Abstract
Oil spill pollution has remained a source of several international litigations in the Niger Delta region of Nigeria. In this paper, we examined the impacts of small recurrent crude oil spills on the physicochemical, microbial and hydrobiological properties of the Nun River, a primary source of drinking water, food and recreational activities for communities in the region. Samples were collected from six sampling points along the stretch of the lower Nun River over a 3-week period. Temperature, pH salinity, turbidity, total suspended solids, total dissolved solids, dissolved oxygen, phosphate, nitrate, heavy metals, BTEX, PAHs and microbial and plankton contents were assessed to ascertain the quality and level of deterioration of the river. The results obtained were compared with the baseline data from studies, national and international standards. The results of the physicochemical parameters indicated a significant deterioration of the river quality due to oil production activities. Turbidity, TDS, TSS, DO, conductivity and heavy metals (Cd, Cr, Cu, Pb, Ni and Zn) were in breach of the national and international limits for drinking water aquatic health. They were also significantly higher than the initial baseline conditions of the river. Also, there were noticeable changes in the phytoplankton, zooplankton and microbial diversities due to oil pollution across the sampling zones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oil spills have the potentials of adversely affecting the coastal and marine habitats, wildlife, fisheries and human activities (Fattal et al. 2010; Allan et al. 2012; Beyer et al. 2016). Crude oil is a highly toxic compound comprising a mixture of up to 10,000 different hydrocarbons which can cause great damage to the aquatic ecosystems (Dawes 1998). Oil spills occur relatively frequently along the Nigerian coastal Niger Delta Region, with an estimated 1.5 million tons of spilled crude oil in the last 50 years (Kadafa 2012; Ordinioha and Brisibe 2013). The Niger Delta has remained a major source of international environmental controversies due to continued environmental disasters caused by oil and gas exploration and production. A United Nations Environment Programme (UNEP 2011) report concluded that oil contamination is wide spread in the area and has severely impacted many components of the environment. However, because of poor management practices and inadequate record keeping, incidences of oil spills and their attendant risks are not well documented within this region. Some of the pollutants introduced into the aquatic system during an oil spill in coastal and marine habitats include polyaromatic hydrocarbons (PAHs), oil and grease (O&G), short-chain alkanes, heavy metals, etc. Heavy metals accumulated in terrestrial and aquatic environments can cause adverse effects on the ecosystem (Bhattacharya et al. 2016; Guo and Yang 2016). Although their adverse health effects have been known for a long time, marine and human exposure to heavy metals is still increasing in some areas (Jarup 2003). Phytoplankton and zooplankton play a significant role in the marine food web dynamics and biogeochemical cycle (Alcaraz et al. 2010; Almeda et al. 2013). The adverse effect of crude oil on these organisms have been extensively studied (Jung et al. 2012; Almeda et al. 2013; Ordinioha and Brisibe 2013).
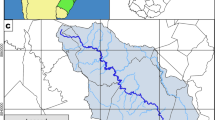
This study evaluated the environmental impacts of spilled crude oil on the aquatic ecosystem of the Nun River in the Niger Delta of Nigeria. The Nun River (Fig. 1) is one of the most important river systems in the Niger Delta providing nursery and breeding grounds for a large variety of fishes (Abowei and Hart 2009) and other wildlife organisms. It is fed by several other smaller rivers and streams within the area. It is a primary source of sustenance for the local communities who depend directly on the river for drinking water, food and sports. The river, like many other river systems in the Niger Delta region, has been plagued with several minor oil spills over several decades of oil and gas exploitation. There are regular small spills in several oil and gas facilities in and around the Nun River. While large oil spills and their effects have been severally documented in the literature, minor and recurrent spills are often not given more attention in literature (Brussaard et al. 2016). This research was therefore designed to assess the impact of small and recurrent crude oil spills on various physicochemical parameters and determine the hydrobiological status of the Nun River following several small spills of an unknown volume of crude oil in 2009. The results were compared with the baseline data previously collected in 2004 and 2008.
Materials and methods
Study area and sample collection
The sampling location was the lower Nun River, which is one of the major river systems of the Niger Delta. The Niger Delta is the most inhabited Delta in the World. It covers 70,000 km2 with a third being wetlands and hosts the largest Mangrove in the World (Nyananyo 1999). The Niger Delta is also the largest river delta in Africa and the third largest in the World. The Nun River is a tributary of the River Niger and was once famous for its serene beauty. It was chosen mainly because of its location in the freshwater swamp forest zone, which is considered as one of the most important ecological zones of the Niger Delta. It is located between latitude 5°011E and 6°17N. The stretch of the river is long with wide meander whose outer concave bank is relatively shallow with minimum and maximum widths of 200 and 250 m, respectively (Sikoki et al. 1998; Abowei and Hart 2009). A systematic sampling pattern (Swaney et al. 1999) was adopted to distribute and locate the sampling zones along a chosen transects. At each of the sampling zone, two sampling points were determined, one each on the left and right sides of the river. The three sampling locations (zones 1, 2 and 3) were distributed downstream of the Nun River Crude Oil Flow Station close to Yenagoa to account for the entire length of the Lower Nun River (Fig. 1), and positioning was carried out using a handheld Global Positioning System. Zone 1 (4°51′00.0″N 6°07′48.0″E) is the upstream location with several upstream petroleum activities including a crude oil flow station and the Nun River/Kolo Creek oil pipeline. There are also several other oil and gas facilities in the upper Nun River north west of Yenagoa. The river flows behind Yenagoa in the south-western end and receives polluted discharges from Epie Creek which runs through the town and has been identified as a major sink for waste generated in the town. (Ezekwe et al. 2013). Zone 2 (4°36′00.0″N 6°04′48.0″E) and zone 3 (4°27′36.4″N 6°03′07.2″E) are the midstream and downstream points, respectively, with less anthropogenic activities and mainly local fishing and recreational activities.
