Abstract
The concentration, source, and ecological risk of 16 polycyclic aromatic hydrocarbons (PAHs) in water and sediment samples in Hoor Al-Azim wetland, a significant freshwater wetland in Lower Mesopotamia, were evaluated. Total PAHs concentrations varied from 15.3 to 160.15 ng/L, and 15.78 to 410.2 μg/Kg in water and sediment, respectively. PAHs pollution levels in sediments compared with sediment quality guidelines (SQG) were found to be moderate in two stations and low in water and the rest of sediment stations. Based on the diagnostic ratio analysis, cluster analysis (CA), and principal component analysis-multiple linear regression (PCA-MLR), the mean percentage contributions were 62.62% for mixed pyrogenic and petrogenic sources (e.g., unburned and combusted fossil fuels from fishing boats and vehicle engines, incomplete combustion, oil leakage), 20.68% for auto emission, and 16.7% for pyrogenic sources (fossil fuels and biomass combustion). According to the sediment risk assessment indices such as mean effects range-median quotient (M-ERM-Q), the ecological risk of multiple PAHs was low. Risk quotient (RQ) calculation of water samples suggested high ecological risk level for Benzo[a]anthracene (BaA), and low to moderate for other individual PAHs and ΣPAHs. The result of PAHs partitioning between sediment and water phases revealed that most PAHs prefer to accumulate in sediment. Sediments probably act as a secondary source for some PAHs in the oil collection and pumping station.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands are generally considered as aquatic and semiaquatic ecosystems, potentially prone to a permanent or periodic waterlogging with low depth. Wetlands are also identified for their ecologically considerable contribution to biogeochemical cycles and as mega-diversity regions (Leguizamo et al. 2017). The majority of wetlands are located within the continents (Mitsch and Gosselink 2015). Freshwater marshes make less than 20% of the total numbers of world’s wetlands. These wetlands are very prolific and supply useful ecosystem services (Keddy et al. 2009; Roebeling et al. 2016). Freshwater wetlands also act as a basis for transforms, filters, and storages of different nutrients and pollutants (Hook 1993; Kadlec and Bevis 1990).
During the last half of the twentieth century and particularly within the last two decades, wetland ecosystem values have suffered a great deal mainly due to anthropogenic stressors, including population growth as well as industrial and economic development, which have reduced the values of wetlands (De Groot et al. 2012; Roebeling et al. 2016).
Polycyclic aromatic hydrocarbons (PAHs) are generally considered as priority pollutants by environmental agencies, including US Environmental Protection Agency (USEPA), due to their carcinogenic, mutagenic, and teratogenic effects (An et al. 2016; Chen et al. 2013; Liang et al. 2008). In general, the high and acute toxicity of PAHs is more related to low molecular weight PAHs (LMWPAHs), while carcinogenicity, mutagenicity, and teratogenicity are more related to high molecular weight PAHs (HMWPAHs) in living organisms (Hussain et al. 2015). Pyrogenic and petrogenic PAHs along with those formed by reduction of biogenic precursors (diagenetic PAHs) are abundant and ubiquitous in the environment (Tongo et al. 2017; Xiao et al. 2014).
In the aquatic environment, PAHs compounds mostly choose to reside in sediments for long spans of time due to their hydrophobic properties and commonly display low biodegradation rate (Wang et al. 2014; Zhang et al. 2015; Giesy et al. 2016). Moreover, direct toxic effects on aquatic biota could be the outcome of PAHs contents in water column which could spread to other areas by water current (McGrath and Di Toro 2009; Li et al. 2015b). PAHs generally enter aquatic environment through several paths including atmospheric fallout, runoff, wastewater discharge, and oil leakage (Hussain et al. 2015; Li et al. 2015a). Thus, decision makers need to estimate PAHs concentration in water and sediment environments to evaluate possible risks (Hussain et al. 2015; Nikolaou et al. 2009).
Hoor Al-Azim is the largest permanent freshwater wetland in Lower Mesopotamia in Iran-Iraq boundary (Mirzaei et al. 2010). The marshland supports a significant population of wildlife (UNEP 2001). About two-thirds of the wetland is located in Iraq and is known as Hoor Al-Hoveizeh, while the remaining is located in Iran (Fuladavand and Sayyad 2015).
In recent decades, part of the wetland has been drained for oil exploration and drilling purposes. So far, 94 oil wells are drilled in this wetland, each bearing an active torch. Also, parts of North and South Azadegan, and North and South Yaran oil fields lie in Hoor Al-Azim. More recently, the development of Azadegan oil field is extended into the Hoor Al-Azim watery section. Despite efforts to bring oil exploration and drilling activities in Hoor Al-Azim wetland in line with environmental regulations, PAHs pollution monitoring is also essential for establishing the ecosystem condition. Thus, the objectives of this research were (1) to determine the pollution levels of 16 priority polycyclic aromatic hydrocarbons (PAHs) in water and surface sediments of the Hoor-Al-Azim wetland, (2) to define possible sources of pollutants in sediments using statistical techniques including diagnostic ratios, cluster analysis (CA), and principal components analysis (PCA) combined with multiple linear regression (MLR) model, (3) to assess the degree of PAHs pollution and ecological risk in water and sediment of the wetland using indices of contamination, and (4) to determine each PAHs compound flux in the Hoor Al-Azim sediment-water perimeter.
Materials and methods
Study area
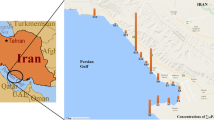
Hoor Al-Azim wetland as a significant wetland in the Lower Mesopotamia is located in North Azadegan Plain, 80 km from Ahvaz City, near Iran-Iraq border. Its geographic coordinates are 47°, 55′ to 47°, 20′ eastern longitude and 30°, 58′ to 31°, 50′ northern latitude (Fig. 1). The areal coverage of the wetland is about 1704 km2, reduced from 3386 km2 in 1985 (Salmabai and Saeedi 2018). Darkhoein, Hoveizeh, and Sousangerd cities are located close to the wetland. The water in northern and central parts of the wetland is perennial, while being largely seasonal in the south. Hoor Al-Azim water depth decreases from north to south, ranging from half a meter to maximum 3 m. The wetland water is supplied by two distributaries from Tigris in Iraq and Karkheh river in Iran which originates from the Zagros Mountain range in the west of the country (UNEP 2001). Flood regimens of Karkheh, and Tigris and Euphrates rivers are effective in wetland revival (Fuladavand and Sayyad 2015). Also, common reed (Phragmites communis) dominates the core of the permanent wetland making up over 50% of the vegetation.
Chemicals and reagents
All used chemicals and reagents were of analytical grade. N-hexane (≥ 98.0% purity), dichloromethane (DCM) (≥ 99.9% purity); acetonitrile (ACN) (≥ 99.9% purity), sodium sulfate (Na2SO4; ≥ 99.99% purity), and silica gel were made by the Merck Group (Darmstadt, Germany). A surrogate standard of Pyr-D10 (98 atom% D) was supplied from Sigma-Aldrich (MO, USA). Also, ERM-CA100 (surface water) and BCR-535 (freshwater harbor sediment), which serve as PAH standard reference materials, were provided by the Institute for Reference Materials and Measurements (IRMM; Geel, Belgium).
Sampling, preparation and analysis
In November 2016, a total of 19 surface sediment samples were taken from the top 5 cm using a Van Veen grab. Each sample was separated into subsamples to measure physicochemical parameters and PAHs concentrations. Also, 2.5 L of water was simultaneously taken above the 5 sediment sampling sites using a pre-cleaned glass bottle. Water samples were passed through a 0.45-μm microporous filter membrane under vacuum to separate dissolved PAHs for analysis. To determine the PAHs concentrations in water and sediments, the collected samples were immediately put in dark glass containers which were already washed with n-hexane and sealed by aluminum foil caps. The collected samples were transported in an icebox at 4 °C and stored at − 20 °C until analysis at the laboratory. It should be mentioned that the samples were collected from monitoring stations set by the Department of Environmental Organization (DEO) and also close to the possible oil polluted areas. The sampling stations information covering the whole study area are presented in Table 1.
