Abstract
Heavy metal pollution in urban cities is now an accepted fact. An understanding of the natural and anthropogenic contributions to heavy metal accumulation in these cities is necessary to develop strategies to mitigate their impacts, particularly on human health. Here, we used multiple records using geological and biological pollution indicators to assess the extent of pollution in the Colombo Metropolitan Region (CMR), Sri Lanka. Elemental concentrations of Cu, Zn, Ni and Pb were determined in four depositories: surface soil (90 samples), canal sediments and canal water (45 samples each) and vegetation (62 samples). These were mapped using GIS overlapping the road network to identify hotspots of heavy metals. While the surface soil, canal sediments and leaves of trees had higher and different amounts than background levels of heavy metals, canal water had low levels. Our results suggest that anthropogenic activities are the major source of heavy metals in an urban city, and unique natural factors, such as coastal conditions, terrain morphology and climate, combine and influence the distribution of these metals. We discuss the possible remediation of metal pollution and the necessity of a holistic multi-proxy approach to understand urban heavy metal contamination in a rapidly populating area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution in different environments such as soil, water and sediments are increasingly studied in urban areas. However, the benefits of combining them in a multi-proxy study remain poorly explored. Each of these features record pollution in its unique perspective of location, sources and distribution method such that their combination yields a better understanding of the overall pollution in the area than any data set could provide on its own. Therefore, despite promising results from separate studies, it is important to use a multi-proxy system for pollution studies.
Heavy metals have a significant place among other common environmental pollutants due to their toxicity, long residence time, non-biodegradability, irreversible nature of contamination and accumulation in food chains (Malik et al. 2010). Further, heavy metal residues in contaminated habitats can enter the human body either directly or indirectly, resulting in serious health hazards (Zhu et al. 2013). Aquatic ecosystems in highly urbanised areas are considered as the most anthropogenically degraded habitat types on Earth (Lytle and Lytle 2001). Therefore, sediments are an important pollutant carrier, absorber and a possible future source of contamination (Li et al. 2000; Zhu et al. 2013). Thus, most environmental pollution studies use water and sediments, which favourably represent the influence of pollution in a relatively small area. Soil, however, can representatively indicate the effects of pollution in a comparatively larger area which attracts a great deal of attention in large-scale pollution studies (Tume et al. 2008). In contrast, use of higher plants in pollution studies is rare. Although plants uptake many heavy metals as micronutrients (Peralta-Videa et al. 2009); excess accumulation of these show negative effects on plants by inhibiting the growth and decreasing the functions (Cheng 2003).
This study focuses on the Colombo Metropolitan Region (CMR), the commercial capital of Sri Lanka, which is rapidly developing and frequently considered as highly vulnerable to pollution. The CMR is the most industrialised city in Sri Lanka and belongs to the new generation of upcoming urban cities in Asia with similar characteristics of the heavy flux of motor vehicles, a transient population and local authorities heavily challenged by waste disposal. We use four depositories of heavy metals (surface soil, canal water, canal sediment and vegetation) and discuss their significance in urban pollution. The results are interpreted using the multi-proxy analysis for heavy metals in the context of climate parameters unique to the urban CMR. These results would assist policymakers to develop strategies to protect the environment of the CMR and implement measures to keep the urban population safe from health hazards. Further, from a global context, this study would act as a case study for the successful use of multi-proxy approach in pollution studies.
Materials and methods
Study area
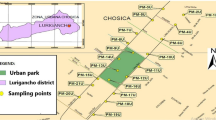
The city of Colombo, located at 6° 54′ N, 79° 52′ E on the west coast of Sri Lanka, has approximately 5.8 million inhabitants with a population density of 3438 persons per square kilometre (Department of Census and Statistics 2011). Due to natural limits to the west (Indian Ocean) and east (extensive marshlands), the city has generally grown in the N–S direction along the national highways (Emmanuel and Johansson 2006) (Fig. 1a). The western coast is mainly a built-up area with government offices while the northwestern region is the centre for rail and passenger transportation. The total number of registered motor vehicles in Sri Lanka was approximately 4 million in 2010 (Department of Census and Statistics 2011) of which, nearly 50% are from the Colombo district. On top of that, CMR experiences a characteristically high traffic flow in and out of the city centre during peak hours of every day.
