Abstract
Phenamacril (JS399-19 with independent intellectual property developed by China), azoxystrobin, and kresoxim-methyl are strobilurin fungicide. Due to their broad spectrum and good control of most of known fungi, strobilurin fungicide has been widely used in agriculture management. Thus, it is important to evaluate their environmental behaviors particularly in soils and underground water. In this study, the sorption/desorption and mobility of strobilurin fungicides in three Chinese soils (Jiangxi red soil, Taihu paddy soil, and Northeast China black soil) were conducted using comprehensively analytic approaches including batch experiment and soil thin-layer chromatography. The strobilurin fungicides were hard to be adsorbed in Jiangxi red soil but had medium adsorption capability in Tanhu paddy soil and Northeast China black soil, while the desorption of three strobilurin fungicides ranked in the order of Jiangxi red soil > Taihu paddy soil > Northeast China black soil. Soil properties including soil organic matter (SOM), pH, and cationic exchange capacity (CEC) affected the adsorption/desorption of the fungicides. Azoxystrobin and kresoxim-methyl had weak mobility in the soils. JS399-19 was moderately mobile in Jiangxi red soil but was not easily moved in Taihu paddy soil and Northeast China black soil. Due to their weak mobility in soils, these strobilurin fungicides tended to remain in the soil phase but not to shift downward to underground water. As azoxystrobin and JS399-19 had a long retention period in soil, there may become persistent residues in the soil environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Strobilurins were first discovered in 1977 by a group of German scientists. Since then, strobilurin fungicides have taken an important position in pesticide chemistry and become one of the most important classes of fungicides due to their stronger biological activities and lower toxicity (Gullino et al. 2002; Wang et al. 2015). Strobilurin is a group of agricultural fungicides for its unique mechanism, good environmental compatibility, and actual application to improve crop yield and quality (de Souza et al. 2009; Xu et al. 2014). They have a wide fungicidal spectrum and show good control of almost all known fungi. Recently, strobilurin fungicides have become the largest fungicide family in production and agronomic practice (Reuveni 2000; Sudisha et al. 2005). For example, azoxystrobin and kresoxim-methyl are among the typical strobilurin fungicides, and phenamacril (or JS399-19) is also a strobilurin fungicide with independent intellectual property developed by China (Li et al. 2008).
As the market share and widespread application of strobilurin fungicides are progressively rising, the negative influence of the fungicides on the environment has been emerging. The residues of some strobilurin fungicides in environment and soil were detected (Flores et al. 2007; Campillo et al. 2010; Dornellas et al. 2013; Abdelraheem et al. 2015). But, little is known about its environmental behaviors (e.g., the adsorption/desorption, leaching, and degradation as well as other environmental factors), particularly for azoxystrobin, kresoxim-methyl, and phenamacril. Hence, there is an urging need to investigate the fate of azoxystrobin, kresoxim-methyl, and phenamacril in agronomic soil and to develop strategies to minimize its residues and toxic effects on crop production.
As more than 80 % of the chemical pesticides eventually reside in soil after field application, even sprayed on the leave of crops and weeds, soil becomes the major sink for bulk of globally applied pesticides (Arias-Estévez et al. 2006; Fang et al. 2009; Pateiro-Moure et al. 2013). The environmental risk of pesticides mainly depends on its mobility in the soil (Rodríguez-Cruz et al. 2006; Jiang et al. 2011). The process of adsorption, desorption, and leaching plays a critical role in the environmental behavior of chemical pesticides in natural environments (Pateiro-Moure et al. 2010; González-Rodríguez et al. 2011). The efficiency of the process is dependent on many factors such as properties of the pesticide used, dissolved organic matter, soil properties, and environmental conditions (Kah and Brown 2006; Margoum et al. 2006; Pateiro-Moure et al. 2009a, b; Chen et al. 2010; Ding et al. 2011). Among the parameters, soil properties such as soil organic matter, clay content, pH, and CEC are predominant (Rodríguez-Cruz et al. 2006; Jiang et al. 2011). In this study, we investigated three Chinese soils with their different capabilities of adsorption, desorption, and mobility of JS399-19, azoxystrobin, and kresoxim-methyl. Influence of soil properties on adsorption and desorption was analyzed. Thus, the aim of the work was to figure out the mobility behavior of three strobilurin fungicides in the soils. These results may help our assessment of environmental risks or safety and efficient employment of the fungicides in agricultural practice.