The extraction of PAHs was performed based on the EPA 3550B (EPA 1996a) and EPA 3510 C (EPA 1996b) standard methods for sediment and water samples, respectively. Finally, following EPA guideline 8310 (EPA 1986), a clean-up procedure (EPA3630 C; EPA 1996c) was carried out. Briefly, approximately 5 g of each dried sediment sample was spiked with appropriate surrogate standards (Pyrene-D10, lot: 10510 semi-volatile internal standards), and an ultrasonic bath (KUDOS, SK3210LHC model, Shanghai, China) was used during the extraction. The samples were then extracted with a 30 mL dichloromethane (DCM) and n-hexane mixture (1:1 v/v) for 30 min at room temperature. Amorphous sodium sulfate was added to the solution and then 2 mL of dried extracts were taken for the next step. Also, 1000 mL of water samples were extracted washing and shaking with 60 mL dichloromethane (DCM). The solution was transferred into a separating funnel and samples were extracted by shaking the funnel for 3 min with periodic venting to release excess pressure. The organic phase containing the PAHs was separated from the water phase for a minimum of 10 min and the DCM extract was collected in a 250 mL Erlenmeyer flask. Another 60 mL portion of DCM was added to the sample bottle and the steps above repeated. The extracts were transferred into the Erlenmeyer flask. The water and sediment extracts were densified to a volume of 10 mL by a rotary vacuum evaporator HeiVAP Advantage (Heidolph Instruments GmbH & CO. KG, Schwabach, Germany) and subsequently to 1 mL under a gentle high purity nitrogen flow. A silica gel column was used to clean up the extract. The 20 μL subsamples were injected manually using syringes.
Sixteen PAHs (including naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fl), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), benzo[ghi]perylene (BghiP), Indeno[1,2,3 cd]pyrene (InP), and dibenz[a,h]anthracene (DahA)) were analyzed in the laboratory of Isfahan University of Technology using a RIGOL L-3000 high-performance liquid chromatographer (HPLC, RIGOL technologies, Inc., Beijing, China) equipped with a RIGOL L-3500 UV–vis detector (RIGOL technologies, Inc., Beijing, China) and a Hewlett-Packard 1046 A fluorescence detector (Agilent Technologies, CA, USA). The separation of analytes was done using a LiChrospher© PAH column (250 mm, 4.6 mm, 5 μm film thickness; Agilent Technologies, CA, USA). The movable fluid was acetonitrile/water in gradient mode at a moving rate of 1 ml/min and the heat set at 35 °C. PAHs concentrations are reported as microgram per kilogram dry weight for the sediments and nanogram per liter for water samples.
In order to evaluate the physiochemical parameters, the sediment samples were dried at room temperature (< 30 °C) and sifted via a 2-mm sieve. Hydrometer method was performed to specify sediment textures (Gee and Bauder 1986; Hakanson and Jansson 1983). Also, the loss on ignition (LOI) procedure was carried out to assess organic matter (OM) and total organic carbon (TOC) (Heiri et al. 2001).
Sediment pH and electrical conductivity (EC) were measured using a combined pH and conductivity meter (CyberScan PCD 6500, Eutech Instruments Pte Ltd., Singapore) based on the methodology outlined by Ryan et al. (2007). Moreover, redox potential (Eh), pH, temperature (T), and electrical conductivity (EC) of water samples were estimated using a multi-parametric portable instrument (CyberScan PCD 650, Eutech Instruments Pte Ltd., Singapore). The measured parameters are presented in Table 1.
Quality assurance/quality control
The QC testing for PAHs analysis relied on procedural blank samples, sample duplicates, samples spiked with surrogate standards (Pyrene-D10, lot: 10510 semi-volatile internal standards) and standard certified reference materials (ERM-CA100 (surface water), and BCR-535 (freshwater harbor sediment)). Pyr-D10 was used as internal standard for the calibration of all species of PAHs. The recovery percentages in water and sediment samples were approximately 90–95% and 88–90% for the 16 measured PAHs, respectively. Procedural blank samples were lack of target PAHs. The detection limits of the target PAHs for surface sediment and water samples ranged between 0.1 (Phe, Ant, BaA, BkF) and 6.4 μg/kg (Acy), 0.2 (Phe, Ant, BkF) and 9.2 ng/L (Acy), respectively.
Ecotoxicological risk assessment
The effect range low (ERL)/effect range median (ERM), threshold effect level (TEL), and probable effect level (PEL) are generally taken as Sediment Quality Guidelines (SQGs). They are commonly used as a practical tool for ecological toxicity assessment of individual PAHs in sediments (Long et al. 1995; Wang et al. 2017). ERLs and TELs indicate infrequent occurrence of adverse biological effects, while ERMs and PELs show the possibility of occurrence of toxicity and adverse consequences. Also, the values between ERLs-ERMs and TELs-PELs show occasional negative consequences (Adeleye et al. 2016; Akhbarizadeh et al. 2016; Wang et al. 2015).
Hence, to assess combined ecological risk and toxicity levels of PAHs in sediments, mean effects range-median quotient (M-ERM-Q), mean probable effect level quotient (M-PEL-Q), and Toxicity equivalent (TEQ) analysis were calculated. The relevant formulas are as follows (Long and MacDonald 1998; Kapsimalis et al. 2014).
where Ci is the contaminant concentration of i PAH in the sediment, ERMi and PELi are the ERM and PEL for compound i, and n is the figure of the considered contaminants. M-ERM-Q and M-PEL-Q contents are classified into four relative priority levels as follows:
-
(a)
High (M-ERM-Q > 1.5 and M-PEL-Q > 2.3);
-
(b)
Medium high (1.5 > M-ERM-Q > 0.51 and 2.3 > M-PEL-Q > 1.51);
-
(c)
Medium low (0.5 > M-ERM-Q > 0.11 and 1.5 > M-PEL-Q > 0.11); and
-
(d)
Low (M-ERM-Q < 0.1 and M-PEL-Q < 0.1) (Long 2006; Long and MacDonald 1998).
TEFi as the toxic equivalency factor relative to BaP for i PAH helps to precisely characterize the carcinogenic properties of PAH mixtures (Fu et al. 2011; Zhang et al. 2012; Zhu et al. 2014), and is considered to be 0.001 for Nap, Ace, Acy, Flu, Phe, Fl, Chr, and Pyr, 0.01 for Ant, BghiP, BkF, 0.1 for BaA, BbF, InP, and 1.00 for BaP and DahA (Kapsimalis et al. 2014; Sarria-Villa et al. 2016).
Furthermore, in order to estimate the PAHs possible ecological risk on aquatic biota, the risk quotient (RQ) was calculated for the wetland (Kalf et al. 1997; Yan et al. 2016) as shown in the following equations:
where CPAHs is the level of individual PAHs in the perimeter, CQV(NCs), and CQV(MPCs) are the quality contents of the NCs (negligible concentrations) and MPCs (maximum permissible concentrations) of PAHs in the medium, respectively. The NCs and MPCs of each PAHs in water are reported in the supporting information of Table A3.
Generally speaking, RQ(NCs) < 1 suggests that the each PAHs ecological risk could be trivial, while RQ(MPCs) > 1 indicates that the each PAHs ecological risk could be extreme. Moreover, RQ(NCs) > 1 and RQ(MPCs) < 1 reveal medium ecological risk of individual PAHs. Risk classification of ∑PAHs is explained as RQNCs < 1 indicating a very low risk; 1 ≤ RQNCs < 800 and RQMPCs < 1 revealing low to moderate risk and RQNCs ≥ 800 and RQMPCs ≥ 1 exhibiting high risk (Cao et al. 2010; Akhbarizadeh et al. 2016; Pheiffer et al. 2018).
Statistical analysis
Statistical analysis of data was carried out using SPSS 18.0 and Excel 2010 software for Windows. Descriptive statistical parameters such as mean, standard deviation (SD), skewness, and kurtosis were computed to reveal the distribution of the variables. The data normality was investigated using the Shapiro-Wilk test (p < 0.05). Since the concentrations of Acy, Ace, Fl, DahA, and InP were below the reporting limits in more than 50% of samples, they were removed from the data before diagnostic ratios determination, CA and PCA-MLR analysis, and water-sediment exchange estimation. For the rest of species with more than 50% detectable data, undetectable values were replaced with 75% of the detection limit. The concentration of omitted PAHs in sediment sampling sites 3 and 14 are significant.
Diagnostic PAH ratios such as LMW/HMW, Ant/(Ant + Phe), and BaA/(BaA + Chr) were used to separate pyrogenic and petrogenic origins (Bortey-Sam et al. 2014). Also, PCA on the basis of similarities and differences of individual PAHs provided useful information regarding the possible sources (Niu et al. 2017). PCA applicability with our nonnormally distributed dataset was verified by the Kaiser-Meyer-Olkin (KMO) and Bartlett’s sphericity test (Ashayeri et al. 2018). Moreover, PCA/MLR as a model receptor quantifies the contribution of different sources. Therefore, MLRA was carried out by PCA factor scores as independent variables, and the standardized normal deviation of total PAH values as dependent variables (Xue et al. 2016). The detailed methods of PCA/MLR are explained in the literatures (Guo et al. 2004; Larsen and Baker 2003). Standardized regression coefficients are applied to determine proportional shares of different origins.
