Abstract
The groundwater quality assessment for the drinking and irrigation purpose is carried out in the Kandivalasa River Sub Basin covered with khondalitic suite (Garneti ferrous, Sillimanite, Gneiss) of rocks, near Cheepurupalli town of Vizianagaram district, Andhra Pradesh, India. The analysis for the groundwater quality for drinking has shown the slightly alkaline nature and high values of alkalinity in the study area. A very high concentration of total dissolved solids value is observed at one pocket where there has been contamination by many fertilizer industries located nearby the study area. The groundwater is highly affected by the nitrate. Higher fluoride values are obtained at few pockets. Most of the samples in the study area are categorized as very hard category. According to the Piper trilinear diagram, it can be observed that the carbonate hardness and secondary salinity have occupied at major part of study area. From the analysis of sodium adsorption ratio, salinity hazard, sodium percentage, residual sodium carbonate, and Kelly’s ratio, all the groundwater samples except at few locations fell under the category of good to excellent for irrigation. The prepared integrated groundwater quality maps for the drinking purpose and agricultural purposes are indicating that, by and large, the low-lying areas are having poor groundwater quality than the uplands for drinking as well as agricultural needs which means that the groundwater quality of the basin is following the topography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is the primary source for drinking water in many parts of the Kandivalasa River Sub Basin (KRSB) as there is no other source available for drinking water for the people. Since agriculture is the main occupation in the basin and the farmers are mainly depending on groundwater for irrigation, over exploitation of groundwater can affect both quantity and quality of groundwater. The quality of water may be altered due to the chemistry of host rock through which groundwater flow below the earth surface and the total time of residence of the water within the host rock apart from over usage of fertilizers in the farm fields. The present study is focused on the quality of groundwater of Kandivalasa River Sub Basin. The important problem in the KRSB is the groundwater table has started declining continuously in pockets of higher abstraction and there is a need to analyze the groundwater quality of the basin. Deterioration of groundwater quality is a problem in the basin partly due to lowering of water table and partly due to anthropogenic pollution.
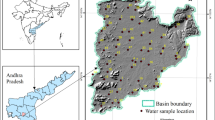
Groundwater quality studies are undertaken in the basin to find out the suitability of groundwater for drinking and irrigation purposes. To analyze the groundwater quality parameters for the said purpose, 42 groundwater samples were collected from the basin. The groundwater samples are tested for determination of major anions namely chloride, fluoride, nitrate, sulfate, and phosphate; the major cations namely sodium, potassium, calcium, and magnesium; and the other parameters including pH, electrical conductivity (EC), alkalinity, total hardness (TH), and total dissolved solids (TDS). The contour maps are prepared from the analyzed data by using Surfer software. For the utilitarian aspect of groundwater for irrigation uses, Piper’s diagram, sodium adsorption ratio (SAR), United States Salinity Laboratory (USSL) diagram, salinity hazard, sodium percentage (Na%), residual sodium carbonate (RSC), magnesium hazard (MH), and Kelly’s ratio (KR) are calculated. The groundwater suitable and unsuitable areas for both drinking and agricultural purposes are identified by preparing the contour maps.
Nordstrom (1987) has stated that the concentration of total dissolved solids determines the use of groundwater for drinking and agricultural purposes. The study of Prasanna et al. (2011) showed that the low chloride concentration in groundwater can be the indication of low surface contamination. According to the study of Karnath (1987), the weathering of silicate minerals from the metamorphic rocks can cause the high concentration of potassium in the groundwater. Lenin and Saseetharan (2008) stated that the sulfate content in the groundwater plays a role in determining the suitability of water for drinking purposes. Suryanarayana (1995) explained that the alkaline nature increases in the groundwater due to the presence of carbonates.
Khodapanah et al. (2009) have stated that the pollution of groundwater depends upon the geochemistry of rocks and soil. Simsek and Gunduz (2007) explained that the reduction of groundwater quality is due to the passage of ions that presented in the groundwater to the soil causing considerable reduction of agricultural yields. Several researchers calculated chemical indices, such as Na%, SAR, RSC, MH, and Kelly’s ratio, to understand the water quality and utilization aspects of groundwater for irrigation (Adhikary et al. 2011; Ramesh and Elango 2012; Vasanthavigar et al. 2012; Al-Taani 2013). However, better results may be obtained by analyzing chemistry of all the anions and cations rather than their individual parameters (Hem 1985). One of the important requirements to undertake irrigation quality assessment is to produce a specialized map to identify areas under pollution threat. Adhikary et al. (2011) have pointed out that the integrated groundwater quality maps are useful for identifying the suitable areas and unsuitable areas for both irrigation and drinking purposes. Irrigation and drinking groundwater quality maps give the valuable information and evaluation of the study area.
Sharma (1982) has observed that in the Eastern Ghat basins of India, a regular increase in the total dissolved salt content with a decrease in elevation. A few pockets of high conductivity zones also observed at lowest elevation where due to absence of movement of groundwater, maximum base exchange takes place in the area. Sharma and Narayanaswamy (1986) have given a hydrochemical approach to the interpretation of groundwater movement in the unconfined condition of the region. They observed that as the topography sloped down along the hill ranges to the plains, the sequence of bicarbonate chloride water is followed by chloride-bicarbonate water again followed by chloride-sulfate water confirming the fact that in general groundwater movement follows the topography.
