partitioning in Coal-fired Power Plants in Japan. Journal of the Japan Institute of Energy, 97, 342–347.
Obrist, D., Kirk, J. L., Zhang, L., Sunderland, E. M., Jiskra, M., & Selin, N. E. (2018). A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio, 47, 116-140.
O'Connor, D., Hou, D., Ok, Y. S., Mulder, J., Duan, L., Wu, Q., Wang, S., Tack, F. M. G., & Rinklebe, J. (2019). Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review. Environment International, 126, 747–761.
Ohki, A., Taira, M., Hirakawa, S., Haraguchi, K., Kanechika, F., Nakajima, T., & Takanashi, H. (2014). Determination of mercury in various coals from different countries by heat-vaporization atomic absorption spectrometry: Influence of particle size distribution of coal. Microchemical Journal, 114, 119–124.
Ojea-Jiménez, I., López, X., Arbiol, J., & Puntes, V. (2012). Citrate-coated gold nanoparticles as smart scavengers for mercury (II) removal from polluted waters. ACS Nano, 6, 2253–2260.
Omine, N., Romero, C. E., Kikkawa, H., Wu, S., & Eswaran, S. (2012). Study of elemental mercury re-emission in a simulated wet scrubber. Fuel., 91, 93–101.
Ostos, C., Pérez-Rodríguez, F., Arroyo, B. M., & Moreno-Rojas, R. (2015). Study of mercury content in wild edible mushrooms and its contribution to the Provisional Tolerable Weekly Intake in Spain. Journal of Food Composition and Analysis, 37, 136–142.
Özkul, C. (2016). Heavy metal contamination in soils around the Tunçbilek thermal power plant (Kütahya, Turkey). Environmental Monitoring and Assessment, 188, 284.
Palmieri, H. E., Nalini, H. A., Jr., Leonel, L. V., Windmöller, C. C., Santos, R. C., & de Brito, W. (2006). Quantification and speciation of mercury in soils from the Tripuí Ecological Station, Minas Gerais, Brazil. Science of the Total Environment, 368, 69–78.
Pant, P., Allen, M., & Tansel, B. (2010). Mercury uptake and translocation in Impatiens walleriana plants grown in the contaminated soil from oak ridge. International Journal of Phytoremediation, 13, 168–176.
Park, K. S., Seo, Y. C., Lee, S. J., & Lee, J. H. (2008). Emission and speciation of mercury from various combustion sources. Powder Technology, 180, 151–156.
Park, C. H., Eom, Y., Lee, L. J. E., & Lee, T. G. (2013). Simple and accessible analytical methods for the determination of mercury in soil and coal samples. Chemosphere, 93, 9–13.
Pastrana-Corral, M. A., Wakida, F. T., Temores-Peña, J., Rodriguez-Mendivil, D. D., García-Flores, E., Piñon-Colin, T. D. J., & Quiñonez-Plaza, A. (2017). Heavy metal pollution in the soil surrounding a thermal power plant in Playas de Rosarito, Mexico. Environmental Earth Sciences, 76, 583.
Patel, K. S., Sharma, R., Dahariya, N. S., Yadav, A., Blazhev, B., Matini, L., & Hoinkis, J. (2015). Heavy metal contamination of tree leaves. American Journal of Analytical Chemistry, 6, 687–693.
Patra, M., Bhowmik, N., Bandopadhyay, B., & Sharma, A. (2004). Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environmental and Experimental Botany, 52, 199–223.
Pavlish, J. H., Sondreal, E. A., Mann, M. D., Olson, E. S., Galbreath, K. C., Laudal, D. L., & Benson, S. A. (2003). Status review of mercury control options for coal-fired power plants. Fuel Processing Technology, 82, 89–165.
Pazos, M., Rosales, E., Alcántara, T., Gómez, J., & Sanromán, M. A. (2010). Decontamination of soils containing PAHs by electroremediation: a review. Journal of Hazardous Materials, 177, 1–11.
Piao, H., & Bishop, P. L. (2006). Stabilization of mercury-containing wastes using sulfide. Environmental Pollution, 139, 498–506.
Pirrone, N., Cinnirella, S., Feng, X., Finkelman, R. B., Friedli, H. R., Leaner, J., Mason, R., Mukherjee, A. B., Stracher, G. B., Streets, D. G., & Telmer, K. (2010). Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmospheric Chemistry and Physics, 10, 5951–5964.
Pöykiö, R., Mäkelä, M., Watkins, G., Nurmesniemi, H., & Olli, D. A. H. L. (2016). Heavy metals leaching in bottom ash and fly ash fractions from industrial-scale BFB-boiler for environmental risks assessment. Transactions of Nonferrous Metals Society of China, 26, 256–264.