Freshwater samples (S1 to S6, two from each zone) were collected in triplicates from the Nun River over a 3-week period in May 2009 following a small spill of an unknown quantity. The sampling period was dry with precipitation and average daily temperatures displaying no trend along the transect. Samples were collected ∼5 to 10 m from the river banks and about 1 m below the river surface. Samples for physicochemical analyses were collected, prewashed and sealed in 2-L plastic container. Samples for total hydrocarbon measurements were placed in 1-L glass containers, acidified with concentrated hydrochloric acid and sealed with aluminium foil. Samples for the analyses of heavy metals we collected in prewashed 150-ml plastic containers acidified with HNO3. The samples were stored at <4 °C until further analysis. The planktons (phytoplanktons and zooplanktons) were collected using plankton net at ∼0–50 cm depth. The phytoplanktons and zooplanktons samples were then rinsed and transferred into a storage bottle; 5% formalin–sucrose solution was then added to preserve them and stored at about 5 °C.
The baseline data used were collected upstream of the current sampling points. The data from 2004 was part of earlier baseline studies carried out by the same sampling team before an oil pipeline installation in the area in May/June 2004. On the other hand, the June 2008 data was from a previous environmental impact assessment (EIA) studies carried by Shell Petroleum Development Company and was used as a baseline for a recent EIA report (SPDC 2015).
Instrumental analysis
Various analytical methods were employed for the physicochemical parameters, heavy metals, benzene, toluene, ethylbenzene and xylene (BTEX), oil and grease and PAHs. All the chemicals used were of analytical grade and were purchased from Sigma-Aldrich (Germany). The standard mixtures of 16 PAHs in methylene chloride–benzene (1:1, v/v) were purchased from Supelco (Bellefonte, PA, USA). Quality control measures were adopted for the laboratory analyses as stipulated by the Department of Petroleum Resources (DPR) Guidelines and Standards for Petroleum Industry in Nigeria (EGASPIN 2002). To maintain analytical accuracy, duplicate and blank samples were included in the analyses. Distilled water used for analysis conforms to ASTM D 1193 Type 1. For all chemical analyses, the samples were evaluated using an internal quality approach and validated if they satisfied the defined internal quality controls. For each experimental run, a blank, certified reference materials and samples were analysed in triplicate to eliminate any batch-specific error.
Physicochemical parameters
Physicochemical parameters (conductivity, pH and temperature and dissolved oxygen) were measured in situ at each of the sampling locations using a portable meter (WTT pH 315I, Germany). Nitrate was determined using a UV-4 Unicam Spectrophotometer (UK) according to APHA (2005); absorbance was measured at 410 nm. Soluble phosphate was determined according to the ascorbic method, as reported by Murphy and Riley (1962), and measured using colorimetric methods. The suspended matter was determined by filtering a known volume of water through a pre-weighed, dried Whatman glass fibre filter.
Heavy metals
Fifty millilitres of the water samples collected was first digested in a Teflon container by adding 1.0 mL of concentrated hydrochloric acid and shaken. The samples were then allowed to cool to room temperature and then filtered. Heavy metals—arsenic (As), barium (Ba), cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), lead (Pb), mercury (Hg) and nickel (Ni) concentrations—were then determined using a Perkin Elmer 3110 Atomic Absorption Spectrophotometer (Perkin Elmer, UK) as have been previously described by Prat et al. 1999 and APHA 2005. The laboratory standards used were checked with standard reference material.
Analysis of oil and grease, BTEX and Total PAH
Oil and grease were analysed by gravimetric methods, as described in APHA 2005. BTEX and polycyclic aromatic hydrocarbons (PAHs) were determined using a Shimadzu GC-17A gas chromatograph equipped with a split/splitless injector, a flame ionization detector. One microliter volume injection of samples (BTEX) was separated using the following oven programme: initially, temperature at 30 °C for 1 min and then ramped to 100 °C at 10 °C/min, held for 5 min and then to 220 °C at 5 °C/min and held for 15 min. Injector and detector temperatures were set at 180 and 220 °C, respectively. Detailed procedures are described in Moliner-Martinez et al. 2013.
Total PAH was extracted and analysed, as has been previously described in Cam et al. 2000. Sixteen priority PAHs were separated and quantified following this oven programme: initial temperature of 40 °C held for 1 min and then ramped to 160 °C at 25 °C/min and then to 300 °C at 5 °C/min and held for 6 min. The total PAH is represented as the summation of all 16 PAHs (naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenzo[a,h]anthracene, benzo[ghi]perylene and indeno[1,2,3-cd]pyrene).