High humidity (75–95%) and high average annual rainfall (2400 mm) are characteristic climatic conditions of the city (Department of Meteorology 2013). Average annual temperature is 25 °C with a small variation in mean monthly temperatures. Colombo is always open to ocean winds flowing into the city, mainly from the southwestern direction which varies depending on monsoonal conditions and moves across the city towards the central parts of the country. The city canal system occupies a total length of 29.2 km and is fed by a catchment area of approximately 100 km2. Most of the canals are artificial which interconnect major streams and surrounded by natural drainage network in the suburbs and wetlands. Undulated morphology in Colombo reduces the flow rate which results in slowly flowing or stagnant water.
Inland water bodies in urban areas in Sri Lanka are increasingly polluted with contaminants coming from industrial, automobile and domestic waste (Senarathne and Pathiratne 2010); therefore, various water quality parameters have occasionally been measured in selected drainage systems in Colombo (De Alwis et al. 1994; Ranasinghe et al. 2006; Senarathne and Pathiratne 2010). However, no study has been done on the surface soil in Colombo concerning heavy metal pollution. Similarly, there are several previous studies which identify the effects of pollution in the country using mosses and lichens (Gunathilaka et al. 2011; Karunaratne et al. 2010), but no studies were done on higher plants in an urban area on the heavy metal pollution. However, trees have been used to assess heavy metal pollution in other urban areas in China and Europe and to serve as biomonitor (Qing et al. 2015; Sawidis et al. 2011). Therefore, this study also establishes baseline data in the absence of previous studies and identify the heavy metals that are important in future pollution studies within the city.
Sampling strategy and analytical protocol
Ninety surface soil samples were collected from urban areas and 10 from the suburbs considering the intensity of traffic, commercial and industrial activities of sampling sites (Fig. 1b). Soil samples were collected using a stainless steel spade. Forty-five sample locations were selected from the drainage network mainly covering the CMR considering the distances between locations and joining of sub-streams to the main (Fig. 1c). Canal sediment samples were collected using a grab sampler while water samples were collected from the same location, acidified and transferred to the laboratory on the same day. Similarly, 62 plant leaves were collected from 26 locations from five plant species (Terminalia catappa, Polyalthia longifolia, Delonix regia, Peltophorum pterocarpum and Cassia fistula) (Fig. 1d). All these plants are found close to main roads as avenue trees in the busiest areas of the city and are vulnerable to heavy metal pollution.
Laboratory analysis
Three subsamples of soil and sediment were collected from each location and combined to obtain ca 1 kg of sample. All samples were air dried, sorted and sieved and pan fractions were selected for geochemical analysis. The total organic matter content of samples was measured by the loss on ignition method as described in Herath et al. (2016). The second set of samples was acid digested by microwave digestion (Milestone, Italy) according to US EPA 3052. A mixture of reagents (7 ml of 90% HNO3, 2 ml of 75% HClO4 and 1 ml of 78% HF) were added to the digestion mixture (Wei et al. 2010). Concentrations of Cu, Zn, Pb and Ni in the acid digested and water samples were measured by Atomic Absorption Spectrophotometer (AAS, Varian AA240FS, Australia).
Fresh plant materials were separated, cleaned, oven-dried (at 85 °C for 15 h), powdered and used for the preparation of samples for total metal concentration analysis. Acid digestion of 0.5 g of plant samples was done by microwave digestion (CEM-MARS-6) (Oliva and Espinosa 2007) with an acid ratio of 10:1 (HNO3:H2O2). Concentrations of Cu, Zn and Pb in acid-digested samples were measured by AAS. For quality control, reagent blanks, three replicates and standard reference materials were incorporated in all analysis to detect contamination and assess precision and bias. Analytical results showed no signs of contamination and revealed that the precision and bias of the analysis were generally < 10%. The recovery rates for the heavy metals in the international standard reference material were around 75–105%.
Geochemical maps of the CMR were prepared by overlaying GIS maps (Arc Map 10.2.2) of the heavy metal distribution of the sample locations onto the surface soil and road network to identify hotspots of the different heavy metals.
Statistical analysis of pollution assessment
Correlation coefficient and coefficient of variation were calculated for measured elements separately to identify similarities between the source and distribution of the metals. To assess the level of heavy metal pollution in the area, Pollution Index (PI) and Integrated Pollution Index (IPI) were calculated based on methods described by Guo et al. (2012). Pollution Index is described as the ratio between the metal concentration and the background value of the metal while IPI is the mean PI value for considered metals from the area. These values were then classified as low (PI or IPI ≤ 1), moderate (1 < PI ≤ 3 or 1 < IPI ≤ 2) or high (PI > 3 or IPI > 2) contamination. The R statistical software (R for windows 3.3.3) was used for all statistical calculations.
