Abstract
This study, performed in Çanakkale-Ezine in NW of Turkey, analyzes the physicochemical properties of 37 groundwater wells. These 37 wells were chosen to represent each geological unit in the study area. The main purpose of the study and its contribution to the literature is to produce information about the resources and availability of groundwater by using multivariate statistical methods and lithology. For determination hydrochemical facies of groundwater, Piper trilinear diagram was used. Gibbs diagram was applied for determining the mechanism of groundwater chemistry and diagram showed that the interaction of rock-water is more dominant in the study area. Multivariate statistics were applied to physicochemical properties for identification origins of waters. According to the Piper diagram, 16 of the wells were identified as Ca-HCO3 type, 13 of them as Ca-Cl type, 5 of them as mixed Ca-Mg-Cl type, 2 of them as Na-Cl type, and 1 as Ca-Na-HCO3 type. In the study with the purpose of determining the resources of groundwater, the physicochemical properties of the wells are analyzed with hierarchical cluster (HCA) and non-hierarchical cluster (K-means) methods, and the resources are associated with the lithology based on these methods. A total of 37 wells are divided into five different clusters through the HCA method. Further, for the interpretation of the resources of the groundwater, the facies of the waters on the Piper diagram are evaluated based on the five clusters generated through the HCA method and on the lithology. In the study, the results obtained from the K-means method are not significant and in line with the lithology for the interpretation of the resources of the groundwater. In conclusion, this study with limited dataset reveals that using HCA method is very effective to identify the origins of groundwater and present the association with lithology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In natural resources, water is a medium that can be highly influenced by the adverse effects of its surroundings. Water has been always a matter of importance given its indispensability for the vital and personal needs of humans and its use for plants where necessary.
Clean groundwater resources are intensely used for consumption and irrigation for all climatic conditions in Turkey. The quality of groundwater depends on environmental pollutants and geochemical characteristics and may change due to the conditions occurred to a water source, hydrological events, and human factors (Vasanthavigar et al. 2010). Water suitability for use is linked to the interaction of many features such as pH, alkalinity, carbonates and bicarbonates, soluble salts, hardness, distribution of anions and cations, biological factors with each other (Will and Faust 2005; Taş and Davarcı 2017). Water quality is of vital importance for humans; it is directly related to human health. Low-quality water adversely affects human health and plant growth (Wilcox 1984; WHO 1984; Thilagavathi et al. 2012).
Monitoring of water quality has gained importance (Nag and Das 2014). There are various studies on groundwater in the literature. Singh et al. (2005), Mondal et al. (2005), and Ullah et al. (2009) investigated the effect of industrial pollutants on groundwater. Babiker et al. (2007), Nobre et al. (2007), and Brhane (2016) examined and formed an index for the quality of groundwater in GIS. Özcan et al. (2007), Subramani et al. (2005), and Irenosen et al. (2012) analyzed groundwater in terms of water quality and presented an evaluation for multi-purpose uses. Vasanthavigar et al. (2010), Han et al. (2010), and Gaury et al. (2018) studied the hydrochemical properties of the waters. Dahiya et al. (2007) assessed different types of waters used for consumption through fuzzy synthetic evaluation. Belkhiri et al. (2010), Chen et al. (2010), and Santos et al. (2017) investigated the physical, chemical, and biological properties of waters by means of multivariate statistical methods.
The selection of appropriate statistical methods in scientific studies, particularly in the data sets based on chemical analysis results, is important for obtaining significant results. There are very different statistical methods for the interpretation of water resources. One of these methods is hierarchical cluster analysis (HCA). HCA is a multivariate classification which detects natural groupings (McNeil et al. 2005). HCA has been used in various studies evaluating surface and ground water resources and analyzing water quality (Güler et al. 2002; McNeil et al. 2005; Belkhiri et al. 2010; Ledesma-Ruiz et al. 2015). Another cluster method is K-means cluster analysis (KMC). K-means clustering is used to classify water samples into different hydrochemical groups (Johnson and Wichern 1992; Güler et al. 2002; Sarkar et al. 2007; Woocay and Walton 2008).
In this study, physicochemical properties of 37 groundwater wells were analyzed in Çanakkale-Ezine NW Turkey. This area is specially selected because it contains many different geological units. Thirty-seven wells in the study area were chosen to represent each geological unit. However, there was no choice in some areas. So that, all 37 wells in the study area were evaluated. Water samples collected from depths which vary from 8 to 110 m wells.
The main purpose of this study is to produce information related to groundwater recharge resources and to evaluate the water quality by using multivariate statistical methods and lithology. For this purpose, multivariate statistical methods such as hierarchical clustering (HCA) and non-hierarchical clustering (K-means) were used to identify the origins of the waters from the analysis results. The study also seeks to assess the suitability of the water in these wells for different purposes such as for consumption and irrigation.