Subba Rao and Krishna Rao (1991) have concluded that in Visakhapatnam basin, India, wells are undesirable at topographic lows (below 15 m contour line) and desirable at topographic highs (above 15 m contour line) as the former contain water with heavy concentrations of total dissolved solids, total hardness, bicarbonates, chlorides, and sulfates associated with corrosion and incrustation of wells, while the latter is free from them. Venateswara Rao (1995) has studied the cationic and anionic composition of groundwater of Kandivalasa River Sub Basin and concluded that the concentrations of all the studied 11 water quality parameters are within the acceptable levels for drinking water, except chlorides and TDS whose concentrations are marginally more than palatable levels. A comparative study of geoelectric resistivity profiles and TDS profiles across the basin shows that there is a general trend of resistivity increase with decrease in TDS. From irrigation point of view, the groundwater in the basin is found to have a low sodium hazard and medium to high salinity hazard.
Central Ground Water Board (CGWB) has noticed that the nitrate values more than 100 mg/L are at Vijayanagaram, Chipurapalli, Payakapadu, Gharbam, and Kanimetta stations in the Vizianagaram district, Andhra Pradesh, India. They opined that chemical pollution by way of applying nitrogenous fertilizers in the agriculture sector is the root cause of high nitrate content in the groundwater (Central Groundwater Board (CGWB) 2013).
Hydrogeology of the study area
The study area is mostly underlain by Archaean group of rocks consisting of khondalites (Fig. 1). Granites and gneissic granites are exposed at Devada, Nadipannapeta, and Koduru villages in the north-western part of the study area. Garnet biotite gneisses are found at Alakanarayanapuram and Chinnanadipalli villages in eastern parts of the basin and sub-recent formations consisting of laterites also exist in some areas. The hydrogeological map of the study area is presented in Fig. 2. The study area is mainly underlain by khonalites. Due to the action of water, the khondalites are altered in two different manners. On the surface, the rock changes into a lateritic soil and the subsurface formation when acted upon by water alter itself into kaoline. In the khondalitic terrain, two types of aquifers are existed. Weathered khondalite is overlain by the fractured khondalite. Hence, the groundwater occurred in two levels, one in weathered kaolinized zone and other in the fractured and fissured zone. Where the weathered and kaolinized zone consists of sandy clay with sufficiently large permeability, the water table continues into the fractured and fissured zone. But at places where the weathered zone is completely kaolinized and clayey in nature, any well located in it shows water at the bottom of this layer, but when it touches the fractured zone, the water table eventually rises to some extent offering semi-confined conditions (Sharma 1982). The average annual rainfall is around 1000 mm. From the topographic maps and field works carried out in the basin, it has been observed that 80% of the land that is covered in the basin is under agricultural activities. The residual hills occupied the 10% of the land. The settlements covered only 5% of the basin area as they are very small villages. Another 5% of the basin area is under miscellaneous uses such as social forest and small industrial units.
On the average, the maximum fluctuation of water table in the Eastern Ghats seems to be 3 m. The water table rise is observed to be linearly related to the amount of rainfall. About a third of the rainfall percolates through the soil surface to recharge the groundwater (Sharma 1982). The average non-capillary porosity of the surface layer is 33%. The water level fluctuations are found to follow the physiography. It is observed that an increase in terrain elevation by 1 m results in a fluctuation of 0.5 m in the groundwater level. Subba Rao and Krishna Rao (1984) have noted that borewells’ tap groundwater in the aquifer while dug wells penetrate only the overlying aquitard. The digital elevation model of KRSB is presented in Fig. 3. The depth to water levels for the post monsoon season of 2013 above mean sea level (a.m.s.l.) are shown in Fig. 4. The groundwater flow directions are modeled with Surfer 8. The flow directions are indicating that the groundwater flow is towards the main stream which is flowing from North to South direction (Fig. 4) (Siva Prasad and Venkateswara Rao 2018).
Methods
To analyze the groundwater quality parameters for the assessment of drinking and irrigation purposes, 42 groundwater samples during post monsoon season of 2013 were collected from Kandivalasa River Sub Basin (Fig. 1). Depth to water levels in these wells are varying from 1.23 to 12.5 m below ground level. The groundwater samples are collected from the study area in 1000 mL clean plastic bottles. The filled bottles are carefully sealed and labeled. Then the bottles are taken for groundwater quality analysis. The chemical analysis of the groundwater samples, except for fluoride and nitrate, is carried out at the Centre for Environment, Jawaharlal Nehru Technological University Hyderabad using standard methods recommended by American Public Health Association (American Public Health Association (APHA) 1998). The chemical analysis of fluoride and nitrate is carried out at Telangana State Groundwater Department, Hyderabad. The quality of groundwater is compared with IS: 10500 (1993) drinking water standards (Table 1). The results of hydrogeochemical studies are presented in Table 2.
All the collected groundwater samples are from borewells and are tested to determine major anions, cations, total hardness, pH, EC, alkalinity, and TDS. In the present study, Surfer software has been used for generating contour maps of groundwater quality maps such as total dissolved solids, fluoride, nitrate, calcium, and magnesium. Surfer software is a contouring and 3D surface mapping software. Contour maps in the Surfer software can display the contour lines, colors, and patterns between the contour lines.