Enrichment factor (EF) for mean metal concentration in soil and sediment were calculated using the following formula:
where X is the metal studied and X/Fe is the ratio of the concentration of element X to iron. Iron was chosen as the element of normalisation because natural sources (98%) vastly dominate its input (Tippie 1984). The crustal abundance data of Wedepohl (1995) were used for all EF values.
The Geoaccumulation Index (Igeo) has been exploited to evaluate the degree of pollution by heavy metals in sediments (Hoque et al. 2011). Igeo can be calculated by the following formula:
where Cn is the concentration of the examined metals, Bn is the geochemical background value of a given metal in the area (data obtained by Dissanayake (1987) were used as the background values) and 1.5 is the background matrix correction factor. The factor 1.5 is used for possible variation in the background due to lithogenic effects. The following classification is given for the index of geoaccumulation by Förstner et al. (1990) and inferences can be drawn accordingly:
-
< 0 Practically unpolluted
-
0–1 Unpolluted to moderately polluted
-
1–2 Moderately polluted
-
2–3 Moderately to strongly polluted
-
3–4 Strongly polluted
-
4–5 Strongly to very strongly polluted
-
> 5 Very strongly polluted
Results
Physicochemical characteristics of surface soil
The surface soil of the study area, in general, was black and of sandy texture. However, some areas were covered with lateritic soil showing yellowish colour. Organic matter content varied broadly within a range of 4.6–19.9% with an average of 10.6%. Similarly, concentrations of Cu, Zn, Pb and Ni also varied widely in the surface soil (Table 1) which is typical for urban soils (Lee et al. 2007). Except for Ni, other element concentrations were high in the northwest and depleted in the east and south (Fig. 2). Interestingly, urban soil samples have characteristically higher concentrations (except Ni) than the suburban soils and lateritic peaty soil. Due to lower concentrations and similarity to the background (suburban) levels, Ni was excluded from further discussion.
Due to lack of official threshold values for heavy metals in Sri Lankan soils, results of this present study were compared with similar studies carried out elsewhere in the world (Table 2). In comparison, it was found that concentrations of Cu and Zn from this study were characteristically high while Pb concentrations were comparable with other countries. Although urbanisation and industrialisation have proceeded rapidly in the CMR in the last five decades, city-based industrial activities are low compared to other larger cities of the world as well as Asia. Therefore, the comparison of heavy metal concentrations in Colombo with other cities indicates that there is a potential threat of metal pollution in the area in the future.
Physical and chemical quality of canal water and sediments
Several water quality parameters were measured in the canal system of the city, which feeds the human-made Beira Lake in the city centre (Fig. 1c). Observed pH values in canal water samples were close to neutral while high pH values (pH = 10.6) were observed only in the Beira Lake. The average temperature was 30 °C which is nearly equal to the daytime temperature of the area. Negative oxidation-reduction potential values were observed at all locations and similar to pH; the lowest values were recorded in the Beira Lake (− 222 mV). Most canal water samples were black, indicating the presence of organic matter in the suspended form which cleared after filtration.
Heavy metal concentrations in canal water were all below the tolerance limits for the discharge of industrial wastewater into inland surface waters in Sri Lanka (Table 3), while in canal sediments concentrations were relatively high. These results indicate that there is a high input of heavy metals into the canal system in Colombo which was not reflected in the canal water analysis. This suggests that the input of heavy metal into the canal system from the surrounding environment is deposited with the suspended particles in the canal beds. Similar to surface soil, heavy metal concentrations in canal sediments were also higher than different types of sediments in other urban environments of the world (Table 4).
Concentration levels in sediments show an increase with the distance from the start of the canal except canal three which experience backwatering from the large-capacity Kelani River and canal four which flows through a marshy land (Fig. 3). Further, concentrations are relatively low in canal six which undergo ocean sediments mixing during high tide.
Elemental contamination of plant leaves
In all sampled tree species, the elemental concentration increased in the order Zn > Pb > Cu (Table 5). T. catappa, which is the most frequently found tree, accumulated the highest level of all elements. Leaves from the same species of vegetation from the suburbs of Colombo with similar geological and climatic conditions indicate lower concentrations than in the urban trees, particularly Zn (Table 5). Highest levels of Zn and Pb were measured in the central bus station where there is a high volume of bus traffic compared to all parts of the country (Fig. 4).
Assessment of heavy metal pollution in the area
All statistical parameters calculated to assess the extent of heavy metal pollution in the CMR are given in Table 6. High correlations between the metals provide information on the similarities in sources and distribution methods of heavy metals. Except for plant samples, others records show a significant positive correlation between Zn and Cu while correlation is also high between Cu and Pb in sediments, suggesting a strong possibility for a common source and/or pathways between these elements.
