Abstract
The Algerian coastline is being exposed to several types of pollution, including that of hydrocarbons. This environment rich in oil could be the source of proliferation of hydrocarbonoclastic bacteria. The objective of the study is to isolate and identify indigenous bacterial strains from marine waters of two ports in the eastern Algerian coast and to test their growth in the presence of hydrocarbons with and without biostimulation throughout the intake of nitrogen and phosphate. Results recorded the highest level of both total hydrocarbons and phosphates in the port of Annaba, followed by El-Kala station and then the control station, while that of total nitrogen was vice versa. Fifty-three bacterial strains were identified from which four were selected to perform the growth tests. Results showed that the growth and the biodegradation differ from one species to another. Thus, the strains tested (Halomonas venusta NY-8, Exiguobacterium aurantiacum NB11-3A, Vibrio alginolyticus Pb-WC11099, and Dietzia sp. CNJ898 PL04) seem very active, in which better growth was obtained with the last two strains during nitrogen and phosphate supplementation. Such strains are suggested to participate a lot in the biodegradation of oil at polluted sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum hydrocarbons are major pollutants of marine environments as a result of terrestrial and freshwater runoff, refuse from coastal oil refineries, offshore oil production, shipping activities, and accidental spills (Arulazhagan et al. 2010). The contamination of marine environments by hydrocarbons represents a global concern with potential consequences on ecosystems and human health (Gong et al. 2013). Marine pollution by hydrocarbons, whether chronic or accidental, raises significant problems to the environment. Indeed, the removal of hydrocarbons by physical and/or chemical methods is often very expensive. Using microorganisms would constitute an alternative for bioremediation and cleaning up hydrocarbons contaminated areas. According to Molina et al. (2009), some microorganisms show a high potential for adaptation to oil pollution in the treatment plants and in the polluted sites as well. Biodegradation by natural populations of microorganisms is the most reliable mechanism by which thousands of pollutants, including crude oil, are removed from the environment (Cappello et al. 2007). In many ecosystems, there is already an adequate indigenous microbial community capable of extensive oil biodegradation (Olajire and Essien 2014). It is necessary to study the indigenous microorganisms capable of degrading pollutants because of their varied effects on the environment (Jain et al. 2005). Numerous microorganisms have been isolated, and their phylogeny and metabolic capacity to degrade a variety of aliphatic and aromatic hydrocarbons have been demonstrated (Olajire and Essien 2014). Microorganisms able to utilize saturated hydrocarbons (n-alkanes) are widely distributed in nature (Atlas and Atlas 1991; Zhang et al. 2011) such as Rhodococcus (Van Hamme and Ward 2001), Alcanivorax (Liu et al. 2010), Pseudomonas (Zhang et al. 2011), Dietzia DQ12–45-1b (Xing Xang-Biao et al. 2011), Acinetobacter lwoffi (Marchal et al. 2003), Exiguobacterium aurantiacum, and Burkholderia capacia (Gita and Suparna 2008a) for the biodegradation of aromatic hydrocarbons. The study of Moxley and Schmidt (2010) reported the ability of Vibrio sp. KM1 to grow with benzoic acid as the sole source of energy and carbon; the metabolic versatility of Halomonas has also been associated with a great biotechnological potential (Simon-Colin et al. 2008); and Halomonas sp. and Marinobacter sp. (Gabet 2004) degrade several polycyclic aromatic hydrocarbons (PAHs) including naphthalene, phenanthrene, anthracene, fluoranthene, fluorine, pyrene, benz[a]anthracene, and benzo [a] pyrene as the sole carbon sources (Dastgheib et al. 2012).
Hydrocarbon molecules are relatively unreactive due to the lack of functional groups and low solubility in water (Hassanshahian et al. 2012). The study of the effects of environmental conditions on microbial degradation of hydrocarbons and the effects of oil contamination on microbial communities are therefore of great interest (Rahman et al. 2004; Cappello et al. 2012). Eliminating environmental oil requires the intervention of various biotic and abiotic factors. Thus, biodegradation by microorganisms, particularly bacteria, is the most important natural process in cleaning up the environment (Soltani 2004). While it is relatively slow, this process allows almost a complete degradation (CO2 conversion) of oil (Sauret 2011). Hydrocarbon-degrading microorganisms usually exist in very low abundance in marine environments. Identification of the key organisms that play roles in pollutant biodegradation is important for understanding, evaluating, and developing in situ bioremediation strategies. Thus, it is highly essential to characterize bacterial communities, to identify responsible degraders, and to elucidate the catalytic potential of these degraders (Sivaraman et al. 2011).