Materials and methods
Materials and instruments
Strobilurin fungicides azoxystrobin, kresoxim-methyl, and phenamacril (or JS399-19) were used with a purity of 95.0, 97.5, and 95.0 %, respectively. Standard stock solutions of the strobilurin fungicides at 1000.0 mg L−1 were prepared with methanol. When used, each stock solution was diluted and merged. The tested soils including Jiangxi red soil, Taihu paddy soil, and Northeast black soil in China were collected, dried, ground, and sieved with 0.25 mm screen. Each type of soil was sterilized. The physical and chemical characteristics of each soil were presented in Table 1.
Analytical grade calcium chloride and ethyl acetate were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Acetonitrile (analytically pure) was purchased from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China). Methanol and acetonitrile of high-performance liquid chromatography (HPLC) grade were purchased from Merck Chemical Co., Ltd. (Darmstadt, Germany).
HPLC measurements were performed using Waters 2695 liquid chromatography with 2996 photodiode array detector (HPLC-PAD) (Waters Technologies, USA). Excella-E24 constant-temperature vibrator (Excella E24 Incubator Shaker Series, USA) and CR22GII high-speed centrifuge (Hitachi, Japan) were employed for sorption/desorption test. N-1001 rotary evaporator (EYELA, Japan) was used to concentrate solvents. Chromatographic tank (5 L) was used for thin-layer chromatography (TLC).
Sorption and desorption experiments
The sorption and desorption experiments were carried out using a batch equilibrium method according to the Organization for Economic Co-operation and Development Testing Guideline 106 (OECD 2000). Five grams of each soil sample was mixed with 50 mL of 0.01 M CaCl2 solution with the three strobilurin fungicide-merged concentrations (C 0) ranging from 0.5, 1.0, 1.5, and 2.0 mg L−1 in 150-mL conical flasks with stopper. The conical flasks with stopper were agitated in a horizontal shaker at 200 rpm and 25 ± 1 °C for 24 h. The soil suspensions were moved into the centrifuge tubes for centrifugation at 6000 rpm min−1 for 20 min. The supernatants were passed through 0.45-μm membrane filters and were determined for contents of pesticides by HPLC. Desorption experiments were performed immediately after the sorption experiments. All supernatants were thoroughly removed immediately. The remaining soils were used for desorption studies. Three strobilurin fungicide-free fresh CaCl2 solution (0.01 M, 40 mL) was added to each remaining soil. Shaking, subsequent separation of soil and aqueous phase, and quantification analyses of three strobilurin fungicides by HPLC were conducted as described above. The sorption and desorption experiments were repeated in triplicate. After sorption/desorption tests, the amount of three strobilurin fungicides sorbed on the soil was calculated from the difference between the initial and final solution concentrations (Cao et al. 2008).
Soil thin-layer chromatography
Ten grams of soil were mixed with 12 mL distilled water and spread onto 20 × 10 cm glass plates. The thickness of the soil layer was within 0.5–0.7 mm. The air-dried plates were marked with two horizontal lines in a distance of 2 cm (baseline) and 18 cm (foreland) from the base. A 10-μL droplet of 300 mg L−1 fungicide-mixed solution prepared in acetone was spotted onto the baseline of plate with the aid of a microsyringe. The plates were placed in closed individual glass chromatographic chambers and with the gradient in about 30°. Distilled water was used as developing solvents. After the developing solvents migrated to the distance of 18 cm from the baseline, the plates were taken out from glass chromatographic chambers and laid flat to dryness at room temperature. Soil thin layer was divided into eight segments from baseline to foreland. The strobilurin residues in each soil segment were extracted and quantified. The mobility distribution coefficient (R f ) and recovery (Re) of each fungicide on plate were calculated as the following formulas:
where L is the moving distance of fungicide from the start point to chromatographic spot center (mm) and L max is the moving distance of developing solvent from the start point (mm).
where m i is the amount of fungicide residue in each segment (mg); M 0 is the total amount of added pesticides (mg); i is the number of segments, i = 1, 2, 3, 4, 5, 6, 7, and 8, representing soil segment at 0∼2, 2∼4, 4∼6, 6∼8, 8∼10, 10∼12, 12∼14, and 14 ∼ 18 cm, respectively.