Further, except in canal water, Pb concentration showed the highest variability among locations while Zn concentrations had a relatively smaller variation. Calculated Pollution Index (PI) and Integrated Pollution Index (IPI) values varied greatly among different environments (Table 6). Samples collected from lateritic soil was used as the background values for the PI calculation of soil, and it indicates that the surface soil in the area is extremely polluted especially with Pb. This was also supported by the high IPI value. Similarly, plant leaves also indicate moderate contamination with heavy metals based on the PI and IPI levels. Contrastingly, canal water indicates low PI and IPI values supporting the earlier findings suggesting low heavy metal pollution in water. Unfortunately, background levels were not available for canal sediment samples to assess their pollution levels. These pollution assessments suggest that the surface soil in the area is heavily polluted by the measured metals which point towards the necessity for immediate remediation methods.
Enrichment Factor (EF) is the extent to which heavy metals exceed pre-anthropogenic concentrations (Birch and Scollen 2003). It is presumed that high EF values indicate an anthropogenic accumulation of heavy metals, mainly from activities such as industrialisation, urbanisation and deposition of domestic wastes (Abrahim and Parker 2008; Mohiuddin et al. 2010). Enrichment factors for mean metal concentration in surface soil and canal sediments are less than 5 except for Cu in sediments (Table 6). This indicates that these toxic metals are moderately enriched in soil and sediments while Cu is significantly enriched in sediments (Barbieri 2016). Similarly, according to the Geoaccumulation Index, Colombo city is moderately polluted in terms of Zn and Cu of surface soils and Zn in sediments while moderately to strongly polluted by Cu in sediments. This reveals that a high amount of contaminants are enriched in the sediments and there is a significant pollution in the studied canal system. Geoaccumulation Index for Pb was not calculated since background Pb concentrations are not available for the region.
Discussion
Sources and accumulation processes of metal pollutants in the area
Statistical analysis, as well as the comparison with other parts of the world, indicated that the heavy metal pollution in urban surface soil from this study is characteristically high. It points to a substantial enrichment of heavy metals in the urban soil, which could be due to long-term accumulation from various sources in the urban environment. In urban cities, the major sources of heavy metal pollutants are natural as well as anthropogenic. As shown by lower concentration values in the suburbs which have similar geological and climatic conditions, natural geochemical sources for heavy metal pollution in the area are negligible. Therefore, pollution from vehicular traffic can be considered as a major source of pollutants since large-scale industries are absent in the CMR (De Alwis et al. 1994). To confirm this, we overlaid geochemical distribution maps of heavy metals on the road and rail network.
Hot spots of Pb were observed in the northwestern part of the study area, which is the centre for rail and passenger transportation of the country with an extensive network of roads and railway yards (Fig. 5a). Interestingly, Pb concentrations were high in the parking areas for motor vehicles than where they are running. This distribution pattern confirms that the major source of Pb in soil is vehicles. Leaded fuel, which was used for vehicles in Sri Lanka until 2005 (Ranasinghe et al. 2006), is a well-documented source for Pb pollution all over the world (Ahmed and Ishiga 2006; de Miguel et al. 1997). Results suggest that although leaded fuel is no longer in use, contaminants continue to remain in the surface soil.
Elevated concentrations of Zn were associated with major highways in the city (Figs. 1a and 5b). High concentrations at road junctions or near major roads indicate the role of vehicles, which add Zn to the environment from wear and tear of tires, especially at junctions by constant braking and halting. Unlike Pb, Zn concentrations were high in areas where vehicles are constantly moving, which suggests that the source of Zn is from moving parts of the vehicles. Other than this, Zn concentration is also high in the built-up area which points towards sources such as electronic parts, paints and plastics which are well known Zn contaminants in the environment (Alloway 1995; Li et al. 2004).
High concentrations of Cu were found north of the international harbour (Colombo harbour) where large amounts of metallurgical parts are used, and large metallic bodies are stored (Fig. 2). The high corrosion rate of these metallurgical parts from exposure to ocean water facilitates the addition of Cu to surface soil. Other than these general trends, several isolated hot spots were found in different geochemical maps. These can be attributed to the point sources associated with these locations. Most elements have low concentration values in the southern and eastern parts of the study area where most residential facilities are concentrated.
