Abstract
Introduction of heavy metals in the environment by various anthropogenic activities has become a potential treat to life. Among the heavy metals, cadmium (Cd) shows relatively high soil mobility and has high phyto-mammalian toxicity. Integration of soil remediation and ecosystem services, such as carbon sequestration in soils through organic amendments, may provide an attractive land management option for contaminated sites. The application of biochar in agriculture has recently received much attention globally due to its associated multiple benefits, particularly, long-term carbon storage in soil. However, the application of biochar from softwood crop residue for heavy metal immobilization, as an alternative to direct field application, has not received much attention. Hence, a pot experiment was conducted to study the effect of pigeon pea biochar on cadmium mobility in a soil-plant system in cadmium-spiked sandy loam soil. The biochar was prepared from pigeon pea stalk through a slow pyrolysis method at 300 °C. The experiment was designed with three levels of Cd (0, 5, and 10 mg Cd kg−1 soil) and three levels of biochar (0, 2.5, and 5 g kg−1 soil) using spinach as a test crop. The results indicate that with increasing levels of applied cadmium at 5 and 10 mg kg−1 soil, the dry matter yield (DMY) of spinach leaf decreased by 9.84 and 18.29 %, respectively. However, application of biochar (at 2.5 and 5 g kg−1 soil) significantly increased the dry matter yield of spinach leaf by 5.07 and 15.02 %, respectively, and root by 14.0 and 24.0 %, respectively, over the control. Organic carbon content in the post-harvest soil increased to 34.9 and 60.5 % due to the application of biochar 2.5 and 5 g kg−1 soil, respectively. Further, there was a reduction in the diethylene triamine pentaacetic acid (DTPA)-extractable cadmium in the soil and in transfer coefficient values (soil to plant), as well as its concentrations in spinach leaf and root, indicating that cadmium mobility was decreased due to biochar application. This study shows that pigeon pea biochar has the potential to increase spinach yield and reduce cadmium mobility in contaminated sandy soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals generally originate from parent material as a consequence of rock weathering. However, soil pollution with heavy metals has increased in recent years due to various human activities such as mining, atmospheric fall out emissions from industrial and vehicular activities, contaminated waste water irrigation (municipal and industrial wastewater), phosphate fertilizers, and sewage sludge application to soil. Among the heavy metals, Cd is widely used in different applications (such as batteries, pigments, stabilizers for PVC, and alloys) and it enters into the soil system by several means. Increased Cd levels can cause nephrotoxicity, osteotoxicity, cancer, and Itai-Itai disease in human beings (WHO 1996). As the above activities are unavoidable for the progress of civilization, the search for effective and feasible remedial measures to address land pollution has become important. Removal of heavy metals from soil and stabilization of heavy metals in soil to minimize their toxicity continues as important remediation approaches. Remedial techniques for cleaning the soil are either very costly, involving engineering approaches (physical and chemical), or very slow (phytoremediation). On the other hand, metal stabilization through the processes of adsorption, binding or co-precipitation with amendments has been widely studied in the last decade (Kumpiene et al. 2008) as it permits profitable and safe utilization of contaminated land (Clemente and Bernal 2006). Of the several amendments used for in situ stabilization of contaminants, organic materials, such as biosolids, manures, and composts, have proved to be successful in reducing the mobility of metal contaminants in multi-metal polluted soils (Clemente and Bernal 2006). Furthermore, increasing interest in integrating remediation and the provision of ecosystem services, such as carbon sequestration in soil, has provided an attractive land management option for contaminated sites using materials rich in carbon.