Determining sediment-water equilibrium partitioning
The lipophilicity or carbon affinity of a chemical is expressed through the octanol-water partition coefficient (Kow). This coefficient which is closely related to the distribution coefficient describes the fate of organic chemicals such as PAHs in the environment (Guo et al. 2009). The higher the Kow value for a chemical, the greater is the propensity of the chemical to partition in organic phase (Sun et al. 2017). The equilibrium partitioning coefficient (Koc) estimates PAHs attraction to sediment and determines the sediment-water partitioning degree (Cao et al. 2015). In order to assess PAHs exchange behavior in the Hoor Al-Azim water perimeter, in situ organic carbon sediment-water partition coefficient (Koc′) are expressed as:
where Cs (μg/kg) is the PAHs content in sediment, Caq (ng∕L) is PAHs content in water, and foc (unitless) is the portion of organic carbon in the sediment (Guo et al. 2009). The difference of contents between log Koc′ and the corresponding log Koc indicates imbalance state of PAHs in the sediment-water system (Ashayeri et al. 2018). Where average log Koc′ for PAHs is smaller than the corresponding log Koc and log Kow, PAH compounds prefer to be sorbed onto the sediment phase, rather than being exchanged with water phase (Sun et al. 2017).
The transmission of a chemical from one perimeter to another is controlled by fugacity. Therefore, the balance status and exchange processes of PAHs between water and sediment were evaluated by fugacity fraction (ff), which depends on the PAHs concentrations and fugacity capacity of the corresponding system (Liu et al. 2013). Hence, ff of sediment-water system in the study area was calculated using the following equation:
The flux direction occurs from water to sediment for ff < 0.3 and sediments act as a reservoir for the contaminants. The 0.3 < ff < 0.7 represent sediment-water equilibrium, while ff > 0.7 indicates flux migration from sediment to water (Harner et al. 2001; Hong et al. 2016; Liu et al. 2013).
Results and discussion
Sediment characterization
Distribution of grain size (sand, silt, and clay), TOC, pH, EC, and CEC in sediment samples are presented in Table 1. TOC contents in Hoor Al-Azim sediment ranged from 3.34 (H8) to 7.81% (H14). Higher TOC occurred at stations 14 (7.81%) and 13 (7.66%), which are believed to be released from exploratory wastes. Also, clay, silt, and sand fraction percentages in surface sediments ranged from 8.2 to 54%, 22 to 57%, and 4.92 to 43.6%, respectively, characterizing “clay, clayey loam, and loam” sediment textures.
A weak correlation was found between ΣPAHs, TOC (r = 0.09) and clay contents (r = 0.003) (Fig. 2). Although there is no general agreement regarding the role of grain size on PAHs sorption, some authors believe in a positive correlation between high PAH concentrations and large particle size (> 500 μm) (Rockne et al. 2002). However, the majority of authors believe that PAHs sorption positively correlates with small size particles (Kim et al. 1999). Also, TOC is a significant controlling factor influencing PAHs sorption on sediments (Liu et al. 2009b; Zakaria et al. 2002), particularly when organic matter content in sediments is > 0.1% (Johnson et al. 2001). However, some authors reported that PAH contents in sediments are independent of TOC percentages (Baniemam et al. 2017; Tam et al. 2001). Moreover, source of pollution could define the concentration and distribution of PAHs (Mostafa et al. 2009). Thus, it may be said that both distribution and concentration of PAHs are mostly controlled by direct entries rather than particle size or sediments’ total organic carbon (TOC) content.
PAHs contents in sediment
The statistical summary of PAHs in collected sediments is presented in Table 2. Among the measured PAHs, Nap was the most abundant species, accounting for 20.75% of ΣPAHs in sediments. The results also revealed that BaA, Ant, Chr, Pyr, and Flu account for 14.65%, 12.4%, 9.44%, 8.9%, and 8.18% of the measured 11 PAHs concentrations, respectively. BkF accounted for only 1.64%.
∑PAHs ranged from 15.78 μg/Kg (in site 11) to 410.2 (in site 14) averaging 51.71 ± 94.86 μg/Kg. The elevated ∑PAHs were measured at sites H14 (410.2 μg/Kg) and H3 (188.62 μg/Kg), respectively. At the first station, borehole drilling waste spread over an area of 40 ha, while at station 3, oil from different wells is collected, separated and pumped to Ahvaz City. Other stations revealed similar ∑PAHs ranging from 15.78 to 29.15 μg/Kg (supporting information in Fig. A1). PAHs contents in Hoor Al-Azim sediment samples can be categorized as moderate in sites 14 and 3, and low for the rest considering the pollutant levels proposed by Baumard et al. (1998).
PAHs concentration in the sediments of Hoor Al-Azim wetland is lower than those reported from Iraq (590–2070 ng/g) (Al-Saad and Al-Timari 1989), South Carolina (69–37,000 ng/g) (Sanger et al. 1999), and Barnegat Bay-Little Egg Harbor Estuary (37–1696 ng/g) (Vane et al. 2008), but higher than those from Chongming wetlands (38.7–136.2 ng/g) (Wang et al. 2012), Gulf of Mexico (229–379 ng/g) (Wang et al. 2014), and marsh/estuarine sediments of Savannah GA, USA (1.2–160 ng/g) (Kumar et al. 2008).
HMW compounds with four and more condensed aromatic rings are frequently found in combustion products, while LMW compounds with two and three aromatic rings result from fossil fuels (Li et al. 2014; Montuori et al. 2016). In this study, ∑2–3 rings PAHs concentration varied from 4.28 to 130 μg/Kg averaging 16.76 ± 31.5 μg/Kg, and ∑4–6 rings PAHs concentration varied from 2.4 to 114 μg/Kg averaging 16.51 ± 29.77 μg/Kg. In general, ∑2–3 rings PAHs with 50.37% of ∑PAHs are similar in abundance to ∑4–6 rings PAHs with 49.63% of ∑PAHs.
Supporting information in Fig. A2 gives a detailed PAHs concentration profile in sediments, showing the concentration of each of the 11 species separately. 4-ring PAHs with 33% of the total are more abundant compared with 3-ring and 2-ring PAHs accounting for 29.62% and 20.75% of the total PAHs, respectively. 5-ring PAHs and 6-ring PAHs exhibit 12.15% and 4.48% of ∑PAHs in the sediment samples, respectively.
PAHs concentration in water
The concentrations of ∑11PAH species detected in the Hoor Al-Azim wetland water ranged from15.3 (site 1) to 160.15 (site 2) ng/L averaging 76.94 ± 62.52 ng/L. Nap is the main PAH species and accounts for 33.7% of the total PAHs. Also, Phe, BaA, Pyr, and Chr are abundant and represent 22.3%, 18.1%, 7.9%, and 7.6% of ∑PAHs, respectively (Table 2).
In the dissolved phase, ∑PAHs contamination could be categorized into four classes: micro-polluted, 10–50 ng/L; lightly polluted, 50–250 ng/L; moderately polluted, 250–1000 ng/L, and highly polluted, > 1000 ng/L (Chen 2008). Consequently, ∑PAHs at 3 stations (2, 3, and 4) are lightly polluted, while in the other two sampling stations are micro-polluted. It is noteworthy that total PAHs concentration in surface water is lower than Yellow river delta (64.8–334.6 ng/L) (Wang et al. 2009) and Qinkenpao Wetland (36.4–23.984.6 ng/L) (Zhang et al. 2014).
The composition patterns of individual PAHs in surface waters of Hoor Al-Azim wetland are shown in supporting information in Fig. A3. Two- and four-ring PAHs are similar, accounting for nearly 33.7% of the ∑PAHs, followed by three-ring PAHs accounting for 29.92% and five- and six-ring PAHs making 1.43 and 1.25% of ∑PAHs, respectively.
∑2–3 and ∑4–6 rings PAHs varied from 8.9 to 128.1 ng/L and 3.37 to 65.3 ng/L, respectively. Also, The LMW/HMW ratios ranged from 0.76 to 4.4, indicating that the two- and three-ring PAHs are predominant in wetland water. The reason is probably the lower solubility of HMW PAHs compared with LMW PAHs; thus, resulting in HMW PAHs tendency towards sediment particles and hence depositing in the bottom sediments.