Variations in concentration of Cu, Zn and Pb along the canal system indicate a parallel pattern (Fig. 3) which shows that there can be one or more common sources or common pathways for these elements in nature. Further, a significant positive correlation between Zn, Cu and Pb in canal sediments also suggests a strong possibility for a common source between these elements. The cause of heavy metal pollution in the canal water is from untreated industrial effluents and domestic wastewater that are directly discharged into surface water bodies and storm water drainage canals in the urban areas of the CMR (Hettiarachchi et al. 2011; Senarathne and Pathiratne 2010). These waste includes raw sewage, household dust, batteries, disposable household materials, plastics, paints and inks, household pesticides and medicines and can act as major sources of heavy metal pollution in surface water bodies.
All measured elements in plant leaves show an increase in concentration in the northwestern part of the study area coinciding with the findings from the surface soil analysis (Fig. 4). This suggests that the major source of elevated heavy metals measured in plant leaves could be the soil these trees grow on. This is further supported by the lower concentrations in the leaves of the same species growing in the suburban areas of Colombo. The reasons for this accumulation are the long lifetimes of these trees and their continuous exposure to the heavy metals in the soil, which are leached from the soil surface to the roots below.
Although anthropogenic practices dominate the sources of heavy metal pollution, unique natural factors involve strongly in the pollutant distribution in CMR. High wind, humidity and rainfall are common weather phenomena in the CMR which are known distributors of soil pollutants in tropical latitudes. Similarly, slow water movement, circulation current and higher clay content can assist the deposition of pollutants and increase the retention time in sediments in urban flowing water. Further, coastal conditions contribute to high metal corrosion which concurrently increases the release of different types of toxic metals into the environment. In addition, during high tide, ocean water with high sulphate concentration can mix with canal waters and reduce environment sulphate to sulphite. The formed sulphite ion (S2−) will enhance the precipitation of heavy metals to sediment and decrease the concentration of heavy metals in water (Fu and Wang 2011). This was evident by the high concentrations in canal sediments compared to canal water in CMR. However, more comprehensive studies have to be conducted in the future to identify the processes responsible for heavy metal distribution in the CMR, which was not attempted during this study.
Mitigating metal pollution
As suggested above, the Colombo Metropolitan region has a unique set of climatic conditions which facilitates the accumulation and distribution of toxic metal pollutants. In areas where natural climate has a high influence over pollution conditions, the best way to prevent environmental pollution is by minimising the creation of pollutants. There are various conventional methods in practice for purification and removing contaminants in soil, sediment and water which can be costly and non-eco-friendly (Dhote and Dixit 2009). However, the use of terrestrial and aquatic plants has been investigated for the remediation of heavy metal-polluted environments as an eco-friendly practice (Peng et al. 2008). The success of phytoremediation depends on the availability of plant species and the ability of the plant to tolerate and accumulate high concentrations of heavy metals (Rajakaruna et al. 2006).
Although none of the plant species which were analysed in this study is recognised as hyper-accumulators, some, for example, T. catappa was used as an environment-friendly adsorbent for pollutants (Jnr and Vicente 2007). This is confirmed by the high elemental concentrations of Zn and Pb in the leaves of this plant observed in this study. The common occurrence of this tree species as avenue trees in the CMR is useful. However, it is necessary to safely dispose of the leaves falling from the trees since, otherwise, the leaf decay would return the heavy metals to the soil.
Many aquatic plants, both living and dead, are heavy metal accumulators and their use for the removal of metals from wastewater is well known (Keskinkan et al. 2004). Different aquatic plant species growing in the canal systems of the CMR such as Eichhornia crassipes, Salvinia molesta and Hydrilla verticillata are recognised as suitable for phytoextraction processes (Dhir and Kumar 2010; Keskinkan et al. 2004; Xia and Ma 2006). Proper use of these submerged aquatic plants can remove heavy metals from aquatic systems in the CMR such as natural and human-made canals followed by their harvest and safe disposal.
Further, Colombo has many freshwater wetlands (mainly marshes) in its vicinity which have provided water and environmental services for the city and suburbs (Hettiarachchi et al. 2011). Similar natural wetlands were used for centuries as a sink for waste, being capable of assimilating large amounts of environmental contaminants (Sheoran and Sheoran 2006). Such wetlands can be used as an inexpensive system for wastewater treatment in the CMR in the future.
Significance of multi-proxy pollution studies
This study assessed the metal pollutants accumulated in the surface soil, canal water and sediments, and surface vegetation. Results showed that contamination of the canal water was comparatively low (Table 3), indicating non-threatening conditions; however, the canal sediments had a higher concentration. Metals accumulated in sediments can be subsequently released into the overlying water column as a result of either physical disturbance or diagenesis, and the sediments can persist as a source of pollutants long after the cessation of direct discharges (Chen et al. 2007). Similarly, with the variation of the physiochemical characteristics, such as pH, oxidation-reduction potential and temperature, elements in the sediment phase will re-enter the overlying water and become available to living organisms (Liaghati et al. 2004; Morrison et al. 2001). Therefore, it is of critical importance to study the chemical characteristics of sediments even though contamination of water in an aquatic system is relatively low.