Material and methods
Study area
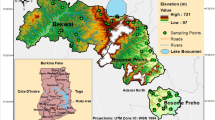
The study area is located between northern latitudes of 39° 54′ 36′′–39° 38′ 42′′ and east longitudes of 26° 10′ 12′′–26° 23′ 42′′ and covers an area of 42,560 ha (Fig. 1). The elevation varies between 0 and 359 m. Soils in the study area are classified according to Soil Taxonomy as Typic Haploustalfs, Typic Ustorthents, Typic Ustifluvents, and Typic Haploustepts. Approximately 1100 ha land is irrigated from sampled wells in the study area. There are no problems the soils of the study are related to irrigation in the current situation. To the west of the study area, there is the Aegean Sea; the city center of Çanakkale is located towards to the north of the study area; and the districts of Ayvacık and Bayramiç are located to the south and the east of the study area, respectively. Ezine is positioned in the transition zone of the Marmara and Aegean Regions, thus, it is also in the transition zone of the climate dominating both regions. The weather is hot and dry in summers, and warm and rainy in winters; precipitation is most often rain and snowfall occurs for a short period of time during the winter months. The coldest months in the region are January and February, with the coldest month average of 3.0 to 3.3 °C. The warmest months are July and August, with the warmest month average of 30.6 to 30.5 °C. In terms of annual precipitation, the months of highest precipitation in the region are November, December, March, and April. The average amount of precipitation is 616.3 mm per year. The months of least precipitation are July and August (TMS 2017).
Geology and hydrogeology
The youngest units in the study area are alluviums formed in the Quaternary period. In the south of the study area, there are granodiorites of the Oligocene epoch. The terrestrial clastics (e.g., conglomerate, sandstone, limestone, and siltstone) formed in the upper Miocene cover a large area in the north and east part of the area. There are andesite, trachandesite, and pyroclastic rocks formed in the lower Miocene in the south-east of Ezine (Fig. 2). There are two rivers in the area named Karamenderes and Akçin. The Karamenderes is born on the northern part of the Ida mountain and passes through the districts of Bayramiç and Ezine, discharge into the Marmara Sea. The River Akçin is born on the southern part of the Ida mountain and passes through the districts of Ayvacık and Ezine, discharge into the Marmara Sea through the harbor Karanlık (Özcan et al. 2007). The irrigation water from the dam of Bayramiç is conveyed to the lands of Ezine through open-system irrigation channels. The irrigation water in the pond Sapanca located on the brook Akçin is conveyed to the south-eastern part of Ezine through a closed irrigation system. Irrigation and domestic waters are also supplied from the wells in the region, in addition to these water resources. All the water resources in the study area are mainly used for irrigation and for livestock farming in order to supply the need for water of animals and, in some locations, for human consumption.
Field studies and laboratory analyses
In the study area, the irrigation practices are intensively performed in July. So samples from the 37 wells were collected in July 2017. The coordinates of each well were recorded with GPS and the land-use type of irrigated fields was noted (Table 1). The pH and EC measurements for the samples were analyzed in situ by using digital pH and EC meters. The samples were stored in a portable refrigerator at 4 °C temperature and brought to the laboratory. Table 2 indicates additional analyses and methods used in the laboratory.
Statistical methods and software
SPSS 20 software program was used for statistical analyses. K-means (Hartigan 1975), hierarchical cluster analysis (Ward Jr. 1963) and regression analysis were done. The evaluation of the data in the Piper (1944) diagram was performed via Water Quality Analyzer (WAQ) software. Gibbs (1970) diagram was used to determine the mechanism of water chemistry.
Results and discussion
Hydrochemical facies of ground waters
The geochemical features of groundwater were determined through the Piper trilinear diagram (Fig. 3). According to the diagram, 16 of the wells in the study area were classified as Ca-HCO3 type, 13 of them as Ca-Cl type, 5 of them as mixed Ca-Mg-Cl type, 2 of them as Na-Cl type, and 1 as Ca-Na-HCO3 type. It was found that alkaline earth metals such as Mg2+ and Ca2+ were more dominant than alkali metals such as Na+ and K+ and that anions HCO3−and Cl− were more dominant compared to SO42− and CO32− in the waters in these wells.
Physicochemical properties of ground waters and their correlation with each other
Table 3 presents the physicochemical properties and some descriptive statistics of the wells. In the study, the EC values varied between 29 μS cm−1 and 2170 μS cm−1, with an average of 460.32 μS cm−1. High EC values were observed generally in the western part of the study area. The pH values of the water samples ranged from 6.68 to 8.84, with an average of 7.68 (Table 3). Analyses indicate that the nature of the groundwater in the region is predominantly slightly alkaline. The abundance of the major ions in groundwater is in the following order: Mg2+ > Ca2+ > Na+ > K+ = HCO3− > Cl− > SO42− > CO32−. In this study for 11 variables, correlation matrices were calculated. The statistical evaluations showed that there were significant positive correlations between Na+ and SAR, between Na+ and EC, between Mg2+ and HCO3−, between Ca2+ and Cl−, between Ca2+ and EC, between Mg2+ and EC, and between Cl− and EC (Table 4).