Hydrocarbons constitute the most type of chronic Algerian coastal pollution during the last decades. Such pollution is related to the degree of proliferation of natural bacterial strains, which are able to degrade these organic molecules. The objective of this work is to isolate and identify the indigenous hydrocarbonoclastic bacteria from two sites of the coastline through the growth kinetics in the presence of known concentrations of different types of hydrocarbons, with or without nitrogen and phosphate.
Materials and methods
Sampling stations
Sampling was carried out in the Algerian eastern coast from the two ports of Annaba and El-Kala (Fig. 1). Annaba City has one of the major industrial ports in Algeria, with a high shipping activity where it is exposed to chronic hydrocarbon pollution. The port of El-Kala is rather characterized by the presence of small fishing boats throughout the year, which use hydrocarbons as a fuel.
About 1 l of surface seawater sample was collected in sterilized containers from sites containing spots of oil at each port. Samples were taken during the period of March–July and September–November. The samples have been stored at 4 °C during the transport.
Physical and chemical analyses
The physico-chemical analyses were performed to determine the total hydrocarbon levels and the concentrations of total nitrogen and phosphates by the methods described in Table 1.
Isolation, identification, and selection of strains
Isolations were carried out on several types of agar culture media, nutrient agar, Chapman, and Mac-Conkey (Marchal et al. 1982). The identification was based on morphological and biochemical analyses (Api 20E and 20NE strips, Biomerieux) and on molecular PCR amplification and sequencing of 16S rDNA gene with primers 27f and 1401r as described in Batisson et al. (2009). Phylogenetic analysis of the strains was also performed (Wang et al. 2007). The evolutionary relationship between different strains was deduced using the neighbor-joining method (Saitsou and Nei 1987).
In order to see their growth and development, the isolated indigenous bacterial strains were then grown on synthetic seawater agar consisting of Tris (2 g L−1), NaCl (23 g L−1), NH4Cl (1 g L−1), MgSO4.7H2O (6 g L−1), MgCl2.6H2O (5 g L−1), CaCl2 2H2O (1 g L−1), and agar (15 g L−1) (Soltani 2004) supplemented with 1 ml of hydrocarbon (crude oil supplied by the Skikda Oil Refinery). The selection of strains was obtained after incubating on the Petri dishes at 30 °C for a period ranging from 24 h to 7 days (Leahy and Colwell 1990).
Tests of bacteria growth in the presence of alkanes and refined hydrocarbons
Among the isolated and identified strains, four were subjected to growth tests in the presence of hydrocarbons and biostimulation with nitrogen and phosphates, respectively, equal to 1.5 g KNO3 and 45 mg NaH2 PO4 in a final volume of 300 ml, respectively. The cultures were carried out in sterile synthetic seawater with added 1 ml alkanes, hexane C6H14, heptanes C7H16, decane C10H22, cyclohexane C6H12, or refined hydrocarbon (gasoline) as the sole carbon and energy source. The media were incubated in a bacteriological incubator at 30 °C with stirring at 150 rpm in aerobic condition. Control cultures were performed without biostimulation. The growth kinetics was estimated through the turbidity media by measuring optical densities at 600 nm (Perry et al. 2004). Finally, the hydrocarbon concentrations were determined (Table 1) before and after bacterial growth. A multivariate analysis of variance (MANOVA) has been performed at α = 0.05 (Hand and Taylor 1987; Kutner et al. 2005).
Results and discussion
Physico-chemical parameters
Results of physicochemical analysis showed that the highest rate of total hydrocarbons (THCs) was registered in the seawater of Annaba port with 111 mg L−1. It is ten times higher than the Algerian standard, which recommends a limit of total hydrocarbons of 10 mg L−1, while the THC of Cape Guard, station considered as a control, is low with only 4.80 mg L−1. This station is situated north-west of the port with a distance of about 10 km. The rate of THC at the fishing port of El-Kala was 11.20 mg L−1 (Table 2). The amounts of nitrogen and phosphates remain low in the three stations. Thus, Algerian standard for water allows a maximum of 30 mg L−1 for total nitrogen and 10 mg L−1 for total phosphates (J.O.R.A 2006). The maximum rate of nitrogen was recorded at the Cape Guard station with 5.4 mg L−1. For phosphates, the maximum concentration was observed at the Port of Annaba with 0.7 mg L−1 (Table 2).