Microbiology analysis
Microbial enumeration
One millilitre of each sample was transferred into 9 mL of sterile distilled water in the next test tube, and serial dilution was made up to 10−3 dilutions to achieve the desired bacterial density. Pure cultures were then isolated by plating 0.1 mL aliquots of the 10−3 dilution onto the appropriate growth media in the Petri dishes for total heterotrophic bacteria, total coliform bacteria and hydrocarbon-utilizing bacteria respectively. Nutrient agar was used for the analysis of total bacteria count at 28 °C for 24 h in the incubator. Pure bacterial isolates were identified by their cultural, morphological and physiological characteristics in accordance with methods described by Cruickshank et al. (1975), and their identification was based on the Bergey’s Manual of Determinative Bacteriology (Buchanan and Gibbons 2001). MacConkey agar was used for the cultivation of total coliform bacteria and incubated at 37 °C for 48 h, while minimal salt agar was used for the cultivation of hydrocarbon-utilizing bacteria at 30 °C in the incubator for 5 days. The inoculations were duplicated and incubated with corresponding un-inoculated plates as control sample plates. The developed colonies were enumerated as total viable organisms at the end of each incubation period. The spread plate inoculation method was used for all the inoculations with the aid of a sterile glass rod.
Plankton analysis
A total of 0.5–1 mL of the sample was placed on a microscope slide and examined under the light microscope at high magnification (×20). Abundance and evenness as a means of characterizing the diversity of the selected species were carried out using the Shannon diversity index (H). This index incorporates species richness as well as equitability of the community, and it is dependent on sample size (Reiss and Kröncke 2005).
Where H = Shannon’s diversity index.
p i = proportion of each species (S) in each sample (made up to the ith species)
Where EH = equitability (evenness).
S = total number of species in the community (richness).
Equitability assumes a value between 0 and 1, with 1 being complete evenness (Heathcote 2009).
Statistical analysis
Statistical analyses were done by descriptive statistics (e.g. mean, median and standard deviation). Analysis of variance was performed to compare the means, and the statistical significance was considered at p < 0.05.
Results and discussion
Physicochemical characteristic of the Nun River
The result for the physicochemical analyses of the freshwater samples from the Nun River is presented in Table 1. pH values are within the range of 5.2 to 6.9, with an average of 5.75 which is below the limit (6.00–9.00) set by the DPR/Federal Ministry of Environment (FMEnv) for effluent wastewater discharges.
pH of water bodies is temperature dependent (Talling 2010). Water pH is a major factor in all chemical reactions associated with the formation, alteration and dissolution of minerals. It affects transformation processes in metals in crude oil and nutrients as well as influencing the toxicity of contaminants. The pH values are within the range previously reported for freshwater rivers in the Niger Delta (Asonye et al. 2007). Freshwater systems in the Niger Delta region are predominantly acidic (Nyananyo et al. 2007; Ezekiel et al. 2011); consequently, drastic changes in pH will affect all other physicochemical parameters of the water, including the aquatic life (Zhang et al. 1995). The pH of the river in zones 1 and 2 was moderately acidic and outside of the DPR limits of 6.0 to 9.0, although there were within the original baseline data for 2004 and 2008. There was a significant difference (P < 0.05) between the pHs of zones 1 and 2 compared to zone 3. The pH values improved further downstream due mainly to dilution effects and possible lower pollution load. Typical biological activities are limited to pHs 6 to 8, and most aquatic organisms do not tolerate wide variations in water pH. The lower pH in zones 1 and 2 could be attributed to either the presence of heavy metals from the oil spills or mainly from humic acid which resulted from the decomposition of forest materials in the river (Beadle 1974).
The mean temperature values ranged between 27.0 and 28.0 °C. These values reflected an optimal condition for the survival of aquatic life. It is also consistent with temperatures recorded in surface water bodies in the tropics and freshwater in the Niger Delta region (Mulusky 1974; Alabaster and Lloyd 1980; Ezekiel et al. 2011, Agedah et al. 2015).
The turbidity values for S1–5 were relatively high. This could be attributed to the presence of a variety of materials, such as silt, and organic matter resulting from the several activities going on along the river. These organic constituents often serve as feed to micro organisms, while the inorganic constituents stimulate algal growth. Turbidity-induced changes, occurring in freshwater body, may alter the composition of an aquatic community. The turbidity levels for zones 1 and 2 are higher than the national limits (P < 0.05) and have significantly deteriorated compared to the earlier baseline conditions of the river in 2004, although lower than reported 2008 values. It is also higher than the general requirements for drinking water, aesthetics and general aquatic health.
Total suspended solids (TSS) are dependent on the nature and makeup of the underlying sediment. S1–S5 all had significant occurrences of total dissolved solids (TDS) and TSS which in most cases were higher than the DPR limit of 500 and 50 mg/L, respectively. The tidal regime may not have influenced these values along the river. The positive effect of the presence of suspended solids in water is that toxic chemicals such as metals tend to adsorb to them or form complex substances with them, making the toxins less bioavailable to living organisms (Kamath et al. 1991).