Determination of three strobilurin fungicides
Water sample was passed through 0.45-μm membrane filters and determined directly for pesticide content by HPLC. Soil sample was added with 30 mL acetonitrile and shaken at 200 rpm and 25 ± 1 °C for 1 h. Soil sample was centrifugated and filtrated. The process was performed for three times. Extracting solution was concentrated by rotary evaporation to remove organic solvent. The rest aqueous phase was extracted with 30 mL ethyl acetate for two times. The extracting solution was concentrated to dry by rotary evaporation. The residue was dissolved with acetonitrile, filtrated through a 0.45-μm microporous membrane, and measured by HPLC.
HPLC measurement of samples was performed using Waters HPLC-PAD with an XterraRRP18 column (250 × 4.6 mm, 5 μm) at 25 ± 5 °C. Acetonitrile/water (55:45, v/v) was used as mobile phase with a constant flow of 0.80 mL min−1. Aliquots of 10 μL sample extract were injected. Under detectable conditions, the retention times and detection wavelengths of JS399–19, azoxystrobin and kresoxim-methyl were 5.77 min and 290 nm, 8.95 min and 220 nm, 8.95 min and 220 nm, respectively.
Results and discussion
Analysis of three strobilurin fungicides in soil
To ensure that JS399-19, azoxystrobin, and kresoxim-methyl were well separated from environment matrix, the spiked recoveries of three strobilurin fungicide samples in soil were investigated. Under optimal operating conditions of HPLC-PAD, the spiked recoveries of JS399-19, azoxystrobin, and kresoxim-methyl at 0.05∼5.00 mg kg−1 were 85.0∼89.8, 85.4∼89.3, and 86.7∼91.0 % and the relative standard deviations (RSDs) were 0.16∼6.01, 2.13∼3.46, and 5.11∼8.02 %, respectively. These results indicated that the developed method could be used for accurate quantification of three strobilurin fungicides in soil.
Adsorption of three strobilurin fungicides
Adsorption of three strobilurin fungicides in different soils
To investigate the environmental behavior and fate of three strobilurin fungicides in different soils, the sorption and desorption behaviors of three strobilurin fungicides were investigated. If a pesticide is weakly adsorbed in soil, it usually has strong mobility and diffusivity in soil and, thus, easily goes downward and contaminates ground water and surrounding environment (Liu et al. 2010; Ding et al. 2011). The batch equilibrium method provides a basis for environmental safety assessment and registration of pesticide and is used to determine the adsorption of three strobilurin fungicides in soil.
Sorption kinetics study indicated that a 24-h period of equilibration was adequate to attain the sorption equilibrium for the three strobilurin fungicides. The isotherm model of Freundlich was chosen to evaluate the sorption process of the strobilurin fungicides on soil, as shown in the following equation:
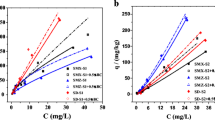
where K d (mL g−1) is the Freundlich sorption coefficient; 1/n is the linearity factor, Cs (mg kg−1) is the adsorption concentration of pesticide on soil; and Ce (mg L−1) is the equilibrium concentration of pesticide in solution. The values of K d and 1/n from Eq. (3) were calculated by regression for the sorption of three strobilurin fungicides on the three different soils. The adsorption isotherms and coefficients of the strobilurin fungicides in three soils were given in Table 2 and Fig. 1. The regression coefficient R 2 ranged from 0.9205 to 0.9857, indicating that Freundlich isotherms were appropriate to describe the sorption of three strobilurin fungicides on the soils.