The pH and EC are measured using Eutech digital portable meters. The instruments are calibrated using the buffer solutions in accordance with the instrument guidelines. The pH scale ranges from 0 to 14 with 7 as neutral, below 7 as acidic, and 7 as alkaline. An upper limit for the EC of 5000 micro-mhos/cm is generally acceptable for drinking water. Hardness of all the groundwater samples is tested by using EDTA-titrimetric method. Estimation of Ca+2 and Mg+2 concentrations in water is made in the laboratory by first measuring the total hardness of water and the calcium hardness of water. The magnesium hardness is the difference of these two hardnesses. Ca+2 and Mg+2 concentrations are related to the respective hardnesses. Chloride (Cl) concentration was measured using argentometric (AgNO3) titration. Carbonate (CO3−) and bicarbonate (HCO3−) are determined by using acid titrimetric method. The fluoride (F−) concentration is estimated by ion-selective electrode method. Concentration of alkali metals like sodium and potassium in groundwater is determined by using the flame photometer. The concentration of sulfate and phosphate ions in the groundwater sample is determined by measuring the absorbance of light energy by the sample in a spectrophotometer after the initial treatment of the sample. All the groundwater samples are tested for total dissolved solids (TDS) in the laboratory using the gravimetric evaporation method. After completion of the analysis of major ions, the ion balance error was calculated and it was within 5%.
Results and discussion
Quality of groundwater for drinking purpose
Total dissolved solids
The salinity in the groundwater occurs due to the total dissolved solids. The distribution of TDS and their variation with time and space in the basin is presented in Fig. 5 for the years of 1993 and 2013 post monsoon seasons. The minimum TDS value of 224 ppm is observed at Chinnanadipally and the maximum TDS of 4716 ppm is observed at Sancham with an average of 833.7 mg/L for post monsoon season of 2013. The average value is higher than permissible limits of BIS standards of 500 mg/L. An unusual TDS value of 4716 mg/L which was absent in the year 1993 but was observed at Sancham village in the year 2013 is due to many fertilizer industries located at Pydibhimavaram of Srikakulam district.
The Bureau of Indian Standards (BIS 1993) described the desirable TDS limit of 500 mg/L and the study area showed 75% of the samples are beyond the desirable limit. The study area is mostly occupied by 500 to 1000 ppm of TDS (58.53%) followed by less than 500 ppm (28.39%) and 1000 ppm to 2000 ppm (14.63%) concentration. From Fig. 5, in the year 2013, the TDS concentration is increasing from North to South in the basin which is indicating that the dissolved minerals, waste water, and landfills by the industrial effluents of many fertilizer industries which are situated at southern region are being seeped into the Kandivalasa River Basin.
In the three TDS profiles across the basin (Fig. 6), it can be observed that TDS concentration is following the same trend for both 1993 and 2013. But at southern part of the study area in the year 2013, high elevated TDS concentration can be observed in Profile-3 and also in the Profile-4 (along the river) (Fig. 6) is partly due to anthropogenic pollution. As studied by Venateswara Rao (1995) in the year 1993, the concentration of TDS in the study area (the TDS profiles of both 1993 and 2013) along the river showed a progressive increase of TDS concentration in the downstream direction that confirmed again the fact of the general belief of identity in the general movement of surface and sub-surface waters.
Fluoride
According to Saralakumari and Rao (1993), the drinking water is the major source for the fluoride in the human body. They also indicated that the fluoride is very much useful for the development of bones and teeth. Fluoride concentration in the basin ranged from 0.36 to 2.34 mg/L (Fig. 7) with an average value of 1.05 mg/L. At six places (14.63% of the total samples), the fluoride values are beyond the acceptable limits of BIS standards (1993) which are not recommended for consumption without treatment (Gopal and Gosh 1985). Higher fluoride value of 2.34 mg/L is observed at Devunipalavala village where the villagers are experiencing health problems, due to consumption of groundwater. Higher concentration of fluoride has effects on the gastrointestinal system, kidneys, liver, and immune system and cause skeletal fluorosis. Fluoride values of less than 0.6 mg/L are found at six places (WS Nos. 10, 21, 26, 28, 38, and 40). At these places, water should be rejected for consumption (BIS: 10500). According to Yadav and Lata (2004), mild dental fluorosis arises due to the consumption of drinking water with fluoride concentration of less than 0.4 mg/L.
Nitrate
In the study area, the nitrate concentration is varying from 3 to 358 mg/L (Fig. 8). The average concentration of 80 mg/L is representing that the groundwater is highly affected by the nitrate in the basin. Twenty-three samples (57.5% of total samples) in the study area are exceeding the limits of BIS standards. Very high nitrate concentrations are found at Velluru (358 mg/L), Boppadam (211 mg/L), Konuru (199 mg/L), and Karkam (196 mg/L) and very low nitrate concentration of 3 mg/L is observed at Girijalapeta. According to Robertson et al. (1991), the nitrate pollution in groundwater occurs mainly due to the wastes of animals and humans, usage of agrochemicals, seepage, and industrial effluents. The high concentrations of nitrates in the study area may be mostly due to effluents of fertilizer industries situated at southern part of the basin and excessive use of fertilizers by the farmers as of most of the people (90%) in the study area are depending on agriculture.