Source apportionment
Diagnostic ratios are effective indices for determining PAH origins (Soclo et al. 2000; Rocher et al. 2004; Liu et al. 2009a; Deng et al. 2013). Even though PAH isomer pair ratios may indicate a specific source, but physical, chemical, and/or biological processes may also change the quantity of each PAHs during transmission and following incorporation in sediments (Viñas et al. 2010).
The dominance of low molecular weight (LMW) two-ring and three-ring PAHs, mostly originating from oil or hydrocarbon spills and low-temperature processes, is indicative of petrogenic origin, while higher contents of HMW PAHs are considered to represent pyrogenic sources (Arias et al. 2010; Ma et al. 2014). Furthermore, Ant/(Ant + Phe) ratio is generally applied to identify petrogenic sources, whereas BaA/(BaA + Chr) is more suitable for identifying pyrolytic sources (Tobiszewski and Namieśnik 2012). Therefore, diagnostic ratios of PAHs including LMW/HMW, Ant/(Ant + Phe) and BaA/(BaA + Chr) (Soclo et al. 2000; Yunker et al. 2002; Barhoumi et al. 2014) for evaluating the petrogenic and pyrolytic sources of PAHs are valuable and are summarized in supporting information in Table A1.
In this study, LMW/HMW PAHs ratio ranges from 0.48 to 2.56, indicating that contamination is probably due to petrogenic-pyrolytic sources (Liu et al. 2008; Soclo et al. 2000). Also, Ant/Ant + Phe ratio ranges from 0.04 to 0.92, which suggests that PAHs in surface sediments probably originated from mixed sources. This ratio in H1, H5, H11, and H13 is 0 < 0.1, and in the rest of the samples > 0.1; thus, the predominance of pyrogenic sources is suggested (Rahmanpoor et al. 2014; Yunker et al. 2002). BaA/(BaA + Chr) ratio varies from 0.13 to 0.86, indicating that the contamination is due to petrogenic source in H10, H11, and H13. Furthermore, mixed sources were found to occur at H2, H13, and H16 stations.
Generally, LMW/HMW, Ant/(Ant+Phe) and BaA/(BaA + Chr) ratios are indicative of petroleum pollution at stations 11 and 13. Moreover, H2, H3, H6, and H8 sampling stations are affected by pyrolytic PAH sources. The rest of the sampling stations revealed mixed pyrogenic and petrogenic sources.
Since Ant/(Ant+Phe) and BaA/(BaA + Chr) ratios are 0.1 to 0.23 and 0.7 to 0.78, respectively, PAHs in Hoor Al-Azim wetland water most likely have originated from pyrolitic sources.
Cluster analysis (CA) is commonly used to distinguish homogenous groups from a data set (Keshavarzi et al. 2015). The cluster analysis result is displayed in the hierarchical dendrogram (supporting information in Fig. A4), which classified the 11 PAHs species into three major groups. Moreover, principal component analysis (PCA), as a mathematical procedure, extracts a small set of factors from the original PAHs data to identify their emission sources (Dudhagara et al. 2016; Malik et al. 2011). The rotated factors of 11 PAHs from the wetland sediments are given as supporting information in Table A2. Three components explained 99.19% of the data variance. The first component making 47.82% of the variance displays high loading values of Nap, Ant, BaA, Chr, BaP, and moderate loadings for Pyr, BbF, and BkF.
Chr, BaA, B(b + k)F, and BaP are commonly recognized as fossil fuels combustion markers, especially diesel engine emissions (Deng et al. 2013; Ma et al. 2014; Feng et al. 2016). In Hoor Al-Azim wetland, diesel engine emissions are produced by boats as well as other vehicles. Also, boat and vehicle engine exhausts are thought to be responsible for both uncombusted fuel and incomplete combustion products (Zeng and Vista 1997). Therefore, Nap as the major component of gasoline and diesel fuels may originate from uncombusted fuel and other incomplete combustion-related sources (Dong and Lee 2009; Soltani et al. 2015; Stogiannidis and Laane 2015). Anthracene (Ant) is present in minute quantities in crude oil, and diesel fuel (Stogiannidis and Laane 2015), and it may also be associated with biomass burning (Larsen and Baker 2003). Moreover, Ant and Pyr are commonly taken as oil combustion markers (Park et al. 2002; Dong and Lee 2009; Soltani et al. 2015). In the Hoor Al-Azim wetland, heavy oil combustion occurs in the burn pit.
Also, BaP emanates from minimal temperature pyrolytic sources, such as wood and vegetation combustion (Freeman and Cattell 1990; Duodu et al. 2017). Moreover, BkF could be the result of wood combustion (Bixiong et al. 2006). Biomass burning (especially Phragmites communis) is a common practice in the study area. Consequently, the first principal component (PC1) is taken as representing mixed pyrogenic and petrogenic sources. Also, based on the CA, the first major group contains BaA, Chr and BaP, high molecular weight PAHs, and Ant and Nap that belong to LMW PAHs. The source of this group is consistent with PC1.
The second factor controls 41.31% of the total variance and is dominated by Flu, Phe, Pyr, BbF, and BkF, along with moderate loadings for BaA and Chr. In addition, Phe is also common in the profiles of pyrogenic sources (Page et al. 2006). Therefore, factor 2 must be allocated to combustion-related sources. It is interesting to note that the second major group in CA including BbF, BkF, Pyr, Flu and Phe represent a mixture of fossil fuel burning and biomass combustion confirming PC2 results.
BghiP as the third factor accounts for 18.88% of the total variance, and it is generally known to be a tracer for automobile emissions (Boonyatumanond et al. 2006; Liu et al. 2009b). Moreover, the third group in CA (Fig. A4) also contains BghiP, confirming PC3 result.
The percentage contributions of these three factors in each sediment sample were calculated by PCA/MLR model. Significance of the regression coefficients (R2 = 0.803 for sediments (N = 19)) were at 95% confidence level (p values < 0.05). Mixed sources, pyrogenic sources, and gasoline and diesel burning vehicle emissions as the main three factor scores were regressed against the standard normalized deviate (Z) of the total PAH concentrations. This linear regression is given as:
where Z is the standardized normal deviate of Σ PAH, Bi is the partial regression coefficient, and Xi is the PCA factor score. For sediment samples, the MLRA equation is presented as:
Then, the regression coefficients were applied to estimate the mean shares of different origins presented in the following equation:
where Bi/ΣBi is the regression coefficient for factor i of the sum of all regression coefficients, and PCi is the factor score for factor i.
Considering the results, a combined source resulting from unburned and combusted fossil fuels from fishing boats and vehicle engines, incomplete combustion, and oil leakage seem to be the dominant source with average contributions being 62.62%. Fossil fuel burning and biomass combustion with pyrogenic origins contribute 16.7% of the total PAHs, and finally the percentage contribution from auto emissions is 20.68%.
Assessing sediment quality and toxicity
Environmental scientists, based on the significant data related to the chemical analyses of the pollutants and their consequences on aquatic biota, developed sediment quality guidelines (SQGs) for assessing the relation between the contaminants concentration and organism toxicity (McCready et al. 2006; Liu et al. 2009a, 2014). The results of this study showed that ∑PAH contents in sediment samples were considerably lower than the ∑PAHs related to TEL and ERL guidelines. The concentration of individual PAHs compared with respective ERM values is low, while the concentration of Acy, Ace, and Flu in station 14 exceeds ERL. Hence, these species have the potential to create occasional detrimental biological consequences at this station. Moreover, among the individual PAHs, the concentration of Acy and Ace in stations 3 and 14 and Nap, Flu, Ant, and DahA in station 14 are above TEL values, even though the values of individual PAHs are lower than respective PEL contents (Table 3). Thus, the input from hydrocarbon spills and incomplete combustion-related sources are considered as elevated levels of some LMW PAHs species in the above-mentioned stations.
An alternative approach for evaluating probable biological consequences or toxicity resulting from exposure to multiple PAHs in sediments is to use M-ERM-Q and M-PEL-Q. The results revealed that in station 14, M-PEL is 0.16 (1.5 > M-PEL-Q > 0.11), indicating potential adverse combined biological effects, while M-PEL-Q in other stations and M-ERM-Q in all sampling stations were below 0.1.