When the units are successfully merged in different levels, they construct a diagram called “dendrogram” (Dibb 1998). The physicochemical properties of the wells were divided into three major classes in the dendrogram (Fig. 4). Na% and the SAR value were included in the cluster of C1; an increase in sodium percentage affects sodium adsorption rate. Later, Na% and the SAR value were together associated with Na+. The use of sodium in the calculation of these two values explains such association. The parameters such as Ca2+-(Cl−-EC), Mg2+-HCO3−, K+-SO42− were included in C2 cluster. The co-classification of Cl− and EC was an indicator that the salinity in these areas resulted from chlorine anion. Later, these two parameters were associated with calcium. These sections in the study area contained sedimentary rocks with limestone sediments and they were also old sea floors. The class where magnesium and bicarbonate were clustered together was an indicator of bicarbonate occurred in the area through the chemical decomposition of magnesium-rich rocks. The co-classification of potassium and sulfate demonstrated potassium occurred in the areas with hydrothermal alteration. In the cluster of C3, carbonate and pH value were classified together. The presence of carbonate in groundwater in case of high pH value is an expected finding. C1 and C3 were classified together to form a cluster; they were later clustered with the cluster of C2, which was a remotely associated cluster, in the dendrogram.
Cations and anions
Na+ concentration
The analysis of groundwater revealed that the values of the concentration of Na+ ions change between 0 and 4.25 me l−1. The average concentration of Na+ ions was around 1.70 me l−1. The concentration of Na+ is the highest in the western part of the study area. The EC values are also high in these part. There is a positive correlation between Na+ and EC at the level of 0.01 (Table 4). The sodium in the waters particularly resulted from the weathering of Na-feldspar and the dissolution of cement or trapped salt minerals in sedimentary rocks. McNeil et al. (2005) mentioned that the sodium source originated from the weathering of feldspars.
HCO3 − concentration
HCO3− was the most dominant anion in the study area. HCO3− concentration varied between 1 and 10.50 me l−1 with an average of 5.06 me l−1. The north-west and north-east parts of the study area were rich in HCO3− ions (Table 3). The chemical composition of these wells was related to lithology. The geological map showed that the region in the north-west of the study area contained carbonate-rich materials such as limestone. Through the chemical decomposition of carbonated rocks, abundant amounts of HCO3− anions moved into these wells. Peiyue et al. (2011) reported that HCO3− anions are derived from interaction with calcareous units and Sadashivaiah et al. (2008) stated that bicarbonate is derived from magnesium and lime-rich rocks. Wedepohl (1978) expressed that the chemical decomposition of lime-rich sedimentary rocks enriches an environment in terms of bicarbonate. The correlation analysis revealed the positive correlation between Mg2+ and HCO3− at the level of 0.01 (Table 4) and the HCA method demonstrated that Mg2+ remained in the same cluster with HCO3− (Fig. 4). A high content of HCO3− in wells in the north-east of the study area was associated with the volcanic rocks located around. The basalt rocks in the area contained abundant amounts of silicate minerals. Ayalew (2009) emphasized that the hydrolysis of silicate minerals transferred bicarbonate minerals to groundwater. McNeil et al. (2005) also support this idea.
Cl− concentration
Following HCO3−, Cl− ion was the second most dominant anion in the study area. Cl− ion concentration varied between 0.20 and 15.00 me l−1 with an average of 3.60 me l−1. The north-west part of the study area was richer compared to other parts in terms of Cl− ion concentration (Table 3). The chlorine in these wells was originated from sea water, due to their proximity to the Aegean Sea, or the dissolution of trapped salt minerals in the sedimentary rocks. Özcan et al. (2007) and Cloutier et al. (2010) also mentioned that some wells in the study area were affected by the seawater intrusion. Rao (2006) highlighted that chlorine was derived from evaporation, a low-slope land and poor drainage conditions in the semi-arid climate as well as the interaction of water and rock. According to the cluster analysis based on HCA method, Cl− and EC were clustered together, which supports the finding that Cl− was of marine origin.
SO4 2− concentration
The SO42− values of the samples varied between 0 and 9.70 me l−1 with an average of about 2.00 me l−1. West and south-west parts of the study area were generally rich in SO42− anions (Table 3). The presence of SO42− anion in the wells may be explained with seasonal precipitation and SO42− anions could be affected by regional geology and geomorphology as well. There were many active fault and alteration zones in the areas rich in SO42− anions. Some studies Özcan et al. (2007), Cooke et al. (2011), and Bastrakov et al. (2010) reported that the source of SO42− anion might be derived from the thermal waters nearby.
Resources of groundwater
Water chemistry influences from reactions between rocks and groundwater and this give an idea the origin of groundwater (Ledesma-Ruiz et al. 2015). The geochemistry of the study area was not homogenous. Carbonate-rich marine sediments such as limestone and clastic-limestone, igneous rocks such as basalt, granite, andesite, and hydrothermal alteration of rocks containing sulfate were the major constituents of the study area. Gibbs (1970) put forward a diagram which used total dissolved solids (TDS) plotted against Na/(Na + Ca) to estimate whether a water sample is dominated by precipitation, evaporation, or rock type. The Gibbs diagram (Fig. 5) showed that the majority of the samples were within the rock interaction dominance field in the study area.