The dissolved oxygen (DO) ranged from 2.3 to 8.6. with the lowest DO recorded in zone 1. The values are lower than those reported by Agedah et al. 2015, who reported DO values of 12.1 to 13.3 mg/L for the upper Nun River. The values are, however, similar to other freshwater studies in the area. Seiyaboh et al. 2007 reported DO values of 4.8 to 7.2 mg/L for the Tombia Bridge construction. Aghoghovwia and Ohimain 2014 reported DO values of 5.0 to 7.92 mg/L for the lower Kolo Creek in Beyelsa State. The DO concentration of the Nun River zone 1 was significantly lower (P < 0.05) when compared to zones 2 and 3 (S4–6). This is due to zone 1 being closer to the source of the crude oil spills. The concentration of DO vary daily and seasonally, depending upon the species of phytoplanktons present, light penetration, nutrient availability, temperature, salinity, water movement, partial pressure of atmospheric oxygen in contact with the water, thickness of the surface film and the bio-depletion rates (Imevbore 1983; Nelson-Smith 1993). The level of DO in water is a major factor that influences the survival of fauna and flora in aquatic environments. For example, while fish species like Ophiocephalus obscura, the snakehead, Clarias gariepinus, and other catfish species can survive with DO that ranged from 0.5 to 4 mg/l which is within the range of DO for zones 1 and 2, other species such as Palaemonetes africana and Paeneus kerathurus (shrimps) will not survive as they require higher DO concentration ranging from 7 to 11 mg/l to thrive (IUCN 2013). The significantly lower DO in all zones sampled, compared to the earlier baseline in 2004 and 2008 (P < 0.05), indicate significant pollution and impact from possibly crude oil pollution from oil operations and urban runoffs received from Epie Creek and nearby towns and villages.
The electrical conductivity is a good indicator of the general chemical richness of the freshwater samples examined. S1 had the highest value (1400 μs/cm) relative to all the freshwater samples collected. This can be attributed to the high values of TSS, TDS and the temperature observed in the samples. The recorded values are higher than values of 64 μs/cm reported by Agedah et al. 2015 for the upper Nun River, but within the values for freshwater systems of the Niger Delta, which has been reported to be usually between 20 and 1500 μs/cm (Abowei 2010).
Nitrate and phosphate are indicators of organic pollution in water mainly from human, plant, animal, sewage and fertilizer sources. Phosphate and nitrate concentrations were significantly higher than the initial baseline conditions (P < 0.05), indicating increased pollution load from urban activities. However, the values remain within the national and international limits and similar to those reported by Agedah et al. 2015. Although they were found to be higher than values reported by Odokuma and Okpokwasili (1993), the relatively low levels of nitrates and phosphate indicate that most of the pollutant loads in the river are likely from hydrocarbon sources, as domestic and urban runoff from the nearby towns is usually associated with high nitrate and phosphate loads.
There was a significant difference (P < 0.05) between the upstream and downstream concentrations for all the physicochemical parameters examined, except for pH and nitrates. This supports the conclusion that the pollutants can be attributed to the oil production activities and other anthropogenic activities upstream of the zone 1.
Hydrocarbon pollution
Values for O&G obtained were within the range of 0.19 to 4.92 mg/L (Table 2), with an average of 2.57 mg/L. S4 reported the highest oil and grease concentration with a value of 4.92 mg/L followed by S1 with a value of 4.1 mg/L, while S6 recorded the lowest concentration of 0.19 mg/L. Comparison with the values set by DPR revealed that the obtained values were below the set standard of 10.00 mg/L. The levels of total PAH and BTEX for all the samples were found to be below the instrument detection limit.
Although the values for O&G obtained for all samples collected were within limits set by DPR, they were significantly higher (P < 0.05) than the initial baseline conditions demonstrating hydrocarbon pollution from the crude oil spills. The relatively high value obtained for S1 may likely be because of the crude oil spill from crude oil exploitation activities upstream of the sample points. Odokuma and Okpokwasili (1993) also observed increases in oil and grease content at sites near areas with large oil-related activities. Surprisingly, quantifiable levels of PAH and BTEX were not obtained in the freshwater samples as their concentration was found to be below the instrument detection limits. This is understandable considering the high levels of TSS reported in the river. In a similar study by IUCN 2013, both BTEX and PAH were below the instrument detection limits from water samples in the same study area. They, however, detected high quantities of PAH in the sediments, indicating greater partitioning of the petroleum hydrocarbons to the river sediments. Sediments studies were outside the scope of this paper.