Adsorption coefficient (K d ) represents adsorption degree and capability of pesticide in soil (Tang et al. 2009; Liu et al. 2010; Gao et al. 2014). The higher K d value indicates the strong pesticide adsorption and weak mobility of the pesticides. The three strobilurin fungicides show much different adsorption capabilities on the three different soils. The adsorption Freundlich coefficients (K d ) for the strobilurin fungicides on the soils were sorted as follows: Northeast China black soil > Taihu paddy soil > Jiangxi red soil. The coefficients varied from 3.65 to 46.5 (Table 2). According to the pesticide adsorption grades stated in Test Guidelines on Environmental Safety Assessment for Chemical Pesticides (USEPA. 2008a; USEPA. 2008b), when K d is less than 5, it is very difficult to adsorb; when K d is 5∼20, it is difficult to adsorb; when K d is 20∼50, it is medium adsorption. Under the conditions, JS399-19 (K d 5.20) and kresoxim-methyl (K d 5.77) were difficult to adsorb on to the Jiangxi red soil and had medium adsorption capability in Tanhu paddy soil and Northeast China black soil (K d 29.4∼46.5). Azoxystrobin was very difficult to adsorb in Jiangxi red soil (K d 3.65) and had medium adsorption capability in Taihu paddy soil (K d 20.0) and Northeast China black soil (K d 35.5) (Table 2).
Effect of soil property on adsorption
The correlation between the pesticide adsorption coefficient (K d ) in soil and the soil physicochemical property (Xu et al. 2005; Porfiri et al. 2015) can be used to predict the influence of soil property on the adsorption of the strobilurin fungicides in different soils. The values of soil pH, soil organic matter (SOM), and cation exchange capacity (CEC) were close to the correlation values of adsorption coefficient (Kd) for the three pesticides (Table 3), suggesting that the influences of soil pH, SOM content, and CEC on the adsorption of three strobilurin fungicides were close to each other in soil. In the strobilurin fungicides, the linear correlation of Kd with SOM, soil pH, and CEC was better for JS39-199 and azoxystrobin than for kresoxim-methyl (Table 3). Compared with soil pH, the correlation of Kd with CEC and SOM was better for the strobilurin fungicides.
Pesticide adsorption in soil was primarily related to soil physicochemical property and pesticide property, of which soil organic matter (SOM) content is an important factor that influences pesticide adsorption (Cao et al. 2008; Liu et al. 2012). SOM usually contains a certain amount of active functional groups, such as carboxyl, phenolic hydroxyl, carbonyl, ethanol-hydroxyl, methyl, etc. Nearly all organic pesticide molecules can react with the active functional groups in SOM to produce hydrogen bonds (Cao et al. 2008; Jiang et al. 2011). Besides, the results of IR, ESR, and fluorometric analyses indicated that there also exists other action mechanisms between pesticides and organic matters, such as covalent bond, ionic bond, coordinate bond, van der Waals’ force, charge dipole-dipole bond, etc. (Deng et al. 2007). In the tested soils, SOM content ranks in the order of Northeast China black soil > Taihu paddy soil > Jiangxi red soil (Table 1). The adsorption of the strobilurin fungicides was strongest in Northeast China black soil, weakest in Jiangxi red soil, and moderate in Taihu paddy soil (Fig. 1). The adsorptions of the strobilurin fungicides were positively related to the SOM content. The results were consistent with the previous reports (Rütters et al. 1999; Schwab et al. 2006; Wang and Keller 2009). In general, if the content of soil clay was higher, the soil particle was smaller and the specific surface area was larger and the adsorption quantity of pesticide was higher (Kim et al. 2012). In our study, the soil clay content was consistent with the ranking of adsorption quantity for Taihu paddy soil and Jiangxi red soil (Table 1 and Fig. 1). However, the soil clay content was lower in Northeast China black soil than that in Jiangxi red soil, but the adsorption capability was stronger in Northeast China black soil than that in Jiangxi red soil (Table 1 and Fig. 1), suggesting that SOM was a more influential factor than the soil clay content for the adsorption of strobilurin fungicides in Northeast China black soil.
Adsorption free energy (△G) of three strobilurin fungicides
Adsorption free energy (△G) is an important parameter that reflects adsorption property in soil. The △G value was used to evaluate the adsorption type of the strobilurin fungicides on soil. The relation between △G of pesticide in soil and organic adsorption coefficient (K oc) is presented by the following equation: △G = −RTlnK oc. The sorption coefficient normalized to the organic carbon content of the soil (K oc) in unit soil was also calculated by the equation: K oc = K f /SOM × 100. When the absolute value of △G is ≤40 kJ mol−1, it implicates a physical adsorption. Otherwise, it is a chemical adsorption. A pesticide with physical adsorption usually has a high adsorption equilibrium speed and its adsorption is reversible, while a pesticide with chemical adsorption has a low adsorption equilibrium speed and its adsorption is irreversible (Gao et al. 2014). When it is chemical adsorption, the adsorption easily passivates and becomes inactive (Porfiri et al. 2015). △G for the strobilurin fungicides in different soils showed negative values (Table 4), indicating that their adsorption belonged to the exothermic reaction and physical adsorption. The lower temperature was beneficial to the adsorption.