K-means and HCA methods were used for the interpretation of the resources of groundwater in the study. In classification through HCA method, a total of 16 wells were grouped in the same cluster (C1) on the dendrogram (Fig. 6). The wells numbered 12, 20, 23, 27, 31, 32, 35, and 37 were taken part in the conglomerate, sandstone, limestone, and siltstone facies (Fig. 2). Based on the Piper diagram, the wells numbered 12 and 23 were classified as Na-Cl type. The EC value and the concentrations of Na+ and Cl− ions of the wells numbered 12 and 23, which were located in a depression, were higher than other wells due to environmental recharges as well as the dissolution of the salt minerals in the cementations substances in the young units of marine origin. According to the Piper diagram, the wells numbered 20 and 37 were classified as mixed Ca-Mg-Cl type. The sources of these wells are different facies. Alluvium, conglomerate, sandstone, limestone, and siltstone have a heterogeneous structure and their contents are different. Based on the Piper diagram, the wells numbered 31, 32 and 35 were classified as Ca-Cl type, since they source from calcareous geological units. The well numbered 27 was classified as Ca-HCO3 type, according to the Piper diagram. This well was located within the limestone units. Through the decomposition of these units, the environment was rich in calcium and in bicarbonate.
The wells numbered 24, 28, 29, 33, and 36 were located within granite, granodiorite, and monzonite facies. The wells numbered 24, 28, 33, and 36 were classified as mixed Ca-Mg-Cl type based on the Piper diagram. The sources of calcium and magnesium in these areas were amphibole and mica group minerals. The chlorine ions in this area were related to the granite rocks. McNeil et al. (2005) stated that chlorine is associated with granitic rocks. The well numbered 29 was classified as Ca-Cl type and affected more from monzonitic lithology.
The well numbered 1 was located in andesite, trachyandesite, and pyroclastic rock facies. This well was classified as Ca-Na-HCO3 type based on the Piper diagram, and affected by the decomposition of silicate minerals (albite and anorthite minerals) in andesite. The calcareous minerals, which were a result of the decomposition of the amphiboles rich in ferromagnesium, led to the formation of bicarbonates. Kukillaya and Narayanan (2014) mentioned that the richness of bicarbonate in the waters in their study was due to the amphiboles.
The well numbered 25 was derived from the conglomerate, limestone, and sandstone facies. This well was classified as Ca-Cl type in the Piper diagram and more affected by limestone lithology.
There are 10 wells in the C4 cluster. The catchment area of the wells numbered 4, 5, 6, 13, 17, and 22 contained conglomerate, sandstone, limestone, and siltstone. The wells numbered 4, 6, 13, and 22 were classified as Ca-Cl type based on the Piper diagram. The resource of calcium in these areas, with a sandstone-dominated lithology, was derived from the cementations substances rich in lime. Also, the wells numbered 5 and 17 were classified as Ca-HCO3 type. These wells were enriched in calcium and bicarbonate due to their interaction with limestone units.
The wells numbered 11, 14, 18, and 21 were taken part in granite, granodiorite, monzonite facies. The wells numbered 14 and 21 were classified as Ca-HCO3 type based on the Piper diagram. The presence of Ca2+ and Na+ in groundwater may result from the weathering of feldspar minerals in magmatic rocks (as mentioned Caritat and Saether 1997). The presence of bicarbonate in these wells might be due to the weathering of amphibole and mica minerals in granites. Dedzo et al. (2017) emphasized that the decomposition of amphiboles results in the delivery of abundant amounts of bicarbonates to the environment.
\( {\mathrm{Ca}}_2{\mathrm{Mg}}_5{\mathrm{Si}}_8{O}_{22}{\left(\mathrm{OH}\right)}_2+14{\mathrm{CO}}_2+22{H}_2{O}_2\to {2\mathrm{Ca}}^{2+}+{5\mathrm{Mg}}^{2+}+14{{\mathrm{HCO}}_3}^{-}+8\mathrm{Si}{\left(\mathrm{OH}\right)}_4 \) The decomposition of amphibole (Dedzo et al. 2017)
The wells numbered 11 and 18 were classified as Ca-Cl type due to the chemical decomposition of monzonite rocks.
In C3 cluster, the wells numbered 3 and 9 were located in granite, granodiorite, and monzonite facies. Based on the Piper diagram, the well numbered 3 was classified as Ca-HCO3 type. The wells numbered 3 and 9 were classified as a Ca-HCO3 type because of the decomposition of plagioclases and amphiboles in granites. The wells numbered 15 and 16 were located in the conglomerate, sandstone, limestone, and siltstone facies. These two wells were classified as Ca-HCO3 type in the Piper diagram. The wells numbered 7 and 8 were located in alluviums. They were also classified as Ca-HCO3 type in the Piper diagram. The wells numbered 10 and 26 were affected from the volcanic rocks (basalt) in the surrounding area. Again, these two wells were classified as Ca-HCO3 type in the Piper diagram. These wells were located on a geological unit of an old seabed site with carbonated sedimentary rocks. The chemical composition of the groundwater in this area was affected by the interaction of water and rock.