Heavy metals
The result for the various heavy metal analyses on the freshwater samples is presented in Table 3. Comparison between the values obtained for the heavy metals and the national limits set by the DPR for freshwater ecosystem shows that cadmium (Cd), chromium (Cr), copper and nickel (Ni) had values above the DPR regulatory limits and higher than the 2004 baseline data (P < 0.05). These metals have been associated with crude oil, and therefore, their high concentrations may be attributed to hydrocarbons pollution in the river. Ni had the overall highest concentration. This is an indication of oil pollution, as Ni is usually at its highest concentration in crude oil (Fiedler et al. 2009, Zhang et al. 2015). The lowest Cd was found in S5 and S6. The maximum concentrations of Cr were recorded in S1 and S4. A higher Pb level was determined in S5, while the highest Ni values were obtained at S2 and S3. The further away from the source of the spill, the lower the metal concentration. This is similar to the trend observed by Zhang et al. 2015, where the concentration of heavy metal of a lake in Louisiana gradually declined with distance from the source of oil pollution.
pH values recorded in Table 1 reported moderately acidic pH conditions with the exception of S6 which was found to be within the neutral range. Acidic values are fundamental indicators of the presence of heavy metals in freshwater samples and acidic conditions accelerate the leaching of heavy metals, hence increasing their availability for absorption in the ecosystem. The value obtained for S6 is not surprising since it was collected from downstream of the river with less indication of crude oil pollution and must have undergone dilution due to the downwards flow of the water and inflow from other tributaries.
Except in S6 downstream of the river, heavy metals in the freshwater of the samples, as shown in Table 3, had high levels of Cd, Cr, Cu, Pb, Ni and Zn which were significantly higher than the 2004 and 2008 baseline conditions. The downstream concentrations are also significantly lower (P < 0.05) than the upstream levels. This can be attibuted to the crude oil pollution upstream of the Nun River. These heavy metals can bioaccumulate in marine organisms which eventually move up the food chain and causes significant health effects to the local communities who depend on the river for drinking water, fishing and other recreational activities (Aloulou et al. 2011; Ordinioha and Brisibe 2013).
Hydrobiological characteristics
The diversity of phytoplanktons in the samples is reported in Table 4. A total of 16 phytoplankton species were noticed in the samples with Melosira moniliformis dominating with the most occurrences, while Cymbella pediculus had the least occurrences. Highest values were recorded in the S6 and S4. The total counts for all the samples were within the range of 32 to 39, the Shannon Weiner Index was within the range of 1.154 to 2.273, while the Equitability Index was within the range of 0.644 to 0.948. S6 was observed to not only have a greater number of species present, but the individuals in the community (biomass) were distributed more equitably among these species. The lower distribution of species in S1 and S2 which are closer to the source of the spills is an indication of the impact of oil pollution on the aquatic ecosystem. The presence of Cyanophytes (as Oscillatoria species) is a good indicator of pollution, as they appear to be abundant in waters high in organic pollutants (Jacob and Al-Muzaini 1995). Their proliferation during a particular season also depends on water temperatures, light penetration and the supply of nutrient to the surface layer. This is well expected as phytoplankton serve as potential food for zooplankton and have shorter and initial higher generation rate. Phyto-planktons are often used as water quality indicators (Brettum and Andersen 2005; DEHP 2016). As a result of the shortness of their life cycle, they tend to respond rapidly to environmental changes; thus, their biomass and species composition indicate the quality of the water in which they are seen. The low biomass in S1, S2 and S3 may be attributed to the levels of turbidity and hydrocarbon contents of these samples as they are collected at the upper end of the river (Selala et al. 2013). On the other hand, the higher biomass in S6, S4 and S5 may be a result of the reducing turbidity and hydrocarbon contents of the samples and tidal action in reducing the turbidity as the water flows down the river, as previously report by Duursma and Dawson (1981). Oscillatoria sp. was widely distributed in the study area and recorded in all the samples. The results in Table 4 showed that the oil spill pollution in the river hindered the growth of certain species of phytoplankton as can been seen with the varying distributions across the sampling zones with lower Shannon Weiner Index in zone 1 compared to zones 2 and 3. Similar patterns were reported by Ozhan and Bargu (2014) while assessing the impact of Macondo oil spill in the Gulf of Mexico.
The zooplankton diversity is reported in Table 5. It can be seen that diversity and evenness in S3 and S6 were higher than those observed in S1, S2, S4 and S5, which exhibited sequentially reducing values. The total counts were within the range of 21 to 29. The Shannon-Weiner index is usually based on density, and it is assumed that the more abundant a particular species, the more important it is (Risser and Rice 1971). The Shannon-Weiner diversity index results ranged between 1.153 and 1.844, while the equitability index was within the 1.153 to 1.844 which is an indication of low–moderate diversity. The highest value was found in S3, and the lowest value was recorded in S1. Centopages typicus, Metridia lucens, Candicia armata and Cyclopoid copepod were all widely distributed in the study area and recorded in all the samples. S3 had a more distribution of all species present in the communities (biomass) and a greater number of species present. A total of eight zooplankton species were observed in the samples where C. armata had the highest occurrence and Temora longicomis the least occurrence. As a result of the absence of data on the diversity of the Nun River before the oil spill, it is not possible to effectively highlight the impact of the oil spill on biodiversity of the study area and compare post-event effects. However, the reduced diversity of the zooplanktons in S1 and S2 closer to the source of oil pollution is an indication of the impact of oil on the ecosystem. Also, zooplanktons are known to metabolize and accumulate hydrocarbons. They therefore play a major role in hydrocarbon cycling within the aquatic ecosystem (Lotufo 1998; Almeda et al. 2013). Overall, while there was a noticeable impact on plankton diversity, the toxic effects were not severe. This could be attributed to the non-detectable levels of PAHs in the surface water which has the major toxic effects of plankton organisms (Barros et al. 2014; Acosta-Gonzalez et al. 2015).