Identification and selection of strains
A total of 53 bacterial strains and consortia (Gram+ and Gram−) as well as some yeasts were isolated and cultured on synthetic seawater agar in the presence of 1 ml crude oil as the unique source of carbon and energy. After incubation at 30 °C for a period of 7 days, 17 strains have been developed on the agar. Four of them were chosen based on the morphological analysis of the colonies and the growth speed on synthetic seawater agar containing hydrocarbons (Table 3).
The morphological, microscopic, and biochemical characteristics of these strains are mentioned in the Table 4.
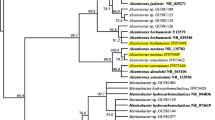
The molecular identification allowed assessing the phylogenetic relationship between the different strains. The optimal tree with the sum of branch length was 0.63253343 (Fig. 2). The shaft is drawn with branch lengths at the same units as those of evolutionary distances used to infer a phylogenetic tree. The evolutionary distances were calculated using the maximum composite likelihood method (Tamura et al. 2004) and are in units of the number of base substitutions per site. The positions of codons were included first + second + third + no coding. All positions containing gaps and missing data were eliminated from the data set (full option to delete). There were a total of 426 positions in the final data set. Phylogenetic analyses were conducted in 5 MEGA (Tamura et al. 2007).
Growth species in the presence of alkanes
According to Das and Chandran (2011), petroleum hydrocarbons in nature are degraded by various groups of microorganisms capable of using hydrocarbons as nutrients. The growth of the strains isolated in the presence of different hydrocarbons as unique carbon source yielded the following results depending on the species tested.
Vibrio alginolyticus PB-11099 WC
The Vibrio species are part of the family Vibrionaceae, class of γ-Proteobacteria (Cappello et al. 2012). They are heterotrophic bacteria part of the most abundant species among the cultivated bacteria in marine environments such as coastal waters, estuaries, sediment, and aquaculture infrastructure (Thompson et al. 2004). Some species such as V. cholerae, V. mimicus, V. hollisae, V. alginolyticus, V. fluviales, and V. damsela are pathogenic to humans and animals (Tantillo et al. 2004).
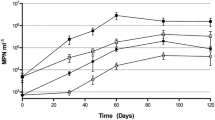
Environmentally, and according to Grossart et al. (2005), Vibrio plays a very important role in the degradation of organic matter. The growth of V. alginolyticus WC PB-11099 shows a very short-latency phase, 2 h in the presence of hexane C6 H14 (Fig. 3a) and 24 h with heptane C7 H16 (Fig. 3b). Overall, the growth was greater in the presence of hexane than heptane. Indeed, the optical density (OD) increased rapidly with hexane from 0.164 to 0.694 within 24 h and the maximum growth heptane was achieved after 72 h with an OD of about 0.49 (Fig. 3b). This growth proves the hypothesis of Ratlege (1978) that some bacteria are able to grow in the presence of short chain of alkanes. However, in the presence of decane, growth was faster with a short-latency phase and it reached an OD of 1.97 after 48 h (Fig. 3c).
The species shows an easy adaptation with longer carbon chains greater than nine carbons (Ratlege 1978). With cyclohexane, bacteria showed an adaptive difficulty which was reflected in a reduction of the number of viable cells and a drop in OD during the first 48 h. Indeed, after 48 h, there was an increase in the OD until 0.36 synonymous of cell growth (Fig. 3d).
E. aurantiacum strain NB11_3A
According to the work of Gita and Suparna (2008b), the species E. aurantiacum NCDO 2321 has a capacity of degrading a wide range of n-alkanes. Furthermore, it can be used for bioremediation and for the treatment of waste oil in the bioreactors.
Generally, E. aurantiacum NB11_3A strain has similar growth whatever the alkane tested (Fig. 4), although the strain reaches a higher OD value (1.12) in the presence of decane after 72 h (Fig. 4a). Despite maladjustments with cyclohexane during the first 2 h of growth, the strain has an exponential phase growth where it reaches a plateau phase after 168 h (Fig. 4d).
Halomonas venusta strain NY-8
According to Rojas et al. (2009), the genus Halomonas is the most abundant bacterial group in marine environments. The species most representative of the genus Halomonas is H. venusta (Wang et al. 2009). H. venusta is a halophilic bacterium of the family Halomonadaceae, belongs to the class of Gammaproteobacteria (Franzmann et al. 1988; Dobson and Franzmann 1996). Many studies have reported that Halomonas shengliensis (Wang et al. 2007), Halomonas sp. C2SS100, and Pseudomonas sp. C450 R (Mnif et al. 2009; Mnif et al. 2011) use crude oil compounds as substrate for growth.