There was a highly close relationship between the wells numbered 2 and 30 in the cluster C2 (Fig. 6). These wells were clustered together both statistic and dendrogram. According to the Piper diagram, the wells numbered 2 and 30 were classified as Ca-HCO3 type. Geological map and DEM data showed that these two wells were located on alluviums. The presence of HCO3− anions has been reported in the groundwater in alluvial areas (Sharp 1988; Singh et al. 2005). Further, these wells were also affected by the calcareous materials in the conglomerate, sandstone, limestone, siltstone facies in their surrounding area. These two wells were located in flood plains and affected both alluviums and clastic material that is in the high lands (Fig. 2).
The well numbered 19 was grouped alone in the cluster C5 in the dendrogram and very weak associated with the other four groups. It was located at the intersection area of conglomerate, sandstone, limestone, siltstone facies and recrystallized limestone, and conglomerate facies. This well was classified as Ca-HCO3 type. Due to some physicochemical properties such as the very low EC value and the low amounts of anions and cations in the well, it was different from the wells in the other four groups. It assumed that Karamenderes river feeds this well and dilution differentiated physicochemical properties of this well (Everest et al. 2017).
The multidimensional scaling (MDS) test was applied for the wells which classified in the same cluster in HCA. But, the result was not satisfactory due to the complex and heterogeneous structure of facies.
In the study, it was remarkable that no significant correlation was observed between the results obtained from the statistics based on the K-means method and lithology. For that reason, the K-means method was not used. Gong and Richman (1995) and McNeil et al. (2005) reported that the K-means method can be successfully used in a very large set of data. As seen in this study as well, the K-means method does not provide accurate results in case of a small limited set of data.
Recent researches in the literature showed that multivariate statistics are intensively used in groundwater studies (Islam et al. 2018; Voutsis et al. 2015; Ghesquière et al. 2015; Varol and Davraz 2015; Owen and Cox 2015; Okiongbo and Douglas 2015; Tziritis et al. 2016; Moya et al. 2015). For example, in 2015 Okiongbo and Douglas studied with 51 groundwater samples in their study area. In this study, all the hydrochemical parameters were detailed interpreted with the lithology. Using factor analyses with lithology identified the major factors affecting the groundwater. The most important differences and novelty of this study from the previously studies like Okiongbo and Douglas (2015), all wells located on different geological units were classified in five different classes by using HCA. With the Piper diagram, the water types were determined. According to Gibbs diagram, it was known that the wells were dominated within the rock interaction. Lithology played a key role after this section. Lithology was used to identification of the origins of rock interacted wells which were classified five different clusters. With the effective interpretation of these four components (multivariate statistical method, Piper diagram, Gibbs diagram, and lithology), it was seen that this study can produce information about the basins of wells. Owen and Cox (2015) also suggested that multivariate statistics are useful for interpreting groundwater composition in complex structures.
Properties of groundwater in terms of different uses
Evaluation of waters for consumption based on total hardness and WHO values
According to total hardness classification (Table 5) and WHO international standards (Table 6), this study cleaned that it is not suitable to use the wells for human consumption in the study area.
Water quality for irrigation
In the evaluation of water quality for irrigation, the EC, Na%, and SAR values provide important insights into both salinity and hazard of alkalinity. In the study, the SAR and Na% values were calculated according to the following formulas:
In view of the EC values, only one of the wells (well numbered 6) in the study area was classified as doubtful, and other wells were within the acceptable limits for irrigation (Table 7). The Na% values of the groundwater in the study area ranged from 0 to 43% with an average of 16%. Based on Wilcox diagram, all the wells are between excellent-permissible classes for irrigation and also were not hazardous in terms of Na% values (Fig. 7). The SAR values of the groundwater in the area varied between 0 and 2.78 with an average of 0.87. In this sense, there was no hazard of alkalinity. Since the SAR value was classified as S1 in determining the irrigation classes for the groundwater in the study area, all the irrigation classes were classified according to the EC values at various levels. Three of the wells were classified as C1S1; 14 of them as C2S1 and 20 of them as C3S1. All the wells in the north-west part of the study area, except for the 3 wells, were suitable for irrigation in terms of HCO3− according to (FAO 1989). Further, all the wells, apart from the 2 wells, were in the allowable range for irrigation in terms of Cl− (Table 7).
Corrosion coefficient
The corrosion coefficient (K) is a significant parameter to asset the water quality. K is a value indicating the extent to which irrigation systems are affected by corrosion. In this study, the K value was calculated as the following formula:
K = (Cl− + SO42−)/HCO3− (Alkalinity) (Larson and Scold 1958).
The neutral pH with dissolved oxygen ratios equal to or below 0.25 indicates generally resistance to corrosion. On the other hand, higher ratios generally damage water supply systems because they are described as corrosive waters (Štambuk-Giljanović 1999; Özcan et al. 2007). According to the analysis of the corrosion coefficient values of the waters (Fig. 8), only 4 of the wells had a value lower than 0.25; and the other wells had a corrosive effect. The corrosive effect has adverse effects in terms of both economy and water quality.
Permeability index
Permeability index is an index aimed at the determination of the suitability of groundwater for irrigation. Permeability index (PI) was calculated based on the formula below (Ragunath 1987).