Total hardness
Prakash and Somashekar (2006) explained about the hardness of water. The hardness is mainly due to dissolved calcium and magnesium salts in the groundwater. Hardness is generally defined as the calcium carbonate equivalent of calcium and magnesium ions.
The groundwater in the study area is classified (Table 3) in terms of degree of hardness. According to the classification, 18 samples (42.85% of total samples) are categorized as hard and 24 samples (57.15%) are in the category of very hard. There is no soft and moderately hard classification from the total samples. A very high hardness of 970 mg/L is found at Gurannapeta village.
Drever (1982) has explained that the groundwater contains calcium and magnesium ions mainly due to the dissolution of salts from the host rocks which is responsible for hardness of water. Calcium (Ca+) ion is varied from 16 to 148 mg/L with a mean of 53.56 mg/L. It is observed that six samples namely Karkam (148 mg/L), Koduru (80 mg/L), Gollapalem (96 mg/L), Itakarlapally (92 mg/L), Alajangi (112 mg/L), and Elakalapeta (120 mg/L) were exceeding the maximum desirable limit of 75 mg/L (Table 2). The high concentration of Ca+2 at few places may be due to the mineral dissolution of Ca+2 in groundwater resources as the crystalline limestone associated with khondalitic rocks is the main source of Ca+2 in the study area (Bohlke 2002). According to Satish Kumar et al. (2014), the low concentration of Ca+2 ion is beneficial in reducing the corrosion in pipes and the high concentration of Ca+2 ion is not desirable for domestic usage. However, all the groundwater samples are not exceeding the maximum permissible limit of 200 mg/L for the drinking water standards of BIS (1993).
Magnesium ion concentration is varied from 11 to 189 mg/L with an average of 50.56 mg/L. Very high concentration of 189 mg/L is observed at Gurannapet village. Itakarlapally and Pothayavalasa villages are having the less Mg+2 ion concentration of 11 mg/L. According to BIS (1993), 33 groundwater samples (80.48%) are exceeding the maximum desirable limits of Mg+2.
Kannan and Sabu (2009) explained the high concentration of the hardness in the study area. According to the study, the geology of the study area plays a significant role in contributing to the total hardness. Geologically, the study area comprises of khondalitic suit of rocks which are having the crystalline limestone. The increase of hardness is due to the leaching action of Ca+2 and Mg+2 ions from these rocks.
Chloride
The Central Groundwater Board (CGWB) (2005) explained that the chloride in the groundwater may occur due to the leaching action of chloride containing minerals and rocks with the water, the usage of huge fertilizers for paddy cultivation, surface salinity, and the discharge of irrigational, industrial, and domestic waste waters. Chloride concentration up to 250 mg/L is desirable though it is permissible up to 1000 mg/L for drinking purpose (BIS 1993). In the study area, chloride concentrations are varying from 7 to 464 mg/L. Chloride values more than 250 mg/L are noticed at only four locations namely Sivaram (464 mg/L), Vellure (421 mg/L), Elakalapeta (312 mg/L), and Devunipalavalasa (276 mg/L) and all other water samples are within the desirable limits. According to Prasanna et al. (2011), less chloride concentration in groundwater is indicating the low surface contamination.
Sodium and potassium
Satish Kumar et al. (2014) have stated that the sodium ion is a very important naturally available cation. The concentration of calcium and magnesium ions in fresh waters is generally higher than that of sodium (Na+). In the present study also, the mean value of sodium ion (44.59 mg/L) is comparatively lower than that of calcium and magnesium ions. According to the Minnesota Pollution Control Agency (1999), it is difficult to determine which aquifers might be sensitive to contamination as there are no drinking water standards for sodium or potassium. Maximum permissible limit given by the guidelines of World Health Organization (World Health Organization 1993) for sodium ion is 200 mg/L. According to WHO standards, all the samples are well within the permissible limit.
Concentrations of potassium (K+) normally found in drinking water are generally low. The concentration of potassium shows within the desirable values in entire study area with the averages of 10.0 mg/L except four locations. The villages namely Parla (54.1 mg/L), Alajangi (32.3 mg/L), Pothayavalasa (26.3 mg/L), and Devarapally (15.9 mg/L) where the people are suffering from pain in the stomach registered values above the drinking water standard of 12 mg/L (World Health Organization 1993). Potassium contamination in groundwater can result from the application of inorganic fertilizer at greater than agronomic rates. According to World Health Organization (1993), in the arid and semiarid regions, the reduction of groundwater quality is due to the loss of nutrients such as potassium from the irrigational lands. Padmanaban et al. (2013) explained that generally minerals contain potassium and through weathering process, the potassium gets dissolved in the groundwater. Consumption of high potassium may lead to adverse effects such as disorder of kidneys, less renal function for the older people, diabetes, heart diseases, and hypertension. The infants can be more vulnerable than adults when consumption of K+ is high.
Sulfate and phosphate
Generally, in the natural waters, one of the major anions is the sulfate ion. The sulfate values in all the groundwater samples are in the range from 0 mg/L at Puraivalasa and Sancham to 84 mg/L at Velluru with a mean of 33.44 mg/L. According to the BIS (1993), the permissible limit of the sulfate ion is 150 mg/L. All groundwater samples are fallen within the prescribed limits of BIS.