Calculated TEQ ranged from 0.25 to 26.1 μg/kg-BaPeq averaging 2.57 μg/kg-BaPeq. The highest TEQ was measured at station 14. Although the eight carcinogenic PAHs (IARC 2018) accounted for 43.5–76.6% of the total PAH concentrations, they were significantly lower than the SQGs of CPAHs recommended by Long et al. (1995). Furthermore, the total TEQcarc contents of sediment samples ranged from 0.24 to 25.24 μg TEQ/kg dw, averaging 2.49 ± 5.88 μg TEQ/kg dw. The higher total TEQcarc occurred at stations 14 and 3. Various CPAHs, share of the total TEQcarc decreased in the following order: BaP (37.35%), DahA (30.38%), BaA (19.54%), BbF (10.29%), Chr (1.26%), IP (0.68%), Nap (0.28%), and BkF (0.22%).
RQNCs of Pyr, BaA, BbF, and BghiP in all stations, Phe, Ant, Chr in W2, W3, and W4, Flu in W2 and W4, and Nap in W3 and W4 were > 1, showing medium ecological risk and necessitating a number of monitoring or corrective actions. Also, RQNCs of BkF and BaP were < 1 in all stations indicating that the ecological risks caused by these individual PAHs are nominal. BaA revealed the highest mean value of RQNCs (139.8), followed by Pyr (8.71) and Phe (5.73).
RQMPCs of individual PAHs varied from 0.0006 to 0.24 except BaA in 3 stations (1.7 in W2, 3.3 in W3, and 1.67 in W4), suggesting high ecological risk for BaA in these stations. RQ(NCs) of ΣPAHs were < 800 in all sampling stations. Also, RQ(MPCs) of ΣPAHs were > 1 at W2, W3, and W4. Based on the above results, Hoor Al-Azim wetland ecosystem is at medium risk in these stations (supporting information in Table. A3).
Surface water-sediment exchange
Most studies only report the concentration levels and contamination status of PAHs in aquatic system, while controlling processes, especially partitioning of PAHs between water and sediment phases, help us to have a better insight into the fate of PAHs and water and sediment quality status (Sun et al. 2017).
The octanol-water partition coefficient (log Kow), sediment-water equilibrium partitioning coefficient (log Koc), in situ sediment-water distribution coefficient (logKoc'), and fugacity fraction (ff) contents of PAHs for the 5 coupled water and sediment data sets are given in Table 4.
The mean log Koc' and ff contents vary from 1.29 to 75.38, and from 0.17 to 0.54, respectively. The contents of log Koc' in the study area illustrated that PAH partitioning behaviors change among different locations. The variations in the organic carbon contents and PAH distributions in various sampling stations may be considered as different PAH partitioning behaviors (Sun et al. 2017).
The results indicated that mean log Koc' for PAHs species (except high ring PAHs: BbF, BkF, and BaP) is lower than corresponding log Koc and log Kow contents; therefore, PAHs species prefer to be sorbed by particle portion, while other three PAH compounds, BbF, BkF, and BaP, prefer to become saturated in sediments; as a result, the trend of PAHs partitioning is from particle phase to water phase.
As shown in Table 4 and supporting information in Fig. A5, most PAH species indicated that the mean ff contents (except high ring PAHs BbF, BkF, BaP, and BghiP) are lower than or equal to 0.3; consequently, these compounds may partition directly from water to sediment. Generally, equilibrium state in the sediment-water system is predicted for PAHs species including BbF, BkF, BaP, and BghiP. Also, sediment seems to be a secondary source for the above mentioned species at North Yaran cluster station, where the collection and pumping of oil to Ahwaz City takes place.
Nevertheless, maximum ff contents of Nap (0.31), Phe (0.38), An (0.53), Pyr (0.41), BaA (0.52), and BghiP (0.34) are close to or fall in the limit of 0.3–0.7, while Flu (0.83), Chr (0.78), BbF (0.85), BkF (0.89), and Bap (0.81) values are more than 0.7, suggesting that these PAH species are close to the balance state between water and sediment, or may cause a flux direction from sediment to water. This research was restricted to surface water of the wetland with less PAHs contamination compared with near-bottom or pore water.
Conclusion
The present study indicated that the sediments and water of Hoor Al-Azim wetland are contaminated with PAHs as a consequence of petroleum exploration and other activities related to oil exploitation. The highest sediment contamination occurs near the storage facilities of oil drilling waste disposal site (H14) and the logistics and transportation center (H3). These two stations proved to be moderately polluted, while other sampling sites displayed low pollution contents.
Since grain size distribution and organic matter content do not affect PAHs sorption, the source of PAHs plays the most important role on PAHs concentration. According to the calculated results of diagnostic ratios, PCA and CA, a mixed pyrolitic and petrogenic source is suggested for elevated PAHs concentrations. Also, PCA-MLR revealed the contribution percentages of different sources as 62.62% for mixed sources, 20.68% for auto emission, and 16.7% for pyrogenic sources (diesel engine emissions, vegetation burning and fossil fuel combustion). PAHs partitioning trend between water and sediment provided substantial information regarding PAHs destination and the quality of water-sediment system. In the Hoor Al-Azim wetland, the net flux direction for most priority PAHs is from surface water to sediment; nevertheless, a few HMW-PAHs including BbF, BkF, BaP, and BghiP fall within the sediment-water equilibrium range or may show a net flux from sediment to water.
Regarding multiple ecological risk estimations, surface sediment in station 14 proved to be moderately polluted, while the rest of the stations proved to be exposed to rare detrimental biological consequences. Also, RQNCs and RQMPCs displayed a moderate ecological risk of PAHs in the wetland water. In general, although pollution indicators suggest low risk to organisms, further monitoring of wetland water and sediment is essential for abatement practices.
References
Adeleye, A. O., Jin, H., Di, Y., Li, D., Chen, J., & Ye, Y. (2016). Distribution and ecological risk of organic pollutants in the sediments and seafood of Yangtze estuary and Hangzhou Bay, East China Sea. Science of the Total Environment, 541, 1540–1548.
Akhbarizadeh, R., Moore, F., Keshavarzi, B., & Moeinpour, A. (2016). Aliphatic and polycyclic aromatic hydrocarbons risk assessment in coastal water and sediments of Khark Island, SW Iran. Marine Pollution Bulletin, 108(1), 33–45.
Al-Saad, H. T., & Al-Timari, A. A. (1989). Distribution of polycyclic aromatic hydrocarbons (PAH’s) in marsh sediments, Iraq. Bulletin of Environmental Contamination and Toxicology, 43(6), 864–869.
An, N., Liu, S., Yin, Y., Cheng, F., Dong, S., & Wu, X. (2016). Spatial distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in the reservoir sediments after impoundment of Manwan dam in the middle of Lancang River, China. Ecotoxicology, 25(6), 1072–1081.
Arias, A. H., Vazquez-Botello, A., Tombesi, N., Ponce-Vélez, G., Freije, H., & Marcovecchio, J. (2010). Presence, distribution, and origins of polycyclic aromatic hydrocarbons (PAHs) in sediments from Bahía Blanca estuary, Argentina. Environmental Monitoring and Assessment, 160(1–4), 301–314.
Ashayeri, N. Y., Keshavarzi, B., Moore, F., Kersten, M., Yazdi, M., & Lahijanzadeh, A. R. (2018). Presence of polycyclic aromatic hydrocarbons in sediments and surface water from Shadegan wetland–Iran: a focus on source apportionment, human and ecological risk assessment and sediment-water exchange. Ecotoxicology and Environmental Safety, 148, 1054–1066.
Baniemam, M., Moradi, A. M., Bakhtiari, A. R., Fatemi, M. R., & Khanghah, K. E. (2017). Seasonal variation of polycyclic aromatic hydrocarbons in the surface sediments of the southern Caspian Sea. Marine Pollution Bulletin, 117(1–2), 478–485.
Barhoumi, B., LeMenach, K., Devier, M. H., Ameur, W. B., Etcheber, H., Budzinski, H., Cachot, J., & Driss, M. R. (2014). Polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the Bizerte lagoon, Tunisia: levels, sources, and toxicological significance. Environmental Monitoring and Assessment, 186(5), 2653–2669.
Baumard, P., Budzinski, H., & Garrigues, P. (1998). Polycyclic aromatic hydrocarbons in sediments and mussels of the western Mediterranean Sea. Environmental Toxicology and Chemistry, 17(5), 765–776.
Bixiong, Y., Zhihuan, Z., & Ting, M. (2006). Pollution sources identification of polycyclic aromatic hydrocarbons of soils in Tianjin area, China. Chemosphere, 64(4), 525–534.