The PI values in the study area varied between 19.28 and 73.28%. According to Doneen’s chart (Domenico and Schwartz 1990) and based on their PI values, 3 of the wells were classified as class II while the other wells were classified as class 1 (Fig. 9).
Conclusion
This study was performed with the 37 samples of the groundwater in the city of Çanakkale, north-west of Turkey. In this study, physicochemical properties of the groundwater were analyzed to provide information for evaluating water resources and quality in regard to their usages.
In the literature, classification of facies which is formed through the Piper diagram has been sufficient for the interpretation of the resources of groundwater. In addition to Piper diagram, Gibbs diagram, multivariate statistics, and the lithology of the region were used to evaluate the resources of the groundwater in this study.
According to Piper diagram, 16 wells were identified as Ca-HCO3 type, 13 of them as Ca-Cl type, 5 of them as mixed Ca-Mg-Cl type, 2 of them as Na-Cl type and 1 as Ca-Na-HCO3 type, Gibbs diagram, HCA and K-means statistical methods were applied to test these results. The results of Piper diagram were not supported by hierarchical cluster analyses. The number of wells in each group was not match. Assessment of Gibbs diagram showed that the effect of lithology on water quality was dominant in the area. Evaluation of HCA and K-means statistical analyses results concluded that the K-means method did not provide significant results with lithology due to a limited set of data. On the other hand, hierarchical cluster analysis was very effective to determine and identify the resource of groundwater based on the lithology. In conclusion, the study put forward that the HCA method is very effective to the interpretation of groundwater resources in areas which are dominated by a heterogeneous lithology with a small scale data set, while the K-means method is failing to provide accurate results with a relatively lower number of samples.
As a result of the study, granite, granodiorite, and monzonite facies have a more homogeneous structure. Interpretations of quality and the resources of the wells in these lithological units were easier than the other facies. The interpretation of water resources and their storage areas were found to be quite difficult in sedimentary rocks. We concluded that the type of cementation materials and environmental recharges changed physicochemical properties of the groundwater so determination and evaluation of the resources and qualities of these waters was too difficult. For this reason, five distinct water types have been determined in the sedimentary facies (conglomerate, sandstone, limestone, and siltstone) in the study area. We have observed that groundwater quality in the area is not suitable for drinking based on WHO standards but it is suitable for all agricultural usages.
We have obtained many valuable data related to water resources and quality that can be easily used by policy makers and users after applying the Piper diagram, Gibbs diagram, and multivariate statistics.
References
APHA-AWWA-WPCF 3110. (1992). Standard methods for examination of water and waste water (18th ed.). Washington, DC: APHA-AWWA-WPCF.
Ayalew, E. (2009). Growing lake with growing problems: integrated hydrogeological investigation on Lake Beseka, Ethiopia. (Published doctoral dissertation). University of Bonn, Germany.
Babiker, I. S., Mohamed, M. A., & Hiyama, T. (2007). Assessing groundwater quality using GIS. Water Resources Management, 21(4), 699–715.
Bastrakov, E. N., Jaireth, S., & Mernagh, T.P. (2010). Solubility of uranium in hydrothermal fluids at 25 to 300 C. Geosci Austral Rec, 29.
Belkhiri, L., Boudoukha, A., Mouni, L., & Baouz, T. (2010). Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater. A- case study: Ain Azel plain (Algeria). Geoderma, 159(3–4), 390–398.
Brhane, G. K., (2016) Irrigation Water Quality Index and GIS Approach based Groundwater Quality Assessment and Evaluation for Irrigation Purpose in Ganta Afshum Selected Kebeles, Northern Ethiopia. International journal of Emerging Trends in Science and Technology
Caritat, D. P., & Saether, O.M. (1997). Chemical changes attending water cycling through a catchment—An overview. In Geochemical processes, weathering and groundwater recharge in catchments (pp. 381–391). Balkema Dordrecht, The Netherlands.
Chen, M., Price, R. M., Yamashita, Y., & Jaffé, R. (2010). Comparative study of dissolved organic matter from groundwater and surface water in the Florida coastal Everglades using multi-dimensional spectrofluorometry combined with multivariate statistics. Applied Geochemistry, 25(6), 872–880.
Cloutier, V., Lefebvre, R., Savard, M. M., & Therrien, R. (2010). Desalination of a sedimentary rock aquifer system invaded by Pleistocene Champlain Sea water and processes controlling groundwater geochemistry. Environmental Earth Sciences, 59(5), 977–994.
Cooke, D. R., Deyell, C. L., Waters, P. J., Gonzales, R. I., & Zaw, K. (2011). Evidence for magmatic-hydrothermal fluids and ore-forming processes in epithermal and porphyry deposits of the Baguio district, Philippines. Economic Geology, 106(8), 1399–1424.
Dahiya, S., Singh, B., Gaur, S., Garg, V. K., & Kushwaha, H. S. (2007). Analysis of groundwater quality using fuzzy synthetic evaluation. Journal of Hazardous Materials, 147(3), 938–946.
Dedzo, M. G., Tsozué, D., Mimba, M. E., Teddy, F., Nembungwe, R. M., & Linida, S. (2017). Importance of rocks and their weathering products on groundwater quality in central-East Cameroon. Hydrology, 4(2), 23.