The phosphate value of all the samples varies in the range of 0.019 at Chinnanadipalli to 0.732 mg/L at Ravivalasa with the mean value of 0.186 mg/L. Since there are no drinking water standards for phosphates in BIS, the permissible limit of the phosphate value in the groundwater is 0.1 mg/L as per WHO standards is taken for the assessment. Rajmohan and Elango (2005) stated that, due to the low solubility of phosphate minerals in the groundwater and less capacity of soil to hold the phosphates, the normal water contains only very low phosphorus values. Out of all the samples, 73% of the samples have slightly higher phosphate values than the permissible value (Table 1).
pH, EC, and alkalinity
pH is a fundamental property that describes the acidity and alkalinity of groundwater. The pH values of the groundwater samples varied from 7.64 to 9.35 indicating that slightly alkaline in nature. All the samples in the entire study area are within the permissible limit of BIS.
Electrical conductivity (EC) is the conducting capacity of water and it can be determined by the presence of dissolved ions. EC of the water samples ranges from 377 to 5040 μS/cm with a mean of 867 μS/cm. All the samples are within the permissible limits except at Sancham. High conductance of more than 1000 μS/cm that was observed in some of the samples may be attributed to high concentration of chloride in groundwaters (Davis and De Wiest 1967) and also due to ion exchange, evaporation, rock water interaction, sulfate reduction, and oxidation process (Ramesh 2008).
Alkalinity in water is due to dissolved carbon dioxide, carbonate, and bicarbonates. The alkalinity varies from 155 at Chinnanadipalli to 1035 at Gurannapet. Higher alkalinity gives unpleasant taste to water. According to World Health Organization (1993), the permissible limit of alkalinity is 120 mg/L and all the samples of the basin are exhibiting the higher alkalinity values. Soman (1977) has explained that, due to the dissolution of crystalline limestone in the khonalitic terrain and also due to the action of the carbonates on the basic material of the soil, the high values of alkalinity in the study area are occurring. According to the National Academy of Science (National Academy of Science (NAS) 1974), the high alkalinity values are also due to the presence of phosphates and nitrates in the water.
Suitability of groundwater for irrigation
The crop productivity reduces when the excess amount of dissolved constituents like carbonate, bi-carbonate, and sodium are present in the groundwater as these constituents affect soil and plants.
Piper’s classification
Piper has given a modified form of the trilinear diagram for the representation of chemical quality data of groundwater. This is considered to be an effective tool for getting an idea of the possible source of the dissolved constituents in the groundwater passing through different geological formations and related geochemical problems (Piper 1953).
As shown in Fig. 9, Piper’s diagram consists of two lower triangular fields, one for cations and another for anions. Plotting in these two fields is done based on the constituent ionic concentrations of the groundwater sample. According to the Piper (1953), groundwater of a particular geochemical character is associated with a unique plotting position in the central diamond-shaped field. The five most important sub-areas of the field, numbers 5, 6, 7, 8, and 9 in Fig. 8, represent the following properties.
-
5: carbonate hardness or secondary alkalinity
-
6: non-carbonate hardness or secondary salinity
-
7: non-carbonate alkali or primary salinity
-
8: carbonate alkali or primary alkalinity
-
9: neutral water
Based on the analysis of the samples, all the 42 observation wells data are plotted on the Piper trilinear diagram (Fig. 10). From Fig. 10, out of 42 samples, 27 samples fell under the area No. 5 which is representing the carbonate hardness. Fifteen samples fell under the area No. 6 belonging to the secondary salinity (non-carbonate hardness) which means that the groundwater is mainly dominated with calcium chloride waters.
Sodium adsorption ratio
The USSL (United States Salinity Laboratory) (1954) recommended the sodium adsorption ratio (SAR) for the suitability of irrigation and it is defined as in Eq. 1.
where all the ionic concentrations are expressed in milliequivalents per liter.
Todd (1959) refers to the importance of the classification of irrigation water because of the fact that sodium reacts with soil and as a result, the permeability of a soil reduces. Soils, high in exchangeable sodium, are considered undesirable for agricultural purposes (Kelly 1940). Wilcox expresses that the extent to which an irrigation water might promote the base exchange from the soil can be estimated on the basis of the sodium percentage and the total salt content. The SAR values during post monsoon season of 2013 obtained for each observation well are presented in Table 4. Todd (1959) classified the groundwater in terms of their use for irrigation according to the ranges of sodium adsorption ratio.
Based on this classification, all the groundwater samples of the study area fall under the category of excellent (Table 4) as far as irrigation purposes are concerned.
United States Salinity Laboratory Diagram
U.S. Salinity Diagram (USSL) denotes the degree to which irrigation water tends to enter into cation-exchange reactions in soil. A graphical classification by the U.S. Salinity Laboratory (Richards 1954) is used for classification of irrigation water in the KRSB. The diagram being widely used for evaluating the groundwater for irrigation on the basis of SAR is published by the USSL (United States Salinity Laboratory) (1954). In this diagram, the sodium adsorption ratio is plotted against the specific electrical conductance. This diagram is divided into 16 areas which are used to rate the degree to which the particular water may give rise to salinity problems and undesirable ion exchange effects (Hem 1985).