Boonyatumanond, R., Wattayakorn, G., Togo, A., & Takada, H. (2006). Distribution and origins of polycyclic aromatic hydrocarbons (PAHs) in riverine, estuarine, and marine sediments in Thailand. Marine Pollution Bulletin, 52(8), 942–956.
Bortey-Sam, N., Ikenaka, Y., Nakayama, S. M. M., Akoto, O., Yohannes, Y. B., Baidoo, E., Mizukawa, H., & Ishizuka, M. (2014). Occurrence, distribution, sources and toxic potential of polycyclic aromatic hydrocarbons (PAHs) in surface soils from the Kumasi Metropolis, Ghana. Science of the Total Environment, 496, 471–478.
Cao, Z., Liu, J., Luan, Y., Li, Y., Ma, M., Xu, J., & Han, S. (2010). Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons in the Luan River, China. Ecotoxicology, 19(5), 827–837.
Cao, Q. M., Wang, H., Qin, J. Q., Chen, G. Z., & Zhang, Y. B. (2015). Partitioning of PAHs in pore water from mangrove wetlands in Shantou, China. Ecotoxicology and Environmental Safety, 111, 42–47.
Chen, Y. Y. (2008). The spatial and temporal distribution, source and bioavailability of PAHs in Qiantang River. PhD thesis, College of Environmental and Resource Science, Zhejiang University, Hangzhou.
Chen, C. F., Chen, C. W., Dong, C. D., & Kao, C. M. (2013). Assessment of toxicity of polycyclic aromatic hydrocarbons in sediments of Kaohsiung Harbor, Taiwan. Science of the Total Environment, 463, 1174–1181.
De Groot, R., Brander, L., Van Der Ploeg, S., Costanza, R., Bernard, F., Braat, L., et al. (2012). Global estimates of the value of ecosystems and their services in monetary units. Ecosystem Services, 1(1), 50–61.
Deng, W., Li, X. G., Li, S. Y., Ma, Y. Y., & Zhang, D. H. (2013). Source apportionment of polycyclic aromatic hydrocarbons in surface sediment of mud areas in the East China Sea using diagnostic ratios and factor analysis. Marine Pollution Bulletin, 70(1), 266–273.
Dong, T. T. T., & Lee, B. K. (2009). Characteristics, toxicity, and source apportionment of polycylic aromatic hydrocarbons (PAHs) in road dust of Ulsan, Korea. Chemosphere, 74(9), 1245–1253.
Dudhagara, D. R., Rajpara, R. K., Bhatt, J. K., Gosai, H. B., Sachaniya, B. K., & Dave, B. P. (2016). Distribution, sources and ecological risk assessment of PAHs in historically contaminated surface sediments at Bhavnagar coast, Gujarat, India. Environmental Pollution, 213, 338–346.
Duodu, G. O., Ogogo, K. N., Mummullage, S., Harden, F., Goonetilleke, A., & Ayoko, G. A. (2017). Source apportionment and risk assessment of PAHs in Brisbane River sediment, Australia. Ecological Indicators, 73, 784–799.
EPA. (1986). Polynuclear Aromatic Hydrocarbons (EPA Method 8310) [WWW Document]. https://www.epa.gov/sites/production/files/201512/documents/8310.pdf. Accessed 18 July 2017.
EPA. (1996a). Ultrasonic extraction (EPA method 3550b) [WWW document]. URL http://www.caslab.com/EPA-Method-3550B. Accessed 18 July 2017.
EPA. (1996b). Separatory Funnel Liquid-Liquid Extraction (EPA 3510c) [WWW Document]. URL http://www.epa.gov/sites/production/files/2015-12/documents/3510c.pdf. Accessed 18 July 2017.
EPA. (1996c). Silica gel cleanup (EPA method 3630c) [WWW document]. URL http://www.caslab.com/EPA-Method-3630C. Accessed 18 July 2017.
Feng, J., Xi, N., Zhang, F., Zhao, J., Hu, P., & Sun, J. (2016). Distributions and potential sources of polycyclic aromatic hydrocarbons in surface sediments from an emerging industrial city (Xinxiang). Environmental Monitoring and Assessment, 188(1), 61.
Freeman, D. J., & Cattell, F. C. R. (1990). Woodburning as a source of atmospheric polycyclic aromatic hydrocarbons. Environmental Science & Technology, 24(10), 1581–1585.
Fu, J., Sheng, S., Wen, T., Zhang, Z. M., Wang, Q., Hu, Q. X., Li, Q. S., An, S. Q., & Zhu, H. L. (2011). Polycyclic aromatic hydrocarbons in surface sediments of the Jialu River. Ecotoxicology, 20(5), 940–950.
Fuladavand, S., & Sayyad, G. A. (2015). The impact of Karkheh dam construction on reducing the extent of wetlands of Hoor-Alazim. Journal of Water Resources and Ocean Science, 4(2), 33–38.
Gee, G. W., & Bauder, J. W. (1986). Particle-size analysis. In A. Klute (Ed.), Methods of soil analysis: Part 1—Physical and mineralogical methods (pp. 383–411). Madison: Soil Science Society of America, American Society of Agronomy.
Giesy, J. P., Tang, Z., & Zhao, X. (2016). Historical record of effects of human activities on absolute and relative concentrations of polycyclic aromatic hydrocarbons (PAHs) in Lake Chao, China. Journal of Environmental Sciences, 46, 1–4.
Guo, H., Wang, T., Simpson, I. J., Blake, D. R., Yu, X. M., Kwok, Y. H., & Li, Y. S. (2004). Source contributions to ambient VOCs and CO at a rural site in eastern China. Atmospheric Environment, 38(27), 4551–4560.
Guo, W., He, M., Yang, Z., Lin, C., Quan, X., & Men, B. (2009). Distribution, partitioning and sources of polycyclic aromatic hydrocarbons in Daliao River water system in dry season, China. Journal of Hazardous Materials, 164(2–3), 1379–1385.
Hakanson, L., & Jansson, U. M. (1983). Principles of lake sedimentology. In Berlin. New York: Springer.
Harner, T., Bidleman, T. F., Jantunen, L. M. M., & Mackay, D. (2001). Soil—air exchange model of persistent pesticides in the United States cotton belt. Environmental Toxicology and Chemistry, 20(7), 1612–1621.
Heiri, O., Lotter, A. F., & Lemcke, G. (2001). Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. Journal of Paleolimnology, 25(1), 101–110.
Hong, W. J., Jia, H., Li, Y. F., Sun, Y., Liu, X., & Wang, L. (2016). Polycyclic aromatic hydrocarbons (PAHs) and alkylated PAHs in the coastal seawater, surface sediment and oyster from Dalian, Northeast China. Ecotoxicology and Environmental Safety, 128, 11–20.
Hook, D. D. (1993). Wetlands: history, current status, and future. Environmental Toxicology and Chemistry, 12(12), 2157–2166.
Hussain, K., Balachandran, S., & Hoque, R. R. (2015). Sources of polycyclic aromatic hydrocarbons in sediments of the Bharalu River, a tributary of the river Brahmaputra in Guwahati, India. Ecotoxicology and Environmental Safety, 122, 61–67.
IARC. (2018). Agents Classified by the IARC Monographs. 1–122 (september). International Agency for Research on Cancer. France. https://monographs.iarc.fr/agents-classified-by-the-iarc/. Accessed 20 Sep 2018.
Johnson, M. D., Huang, W., & Weber, W. J. (2001). A distributed reactivity model for sorption by soils and sediments. 13. Simulated diagenesis of natural sediment organic matter and its impact on sorption/desorption equilibria. Environmental Science & Technology, 35(8), 1680–1687.
Kadlec, R. H., & Bevis, F. B. (1990). Wetlands and wastewater: Kinross, Michigan. Wetlands, 10(1), 77–92.
Kalf, D. F., Crommentuijn, T., & Van De Plassche, E. J. (1997). Environmental quality objectives for 10 polycyclic aromatic hydrocarbons (PAHs). Ecotoxicology and Environmental Safety, 36(1), 89–97.
Kapsimalis, V., Panagiotopoulos, I. P., Talagani, P., Hatzianestis, I., Kaberi, H., Rousakis, G., Kanellopoulos, T. D., & Hatiris, G. A. (2014). Organic contamination of surface sediments in the metropolitan coastal zone of Athens, Greece: Sources, degree, and ecological risk. Marine Pollution Bulletin, 80(1), 312–324.
Keddy, P. A., Fraser, L. H., Solomeshch, A. I., Junk, W. J., Campbell, D. R., Arroyo, M. T. K., & Alho, C. J. R. (2009). Wet and wonderful: the world’s largest wetlands are conservation priorities. BioScience, 59(1), 39–51.