Dibb, S. (1998). Market segmentation: strategies for success, marketing. Intelligence & Planning, 16/7, 394–406.
Domenico, P. A., & Schwartz, F. W. (1990). Physical and chemical hydrogeology (pp. 410–420). New York: Wiley.
Everest, T., Taslı, T. C., Akbulak, C., & Sungur, A. (2017). Ecological risk assessment for protected areas: case of Troia historical national park, Canakkale–Turkey. FEB-Fresenius Environmental Bulletin. (26), 7463-7472.
FAO. (1989). Water quality for agriculture. Food and Agricultural Organization (FAO) of the United Nations. FAO, Irrigation and Drainage Paper 29, Rome.
Gaury, K. P., Meena, K. N., & Mahajan, A. K. (2018). Hydrochemistry and water quality of Rewalsar Lake of Lesser Himalaya, Himachal Pradesh, India. Environmental Monitoring and Assessment, 190, 84.
Ghesquière, O., Walter, J., Chesnaux, R., Rouleau, A.(2015) Scenarios of groundwater chemical evolution in a region of the Canadian Shield based on multivariate statistical analysis. Journal of Hydrology: Regional Studies 4:246-266
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 170, 1088–1090.
Gong, X., & Richman, M. B. (1995). On the application of cluster analysis to growing season precipitation data in North America east of the Rockies. Journal of Climate, 8(4), 897–931.
Güler, C., Thyne, G. D., McCray, J. E., & Turner, K. A. (2002). Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeology Journal, 10(4), 455–474.
Han, D. M., Liang, X., Jin, M. G., Currell, M. J., Song, X. F., & Liu, C. M. (2010). Evaluation of groundwater hydrochemical characteristics and mixing behavior in the Daying and Qicun geothermal systems, Xinzhou Basin. Journal of Volcanology and Geothermal Research, 189(1–2), 92–104.
Hartigan, J. A. (1975). Clustering algorithms.
Irenosen, O. G., Festus, A. A., & Coolborn, A. F. (2012). Water quality assessment of the Owena multi-purpose Dam, Ondo State, southwestern Nigeria. Journal of Environmental Protection, 3(01), 14–25.
Islam, A., R., M., T., Shen, S., Haque, M., A., Bodrud-Doza, Md., Maw, K., W., Habib, Md., A. (2018) Assessing groundwater quality and its sustainability in Joypurhat district of Bangladesh using GIS and multivariate statistical approaches. Environment, Development and Sustainability 20 (5):1935-1959
Johnson, R. A., & Wichern, D. W. (1992). Applied multivariate statistical analysis. Englewood Cliffs, NJ: Prentice Hall.
Kukillaya, J. P., & Narayanan, T. (2014). Role of weathering of ferromagnesian minerals and surface water irrigation in evolving and modifying chemistry of groundwater in Palakkad district, Kerala, with special reference to its fluoride content. Journal of the Geological Society of India, 84(5), 579–589.
Larson, T. S., & Scold, R. W. (1958). Laboratory studies relating mineral quality of water to corrosion of steel and cast iron. Corrosion, 16, 285.
Ledesma-Ruiz, R., Pastén-Zapata, E., Parra, R., Harter, T., & Mahlknecht, J. (2015). Investigation of the geochemical evolution of groundwater under agricultural land: a case study in northeastern Mexico. Journal of Hydrology, 521, 410–423.
McNeil, V. H., Cox, M. E., & Preda, M. (2005). Assessment of chemical water types and their spatial variation using multi-stage cluster analysis, Queensland, Australia. Journal of Hydrology, 310(1–4), 181–200.
Mondal, N. C., Saxena, V. K., & Singh, V. S. (2005). Assessment of groundwater pollution due to tannery industries in and around Dindigul, Tamilnadu, India. Environmental Geology, 48(2), 149–157.
Moya, C., E., Raiber, M., Taulis, M., Cox, M., E. (2015) Hydrochemical evolution and groundwater flow processes in the Galilee and Eromanga basins, Great Artesian Basin, Australia: A multivariate statistical approach. Science of The Total Environment 508:411-426
Nag, S. K., & Das, S. (2014). Quality assessment of groundwater with special emphasis on irrigation and domestic suitability in Suri I & II Blocks, Birbhum District, West Bengal, India. American Journal of Water Resources, 2(4), 81–98.
Nobre, R. C. M., Rotunno Filho, O. C., Mansur, W. J., Nobre, M. M. M., & Cosenza, C. A. N. (2007). Groundwater vulnerability and risk mapping using GIS, modeling and a fuzzy logic tool. Journal of Contaminant Hydrology, 94(3–4), 277–292.
Okiongbo, K. S., Douglas, R. K. (2015) Evaluation of major factors influencing the geochemistry of groundwater using graphical and multivariate statistical methods in Yenagoa city, Southern Nigeria. Applied Water Science 5 (1):27-37
Özcan, H., Ekinci, H., Baba, A., Kavdır, Y., Yüksel, O., & Yiğini, Y. (2007). Assessment of the water quality of Troia for the multipurpose usages. Environmental Monitoring and Assessment, 130(1–3), 389–402.