The principal problem with high heavy metal concentrations in the surface soil is that biochemical processes can promote the translation of metal ions from a solid phase to the root system in plants (Relić et al. 2010). Plants will then move these non-biodegradable heavy metals up in the food chain, finally adding them to the human diet. Thus, for better application of surface soil pollution data, they have to be combined with vegetation analysis. The present study indicates higher concentrations of heavy metals in the studied plant leaves in polluted surface soils than the unpolluted suburban soils (Table 5 and Fig. 4). This implies that surface soil pollution affects the vegetation in the area and presents the risk of moving heavy metals into the vegetation. More importantly, edible green leaf vegetables cultivated in the area face the same risk from heavy metals which impose a greater health hazard.
Our results also show that sources of heavy metal pollutants in the area are mainly anthropogenic while unique natural weather conditions facilitate the distribution of these contaminants. This indicates that even though the anthropogenic sources of environmental pollutants are similar in all parts of the world, distribution of these pollutants can vary from place to place. When natural and anthropogenic factors interact with each other, it can create complex situations where the behaviour of elements can be misleading. Therefore, in an urban setting, it is of vital importance to study all possible indicators of environmental pollution for a better understanding.
These results suggest that multi-proxy studies can be helpful in verifying pollution from different records, identifying the movements of heavy metals through different phases and assist in interpreting data derived from complex locations. Therefore, it is of vital importance to conduct comprehensive studies involving characteristically different geological and biological recorders of environmental pollution.
Conclusions
High concentrations of heavy metals in the CMR show the influence of urban anthropogenic processes on contamination of the environment. Observed concentration values and patterns of distribution indicate that automobiles are the main source of heavy metals in the surface soil. Further, discharge of domestic and industrial waste into canals is responsible for the addition of heavy metals into canal sediments. The trees accumulated an excess of Zn and Pb in their leaves which are closely related to the surface soil, and it can be assumed that plants uptake elements in excessive amounts when they are exposed to high-pollution conditions.
In addition, the unique natural weather conditions in an urban area can affect the pollution level. Therefore, pollution control in an area has to be designed according to the natural and anthropogenic factors peculiar to the selected area. Further, this study identified the importance of studying different pollution recorders which will collectively yield a better understanding of the overall pollution in the area.
References
Abrahim, G., & Parker, R. (2008). Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environmental Monitoring and Assessment, 136(1–3), 227–238.
Ahmed, F., & Ishiga, H. (2006). Trace metal concentrations in street dusts of Dhaka city, Bangladesh. Atmospheric Environment, 40(21), 3835–3844.
Alloway, B. (1995). Heavy metals in soils. Springer Science & Business Media.
Barbieri, M. (2016). The importance of enrichment factor (EF) and geoaccumulation index (Igeo) to evaluate the soil contamination. Journal of Geology and Geophysics, 5(237), 2.
Birch, G., & Scollen, A. (2003). Heavy metals in road dust, gully pots and parkland soils in a highly urbanised sub-catchment of Port Jackson, Australia. Soil Research, 41(7), 1329–1342.
Chen, C. W., Kao, C. M., Chen, C. F., & Dong, C. D. (2007). Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere, 66(8), 1431–1440.
Cheng, S. (2003). Effects of heavy metals on plants and resistance mechanisms. Environmental Science and Pollution Research, 10(4), 256–264.
Chung, H., & Lee, M. (2006). Metal contamination on, and adjacent to, road pavement at two tunnel sites in Korea.
De Alwis, P., Ariyaratna, S., Azmy, S., & Dassanayaka, N. (1994). Environmental pollution and its impact on fishery management in Lunawa Lagoon. In Sri Lanka/FAO national workshop on development of community-based fishery management, Colombo (Sri Lanka), 3–5 Oct 1994.
de Miguel, E., Llamas, J. F., Chacón, E., Berg, T., Larssen, S., Røyset, O., et al. (1997). Origin and patterns of distribution of trace elements in street dust: unleaded petrol and urban lead. Atmospheric Environment, 31(17), 2733–2740.
Department of Census and Statistics (2011). Available at http://www.statistics.gov.lk/PopHouSat/CPH2011/index.php?fileName=Activities/TentativelistofPublications. Accessed 1 Jan 2018.
Department of Meteorology (2013). Available at http://www.meteo.gov.lk/index.php?option=com_content&view=article&id=13&Itemid=132&lang=en. Accessed 1 Jan 2018.