The genus Halomonas was able to assimilate a wide variety of carbon source. Several studies (Okamoto et al. 2004; Mnif et al. 2011) have isolated Halomonas sp. C2SS100 and Pseudomonas. sp. C450R, which degrade from 39 to 96% of the aliphatic fraction C13–C29 of crude oil. H. venusta NY-8 showed similar growth kinetics to other strains, with a very long-latency period of 216 h with hexane (Fig. 5a) and an adaptive difficulty in the early stage (2 h) with heptane (Fig. 5b) and cyclohexane (Fig. 5d). The growth rate was with slow exponential phases and low OD of 0.38, 0.097, and 0.189, respectively for hexane, heptanes, and cyclohexane (Fig. 5a, b, d). The growth of H. venusta NY-8 in the presence of decane seemed faster with an OD of 0.46 (Fig. 5c) after 4 days of culture.
Dietzia sp. CNJ898 PLO4
Dietzia spp. was isolated in different environments like tropical soils (Von der Weid et al. 2007), alkaline lakes (Duckworth et al. 1998), contaminated land with oil (Borzenkov et al. 2006), deep marine sediments (Colquhoun et al. 1998), and the skin and the intestinal tract of marine fish (Yumoto et al. 2002). Nowadays, many studies have reported that microorganisms as Dietzia sp. (Riis et al. 2003) and Dietzia maris and Rhodococcus erythropolis (Zvyagintseva et al. 2001) can use crude oil compounds as a growth substrate.
Dietzia sp. CNJ898 PLO4 easily degrades decane and to a lesser extent hexane. The cell adaptation was short with a rapid increase in OD of 0.86 (Fig. 6a) in hexane after 240 h of culture and 1.8 with decane after 48 h (Fig. 6c). The growth rate was lower with heptane and cyclohexane, respectively, with a maximum OD of 0.097 0.43 (Fig. 6b) and 0.32 (Fig. 6d) after 192 h of culture. Lower ODs in early stage with both alkanes were recorded. A resumption of growth was observed after 24 h in heptanes (Fig. 6b) and 48 h in cyclohexane (Fig. 6d), unlike the controls which have a low OD.
Several species of Dietzia described to date have shown their ability to degrade aliphatic hydrocarbons (Yumoto et al. 2002). For example, D. maris DSM 43672 is able to grow with C6 n-alkanes C17, C19, and C23 (Raeiney et al. 1995); Dietzia psychralcaliphila uses n-alkanes C13, C15, C16, C20, and C24 and pristine but does not use C10 or C32 (Yumoto et al. 2002). Dietzia sp. E1 uses n-alkanes with lengths chain ranging from C6 to C30 (Bihari et al. 2010). The strain of Dietzia 12–45-1b DQ is able to use a wide range of n-alkanes (C6–C40), aromatics, and crude oil as a unique carbon source for growth. Xing Xang-Biao et al. (2011) and Von der Weid et al. (2007) confirmed the ability of Dietzia cinnamae p4 to grow in a wide range of n-alkanes of different sizes (C11 to C36), crude oil, and aromatic compounds (e.g., benzene), revealing that this microorganism can be used in bioremediation of petroleum hydrocarbons.
Effect of biostimulation in the presence of refined hydrocarbons
Petroleum products are introduced into the marine environment in the form of refined products. Fuels and oils have compositions that depend on the origin of oil and sudden operations during refining. There are about 230 components for gasoline and 2000 for heavy fuel oil (Soltani 2004). Rejection of products in marine and terrestrial environments causes a proliferation of microorganisms capable to grow on hydrocarbons and their degradation products. Their number is much higher in chronically polluted areas and increases after an intake of hydrocarbons in contaminated sites (Bartha and Atlas 1977). In polluted environments, specialized microorganisms are abundant because of their adaptation to pollutants (Marchal et al. 2003). The amounts of nitrogen and phosphates in water-selected sites were low initially, and their addition to the culture media had some effects on growth kinetics.
V. alginolyticus PB-11099 WC
The growth of V. alginolyticus PB-11099 WC without nitrogen and phosphates shown in (Fig. 7a) was very long. A stationary phase of 16 days was observed before the start of the exponential phase with an OD of 0.226. By contrast, when V. alginolyticus WC PB-11099 was cultured in a medium supplemented with nitrogen and phosphate (Fig. 7b), the growth curve showed a shorter-latency period of about 24 h, and the exponential phase growth achieved an OD of 1.716 after 322-h cultivation. V. alginolyticus PB-11099 WC seems more active and rapidly assimilates and metabolizes gasoline as the only source of carbon and energy for growth in the presence of nitrogen and phosphate.