Pudasainee, D., Kim, J. H., & Seo, Y. C. (2009). Mercury emission trend influenced by stringent air pollutants regulation for coal-fired power plants in Korea. Atmospheric Environment, 43, 6254–6259.
Qiu, G., Feng, X., Wang, S., & Xiao, T. (2006). Mercury contaminations from historic mining to water, soil and vegetation in Lanmuchang, Guizhou, southwestern China. Science of the Total Environment, 368, 56–68.
Rahman, Z., & Singh, V. P. (2019). The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environmental Monitoring and Assessment, 191, 419.
Rai, V. K., Raman, N. S., & Choudhary, S. K. (2013). Mercury in thermal power plants–a case study. International Journal of Pure &Applied Bioscience, 1, 31–37.
Raj, D., & Maiti, S. K. (2019). Bioaccumulation of potentially toxic elements in tree and vegetable species with associated health and ecological risks: a case study from a thermal power plant, Chandrapura, India. Rendiconti Lincei. Scienze Fisiche e Naturali. https://doi.org/10.1007/s12210-019-00831-7.
Raj, D., Chowdhury, A., & Maiti, S. K. (2017). Ecological risk assessment of mercury and other heavy metals in soils of coal mining area: A case study from the eastern part of a Jharia coal field, India. Human and Ecological Risk Assessment: An International Journal, 23, 767–787.
Raju, A., Singh, A., Srivastava, N., Singh, S., Jigyasu, D. K., & Singh, M. (2019). Mapping human health risk by geostatistical method: a case study of mercury in drinking groundwater resource of the central Ganga alluvial plain, northern India. Environmental Monitoring and Assessment, 191, 298.
Rasulov, O., Zacharová, A., & Schwarz, M. (2017). Determination of total mercury in aluminium industrial zones and soil contaminated with red mud. Environmental Monitoring and Assessment, 189, 388.
Reis, A. T., Rodrigues, S. M., Davidson, C. M., Pereira, E., & Duarte, A. C. (2010). Extractability and mobility of mercury from agricultural soils surrounding industrial and mining contaminated areas. Chemosphere, 81, 1369–1377.
Reis, A. T., Lopes, C. B., Davidson, C. M., Duarte, A. C., & Pereira, E. (2015). Extraction of available and labile fractions of mercury from contaminated soils: The role of operational parameters. Geoderma, 259, 213–223.
Ren, D. Y., Zhao, F. H., Dai, S. F., Zhang, J. Y., & Luo, K. L. (2006). Geochemistry of trace elements in coals (pp. 268–279). Beijing: The Science Press.
Renneberg, A. J., & Dudas, M. J. (2001). Transformations of elemental mercury to inorganic and organic forms in mercury and hydrocarbon co-contaminated soils. Chemosphere, 45, 1103–1109.
Rodriguez, L., Rincón, J., Asencio, I., & Rodríguez-Castellanos, L. (2007). Capability of selected crop plants for shoot mercury accumulation from polluted soils: phytoremediation perspectives. International Journal of Phytoremediation, 9, 1–13.
Sahi, C., Singh, A., Kumar, K., Blumwald, E., & Grover, A. (2006). Salt stress response in rice: genetics, molecular biology, and comparative genomics. Functional & Integrative Genomics, 6, 263–284.
Salt, D. E., Blaylock, M., Kumar, N. P., Dushenkov, V., Ensley, B. D., Chet, I., & Raskin, I. (1995). Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology, 13, 468.
Sanchez-Rodas, D., Corns, W. T., Chen, B., & Stockwell, P. B. (2010). Atomic fluorescence spectrometry: a suitable detection technique in speciation studies for arsenic, selenium, antimony and mercury. Journal of Analytical Atomic Spectrometry, 25, 933–946.
Shiyab, S., Chen, J., Han, F. X., Monts, D. L., Matta, F. B., Gu, M., & Su, Y. (2009). Phytotoxicity of mercury in Indian mustard (Brassica juncea L.). Ecotoxicology and Environmental Safety, 72, 619–625.
Sierra, C., Gallego, J. R., Afif, E., Menéndez-Aguado, J. M., & González-Coto, F. (2010). Analysis of soil washing effectiveness to remediate a brownfield polluted with pyrite ashes. Journal of Hazardous Materials, 180, 602–608.
Soares, L. C., Egreja Filho, F. B., Linhares, L. A., Windmoller, C. C., & Yoshida, M. I. (2015). Accumulation and oxidation of elemental mercury in tropical soils. Chemosphere, 134, 181–191.