The total heterotrophic bacteria were found to be within 1.51 × 106 and 0.78 × 106 cfu/mL, the total coliform bacteria was within the range of 0.43 × 105 and 1.16 × 105 cfu/mL, while the hydrocarbon-utilizing bacteria (HUB) values were within the range of 3.5 × 102 in the S4 to 7 × 102 in S2 (Table 6). Hydrocarbon-utilizing bacteria increases in abundance usually following oil spills and during the oil biodegradation process (Yakimov et al. 2007, Hazen et al. 2010; Dubinsky et al. 2013). From Table 6, it can be seen that there were more counts of the HUB in zone 1 closer to the source of the spill compared to the other samples downstream of the Nun River, indicating higher levels of hydrocarbon pollution upstream.
Conclusion
The effects of oil pollution on the water quality of the Nun River were investigated. While some of the physicochemical parameters were within national regulatory limits, they were significantly higher than previous baseline conditions of the river. turbidity, TDS, TSS, DO, conductivity and heavy metals (Cd, Cr, Cu, Pb, Ni and Zn) were in breach of the national and international limits for drinking water and ecological health. Microbial and plankton populations and diversities in all freshwater samples were also found to be relatively impacted by the recurrent small spills reaching the river. The pollutants detected in the river will pose serious health risk to the local communities using the river as a source of sustenance.
References
Abowei, F. N., & Hart, A. I. (2009). Some morphormetric parameters of ten finfish species from the lower Nun River, Niger Delta Nigeria. Research Journal of Biological Sciences, 4(3), 282–288.
Abowei, J. F. N. (2010). Salinity, dissolved oxygen, pH and surface water temperature conditions in Nkoro River, Niger Delta, Nigeria. Advance Journal of Food Science and Technology, 2(1), 36–40.
Agedah, E. C., Ineyougha, E. R., Izah, S. C., & Orutugu, L. A. (2015). Enumeration of total heterotrophic bacteria and some physico-chemical characteristics of surface water used for drinking sources in Wilberforce Island, Nigeria. Journal of Environmental Treatreatment Techniques, 3(1), 28–34.
Alcaraz, M., Almeda, R., Calbet, A., Saiz, E., Duarte, C. M., Lasternas, S., Agustí, S., Santiago, R. D., Movilla, J., & Alonso, A. (2010). The role of arctic zooplankton in biogeochemical cycles: respiration and excretion of ammonia and phosphate during summer. Polar Biology, 33(12), 1719–1731.
Acosta-González, A., Martirani-von Abercron, S. M., Rosselló-Móra, R., Wittich, R. M., & Marqués, S. (2015). The effect of oil spills on the bacterial diversity and catabolic function in coastal sediments: a case study on the prestige oil spill. Environmental Science & Pollution Research, 22(20), 15200–15214.
Aghoghovwia, O. A., & Ohimain, E. I. (2014). Physicochemical characteristics of lower Kolo creek, Otuogidi, Bayelsa state. Nigerian Journal of Agriculture, Food and Environment, 10(1), 23–26.
Alabaster, J. S., & Lloyd, R. (1980). Water quality criteria for freshwater fish. Internal Review of Hydrobiology, 66(3), 297.
Allan, S. E., Smith, B. W., et al. (2012). Impact of the deepwater horizon oil spill on boavailable polycyclic aromatic hydrocarbons in Gulf of Mexico coastal waters. Environmental Science & Technology, 46(4), 2033–2039.
Aloulou, F., Kallell, M., & Belayouni, H. (2011). Impact of oil field-produced water discharges on sediments: a case study of Sabkhat Boujemal, Sfax, Tunisia. Environmental Forensics, 12(3), 290–299.
Almeda, R., Wambaugh, Z., Chai, C., Wang, Z., Liu, Z., & Buskey, E. J. (2013). Effects of crude oil exposure on bioaccumulation of polycyclic aromatic hydrocarbons and survival of adult and larval stages of gelatinous zooplankton. PloS One, 8(10), e74476.
APHA. (2005). Standard methods for examination of water and wastewater. Washington DC: American Public Health Association.
Asonye, C. C., Okolie, N. P., Okenwa, E. E., & Iwuanyanwu, U. G. (2007). Some physico-chemical characteristics and heavy metal profiles of Nigerian rivers, streams and waterways. African Journal of Biotechnology, 6(5), 617.
Barros, Á., Álvarez, D., & Velando, A. (2014). Long-term reproductive impairment in a seabird after the prestige oil spill. Biology Letters, 10(4), 20131041.
Beadle, L.C. (1974). The inland waters of tropical Africa. An introduction to tropical limnology. Longman Group Ltd, Publishers, 74 Grosvenor Street, London W. 1.
Bhattacharya, D., Clement, T. P., & Dhanasekaran, M. (2016). Evaluating the neurotoxic effects of Deepwater Horizon oil spill residues trapped along Alabama’s beaches. Life Sciences, 155, 161–166.
Beyer, J., Trannum, H. C., Bakke, T., Hodson, P. V., & Collier, T. K. (2016). Environmental effects of the Deepwater Horizon oil spill: a review. Marine Pollution Bulletin. doi:10.1016/j.marpolbul.2016.06.027.