E. aurantiacum strain NB11_3A
The growth of E. aurantiacum NB11_3A with or without biostimulation (Fig. 8a, b) in the presence of gasoline required a very long time to adapt with a latency period that lasted more than 9 days. Nevertheless, nitrogen and phosphate had the effect of stimulating the growth and maintained the exponential phase for a period of several days, allowing to reach an OD of 1.03 after 504 h of culture. In the absence of biostimulation, exponential phase was very short with a maximum OD of 0.227 at 298 h.
H. venusta strain NY-8
H. venusta NY-8 was hardly grown in a medium containing gasoline as a unique source of carbon and energy (Fig. 9a). The addition of nitrogen and phosphate in the medium accelerated the growth of bacteria and the OD increased rapidly, reaching a maximum of 1.89 after 4 days of culture.
Dietzia sp. CNJ898 PLO4
The multiplication of Dietzia sp. CNJ898 PLO4 appeared after 24 h of incubation in a biostimulated medium (Fig. 10b) and only after 48 h when it was not biostimulated (Fig. 10a) by nitrogen and phosphate. In addition, at the end of the exponential phase, the OD was 2.193 and 0.436, respectively, for the biostimulated and the nonstimulated mediums.
Concentrations of hydrocarbons
The hydrocarbon concentrations were determined before and after bacterial growth. Table 5 expresses the initial concentration and the different results obtained with the four strains.
All strains appeared to degrade hydrocarbons with different rates. Thus, the maximum degradation was obtained with H. venusta strain NY-8 in the presence of heptane 3.12 mg L−1. With E. aurantiacum strain NB11_3A, the lowest rate of degradation was observed in the presence of 21.23 mg L−1 of cyclohexane. The V. alginolyticus PB-WC 11099 gave the best results with all tested n-alkanes, but Dietzia sp. CNJ898 PLO4 appeared more effective for the degradation of gasoline with a rate of 7.93 mg L−1 (Table 5).
The MANOVA analysis at the threshold α = 0.05 shows that the values of p are generally less than α we reject at the null hypothesis. Thus, using the Scheffe test with strains E. aurantiacum NB11_3A and V. alginolyticus PB-WC 11099, there are no significant differences compared with heptane, the same for H. venusta NY-8 with hexane.
For a threshold α = 0.01, as the majority of the values of p are less than 0.01, we have sufficient proof that at α = 0.01, the correlations are not zero. There are very significant differences in the degradation of hydrocarbons with the E. aurantiacum NB11_3A (using Fisher’s test). The results were confirmed by partial correlations for each variable (E. aurantiacum NB11_3A, r = 0.971; V. alginolyticus PB-WC 11099, r = 0.968; H. venusta NY-8, r = 0.971; and Dietzia sp. CNJ898 PLO4, r = 0.922).
Conclusion
In the current study, several bacterial strains were isolated and identified from the seawaters of two ports subjected to chronic oil pollution. The growth test carried out with four selected strains, Dietzia sp. CNJ898 PLO4, E. aurantiacum NB11_3A, H. venusta NY-8, and V. alginolyticus WC PB-11099, have shown that all the strains are able to grow in the presence of hydrocarbons, but with a different kinetic growth, suggesting that they are able to use these molecules as a source of carbon and energy. In addition, nitrogen and phosphate input accelerate the growth of the studied strains. The tested strains appeared to be very active, in which the best results of biodegradation were obtained with V. alginolyticus PB-11099 WC. These bacteria could thus be used for bioremediation to fight against marine oil pollution.
References
Arulazhagan, P., Vasudevan, N., & Yeom, I. T. (2010). Biodegradation of polycyclic aromatic hydrocarbon by a halotolerant bacterial consortium isolated from marine environment. Int. J. Environ. Sci. Tech., 7(4), 639–652.
Atlas, R. M., & Atlas, M. C. (1991). Biodegradation of oil and bioremediation of oil spills. Current Opinion in Biotechnology, 2, 440–443.
Bartha, R., & Atlas, R. M. (1977). The microbiology of aquatic oil spills. Advances in Applied Microbiology, 22, 225–266.
Batisson, I., Crouzet, O., Hoggan, P. B., Sancelme, M., Mangot, J. F., Mallet, C., & Bohatier, J. (2009). Isolation and characterization of mesotrione-degrading Bacillus sp. from soil. Environmental Pollution, 157, 1195–1201.