Sorkhoh, N. A., Ali, N., Al-Awadhi, H., Dashti, N., Al-Mailem, D. M., Eliyas, M., & Radwan, S. S. (2010). Phytoremediation of mercury in pristine and crude oil contaminated soils: Contributions of rhizobacteria and their host plants to mercury removal. Ecotoxicology and Environmental Safety, 73, 1998–2003.
Spahić, M. P., Sakan, S., Cvetković, Ž., Tančić, P., Trifković, J., Nikić, Z., & Manojlović, D. (2018). Assessment of contamination, environmental risk, and origin of heavy metals in soils surrounding industrial facilities in Vojvodina, Serbia. Environmental Monitoring and Assessment, 190, 208.
Spahić, M. P., Manojlović, D., Tančić, P., Cvetković, Ž., Nikić, Z., Kovačević, R., & Sakan, S. (2019). Environmental impact of industrial and agricultural activities to the trace element content in soil of Srem (Serbia). Environmental Monitoring and Assessment, 191, 133.
Steinnes, E. (1995). Mercury. In B. J. Alloway (Ed.), Heavy Metals in Soils (2nd ed.). London: Blackie Academic & Professional..
Streets, D. G., Devane, M. K., Lu, Z., Bond, T. C., Sunderland, E. M., & Jacob, D. J. (2011). All-time releases of mercury to the atmosphere from human activities. Environmental Science & Technology, 45, 10485–10491.
Streets, D. G., Lu, Z., Levin, L., ter Schure, A. F., & Sunderland, E. M. (2018). Historical releases of mercury to air, land, and water from coal combustion. Science of the Total Environment, 615, 131-140.
Su, Y., Han, F. X., Chen, J., Sridhar, B. M., & Monts, D. L. (2008). Phytoextraction and accumulation of mercury in three plant species: Indian mustard (Brassica juncea), beard grass (Polypogon monospeliensis), and Chinese brake fern (Pteris vittata). International Journal of Phytoremediation, 10, 547–560.
Subirés-Muñoz, J. D., García-Rubio, A., Vereda-Alonso, C., Gómez-Lahoz, C., Rodríguez-Maroto, J. M., García-Herruzo, F., & Paz-Garcia, J. M. (2011). Feasibility study of the use of different extractant agents in the remediation of a mercury contaminated soil from Almaden. Separation and Purification Technology, 79, 151–156.
Tan, Y., Mortazavi, R., Dureau, B., & Douglas, M. A. (2004). An investigation of mercury distribution and speciation during coal combustion. Fuel, 83, 2229–2236.
Tang, X. Y., & Huang, W. H. (2004). Trace elements in Chinese coal. Beijing: The commercial press (In Chinese).
Tomašević, M., Rajšić, S., Đorđević, D., Tasić, M., Krstić, J., & Novaković, V. (2004). Heavy metals accumulation in tree leaves from urban areas. Environmental Chemistry Letters., 2, 151–154.
Toole-O'Neil, B., Tewalt, S. J., Finkelman, R. B., & Akers, D. J. (1999). Mercury concentration in coal—unraveling the puzzle. Fuel, 78, 47–54.
UN. (1997). Glossary of environment statistics, studies in methods. NY: United Nations New York.
UNEP. (2013). Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport. Geneva: United Nations Environment Programme.
UNEP. (2014). Assessment of the Mercury Content in Coal fed to Power Plant and study of Mercury Emissions from the Sector in India. UNEP Chemicals Branch, Geneva, Switzerland. http://www.unep.org/chemicalsandwaste/Portals/9/Mercury/REPORT%20FINAL%2019%20March%202014.pdf. Accessed 11 Feb 2019.
USEPA. (1991). Determination of Mercury in Tissues by Cold Vapor Atomic Absorption Spectrometry. Cincinnati, Ohio. 906R1102, Accessed 11 Feb 2019.
USEPA. (2002). Control of Mercury Emissions From Coal-fired Electric Utility Boilers, Interim Report Including Errata Data, 3-21-02, EPA-600/R-01-109, Accessed 11 Feb 2019.
USEPA. (2007). Treatment technologies for mercury in soil, waste, and water. US EPA, Office of Superfund Remediation and Technology Innovation Washington, DC 20460, EPA-542-R-07-003. Accessed 11 Feb 2019.
USEPA. (2007a). Method 7471B, Mercury in Solid or Semisolid Waste (Manual Cold Vapor Technique), revision 2. https://www.epa.gov/sites/production/files/2015-12/documents/7471b.pdf. Accessed 11 Feb 2019.
Virkutyte, J., Sillanpää, M., & Latostenmaa, P. (2002). Electrokinetic soil remediation—critical overview. Science of the Total Environment, 289, 97–121.
Wang, S., & Luo, K. (2017). Atmospheric emission of mercury due to combustion of steam coal and domestic coal in China. Atmospheric Environment, 162, 45–54.