The SAR values of all the 42 observation wells are plotted against the corresponding EC values. Figure 11 shows the classification of well waters into various areas of sodium alkali hazard for the post monsoon season of 2013. Out of 42 observation wells, one well is fallen in the area of C1S1. The 20 wells in the area of C2S1 representing the irrigation water have medium salinity/low sodium type. That means, without any salinity control, the groundwater at these locations can be used for irrigation activities. The 18 wells are belonging to C3S1 class indicating that the irrigation water has the high salinity/low sodium type. According to Khodapanah et al. (2009), at these locations, the irrigation water can be detrimental to crops. Based on USSL classification, in the overall context, all the groundwater samples in the study area are suitable for irrigation.
Salinity hazard
According to Ravikumar et al. (2011), the salinity index or hazard which is computed by electrical conductivity (EC) values is the very important groundwater quality criteria for productivity of crops. Simsek and Gunduz (2007) have concluded that the dissolved minerals contribute many salts in the groundwater. The electrical conductance values of less than 750 μS/cm in the groundwater are categorized as excellent to good quality for the agricultural needs (Table 5).
From Table 5, 50% of the groundwater samples (21 groundwater samples) are categorized as class І (low to medium salinity) which are suitable for high salt-tolerant crops (Ravikumar et al. 2011). The 21 samples are classified as class II. From Fig. 12, the southern, north-western, and north-eastern parts of the basin are having the class II of water with permissible quality. Only one sample at the Sancham village belongs to very high class of salinity where the high TDS values are noted.
Sodium percentage
The sodium percentage in the groundwater means the concentration of the sodium in the groundwater. The sodium percentage is useful for classifying the chemical composition of the groundwater. Nishanthiny et al. (2010) have stated that change of the soil structure and reduction of the soil permeability are due to the presence of excess sodium in the groundwater. Ravikumar et al. (2011) have explained that, due to adsorption of clay, the replacement of Mg+2 and Ca+2 takes place in the clay grains with high concentrations of sodium, resulting in the reduction of the permeability of soils. According to Wilcox (1955), the sodium percentage is obtained using Eq. 2.
where all ionic concentrations are represented in milliequivalents per liter.
From Table 6, the Wilcox (1955) classification of groundwater samples indicates that 57.14% of samples (24 samples) are under the category of excellent water quality for irrigation purposes. 28.57% of groundwater samples (12 samples) are under the category of good quality.
According to the sodium percentage of all the samples, the concentration of sodium in the groundwater is excellent to permissible limits and the groundwater quality is suitable for the irrigation.
Residual sodium carbonate
Eaton (1950) has explained that when the high concentration of bicarbonate is present in the groundwater, the tendency for calcium and magnesium to precipitate as carbonate increases. To measure this effect, the indicator namely residual sodium carbonate (RSC) is used. Hence, the bicarbonate hazard is generally described in terms of RSC. RSC is obtained from Eq. 3.
where all ionic concentrations are represented in milliequivalents per liter.
Thirty-five samples in the study area are analyzed for RSC. Out of 35 samples, except one sample which is at Buchannapeta, the remaining samples show the RSC values less than 1.25 (Table 7), indicating that the groundwater is suitable for irrigation.
Magnesium hazard
Ravikumar et al. (2011) have explained that the Mg2+ and Ca2+ ions are very important for growth of plant but high concentrations of Mg2+ in the groundwater affect the soil and crop production. A high concentration of Mg2+ is usually because of the presence of exchangeable Na+ in irrigated soils. For specifying the magnesium hazard (MH) for irrigation water, the following Eq. 4 was suggested by Doneen (1964).
where all ionic concentrations are represented in milliequivalents per liter.
Spatial distribution of MH in percent is shown in Fig. 13. In the KRSB, around 70% of groundwater samples (29 samples) showed MH% is more than 50, representing the adverse effects on crop production (Doneen 1964). The MH ratio of the remaining 13 samples is less than 50% denoting the suitability of groundwater for irrigation.
Kelly’s ratio
The irrigation water quality in terms of composition of Na+, Mg2+, and Ca2+ is classified by Kelly’s ratio (Kelly 1940). The Kelly’s ratio (KR) for irrigation water can be calculated by Eq. 5.
where all ionic concentrations are expressed in milliequivalents per liter.
KR of greater than “1” indicates an excess level of Na+ ion in groundwater which is undesirable for agricultural needs. In the study area, except two locations namely Chinnanadipally and Velpurai, the remaining villages have the KR value less than 1, indicating that the groundwater is suitable for irrigation.
Preparation of groundwater quality maps
The integrated groundwater quality map (Fig. 14) is prepared for drinking purpose by spatially integrating the grid maps of total dissolved solids, nitrate, total hardness, and chloride in the basin scale. This integrated groundwater quality map is representing the suitable area for drinking and unsuitable area for drinking in the study area. The map is also indicating that, by and large, the low-lying area is having poor groundwater quality for drinking when compared to the uplands.
The irrigation groundwater quality map (Fig. 15) for representing the suitability and unsuitability of the irrigation water in the study area is prepared by integrating the thematic maps of EC, sodium percentage, magnesium hazard, and Kelly’s ratio. Figure 14 shows that the irrigation groundwater has poor quality in the most of the southern, north-western, and north-eastern parts of the study area. Suitable irrigation groundwater quality has been found in the remaining part of the basin. It is also observed that the irrigation groundwater quality map is following the topography of the basin (Fig. 3), which means that, irrigation water quality is reducing from the higher elevated areas to the lower elevated areas.