Keshavarzi, B., Mokhtarzadeh, Z., Moore, F., Rastegari Mehr, M., Lahijanzadeh, A., Rostami, S., & Kaabi, H. (2015). Heavy metals and polycyclic aromatic hydrocarbons in surface sediments of Karoon River, Khuzestan Province, Iran. Environmental Science and Pollution Research, 22(23), 19077–19092.
Kim, G. B., Maruya, K. A., Lee, R. F., Lee, J. H., Koh, C. H., & Tanabe, S. (1999). Distribution and sources of polycyclic aromatic hydrocarbons in sediments from Kyeonggi Bay, Korea. Marine Pollution Bulletin, 38(1), 7–15.
Kumar, K. S., Sajwan, K. S., Richardson, J. P., & Kannan, K. (2008). Contamination profiles of heavy metals, organochlorine pesticides, polycyclic aromatic hydrocarbons and alkylphenols in sediment and oyster collected from marsh/estuarine Savannah GA, USA. Marine Pollution Bulletin, 56(1), 136–149.
Larsen, R. K., & Baker, J. E. (2003). Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: a comparison of three methods. Environmental Science & Technology, 37(9), 1873–1881.
Leguizamo, M. A. O., Gómez, W. D. F., & Sarmiento, M. C. G. (2017). Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands—a review. Chemosphere, 168, 1230–1247.
Li, J. L., Wang, Y. X., Zhang, C. X., Dong, Y. H., Du, B., & Liao, X. P. (2014). The source apportionment of polycyclic aromatic hydrocarbons (PAHs) in the topsoil in Xiaodian sewage irrigation area, north of China. Ecotoxicology, 23(10), 1943–1950.
Li, C. H., Wong, Y. S., Wang, H. Y., & Tam, N. F. Y. (2015a). Anaerobic biodegradation of PAHs in mangrove sediment with amendment of NaHCO3. Journal of Environmental Sciences, 30, 148–156.
Li, P., Cao, J., Diao, X., Wang, B., Zhou, H., Han, Q., Zheng, P., & Li, Y. (2015b). Spatial distribution, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in surface seawater from Yangpu Bay, China. Marine Pollution Bulletin, 93(1), 53–60.
Liang, Y., Fung, P. K., Tse, M. F., Hong, H. C., & Wong, M. H. (2008). Sources and seasonal variation of PAHs in the sediments of drinking water reservoirs in Hong Kong and the Dongjiang River (China). Environmental Monitoring and Assessment, 146(1–3), 41–50.
Liu, Y., Chen, L., Jianfu, Z., Qinghui, H., Zhiliang, Z., & Hongwen, G. (2008). Distribution and sources of polycyclic aromatic hydrocarbons in surface sediments of rivers and an estuary in Shanghai, China. Environmental Pollution, 154(2), 298–305.
Liu, A., Lang, Y., Xue, L., & Liu, J. (2009a). Ecological risk analysis of polycyclic aromatic hydrocarbons (PAHs) in surface sediments from Laizhou Bay. Environmental Monitoring and Assessment, 159(1), 429–436.
Liu, Y., Chen, L., Huang, Q., Li, W., Tang, Y., & Zhao, J. (2009b). Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Huangpu River, Shanghai, China. Science of the Total Environment, 407(8), 2931–2938.
Liu, X., Jia, H., Wang, L., Qi, H., Ma, W., Hong, W., Guo, J., Yang, M., Sun, Y., & Li, Y. F. (2013). Characterization of polycyclic aromatic hydrocarbons in concurrently monitored surface seawater and sediment along Dalian coast after oil spill. Ecotoxicology and Environmental Safety, 90, 151–156.
Liu, J. L., Zhang, J., Liu, F., & Zhang, L. L. (2014). Polycyclic aromatic hydrocarbons in surface sediment of typical estuaries and the spatial distribution in Haihe river basin. Ecotoxicology, 23(4), 486–494.
Long, E. R. (2006). Calculation and uses of mean sediment quality guideline quotients: a critical review. Environmental Science & Technology, 40(6), 1726–1736.
Long, E. R., & MacDonald, D. D. (1998). Recommended uses of empirically derived, sediment quality guidelines for marine and estuarine ecosystems. Human and Ecological Risk Assessment, 4(5), 1019–1039.
Long, E. R., Macdonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19(1), 81–97.
Ma, C., Ye, S., Lin, T., Ding, X., Yuan, H., & Guo, Z. (2014). Source apportionment of polycyclic aromatic hydrocarbons in soils of wetlands in the Liao River Delta, Northeast China. Marine Pollution Bulletin, 80(1), 160–167.
Malik, A., Verma, P., Singh, A. K., & Singh, K. P. (2011). Distribution of polycyclic aromatic hydrocarbons in water and bed sediments of the Gomti River, India. Environmental Monitoring and Assessment, 172(1–4), 529–545.
McCready, S., Birch, G. F., Long, E. R., Spyrakis, G., & Greely, C. R. (2006). Relationships between toxicity and concentrations of chemical contaminants in sediments from Sydney Harbour, Australia, and vicinity. Environmental Monitoring and Assessment, 120(1), 187–220.
McGrath, J. A., & Di Toro, D. M. (2009). Validation of the target lipid model for toxicity assessment of residual petroleum constituents: monocyclic and polycyclic aromatic hydrocarbons. Environmental Toxicology and Chemistry, 28(6), 1130–1148.
Mirzaei, R., Conroy, J., & Yoxon, P. (2010). Otters in the Hawr al Azim wetland, Iran. Hystrix, the Italian Journal of Mammalogy, 21(1), 83–88.
Mitsch, W. J., & Gosselink, J. G. (2015). Wetlands. Hoboken: John Wiley & Sons.
Montuori, P., Aurino, S., Garzonio, F., Sarnacchiaro, P., Nardone, A., & Triassi, M. (2016). Distribution, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in water and sediments from Tiber River and estuary, Italy. Science of the Total Environment, 566, 1254–1267.
Mostafa, A. R., Wade, T. L., Sweet, S. T., Al-Alimi, A. K. A., & Barakat, A. O. (2009). Distribution and characteristics of polycyclic aromatic hydrocarbons (PAHs) in sediments of Hadhramout coastal area, Gulf of Aden, Yemen. Journal of Marine Systems, 78(1), 1–8.
Nikolaou, A., Kostopoulou, M., Lofrano, G., & Meric, S. (2009). Determination of PAHs in marine sediments: analytical methods and environmental concerns. Global NEST Journal, 11(4), 391–405.
Niu, L., Cai, H., Van Gelder, P., Luo, P., Liu, F., & Yang, Q. (2017). Dynamics of polycyclic aromatic hydrocarbons (PAHs) in water column of Pearl River estuary (China): Seasonal pattern, environmental fate and source implication. Applied Geochemistry, 90, 39–49.
Page, D. S., Brown, J. S., Boehm, P. D., Bence, A. E., & Neff, J. M. (2006). A hierarchical approach measures the aerial extent and concentration levels of PAH-contaminated shoreline sediments at historic industrial sites in Prince William Sound, Alaska. Marine Pollution Bulletin, 52(4), 367–379.
Park, S. S., Kim, Y. J., & Kang, C. H. (2002). Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmospheric Environment, 36(17), 2917–2924.
Pheiffer, W., Quinn, L. P., Bouwman, H., Smit, N. J., & Pieters, R. (2018). Polycyclic aromatic hydrocarbons (PAHs) in sediments from a typical urban impacted river: application of a comprehensive risk assessment. Ecotoxicology, 27(3), 336–351.
Rahmanpoor, S., Ghafourian, H., Hashtroudi, S. M., & Bastami, K. D. (2014). Distribution and sources of polycyclic aromatic hydrocarbons in surface sediments of the Hormuz strait, Persian gulf. Marine Pollution Bulletin, 78(1), 224–229.
Rocher, V., Azimi, S., Moilleron, R., & Chebbo, G. (2004). Hydrocarbons and heavy metals in the different sewer deposits in the “Le Marais” catchment (Paris, France): stocks, distributions and origins. Science of the Total Environment, 323(1), 107–122.
Rockne, K. J., Shor, L. M., Young, L. Y., Taghon, G. L., & Kosson, D. S. (2002). Distributed sequestration and release of PAHs in weathered sediment: the role of sediment structure and organic carbon properties. Environmental Science & Technology, 36(12), 2636–2644.