Influence of three strobilurin fungicides on ground water
In rural areas, the quality of drinking water is an important indicator of social and economic development. In many rural areas of China, drinking water is still directly taken from ground water without any treatment (Ding et al. 2015). As pesticides have been widely used, more attention has been paid to pesticide pollution to ground water in rural areas. The mobility and durability of pesticide are some principal factors of pesticide pollution for the ground water (Arias-Estévez et al. 2008). The ground ubiquity score (GUS) was chosen to evaluate the possibility of pesticide leaching, as presented by the following equation: GUS = lgT 1/2 (4 − lg K OC). According to the method (Michael 1995), pesticides are easy to leach when GUS is more than 2.8; pesticides have medium leaching property when GUS is 1.8∼2.8, whereas pesticides is not easy to leach when GUS is less than 1.8.
In our research, the GUS values of three strobilurin fungicides were less than 1.8 in Taihu Paddy Soil and Northeast China black soil (Table 5), suggesting that they were not easy to leach in the two soils. In Jiangxi red soil, the GUS values of JS399-19 and azoxystrobin were 2.63 and 3.13, respectively, indicating that JS399-19 had medium leaching property in the soil and some risk of pollution to the ground water. Azoxystrobin was easy to leach in the soil, which was likely to have a high risk of pollution to the ground water. As kresoxim-methyl had a very short half-life period in the three soils, it could be less likely to pollute the ground water.
Desorption of JS399-19, azoxystrobin, and kresoxim-methyl
Desorption of three strobilurin fungicides in different soils
To further investigate the soil environmental behavior of three fungicides, desorption of JS399-19, azoxystrobin, and kresoxim-methyl was tested after their adsorption in soils. The average desorption rates of JS399-19, azoxystrobin, and kresoxim-methyl were 45.6, 36.7, and 46.1 % in Jiangxi red soil; 30.2, 16.2, and 17.0 % in Taihu paddy soil; and 27.0, 10.1, and 12.8 % in Northeast China black soil, respectively (Table 6). These results indicated that three strobilurin fungicides were hard to be desorbed in Northeast China black soil and Taihu paddy soil but desorbed relatively easy in Jiangxi red soil. In Jiangxi red soil, the three fungicides show strong desorption performance.
Influence of soil property on desorption of three strobilurin fungicides
Analysis of the linear correlation between desorption of three strobilurin fungicides in the soils, along with their soil physicochemical properties, was conducted. The soil pH, SOM, and CEC were close to the desorption rate (Table 7), consistent with the correlation between the adsorption and soil properties. SOM was an important factor in the desorption of three fungicides. However, when SOM content was lower, the other soil properties such as soil types, pH, clay particle, and CEC also affected the adsorption/desorption of pesticides (Bresnahan et al. 2002; Singh 2002). Soil CEC was closely correlated with the desorption of three fungicides, with the correlation coefficient (R 2) being 0.9163–0.9323 (Table 7).
Mobility of three strobilurin fungicides in different soils
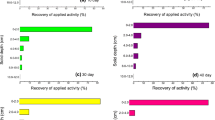
To assess the pesticide pollution to soil and ground water, soil thin-layer chromatography was employed to dissect the mobility and distribution of three fungicides in soil. The profiling of three fungicides in the different soils was shown in Table 8 and Fig. 2. JS399-19 in Jiangxi red soil, Taihu paddy soil, and Northeast China black soil moved a distance of 6–8, 4–6, and 4–6 cm in soil thin layer, respectively, indicating that the mobility of JS399-19 was faster in Jiangxi red soil than in other two soils. Azoxystrobin in Jiangxi red soil, Taihu paddy soil, and Northeast China black soil moved forward 4–6, 4–6, and 2–4 cm in soil thin layer, respectively. The maximum concentration of JS399-19 and azoxystrobin in three soils was all found in 0–2 cm. These results suggested that JS399-19 and azoxystrobin had weaker mobility in Northeast China black soil than in other two soils. Kresoxim-methyl in Jiangxi red soil, Taihu paddy soil, and Northeast China black soil moved for 8–10, 6–8, and 2–4 cm in soil thin layer, respectively, indicating that its mobility was slowest in Northeast China black soil and fastest in Jiangxi red soil.