Conclusions
The groundwater quality assessment of Kandivalasa River Sub Basin for the drinking purpose has shown the slightly alkaline nature and the high values of alkalinity in the study area. The TDS profiles of both 1993 and 2013 along the river in the study area showed a progressive increase of TDS concentration in the downstream direction. A very high concentration of TDS is observed near the fertilizer industries and 75% of the samples in the study area were exceeding permissible limit of TDS. The groundwater in the study area is highly affected by the nitrate. It is found that most of the groundwater in the study area belong to hard category. Less chloride concentration in groundwater is indicating the evidence of low surface contamination in the study area. According to the Piper trilinear diagram, it can be observed that the carbonate hardness (secondary alkalinity) and secondary salinity have occupied at major parts of study area. According to the sodium percentage, most of the water samples are belonging to the excellent to good groundwater quality for the agricultural needs. Spatial distribution of magnesium hazard is representing that around 70% of groundwater samples indicated the magnesium ratio of more than 50%. From the analysis of sodium adsorption ratio, salinity hazard, sodium percentage, residual sodium carbonate, and Kelly’s ratio, all the groundwater samples except at few locations are of good to excellent category for irrigation. It is observed by and large that the irrigation groundwater quality map is following the topography of the basin. That means, both groundwater quality and irrigation water quality are reducing from the higher elevated areas to the lower elevated areas.
References
Adhikary, P., Dash, C., Chandrasekharan, H., Rajput, T., Dubey, S., (2011). Evaluation of groundwater quality for irrigation and drinking using GIS and geostatistics in a peri-urban area of Delhi, India. Arabian Journal of Geosciences. https://doi.org/10.1007/s12517-011-0330-7
Al-Taani, A. (2013). Seasonal variations in water quality of Al-Wehda Dam north of Jordan and water suitability for irrigation in summer. Arabian Journal of Geosciences, 6(4), 1131–1140.
American Public Health Association (APHA). (1998). Standard methods for the examination of water and wastewater (20th ed.). Baltimore: United Book Press, Inc..
Bohlke, J. K. (2002). Groundwater recharge and agricultural contamination. Hydrogeology Journal, 10, 153–179.
Central Groundwater Board (CGWB), 2005). District groundwater management studies of Palghat District, Kerala, Technical Report Series Report No.25/KR/CGWB/2004–05.
Bureau of Indian Standards (BIS). (1993). Guidelines for Drinking-water Quality, Indian Standard (IS: 10500), New Delhi, India.
Central Groundwater Board (CGWB), (2013). Groundwater brochure, Vizianagaram district, Andhra Pradesh. A CGWB report, Ministry of Water Resources, New Delhi, India.
Davis, S.N. and De Wiest, R.J.M. (1967). Hydrogeology, 2nd ed. New York, London, Sydney, Wiley. p. 463.
Doneen, L. D. (1964). Notes on water quality in agriculture. Water science and engineering. Davis: University of California.
Drever, J. I. (1982). The geochemistry of natural waters. New Jersey: Prentice-Hall.
Eaton, E. M. (1950). Significance of carbonate in irrigation water. Soil Science, 69, 12–133.
Gopal, R., & Gosh, P. K. (1985). Fluoride in drinking water—its effects and removal. Defence Science Journal, 35(1), 71–88.
Handa, B. K. (1964). Modified classification procedure for rating irrigation waters. Soil Science, 98, 264–269.
Hem, J. D. (1985). Study and interpretation of the chemical characteristics of natural water. USGS water supply paper, 2254, 117–120.
Kannan, N., & Sabu, J. P. (2009). Quality of groundwater in the shallow aquifers of a paddy dominated agricultural river basin, Kerala, India. World Academy of Science, Engineering and Technology, 52, 475–482.
Karnath, K. R. (1987). Groundwater assessment, development and management. New Delhi: Tata McGraw Hill.
Kelly, W.P., 1940. Alkali soils—their formation, properties and reclamation. Reinhold, New York.
Khodapanah, L., Sulaiman, W. N. A., & Khodapanah, N. (2009). Groundwater quality assessment for different purposes in Eshtehard District, Tehran, Iran. European Journal of Scientific Research, 36, 543–553.
Lenin, S. M., & Saseetharan, M. K. (2008). Groundwater quality in Coimbatore, Tamil Nadu along Noyyal River. Journal of Environmental Science and Engineering, 50(3), 187–190.
Minnesota Pollution Control Agency (MPCA), 1999. Effects of Land Use on Ground Water Quality. St. Cloud Area, Minnesota. 1998 Results. St. Paul, MN 46 p.
National Academy of Science (NAS). (1974). Water quality criteria. National Academy of Sciences, 23, 105.
Nishanthiny, C. S., Thushyanthy, M., Barathithasan, T., & Saravanan, S. (2010). Irrigation water quality based on hydro chemical analysis, Jaffna, Sri Lanka. American-Eurasian Journal of Agricultural & Environmental Sciences, 7(1), 100–102.
Nordstrom, P.L., 1987. Groundwater resource of the antlers and Travis peak formations in the outcrop area of north central Texas, Texas Water Development Board, Rep. No. 298, P 280.
Padmanaban, R., Dharmendira Kumar, M., Sakthivel, P. B., & Elangovan, N. S. (2013). A case study on chemical properties of ground water in Madurai District, Tamil Nadu, India. International Journal of Engineering and Advanced Technology, 2(4), 715–718.