Wang, D., Shi, X., & Wei, S. (2003). Accumulation and transformation of atmospheric mercury in soil. Science of the Total Environment, 304, 209–214.
Wang, J., Feng, X., Anderson, C. W., Xing, Y., & Shang, L. (2012). Remediation of mercury contaminated sites–a review. Journal of Hazardous Materials, 221, 1–18.
Wang, X., Liu, X., Han, Z., Zhou, J., Xu, S., Zhang, Q., Chen, H., Bo, W., & Xia, X. (2015). Concentration and distribution of mercury in drainage catchment sediment and alluvial soil of China. Journal of Geochemical Exploration, 154, 32–48.
Wang, S., Zhong, T., Chen, D., & Zhang, X. (2016). Spatial distribution of mercury (Hg) concentration in agricultural soil and its risk assessment on food safety in China. Sustainability, 8, 795.
WHO (1993). Guidelines for Drinking-Water Quality. Vol. 1: Recommendations. 2d ed. Geneva. Accessed 11 Feb 2019.
WHO (2003). Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects. http://www.who.int/ipcs/publications/cicad/en/cicad50.pdf. Accessed 11 Feb 2019.
WHO (2004). Guidelines for drinking-water quality (Vol. 1). World Health Organization. Accessed 11 Feb 2019.
Xinmin, Z., Kunli, L., Xinzhang, S., Jian'an, T., & Yilun, L. (2006). Mercury in the topsoil and dust of Beijing City. Science of the Total Environment, 368, 713–722.
Xu, J., Bravo, A. G., Lagerkvist, A., Bertilsson, S., Sjöblom, R., & Kumpiene, J. (2015). Sources and remediation techniques for mercury contaminated soil. Environment International, 74, 42–53.
Xun, Y., Feng, L., Li, Y., & Dong, H. (2017). Mercury accumulation plant Cyrtomium macrophyllum and its potential for phytoremediation of mercury polluted sites. Chemosphere, 189, 161–170.
Yao, D. X., Meng, J., & Zhang, Z. G. (2010). Heavy metal pollution and potential ecological risk in reclaimed soils in Huainan mining area. Journal of Coal Science and Engineering (China), 16, 316-319.
Yin, Y., Allen, H. E., Li, Y., Huang, C. P., & Sanders, P. F. (1996). Adsorption of mercury (II) by soil: effects of pH, chloride, and organic matter. Journal of Environmental Quality, 25, 837–844.
Yu, J. G., Yue, B. Y., Wu, X. W., Liu, Q., Jiao, F. P., Jiang, X. Y., & Chen, X. Q. (2016). Removal of mercury by adsorption: a review. Environmental Science and Pollution Research, 23, 5056–5076.
Yudovich, Y. E., & Ketris, M. P. (2005a). Mercury in coal: a review. Part 1. Geochemistry. International Journal of Coal Geology, 62, 107–134.
Yudovich, Y. E., & Ketris, M. P. (2005b). Mercury in coal: a review Part 2. Coal use and environmental problems. International Journal of Coal Geology, 62, 135–165.
Zalups, R. K. (2000). Molecular interactions with mercury in the kidney. Pharmacological Reviews., 52, 113–144.
Zhang, H., Chen, J., Zhu, L., Yang, G., & Li, D. (2014). Anthropogenic mercury enrichment factors and contributions in soils of Guangdong Province, South China. Journal of Geochemical Exploration, 144, 312–319.
Zhang, L., Wang, S., Wang, L., Wu, Y., Duan, L., Wu, Q., Wang, F., Yang, M., Yang, H., Hao, J., & Liu, X. (2015). Updated emission inventories for speciated atmospheric mercury from anthropogenic sources in China. Environmental Science & Technolog, 49, 3185–3194.
Zhao, S., Duan, Y., Yao, T., Liu, M., Lu, J., Tan, H., Wang, X., & Wu, L. (2017). Study on the mercury emission and transformation in an ultra-low emission coal-fired power plant. Fuel, 199, 653–661.
Zheng, L., Liu, G., & Chou, C. L. (2007). The distribution, occurrence and environmental effect of mercury in Chinese coals. Science of the Total Environment, 384, 374–383.
Acknowledgements
The first author is grateful to the Ministry of Human Resource and Development (MHRD), Government of India, for providing scholarship. The authors also acknowledge the Indian Institute of Technology (Indian School of Mines), Dhanbad (India), for providing basic research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raj, D., Maiti, S.K. Sources, toxicity, and remediation of mercury: an essence review. Environ Monit Assess 191, 566 (2019). https://doi.org/10.1007/s10661-019-7743-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7743-2