The calculated mobility distribution coefficients (R fs ) of three fungicides were shown in Table 9. The R f values of JS399-19 in Jiangxi red soil, Taihu paddy soil, and Northeast China black soil were 0.08, 0.06, and 0.06, respectively. The R f values of azoxystrobin were 0.06 in three soils. The R f values of kresoxim-methyl in Jiangxi red soil, Taihu paddy soil, and Northeast China black soil were 0.17, 0.17, and 0.08, respectively. These results indicated that kresoxim-methyl in Jiangxi red soil and Taihu paddy soil was not easy to move, and JS399-19 and azoxystrobin in three soils and kresoxim-methyl in Northeast China black soil did not move at all.
Conclusions
Under the experimental condition, three fungicides JS399-19, azoxystrobin, and kresoxim-methyl were uneasy to be adsorbed in Jiangxi red soil. But they had a medium adsorption capability in Tanhu paddy soil and Northeast China black soil. Soil pH, SOM, and CEC were positively correlated with the adsorption and desorption of three fungicides. The strobilurin fungicides had weak mobility in soil. As azoxystrobin and JS399-19 had good stability in soil, they might cause pollution to soil. Further studies are required to track and monitor residues of the fungicides in soils and underground water.
References
Abdelraheem, E. M. H., Hassan, S. M., Arief, M. M. H., & Mohammad, S. G. (2015). Validation of quantitative method for azoxystrobin residues in green beans and peas. Food Chemistry., 182, 246–250.
Arias-Estévez, M., López-Periago, E., Martínez-Carballo, E., & Simal-Gándara, J. (2006). Carbofuran sorption kinetics by corn crop soils. Bulletin of Environmental Contamination and Toxicology, 77, 267–273.
Arias-Estévez, M., López-Periago, E., Martínez-Carballo, E., Simal-Gándara, J., Mejuto, J. C., & García-Río, L. (2008). The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agriculture, Ecosystems & Environment, 123, 247–260.
Bresnahan, G., Dexter, A., Koskinen, W., & Lueschen, W. (2002). Influence of soil pH-sorption interactions on the carry-over of fresh and aged soil residues of imazamox. Weed Research, 42, 45–51.
Campillo, N., Vinas, P., Aguinaga, N., Ferez, G., & Cordoba, M. H. (2010). Stir bar sorptive extraction coupled to liquid chromatography for the analysis of strobilurin fungicides in fruit samples. Journal of Chromatography. A, 1217, 4529–4534.
Cao, J., Guo, H., Zhu, H. M., Jiang, L., & Yang, H. (2008). Effects of SOM, surfactant and pH on the sorption–desorption and mobility of prometryne in soils. Chemosphere, 70, 2127–2134.
Chen, G., Lin, C., Chen, L., & Yang, H. (2010). Effect of size-fractionation dissolved organic matter on the mobility of prometryne in soil. Chemosphere, 79, 1046–1055.
de Souza, C. F., da Cunha, A. L. M. C., & Aucélio, R. Q. (2009). Determination of picoxystrobin and pyraclostrobin by MEKC with on-line analyte concentration. Journal of Chromatography. A, 70, 1461–1465.
Deng, J. C., Jiang, X., Lu, X., Yu, G. F., Wang, F., & Zhang, B. (2007). Atrazine adsorption behavior on a fluvo-aquic soil as influenced by contract periods. Pedosphere, 17(6), 786–791.
Ding, Q., Wu, H. L., Xu, Y., Guo, L. J., Liu, K., Gao, M. H., et al. (2011). Impact of low molecular weight organic acids and dissolved organic matter on sorption and mobility of isoproturon in two soils. Journal of Hazardous Materials, 190, 823–832.
Ding, J. J., Shen, X. L., Liu, W. P., Covaci, A., & Yang, F. X. (2015). Occurrence and risk assessment of organophosphate esters in drinking water from Eastern China [J]. The Science of the Total Environment, 538, 959–965.