Piper, A. M. (1953). A graphic procedure in the geochemical interpretation of water analysis. USGS Ground Water Note, 12, 63.
Prakash, K. L., & Somashekar, R. K. (2006). Groundwater quality—assessment on Anekal Taluk, Bangalore urban district, India. Journal of Environmental Biology, 27(4), 633–637.
Prasanna, M. V., Chidambaram, S., Gireesh, T. V., & Jabir Ali, T. V. (2011). A study on hydrochemical characteristics of surface and sub-surface water in and around Perumal Lake, Cuddalore District, Tamil Nadu. South India. Environ. Earth Sci., 64(5), 1419–1431.
Rajmohan, N., & Elango, L. (2005). Nutrient chemistry of groundwater in an intensively irrigated region of southern India. Journal of Environmental Geology, 47, 820–830.
Ramesh, K., (2008). Hydrochemical studies and effect of irrigation on groundwater quality Tondiar basin Tamil Nadu. Dissertation, Anna University, Chennai, Tamilnadu.
Ramesh, K., & Elango, L. (2012). Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin, Tamil Nadu, India. Environmental Monitoring and Assessment, 184(6), 3887–3899.
Ravikumar, P., Somashekar, R., & Angami, M. (2011). Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgaum District, Karnataka State, India. Environmental Monitoring and Assessment, 173(1), 459–487.
Richards, L.A., (1954). Diagnosis and improvement of saline and alkaline soils. US Department of Agriculture hand book, US Salinity Laboratory, USA.
Robertson, W. D., Cherry, J. A., & Sudicky, E. A. (1991). Groundwater contamination from two small septic systems in sand aquifers. Groundwater, 29(1), 82–91.
Saralakumari, D., Rao, P.R., 1993. Endemic fluorosis in the village Ralla, Anantapuram in Andra Pradesh. An epidemiological study. Fluoride, 26(3), 177–180.
Satish Kumar, V., Amarender, B., Dhakate, R., Sankaran, S., & Raj Kumar, K. (2014). Assessment of groundwater quality for drinking and irrigation use in shallow hard rock aquifer of Pudunagaram, Palakkad District Kerala. Applied Water Science, 6, 149–167.
Sharma V. V. J., (1982). Ground water resources of northern eastern Ghats, Procs. of the Seminar on Resources Development and Environment in the Eastern Ghats, Visakhapatnam, 69–75.
Sharma, V.V.J., Narayanaswamy, A., 1986. Regional groundwater flow investigations in Visakhapatnam Basin, Andhra Pradesh. Journal of Geological Society of India, 27, 386–391.
Simsek, C., & Gunduz, O. (2007). IWQ index: a GIS-integrated technique to assess irrigation water quality. Environmental Monitoring and Assessment, 128(1), 277–300.
Siva Prasad, Y., & Venkateswara Rao, B. (2018). Groundwater depletion and groundwater balance studies of Kandivalasa River Sub Basin, Vizianagaram District, A.P., India. Groundwater for Sustainable Development, 6, 71–78.
Soman, K. (1977). Geology of Kerala. Geological Society of India, 280.
Subba Rao, N., & Krishna Rao, G. (1984). Dug wells and bore wells in hard rock aquifers of Visakhapatnam District, Andhra Pradesh—a comparative study. Association of Exploration Geophysics, 4, 11–18.
Subba Rao, N., & Krishna Rao, G. (1991). Groundwater chemistry for location of wells in Visakapatnam area, Andhra Pradesh. Bhujal News, 10, 7–10.
Suryanarayana, K. (1995). Effect of groundwater quality on health hazards in parts of eastern ghats. Indian Journal of Environmental Protection, 15(7), 497–500.
Todd, D. K. (1959). Groundwater hydrology. New York: Wiley.
USSL (United States Salinity Laboratory) (1954). Diagnosis and improvement of saline and alkali soils. United States Development Agency handbook 60. Government Printing Office, Washington, DC, 147p.
Vasanthavigar, M., Srinivasamoorthy, K., Rajiv Ganthi, R., Vijayaraghavan, K., & Sarma, V. S. (2012). Characterisation and quality assessment of groundwater with a special emphasis on irrigation utility: Thirumanimuttar sub-basin, Tamil Nadu, India. Arabian Journal of Geosciences, 5(2), 245–258.
Venateswara Rao B., 1995. Integrated studies for evaluation of groundwater potential in a typical khondalitic terrain, Dissertation, JNT University Hyderabad, India.
Wilcox, L. V. (1955). Classification and use of irrigation waters. Washington: US Department of Agriculture.
World Health Organization (Ed.). (1993). Guidelines for drinking-water quality recommendations (2nd ed.). Geneva: World Health Organization.
Yadav, J.P., Lata S. 2004. Fluoride levels in drinking water sources in rural areas of block Jhajjar, district Jhajjar, Haryana. Journal of Indian Water Works Association, 131–136.
Acknowledgments
The authors are thankful to the Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India for sponsoring the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prasad, Y.S., Rao, B.V. Monitoring and assessment of groundwater quality in a khondalitic terrain, Andhra Pradesh, India. Environ Monit Assess 190, 426 (2018). https://doi.org/10.1007/s10661-018-6757-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6757-5