Bihari, Z., Szabo, Z., Szvetnika, A., Balazs, M., Bartos, P., Tolmacsov, P., Zambou, Z., & Kiss, T. (2010). Characterization of a novel log chain n-alkane degrading strain, Dietzia sp.E1. Z. Naturforsh.c, 65, 693–700.
Borzenkov, I. A., Milekhina, E. I., Gotoeva, M. T., Rozanova, E. P., & Beliaev, S. S. (2006). The properties of hydrocarbon-oxidizing bacteria isolated from the oilfields of Tatarstan, western Siberia, and Vietnam. Microbiology, 75, 82–89.
Cappello, S., Caruso, G., Zampino, D., Monticelli, L. S., Maimone, G., Denaro, R., Tripodo, B., Troussellier, M., Yakimov, M. M., & Giuliano, L. (2007). Microbial community dynamics during assays of harbour oil spill bioremediation: a microscale simulation study. Journal of Applied Microbiology, 102(1), 184–194.
Cappello, S., Genovese, M., Della Torre, C., Crisari, A., Hassanshahian, M., Santisi, S., Calogero, R., & Yakimov, M. M. (2012). Effect of bioemulsificant exopolysaccharide EPS 2003 on microbial community dynamics during assays of oil spill bioremediation: a microcosm study. Marine Pollution Bulletin, 64(12), 2820–2828.
Colquhoun, J. A., Mexson, J., Goodfellow, M., Ward, A. C., Horikoshi, K., & Bull, A. T. (1998). Novel rhodococci and other mycolate actinomycetes from the deep sea. Antonie Van Leeuwenhoek, 74, 27–40.
Das, N. & Chandran, P. (2011). Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnology Research International, 1–13.
Dastgheib, S. M. M., Amoozegar, M. A., Khajeh, K., Shavandi, M., & Ventosa, A. (2012). Biodegradation of polycyclic aromatic hydrocarbons by a halophilic microbial consortium. Applied Microbiology and Biotechnology, 95, 789–798.
Dobson, S. J., & Franzmann, P. D. (1996). Unification of the genera Deleya, Halomonas, and Halovibrio and the species Paracoccus halodenitrificus into a single genus Halomonas, and placement of the genus Zymobacter in the family Halomonadaceae. International Journal of Systematic Bacteriology, 46, 550–558.
Duckworth, A. W., Grant, S., Grant, W. D., Jones, B. E., & Meijer, D. (1998). Dietzia natronolimnaios sp. nov., a new member of the genus Dietzia isolated from an East African soda lake. Extremophile, 2, 359–366.
Franzmann, P. D., Wehmeyer, U., & Stackebrandt, E. (1988). Halomonadaceae fam. nov., a new family of the class Proteobacteria to accomodate the genera Halomonas and Deleya. Systematic and Applied Microbiology., 11, 16–19.
Gabet, S. (2004). Remobilisation d’Hydrocarbures Aromatiques Polycycliques (HAP) présents dans les sols contaminés à l’aide d’un tensioactif d’origine biologique. Thèse de doctorat de l’université de Limoges, spécialité Chimie et Microbiologie de l’Eau, p. 177.
Gita, M., & Suparna, M. (2008a). Biodegradation rate of diesel range n-alkanes by bacterial cultures Exiguobacterium aurantiacum and Burkholderia cepacia. Indian Journal of Biotechnology, 7, 295–306.
Gita, M., & Suparna, M. (2008b). Biodegradation rate of diesel range n-alkanes by bacterial cultures Exiguobacterium aurantiacum and Burkholderia cepacia. International Biodeterioration & Biodegradation, 61, 240–250.
Gong, Y., Zhao, X., Cai, Z., O’Riely, S., Hao, X., & Zhao, D. (2013). A review of oil, dispersed oil and sediment interactions in the aquatic environment: influence on the fate, transport and remediation of oil spills. Marine Pollution Bulletin. doi:10.1016/j.marpolbul.2013.12.024.
Grossart, H. P., Levold, F., Allgaier, M., Simon, M., & Brinkhoff, T. (2005). Marine diatom species harbour distinct bacterial communities. Environmental Microbiology, 7, 860–873.
Hand, D. J. & Taylor, C. C. (1987). Multivariate analysis of variance and repeated measures: a practical approach to behavioural scientists. Chapman & Hall (Ed), p. 340.
Hassanshahian, M., Imtiazi, G., & Cappello, S. (2012). Isolation and characterization of crude-oil-degraded bacateria of the Persian Gulf and the Caspian Sea. Marine Pollution Bulletin, 64, 7–12.