Dhir, B., & Kumar, R. (2010). Adsorption of heavy metals by Salvinia biomass and agricultural residues.
Dhote, S., & Dixit, S. (2009). Water quality improvement through macrophytes—a review. Environmental Monitoring and Assessment, 152(1–4), 149–153.
Dissanayake C. (1987). Metals in a lateritic peat deposit - a case study from Sri Lanka. Chemical Geology (60), 137−143.
Emmanuel, R., & Johansson, E. (2006). Influence of urban morphology and sea breeze on hot humid microclimate: the case of Colombo, Sri Lanka. Climate Research, 30(3), 189–200.
Förstner, U., Ahlf, W., Calmano, W., & Kersten, M. (1990). Sediment criteria development. In Sediments and environmental geochemistry (pp. 311–338). Springer.
Fu, F., & Wang, Q. (2011). Removal of heavy metal ions from wastewaters: a review. Journal of Environmental Management, 92(3), 407–418.
Gunathilaka, P. A. D. H. N., Ranundeniya, R. M. N. S., Najim, M. M. M., & Seneviratne, S. (2011). A determination of air pollution in Colombo and Kurunegala, Sri Lanka, using energy dispersive X-ray fluorescence spectrometry on Heterodermia speciosa. Turkish Journal of Botany, 35(4), 439–446.
Guo, G., Wu, F., Xie, F., & Zhang, R. (2012). Spatial distribution and pollution assessment of heavy metals in urban soils from southwest China. Journal of Environmental Sciences, 24(3), 410–418.
Herath, D., Pitawala, A., & Gunatilake, J. (2016). Heavy metals in road deposited sediments and road dusts of Colombo Capital, Sri Lanka. Journal of the National Science Foundation of Sri Lanka, 44(2), 193.
Hettiarachchi, M., Anurangi, J., & De Alwis, A. (2011). Characterisation and description of surface water quality in the threatened urban wetlands around the city of Colombo. Journal of Wetlands Ecology, 5, 10–19.
Hoque, R. R., Goswami, K., Kusre, B., & Sarma, K. (2011). Distribution and solid-phase speciation of toxic heavy metals of bed sediments of Bharali tributary of Brahmaputra River. Environmental Monitoring and Assessment, 177(1–4), 457–466.
Jnr, M. H., & Vicente, J. L. (2007). Kinetic study of liquid-phase adsorptive removal of heavy metal ions by almond tree (Terminalia catappa L.) leaves waste. Bulletin of the Chemical Society of Ethiopia, 21(3), 349–362.
Karunaratne, V., Bombuwela, K., Kathirgamanathar, S., & Thadhani, V. M. (2010). Lichens: a chemically important biota. Journal of the National Science Foundation of Sri Lanka, 33(3).
Keskinkan, O., Goksu, M., Basibuyuk, M., & Forster, C. (2004). Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresource Technology, 92(2), 197–200.
Lee, C. S., Li, X.-D., Zhang, G., Li, J., Ding, A.-J., & Wang, T. (2007). Heavy metals and Pb isotopic composition of aerosols in urban and suburban areas of Hong Kong and Guangzhou, South China—evidence of the long-range transport of air contaminants. Atmospheric Environment, 41(2), 432–447.
Li, X., Wai, O. W., Li, Y., Coles, B. J., Ramsey, M. H., & Thornton, I. (2000). Heavy metal distribution in sediment profiles of the Pearl River estuary, South China. Applied Geochemistry, 15(5), 567–581.
Li, X., Lee, S.-L., Wong, S.-C., Shi, W., & Thornton, I. (2004). The study of metal contamination in urban soils of Hong Kong using a GIS-based approach. Environmental Pollution, 129(1), 113–124.
Liaghati, T., Preda, M., & Cox, M. (2004). Heavy metal distribution and controlling factors within coastal plain sediments, Bells Creek catchment, southeast Queensland, Australia. Environment International, 29(7), 935–948.
Liu, X., Wu, J., & Xu, J. (2006). Characterizing the risk assessment of heavy metals and sampling uncertainty analysis in paddy field by geostatistics and GIS. Environmental Pollution, 141(2), 257–264.
Lytle, J. S., & Lytle, T. F. (2001). Use of plants for toxicity assessment of estuarine ecosystems. Environmental Toxicology and Chemistry, 20(1), 68–83.
Malik, R. N., Jadoon, W. A., & Husain, S. Z. (2010). Metal contamination of surface soils of industrial city Sialkot, Pakistan: a multivariate and GIS approach. Environmental Geochemistry and Health, 32(3), 179–191.