Dornellas, R. M., Franchini, R. A. A., da Silva, A. R., Matos, R. C., & Aucelio, R. Q. (2013). Determination of the fungicide kresoxim-methyl in grape juices using square-wave voltammetry and a boron-doped diamond electrode. Journal of Electroanalytical Chemistry, 708, 46–53.
Fang, H., Yu, Y. L., Chu, X. Q., Wang, X. G., Yang, X. E., & Yu, J. Q. (2009). Degradation of chorpyrifos in laboratory soil and its impact on soil microbial functional diversity. Journal of Environmental Sciences, 21, 380–386.
Flores, J. L., Díaz, A. M. D., & Crdova, M. F. (2007). Determination of azoxystrobin residues in grapes, musts and wines with a multicommuted flow-through optosensor implemented with photochemically induced fluorescence. Analytica Chimica Acta, 585, 185–191.
Gao, M. X., Li, Y. Y., Yang, H., & Gu, Y. C. (2014). Sorption and desorption of pymetrozine on six Chinese soils. Frontiers of Environmental Science & Engineering, 4, 1–9.
González-Rodríguez, R. M., Rial-Otero, R., Cancho-Grande, B., Gonzalez-Barreiro, C., & Simal-Gándara, J. (2011). A review on the fate of pesticides during the processes within the food production chain. Critical Reviews in Food Science and Nutrition, 51, 99–114.
Gullino, M. L., Minuto, A., Gilardi, G., & Garibaldi, A. (2002). Efficacy of azoxystrobin and other strobilurins against Fusarium wilts of carnation, cyclamen and Paris daisy. Crop Protection, 21, 57–61.
Jiang, L., Ma, L., Sui, Y., Han, S. Q., & Yang, H. (2011). Mobilization and plant accumulation of prometryne in soil by two different sources of organic matter. Journal of Environmental Monitoring, 13, 1935–1943.
Kah, M., & Brown, C. D. (2006). Adsorption of ionisable pesticides in soils. Reviews of Environmental Contamination and Toxicology, 188, 149–217.
Kim, Y. K., Lim, S. J., Han, M. H., & Cho, J. Y. (2012). Sorption characteristics ofoxytetracycline, amoxicillin, and sulfathiazole in two different soil types. Geoderma, 185–186, 97–101.
Li, H. K., Diao, Y. M., Wang, J. X., Chen, C. J., Ni, J. P., & Zhou, M. G. (2008). JS399-19, a new fungicide against wheat scab. Crop Protection, 27, 90–95.
Liu, Y. H., Xu, Z. Z., Wu, X. G., Gui, W. J., & Zhu, G. N. (2010). Adsorption and desorption behavior of herbicide diuron on various Chinese cultivated soils. Journal of Hazardous Materials, 178, 462–468.
Liu, Z. Y., Guo, H. Q., He, H., & Sun, C. (2012). Sorption and cosorption of the nonionic herbicide mefenacet and heavy metals on soil and its components. Journal of Environmental Sciences, 24, 427–434.
Margoum, C., Malessard, C., & Gouy, V. (2006). Investigation of various physicochemical and environmental parameter influence on pesticide sorption to ditch bed substratum by means of experimental design. Chemosphere, 63, 1835–1841.
Michael, R.B. (1995). The environmental impact of pesticide degrades in groundwater. Herbicide metabolites in surface water and groundwater. ACE Symposium 630.
OECD (2000). Guidelines for the testing of chemicals. Test no. 106: adsorption desorption using a batch equilibrium method. Paris: OECD Organisation for Economic Co-Operation and Development.
Pateiro-Moure, M., Nóvoa-Muñoz, J. C., Arias-Estévez, M., López-Periago, E., Martínez-Carballo, E., & Simal-Gándara, J. (2009a). Quaternary herbicides retention by the amendment of acid soils with a bentonite-based waste from wineries. Journal of Hazardous Materials, 164, 769–775.
Pateiro-Moure, M., Pérez-Novo, C., Arias-Estévez, M., Rial-Otero, R., & Simal-Gándara, J. (2009b). Effect of organic matter and iron oxides on quaternary herbicide sorption-desorption in vineyard-devoted soils. Journal of Colloid and Interface Science, 333, 431–438.