Jain, R. K., Kapur, M., Labana, S., Lal, B., Sarma, P. M., Bhattacharya, D., & Thakur, I. S. (2005). Microbial diversity: application of microorganisms for the biodegradation of xenobiotics. Current Science, 89(1), 101–112.
J.O.R.A.: Journal Officiel de la République Algérienne n° 26 (2006).
Kutner, M. H., Nachtsheim, C. J., Neter, J., & Li, W. (2005). Applied linear statistical models. McGraw-Hill (5 edn), p. 1396.
Leahy, J. G., & Colwell, R. (1990). Microbial degradation of hydrocarbons in the environment. Microbial Reviews, 54, 305–315.
Liu, Y., Li, I., Wu, Y., Tian, W., Zhang, L., Xu, I., Shen, Q., & Shen, B. (2010). Isolation of an alkane-degrading Alcanivorax sp. strain 2B5 and cloning of the alkB gene. Bioresource Technology, 101, 310–316.
Marchal, N., Bourdon, J.L., & Richard, C.L. (Eds.) (1982). Les milieux de culture pour l’isolement et l’identification biochimique des bactéries. Doin. 482 pp.
Marchal, R., Perret, S., Solano-Serena, F., & Vandecasteele, J. P. (2003). Gasoline and diesel oil biodegradation. Oil & Gas Science and Technology Review, IFP, 58(4), 441–448.
Mnif, S., Rojas, R., Miranda, C. D., Amaro, A. M., et al. (2009). Pathogenicity of a highly exopolysaccharide-producing Halomonas strain causing epizootics in larval cultures of the Chilean scallop Argopecten purpuratus. Microbiol Ecology., 57, 129–139.
Mnif, S., Chamkha, M., Labat, M., & Sayadi, S. (2011). Simultaneous hydrocarbon biodegradation and biosurfactant production by oil field-selected bacteria. Journal of Applied Microbiology, 111, 525–536.
Molina, M., Gonzalez, N., Bautista, L., Sanz, R., Simarro, R., Sanchez, I., & Sanz, J. (2009). Isolation and genetic identification of PAH degrading bacteria from microbial consortium. Biodegradation, 20(6), 789–800.
Moxley, K., & Schmidt, S. (2010). Preliminary characterization of an estuarine, benzoate-utilizing Vibrio sp. isolated from Durban harbour, South Africa. In A. Mendez-Vilas (Ed.), Current research, technology and education topics in applied microbiology and microbial biotechnology (Vol. 2, pp. 1249–1254).
Okamoto, T., Maruyama, A., Imura, S., Takeyama, H., & Naganuma, T. (2004). Comparative phylogenetic analyses of Halomonas variabilis and related organisms based on 16 r RNA, gyrB and ect BC. Gene sequences. Systematic and Applied Microbiology, 27, 323–333.
Olajire, A. A., & Essien, J. P. (2014). Aerobic degradation of petroleum components by microbial consortia. Petroleum & Environmental Biotechnology., 5, 195. doi:10.4172/2157-7463.1000195.
Perry, J. J., Staley, T. J., & Lory, S. (2004). Croissance des micro-organismes. In A. Sinauer (Ed.), Microbiologie, Inc, sous le titre Microbial Life ©2002, USA (p. 136).
Raeiney, F. A., Klatte, S., Kroppenstedt, R. M., & Stackebrandt, E. (1995). Dietzia, new genus including Dietzia maris comb. nov., formerly Rhodococcus maris. International Journal of Systematic and Evolutionary Microbiology, 45, 32–36.
Rahman, K. S. M., Thahira-Rahman, J., Lakshmanaperumalsamy, P., & Banat, I. M. (2004). Towards efficient crude oil degradation by a mixed bacterial consortium. Bioresource Technology, 85, 257–261.
Ratlege, C. (1978). Degradation of aliphatic hydrocarbons. In R. J. Atkinson (Ed.), Development in biodegradation of hydrocarbons (Vol. 1, pp. 1–46). London: Applied Sciences Publishers.
Riis, V., Kleinsteuber, S., & Babel, W. (2003). Influence of high salinities on the degradation of diesel fuel by bacteria consortia. Canadian Journal of Microbiology, 49, 713–772.
Rojas, R., Miranda, C. D., & Amaro, A. M. (2009). Pathogenicity of a highly exopolysaccharide-producing Halomonas strain causing epizootics in larval culture of the Chilean scallop Argopecten purpuratus. Microbial Ecology, 57, 129–139.