Mohiuddin, K., Zakir, H., Otomo, K., Sharmin, S., & Shikazono, N. (2010). Geochemical distribution of trace metal pollutants in water and sediments of downstream of an urban river. International Journal of Environmental Science & Technology, 7(1), 17–28.
Morrison, G., Fatoki, O., Persson, L., & Ekberg, A. (2001). Assessment of the impact of point source pollution from the Keiskammahoek Sewage Treatment Plant on the Keiskamma River-pH, electrical conductivity, oxygen-demanding substance (COD) and nutrients. Water SA, 27(4), 475–480.
Oliva, S. R., & Espinosa, A. F. (2007). Monitoring of heavy metals in topsoils, atmospheric particles and plant leaves to identify possible contamination sources. Microchemical Journal, 86(1), 131–139.
Peng, K., Luo, C., Lou, L., Li, X., & Shen, Z. (2008). Bioaccumulation of heavy metals by the aquatic plants Potamogeton pectinatus L. and Potamogeton malaianus Miq. and their potential use for contamination indicators and in wastewater treatment. Science of the Total Environment, 392(1), 22–29.
Peralta-Videa, J. R., Lopez, M. L., Narayan, M., Saupe, G., & Gardea-Torresdey, J. (2009). The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. The International Journal of Biochemistry & Cell Biology, 41(8), 1665–1677.
Qing, X., Yutong, Z., & Shenggao, L. (2015). Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicology and Environmental Safety, 120, 377–385.
Rajakaruna, N., Tompkins, K. M., & Pavicevic, P. G. (2006). Phytoremediation: an affordable green technology for the clean-up of metal contaminated sites in Sri Lanka. Ceylon Journal of Science (Biological Sciences), 35, 25–39.
Ranasinghe, P. N., Siriwardana, Y. P. S., & Wanasinghe, V. R. (2006). Heavy metal pollution in drainage network of Colombo City and suburbs of Sri Lanka. Chinese Journal of Geochemistry, 25, 84–85.
Relić, D., Đorđević, D., Popović, A., Jadranin, M., & Polić, P. (2010). Fractionation and potential mobility of trace metals in Danube alluvial aquifer within an industrialized zone. Environmental Monitoring and Assessment, 171(1–4), 229–248.
Sawidis, T., Breuste, J., Mitrovic, M., Pavlovic, P., & Tsigaridas, K. (2011). Trees as bioindicator of heavy metal pollution in three European cities. Environmental Pollution, 159(12), 3560–3570.
Senarathne, P., & Pathiratne, K. (2010). Accumulation of heavy metals in a food fish, Mystus gulio inhabiting Bolgoda Lake, Sri Lanka. Sri Lanka Journal of Aquatic Sciences, 12, 61.
Sheoran, A., & Sheoran, V. (2006). Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Minerals Engineering, 19(2), 105–116.
Singh, K. P., Mohan, D., Singh, V. K., & Malik, A. (2005). Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganges, India. Journal of Hydrology, 312(1), 14–27.
Tippie, V. (1984). Environmental characterization of Chesapeake Bay and a framework for action, The Estuary as a filter (pp. 467–487). Orlando: Academic Press 7 fig, 2 tab, 4 ref.
Tume, P., Bech, J., Sepulveda, B., Tume, L., & Bech, J. (2008). Concentrations of heavy metals in urban soils of Talcahuano (Chile): a preliminary study. Environmental Monitoring and Assessment, 140(1–3), 91–98.
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica et Cosmochimica Acta, 59(7), 1217–1232.
Wei, B., Jiang, F., Li, X., & Mu, S. (2010). Heavy metal induced ecological risk in the city of Urumqi, NW China. Environmental Monitoring and Assessment, 160(1–4), 33–45.
Xia, H., & Ma, X. (2006). Phytoremediation of ethion by water hyacinth (Eichhornia crassipes) from water. Bioresource Technology, 97(8), 1050–1054.
Zhu, X., Ji, H., Chen, Y., Qiao, M., & Tang, L. (2013). Assessment and sources of heavy metals in surface sediments of Miyun Reservoir, Beijing. Environmental Monitoring and Assessment, 185(7), 6049–6062.
Acknowledgements
This work was financially supported by the National Research Council, Sri Lanka (Grant No.: NRC-11-142).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herath, D., Pitawala, A., Gunatilake, J. et al. Using multiple methods to assess heavy metal pollution in an urban city. Environ Monit Assess 190, 657 (2018). https://doi.org/10.1007/s10661-018-7016-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-7016-5