Brettum, P., Andersen, T. (2005). The use of phytoplankton as indicators of water quality Norwegian Institute for Water Research Report.
Brussaard, C. P., Peperzak, L., Beggah, S., Wick, L.Y., Wuerz, B., Weber, J., Arey, J.S., van der Burg, B., Jonas, A., Huisman, J. and van der Meer, J.R. (2016). Immediate ecotoxicological effects of short-lived oil spills on marine biota. Nature Communication, 7.
Buchanan, R. E., & Gibbons, N. E. (2001). Bergey’s manual of determinative bacteriology. Baltimore: Williams and Wilkins Co.
Cam, D., Gagni, S., Meldolesi, L., & Galletti, G. (2000). Determination of polycyclic aromatic hydrocarbons in sediment using solid-phase microextraction with gas chromatography—mass spectrometry. Journal of Chromatographic Science, 38(2), 55–60.
Cruickshank, R., Duguid, J. P., et al. (1975). Medical microbiology, the practise of medical microbiology. Edinburgh, London and New York: Churchill Livingstone Limited.
Dawes, C. J. (1998). Marine Botany. New York: Wiley.
DEHP. (2016) Ecosystem health indicators’ department for environment and heritage protection Queensland government.
Department of Petroleum Resources (DPR) (2002). Environmental Guidelines and Standards for the Petroleum Industry in Nigeria (EGASPIN), pp 59–66.
Dubinsky, E. A., Conrad, M. E., Chakraborty, R., Bill, M., Borglin, S. E., Hollibaugh, J. T., Mason, O. U., Piceno, M. Y., Reid, F. C., Stringfellow, W. T., & Tom, L. M. (2013). Succession of hydrocarbon-degrading bacteria in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environmental Science & Technology, 47(19), 10860–10867.
Duursma, E. K., & Dawson, R. (1981). Marine organic chemistry: evolution, composition, interaction and chemistry of organic matter in seawater. Amsterdam; New York: Elsevier.
Ezekiel, E. N., Hart, A. I., & Abowei, F. F. N. (2011). The sediment physical and chemical characteristics in Sombreiro River, Niger Delta, Nigeria. Research Journal of Environmental and Earth Science, 3(4), 341–349.
Ezekwe, I. C., Chima, G. N., & Ikogori, G. (2013). An investigation of selected microbial pollutants in groundwater sources in Yenegoa town, Bayelsa, Nigeria. Estudos de Biologia, 35(84), 77–84.
Fattal, P., Maanan, M., Tillier, I., Rollo, N., Robin, M., & Pottier, P. (2010). Coastal vulnerability to oil spill pollution: the case of Noirmoutier Island (France). Journal of Coastal Research, 26(5), 879–887.
Fiedler, S., Siebe, C., Herre, A., Roth, B., Cram, S., & Stahr, K. (2009). Contribution of oil industry activities to environmental loads of heavy metals in the Tabasco Lowlands, Mexico. Water Air and Soil Pollution, 197(1–4), 35–47.
Guo, Y., & Yang, S. (2016). Heavy metal enrichments in the Changjiang (Yangtze River) catchment and on the inner shelf of the East China Sea over the last 150 years. Science of the Total Environment, 543, 105–115.
Hazen, T. C., Dubinsky, E. A., DeSantis, T. Z., Andersen, G. L., Piceno, Y. M., Singh, N., Jansson, J. K., Probst, A., Borglin, S. E., Fortney, J. L., & Stringfellow, W. T. (2010). Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science, 330(6001), 204–208.
Heathcote, I. W. (2009). Integrated watershed management: principles and practise. New Jersey: John Wiley & Sons.
Imevbore, A.M.A. (1983). The investigation of faecal pollution in the surface water of the Niger Delta of Nigeria: final report Niger Delta Basin Development Authority, Niger Delta Basin Development Authority.
IUCN Niger – Delta Panel. 2013. Sustainable Remediation and Rehabilitation of Biodiversity and Habitats of Oil Spill Sites in the Niger Delta. Main Report including recommendations for the future. A report by the independent IUCN—Niger Delta Panel (IUCN-NDP) to the Shell Petroleum Development Company of Nigeria (SPDC). January 2013. Gland, Switzerland: IUCN.
Jacob, P. G., & Al-Muzaini, S. (1995). Marine plants of the Arabian Gulf and effects of oil pollution. Mahasagar, 28(1–2), 83–101.
Jarup, L. (2003). Hazard of heavy metal contamination. British Medical Bulletin, 68(1), 167–182.
Jung, S. W., Kwon, O. Y., Joo, C. K., Kang, J. H., Kim, M., Shim, W. J., & Kim, Y. O. (2012). Stronger impact of dispersant plus crude oil on natural plankton assemblages in short-term marine mesocosms. Journal of Hazardous Materials, 217, 338–349.
Kadafa, A. A. (2012). Oil exploration and spillage in the Niger Delta of Nigeria. Civil and Environmental Research, 2(3), 38–51.
Kamath, S., Sabatini, D. A., & Canter, L. W. (1991). Biological nitrification/denitrification of high sodium nitrite (navy shipyard) wastewater. Environmental Pollution, 69(1), 25–38.