Saitsou, N., & Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406–425.
Sauret, C. (2011). Ecologie des communautés bactériennes marines soumises à une pollution pétrolière. Influence des facteurs environnementaux, de la prédation et de la réccurence des pollutions. Thèse de Doctorat. Université Pierre et Marie Curie. Paris 6. p 167.
Simon-Colin, C., Raguénès, G., Cozien, J., Guezennec, J. G. (2008). Halomonas profundus sp. nov, a new PHA-producing bacterium isolated from deep-sea hydrothermal vent shrimp. Journal of Applied Microbiology, 104, 1425–1432.
Sivaraman, C., Ganguly, A., Nikolausz, M., & Mutnuri, S. (2011). Isolation of hydrocarbonoclastic bacteria from bilge oil contaminated water. International journal of Environmental Science and Technology, 8(3), 461–470.
Soltani, M. (2004). Distribution lipidique et voies métaboliques chez quatre bactéries Gram-négatives hydrocarbonoclastes variation en fonction de la source de carbone. Thèse de Doctorat, University of Pierre et Marie curie. Paris 6. p 284.
Tamura, K., Nei, M., & Kumar, S. (2004). Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences (USA), 101, 11030–11035.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution., 24, 1596–1599.
Tantillo, G. M., Fontanarosa, M., Di Pinto, A., & Musti, M. (2004). Updated perspectives on emerging vibrios associated with human infections. Letters in Applied Microbiology., 39, 117–126.
Thompson, F. L., Lida, T., & Swings, J. (2004). Biodiversity of vibrios. Microbiology and Molecular Biology Reviews, 68, 403–431.
Van Hamme, J. D., & Ward, O. P. (2001). Physical and metabolic interactions of Pseudomonas sp. strain ja 5-b45 and Rhodococcus sp. strain f9-79 during growth on crude oil and effect of chemical surfactant on them. Appl. Environ Mirobiol., 67(10), 4874–4879.
Von der Weid, I., Marques, J. M., Cunha, C. D., Lippi, R. K., Dos Santos, S. C., Rosado, A. S., Lins, U., & Seldin, L. (2007). Identification and biodegradation potential of a novel strain of Dietzia cinnamea isolated from a petroleum-contaminated tropical soil. Systematic and Applied Microbiology., 30, 331–333.
Wang, Y. N., Cai, H., Chi, C. Q., Lu, A. H., Lin, X. G., Jian, Z. F., et al. (2007). Halomonas shengliensis sp. nov., a moderately halophilic, denitrifying, crude-oil-utilizing bacterium. International Journal of Systematic and Evolutionary Microbiology., 57, 1222–1226.
Wang, Z. F., Xiao, T., Pang, S., Liu, M., & Yue, H. (2009). Isolation and identification of bacteria associated with the surfaces of several algal spices. Chinese Journal of Oceanography and Limnology., 27, 487–492.
Xing Xang-Biao, W., Chang-Qiao, C., Yong, N., Yue-Qin, T., Yan, T., Gang, W., & Xiao-Lei, W. (2011). Degradation of petroleum hydrocarbons (C6–C40) and crude oil by a novel Dietzia strain. Bioresource Technology., 102, 7755–7761.
Yumoto, L., Nakamura, A., Iwata, H., Kojima, K., Kusumoto, K., Nodasaka, Y., & Matsuyama, H. (2002). Dietzia psychralcaliphila sp. nov., a novel facultatively psychrophilic alkaliphile that grows on hydrocarbons. International Journal of Systematic and Evolutionary Microbiology, 52, 85–90.
Zhang, Z., Hou, Z., Yang, C., Ma, C., Tao, F., & Xu, P. (2011). Degradation of n-alkanes and polycyclic aromatic hydrocarbons in petroleum by a newly isolated Pseudomonas aeruginosa DQ8. Bioresource. Technol., 102, 4111–4116.
Zvyagintseva, I. S., Poglasova, M. N., Gotoeva, M. T., & Belyaev, S. S. (2001). Effect of the medium salinity on oil degradation by Nocardioform bacteria. Microbiology, 70, 652–656.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 312 kb)
Rights and permissions
About this article
Cite this article
Feknous, N., Branes, Z., Rouabhia, K. et al. Isolation characterization and growth of locally isolated hydrocarbonoclastic marine bacteria (eastern Algerian coast). Environ Monit Assess 189, 49 (2017). https://doi.org/10.1007/s10661-016-5758-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5758-5