Roebeling, P., Abrantes, N., Ribeiro, S., & Almeida, P. (2016). Estimating cultural benefits from surface water status improvements in freshwater wetland ecosystems. Science of the Total Environment, 545, 219–226.
Ryan, J., Estefan, G., & Rashid, A. (2007). Soil and plant analysis laboratory manual. Aleppo: International Center for Agricultural Research in the dryland areas (ICARDA).
Salmabai, H., Saeedi, M. (2018). Areal fluctuations monitoring of Al-Azim/Al-Havizeh wetland during the 1986–2017 period, using time-series Landsat data. The 2nd international conference on strategic ideas for architecture urbanism, geography, and the environment, Mashhad, Iran.
Sanger, D. M., Holland, A. F., & Scott, G. I. (1999). Tidal creek and salt marsh sediments in South Carolina coastal estuaries: II. Distribution of organic contaminants. Archives of Environmental Contamination and Toxicology, 37(4), 458–471.
Sarria-Villa, R., Ocampo-Duque, W., Páez, M., & Schuhmacher, M. (2016). Presence of PAHs in water and sediments of the Colombian Cauca River during heavy rain episodes, and implications for risk assessment. Science of the Total Environment, 540, 455–465.
Soclo, H. H., Garrigues, P. H., & Ewald, M. (2000). Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: case studies in Cotonou (Benin) and Aquitaine (France) areas. Marine Pollution Bulletin, 40(5), 387–396.
Soltani, N., Keshavarzi, B., Moore, F., Tavakol, T., Lahijanzadeh, A. R., Jaafarzadeh, N., & Kermani, M. (2015). Ecological and human health hazards of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in road dust of Isfahan metropolis, Iran. Science of the Total Environment, 505, 712–723.
Stogiannidis, E., & Laane, R. (2015). Source characterization of polycyclic aromatic hydrocarbons by using their molecular indices: an overview of possibilities. In D. M. Whitacre (Ed.), Reviews of environmental contamination and toxicology (pp. 49–133). Cham: Springer.
Sun, C., Zhang, J., Ma, Q., Chen, Y., & Ju, H. (2017). Polycyclic aromatic hydrocarbons (PAHs) in water and sediment from a river basin: sediment–water partitioning, source identification and environmental health risk assessment. Environmental Geochemistry and Health, 39(1), 63–74.
Tam, N. F. Y., Ke, L., Wang, X. H., & Wong, Y. S. (2001). Contamination of polycyclic aromatic hydrocarbons in surface sediments of mangrove swamps. Environmental Pollution, 114(2), 255–263.
Tobiszewski, M., & Namieśnik, J. (2012). PAH diagnostic ratios for the identification of pollution emission sources. Environmental Pollution, 162, 110–119.
Tongo, I., Ezemonye, L., & Akpeh, K. (2017). Distribution, characterization, and human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in Ovia River, southern Nigeria. Environmental Monitoring and Assessment, 189(6), 247.
UNEP. (2001). The Mesopotamian marshlands: demise of an ecosystem. In H. Partow (Ed.), Early warning and assessment technical report, UNEP/DEWA/TR.01–3 rev. 1. Nairobi: Division of Early Warning and Assessment United Nations Environment Programme Nairobi.
Vane, C. H., Harrison, I., Kim, A. W., Moss-Hayes, V., Vickers, B. P., & Horton, B. P. (2008). Status of organic pollutants in surface sediments of Barnegat bay-little Egg Harbor estuary, New Jersey, USA. Marine Pollution Bulletin, 56(10), 1802–1808.
Viñas, L., Franco, M. A., Soriano, J. A., González, J. J., Pon, J., & Albaigés, J. (2010). Sources and distribution of polycyclic aromatic hydrocarbons in sediments from the Spanish northern continental shelf. Assessment of spatial and temporal trends. Environmental Pollution, 158(5), 1551–1560.
Wang, L., Yang, Z., Niu, J., & Wang, J. (2009). Characterization, ecological risk assessment and source diagnostics of polycyclic aromatic hydrocarbons in water column of the Yellow River Delta, one of the most plenty biodiversity zones in the world. Journal of Hazardous Materials, 169(1), 460–465.
Wang, Z., Liu, Z., Yang, Y., Li, T., & Liu, M. (2012). Distribution of PAHs in tissues of wetland plants and the surrounding sediments in the Chongming wetland, Shanghai, China. Chemosphere, 89(3), 221–227.
Wang, Z., Liu, Z., Xu, K., Mayer, L. M., Zhang, Z., Kolker, A. S., & Wu, W. (2014). Concentrations and sources of polycyclic aromatic hydrocarbons in surface coastal sediments of the northern Gulf of Mexico. Geochemical Transactions, 15(1), 2.
Wang, M., Wang, C., Hu, X., Zhang, H., He, S., & Lv, S. (2015). Distributions and sources of petroleum, aliphatic hydrocarbons and polycyclic aromatic hydrocarbons (PAHs) in surface sediments from Bohai Bay and its adjacent river, China. Marine Pollution Bulletin, 90(1), 88–94.
Wang, M., Wang, C., & Li, Y. (2017). Petroleum hydrocarbons in a water-sediment system from Yellow River estuary and adjacent coastal area, China: distribution pattern, risk assessment and sources. Marine Pollution Bulletin., 122, 139–148.
Xiao, R., Bai, J., Wang, J., Lu, Q., Zhao, Q., Cui, B., & Liu, X. (2014). Polycyclic aromatic hydrocarbons (PAHs) in wetland soils under different land uses in a coastal estuary: toxic levels, sources and relationships with soil organic matter and water-stable aggregates. Chemosphere, 110, 8–16.
Xue, R., Chen, L., Lu, Z., Wang, J., Yang, H., Zhang, J., & Cai, M. (2016). Spatial distribution and source apportionment of PAHs in marine surface sediments of Prydz Bay, East Antarctica. Environmental Pollution, 219, 528–536.
Yan, J., Liu, J., Shi, X., You, X., & Cao, Z. (2016). Polycyclic aromatic hydrocarbons (PAHs) in water from three estuaries of China: distribution, seasonal variations and ecological risk assessment. Marine Pollution Bulletin, 109(1), 471–479.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33(4), 489–515.
Zakaria, M. P., Takada, H., Tsutsumi, S., Ohno, K., Yamada, J., Kouno, E., & Kumata, H. (2002). Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: a widespread input of petrogenic PAHs. Environmental Science & Technology, 36(9), 1907–1918.
Zeng, E. Y., & Vista, C. L. (1997). Organic pollutants in the coastal environment off San Diego, California. 1. Source identification and assessment by compositional indices of polycyclic aromatic hydrocarbons. Environmental Toxicology and Chemistry, 16(2), 179–188.
Zhang, L., Dong, L., Ren, L., Shi, S., Zhou, L., Zhang, T., & Huang, Y. (2012). Concentration and source identification of polycyclic aromatic hydrocarbons and phthalic acid esters in the surface water of the Yangtze River Delta, China. Journal of Environmental Sciences, 24(2), 335–342.
Zhang, Y., Liu, M., Chen, H., & Hou, G. (2014). Source identification of polycyclic aromatic hydrocarbons in different ecological wetland components of the Qinkenpao wetland in Northeast China. Ecotoxicology and Environmental Safety, 102, 160–167.
Zhang, J. D., Wang, Y. S., Cheng, H., Jiang, Z. Y., Sun, C. C., & Wu, M. L. (2015). Distribution and sources of the polycyclic aromatic hydrocarbons in the sediments of the Pearl River estuary, China. Ecotoxicology, 24(7–8), 1643–1649.
Zhu, Y., Yang, L., Yuan, Q., Yan, C., Dong, C., Meng, C., et al. (2014). Airborne particulate polycyclic aromatic hydrocarbon (PAH) pollution in a background site in the North China plain: concentration, size distribution, toxicity and sources. Science of the Total Environment, 466, 357–368.
Acknowledgments
The authors gratefully acknowledge Shiraz University Research Committee and medical geology research center of Shiraz University for supporting this research.
Funding
This work was financially supported by the Khuzestan Environmental Protection Office.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 185 kb)
Rights and permissions
About this article
Cite this article
Sheikh Fakhradini, S., Moore, F., Keshavarzi, B. et al. Polycyclic aromatic hydrocarbons (PAHs) in water and sediment of Hoor Al-Azim wetland, Iran: a focus on source apportionment, environmental risk assessment, and sediment-water partitioning. Environ Monit Assess 191, 233 (2019). https://doi.org/10.1007/s10661-019-7360-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7360-0