Lotufo, G. R. (1998). Bioaccumulation of sediment-associated fluoranthene in benthic copepods: uptake, elimination and biotransformation. Aquatic Toxicology, 44(1), 1–15.
Moliner-Martínez, Y., Herraez-Hernandez, R., Verdú-Andres, J., Campíns-Falcó, P., Garrido-Palanca, C., Molins-Legua, C., & Seco, A. (2013). Study of the influence of temperature and precipitations on the levels of BTEX in natural waters. Journal of Hazardous Materials, 263, 131–138.
Mulusky, D. S. (1974). Ecology of estuaries, Heinemann educational books.
Murphy, J., & Riley, J. P. (1962). A modified single solution method for the determination of soluble phosphate in natural waters. Analytica Chimica Acta, 26, 31–36.
Nyananyo, B. L. (1999). Vegetation. In: Alagoa E. J (ed). The land and people of Bayelsa State: central Niger Delta. Onyoma Publications P44.
Nyananyo, B. L., Gijo, A. H., & Ogamba, E. N. (2007). The physico-chemistry and distribution of water hyacinth (Eichhornia cressipes) on the river Nun in the Niger Delta. Journal of Applied Science and Environmental Management., 11(3), 133–137.
Nelson-Smith, A. (1993). Oil pollution and marine ecology. New York: Plenum Press.
Odokuma, L. O., & Okpokwasili, G. C. (1993). Seasonal influences on inorganic anion monitoring of the new Calabar River, Nigeria. Environmental Management Journal, 17(4), 491–496.
Ordinioha, B., & Brisibe, S. (2013). The human health implications of crude oil spills in the Niger Delta, Nigeria: An interpretation of published studies. Nigerian Medical Journal, 54(1), 10.
Ozhan, K., & Bargu, S. (2014). Distinct responses of Gulf of Mexico phytoplankton communities to crude oil and the dispersant Corexit® Ec9500A under different nutrient regimes. Ecotoxicology, 23(3), 370–384.
Prat, N., Toja, J., Sola, C., Burgos, M. D., Plans, M., & Rieradevall, M. (1999). Effect of dumping and cleaning activities on the aquatic ecosystems of the Guadiamar River following a toxic flood. Science of the Total Environment, 242(1), 231–248.
Reiss, H., & Kröncke, I. (2005). Seasonal variability of benthic indices: an approach to test the applicability of different indices for ecosystem quality assessment. Marine Pollution Environment, 50(12), 1490–1499.
Risser, P. G., & Rice, E. L. (1971). Diversity in tree species in Oklahoma Upland. Ecology, 52(5), 876–880.
Seiyaboh, E. I., Ekweozor, I. K. E., Ogamba, E. N., & Alagha, W. E. (2007). Effect of Tombia bridge construction on the water quality of Nun River in Central Niger Delta, Nigeria. Journal of Applied Sciences, 10(3), 7148–7156.
Selala, M.C., Oberholster, P.J., Surridge, K.A., de Klerk, A.R. & Botha, A.M. (2013). Responses of selected biota after biostimulation of a vegetable oil spill in the Con Joubert Bird Sanctuary wetland: a pilot study. African Journal of Biotechnology, 12 (4).
Sikoki, F. D., Hart, A. I., & Abowei, J. F. N. (1998). Gill net selectivity and fish abundance in the lower Nun river. Nigeria Journal of Applied Science and Environmental Management, 1, 13–19.
SPDC (2015). Final Environmental Impact Assessment (EIA) report for the Adibawa-Gbaran 3D Reshoot Seismic Data Acquisition Project in Bayelsa and Rivers States, November 2015.
Swaney, D. P., Howarth, R. W., & Butler, T. J. (1999). A novel approach for estimating ecosystem production and respiration in estuaries: application to the oligohaline and mesohaline Hudson River. Limnology and Oceanography, 44(6), 1509–1521.
Talling, J. F. (2010). pH, the CO2 system and freshwater science. Freshwater Reviews, 3(2), 133–146.
United National Environmental Programme (UNEP) (2011). Environmental assessment of Ogoniland. Nairobi pp. 1–262.
Yakimov, M. M., Timmis, K. N., & Golyshin, P. N. (2007). Obligate oil-degrading marine bacteria. Current Opinion in Biotechnology, 18(3), 257–266.
Zhang, Z., Wang, J. J., Tang, C., & DeLaune, R. D. (2015). Heavy metals and metalloids content and enrichment in Gulf Coast sediments in the vicinity of an oil refinery. Journal of Geochemical Exploration, 159, 93–100.
Zhang, J., Yan, J. & Zhang, Z. F. (1995). Nationwide river chemistry trends in China: Huanghe and Changjiang. Ambio, 275–279.
Acknowledgements
The authors would like to thank Dr. Alex Onojeghuo of Lancaster Environment Centre and Southsea Datcomm Laboratory Limited for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ifelebuegu, A.O., Ukpebor, J.E., Ahukannah, A.U. et al. Environmental effects of crude oil spill on the physicochemical and hydrobiological characteristics of the Nun River, Niger Delta. Environ Monit Assess 189, 173 (2017). https://doi.org/10.1007/s10661-017-5882-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-5882-x