Owen, D., D. R., Cox, M., E., (2015) Hydrochemical evolution within a large alluvial groundwater resource overlying a shallow coal seam gas reservoir. Science of The Total Environment 523:233-252
Peiyue, L., Hui, Q., & Jianhua, W. U. (2011). Hydrochemical formation mechanisms and quality assessment of groundwater with improved TOPSIS method in Pengyang County Northwest China. Journal of Chemistry, 8(3), 1164–1173.
Piper, A. M. (1944). A graphical procedure in the geochemical interpretation of wateranalysis. Transactions American Geophysical Union, 25, 914–928.
Ragunath, H. M. (1987). Groundwater (p. 563). New Delhi: Wiley Eastern Ltd.
Rao, N. S. (2006). Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environmental Geology, 49(3), 413–429.
Sadashivaiah, C., Ramakrishnaiah, C. R., & Ranganna, G. (2008). Hydrochemical analysis and evaluation of groundwater quality in Tumkur Taluk, Karnataka State, India. International Journal of Environmental Research and Public Health, 5(3), 158–164.
Santos, G. O., Hernandez, F. B., Ferraudo, A. S., Vanzela, L. S., & Santos, D. J. (2017). A study of the impact of land use and occupation on basin water quality through multivariate statistics. Engenharia Agrícola, 37(3), 453–462.
Sarkar, B. C., Mahanta, B. N., Saikia, K., Paul, P. R., & Singh, G. (2007). Geo-environmental quality assessment in Jharia coalfield, India, using multivariate statistics and geographic information system. Environmental Geology, 51(7), 1177–1196.
Sharp, J. M. (1988). Alluvial aquifers along major rivers. Hydrogeology. The Geological Society of North America, Boulder Colorado. 1988. p 273–282. 8 fig, 1 tab, 56 ref.
Singh, K. P., Malik, A., Singh, V. K., Mohan, D., & Sinha, S. (2005). Chemometric analysis of groundwater quality data of alluvial aquifer of Gangetic plain, North India. Analytica Chimica Acta, 550(1), 82–91.
Štambuk-Giljanović, N. (1999). Water quality evaluation by index in Dalmatia. Water Research, 33(16), 3423–3440.
Subramani, T., Elango, L., & Damodarasamy, S. R. (2005). Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environmental Geology, 47(8), 1099–1110.
Taş, İ., & Davarcı, B. (2017). Variation in groundwater quality of Bursa- İnegöl Plain throughout ten years period. Mediterranean Agricultural Sciences, 30(2), 143–149.
Thilagavathi, R., Chidambaram, S., Prasanna, M. V., Thivya, C., & Singaraja, C. (2012). A study on groundwater geochemistry and water quality in layered aquifers system of Pondicherry region, southeast India. Applied Water Science, 2(4), 253–269.
TMS. (2017). Turkey Meteorology Services. Meteorology General Directorate, Meteorological statistics data. www.mgm.gov.tr (Access date: 13.04.2018).
Ullah, R., Malik, R. N., & Qadir, A. (2009). Assessment of groundwater contamination in an industrial city, Sialkot, Pakistan. African Journal of Environmental Science and Technology, 3(12).
Varol, S., Davraz, A., (2015) Evaluation of the groundwater quality with WQI (Water Quality Index) and multivariate analysis: a case study of the Tefenni plain (Burdur/Turkey). Environmental Earth Sciences 73 (4):1725-1744
Vasanthavigar, M., Srinivasamoorthy, K., Vijayaragavan, K., Ganthi, R. R., Chidambaram, S., Anandhan, P., Manivannan, R., & Vasudevan, S. (2010). Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environmental Monitoring and Assessment, 171(1–4), 595–609.
Voutsis, N., Kelepertzis, E., Tziritis, E., Kelepertsis, A., (2015) Assessing the hydrogeochemistry of groundwaters in ophiolite areas of Euboea Island, Greece, using multivariate statistical methods. Journal of Geochemical Exploration 159:79-92
Ward, J. H., Jr. (1963). Hierarchical grouping to optimise an objective function. Journal of The American Statistical Association, 58, 236244.
Wedepohl, K. H. (1978). Handbook of geochemistry 11, sections 73. Berlin: B-G. Springer-Verlag.
WHO. (1971). International standards for drinking water. Geneva: World Health Organization.
WHO. (1983). Guidelines to drinking water quality. Geneva: World Health Organization.
WHO. (1984). Guidelines for drinking water quality. Geneva: World Health Organization.
Wilcox, L. V. (1984). The quality of water for irrigation uses. US Department of Agricultural Technical Bulletin 1962, Washington.
Will, E., & Faust, J. E. (2005). Irrigation water quality for greenhouse production. Agricultural Extension Service, PB 1617, The University of Tennesse, USA.
Woocay, A., & Walton, J. (2008). Multivariate analyses of water chemistry: surface and ground water interactions. Groundwater, 46(3), 437–449.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Everest, T., Özcan, H. Applying multivariate statistics for identification of groundwater resources and qualities in NW Turkey. Environ Monit Assess 191, 47 (2019). https://doi.org/10.1007/s10661-018-7165-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-7165-6