Pateiro-Moure, M., Arias-Estévez, M., & Simal-Gándara, J. (2010). Competitive and non-competitive adsorption/desorption of paraquat, diquat and difenzoquat in vineyard-devoted soils. Journal of Hazardous Materials, 178, 194–201.
Pateiro-Moure, M., Arias-Estévez, M., & Simal-Gándara, J. (2013). Critical review on the environmental fate of quaternary ammonium herbicides in soils devoted to vineyards. Environmental Science & Technology, 47, 4984–4998.
Porfiri, C., Montoya, J. C., Koskinen, W. C., & Azcarate, P. (2015). Adsorption and transport of imazapyr through intact soil columns taken from two soils under two tillage systems. Geoderma, 251-252, 1–9.
Reuveni, M. (2000). Efficacy of trifloxystrobin (Flint), a new strobilurin fungicide, in controlling powdery mildews on apple, mango and nectarine, and rust on prune trees. Crop Protection, 19, 335–341.
Rodríguez-Cruz, M. S., Sánchez-Martín, M. J., Andrades, M. S., & Sánchez-Camazano, M. (2006). Comparison of pesticide sorption by physicochemically modified soils with natural soils as a function of soil properties and pesticide hydrophobicity. Soil and Sediment Contamination, 15, 401–415.
Rütters, H., Rosta, A. H., Kreuzig, R., & Bahadir, M. (1999). Sorption behavior of prochloraz in different soils. Journal of Agricultural and Food Chemistry, 47, 1242–1246.
Schwab, A. P., Splichal, P. A., & Banks, M. K. (2006). Absorption of atrazine and alachlor to aquifer material and soil. Water, Air, and Soil Pollution, 177, 119–134.
Singh, N. (2002). Sorption behavior of triazole fungicides in Indian soils and its correlation with soil properties. Journal of Agricultural and Food Chemistry, 50, 6434–6439.
Sudisha, J., Amruthesh, K. N., Deepak, S. A., Shetty, N. P., Sarosh, B. R., & Shetty, H. S. (2005). Comparative efficacy of strobilurin fungicides against downy mildew disease of pearl millet. Pesticide Biochemistry and Physiology, 81, 188–197.
Tang, Z. W., Zhang, W., & Chen, Y. M. (2009). Adsorption and desorption characteristics of monosulfuron in Chinese soils. Journal of Hazardous Materials, 166, 1351–1356.
USEPA (2008a). OPPTS 835.4100 and OPPTS 835.4200. Fate, transport and transformation test guidelines: aerobic soil metabolism and anaerobic soil metabolism. Washington DC: Environmental Protection Agency.
USEPA (2008b). OPPTS 835.1230. Fate, transport and transformation test guidelines: adsorption/desorption (batch equilibrium). Washington DC: Environmental Protection Agency.
Wang, P., & Keller, A. A. (2009). Sorption and desorption of atrazine and diuron onto water dispersible soil primary size fractions. Water Research, 43, 1448–1456.
Wang, C., Wu, J. X., Zhang, Y., Wang, K., & Zhang, H. Y. (2015). Field dissipation of trifloxystrobin and its metabolite trifloxystrobin acid in soil and apples. Environmental Monitoring and Assessment, 187, 4100.
Xu, D. P., Xu, Z. H., Zhu, S. Q., Cao, Y. Z., Wang, Y., & Du, X. M. (2005). Adsorption behavior of herbicide butachlor on typical soils in China and humic acids from the soil samples. Journal of Colloid and Interface Science, 285, 27–32.
Xu, C. Y., Hou, Y. P., Wang, J. X., Yang, G. F., Liang, X. Y., & Zhou, M. G. (2014). Activity of a novel strobilurin fungicide benzothiostrobin against Sclerotinia sclerotiorum. Pesticide Biochemistry and Physiology, 115, 32–38.
Acknowledgments
The authors acknowledge the financial support of the Special Fund for Agro-scientific Research in the Public Interest (No. 201203022) from the Ministry of Agriculture of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, P., Wu, W.Z., Han, Z.H. et al. Desorption and mobilization of three strobilurin fungicides in three types of soil. Environ Monit Assess 188, 363 (2016). https://doi.org/10.1007/s10661-016-5372-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5372-6