Biochar, carbonaceous residues of incomplete burning of carbon-rich biomass, has gained significant importance in recent days as a soil amendment because of its potential benefits in carbon sequestration in soil (Lehmann 2007). Recent studies have further indicated that it is an effective soil amendment to improve the fertility of soil (Asai et al. 2009; Hossain et al. 2010; Laird et al. 2010) and to immobilize organic and inorganic contaminants (Brennan et al. 2014; Fellet et al. 2014; Kumari et al. 2014; Mendez et al. 2014). The mechanisms involved in immobilization of inorganic pollutants by biochar have been reviewed recently (Paz-Ferreiro et al. 2014). Among these mechanisms, the contribution to pH changes was highly specific to biochar to decrease the mobility of the pollutant (Lu et al. 2014). Also, biochar is known to have a highly porous structure, high specific surface area (Downie et al. 2009), and cation exchange capacity (Glaser and Birk 2012) as well as various functional groups (Mumme et al. 2011; Libra et al. 2011), leading to the assumption that biochar added to the soils may provide negatively charged surfaces for ion exchange that are responsible for the ability to sorb heavy metals. Hence, biochar plays an important role in controlling levels of organic and inorganic pollutants in the environment. The importance of Cd over other heavy metals is that the transfer rate (from soil to plant) is relatively high due to lower interaction of Cd with soil components. The immobilization of cadmium by biochar has been recently studied by many authors (Uchimiya et al. 2010; Houben et al. 2013; Lu et al. 2014, 2015a, b; Brendova et al. 2015) and they have mainly focused on the potential of a combination of biochar amendment and phytoremediation technologies. However, the effect of biochar produced particularly from crop residues (pigeon pea) on plant growth and heavy metal (Cd) immobilization has not been studied. The behavior of biochar in the soil and its capacity to immobilize heavy metals may governed by many factors and more importantly the feedstock used for biochar production (Mukherjee et al. 2014). Hence, the present investigation aims to determine the effects of pigeon pea biochar on cadmium (Cd) mobility in a soil-plant system and spinach yield.
Materials and methods
Preparation of materials used in pot culture experiment and their characterization
A greenhouse experiment was conducted to evaluate the effects of biochar on spinach yield and on cadmium mobility in a soil-plant system. The biochar was prepared at the Central Institute of Agricultural Engineering (CIAE), Bhopal, using pigeon pea stalk as a feedstock material. The pigeon pea stem was cut into pieces (10–20 cm) and after drying, the biomass was pyrolyzed at 300 °C for 2 h, followed by quenching and subsequent drying in an oven at 105 °C. The biochar was then crushed in a 24 blade Rotar Mill (Model. No. Pulversittee 14) and sieved to obtain a uniform 53–75 μm particle size. It was then analyzed for plant nutrients and heavy metal content (Table 1).
The experimental soil for pot culture experiment was collected from the surface soil (0–15 cm) layer of an agricultural field in village Nipaniya Jatkhedi, Bhopal, processed and passed through a 2 mm sieve. A representative sieved soil sample was analyzed for its physico-chemical properties by using standard methods (Table 2). The experimental soil was sandy loam in texture had pH 7.9 and electrical conductivity (EC) 0.12 dSm−1. The soil was low in organic carbon (OC), available nitrogen (N), available phosphorus (P), available potassium (K), and available sulfur (S) content, indicating nutrient-poor status. The diethylene triamine pentaacetic acid (DTPA)-extractable heavy metal content was 9.30, 0.03, 0.34, 0.09, 0.60, and 0.32 mg kg−1 for Cu, Cd, Pb, Cr, Ni, and Zn, respectively (Table 2).
Treatment details, imposition, sowing, and aftercare
Pot experiment was carried out with spinach as a test crop in sandy loam soil. Five kilograms of processed soil were filled in wide-mouthed glazed pots of 7 kg capacity. The experiment was designed with three levels of Cd (0, 5, and 10 mg Cd kg−1 soil) and three levels of biochar (0, 2.5, and 5 g kg−1 soil) applied in every possible combination. The required amount of cadmium was added in solution form as Cd (NO3)2.4H2O, whereas the biochar was applied in powder form, and both were mixed thoroughly with soil. Potted soils treated with biochar and/or cadmium were allowed to equilibrate for 1 week in a moist condition prior to sowing spinach seeds.
Uniform doses of N (0.03 g N kg−1 soil), P (0.018 g P2O5 kg−1 soil) and K (0.018 g K2O kg−1 soil) were supplied in the form of urea, di-ammonium phosphate, and potassium chloride, respectively, in order to ensure adequate supply of nutrients. The entire dose of P, K, and half of the dose of N were applied basally before sowing, while the remaining N was top-dressed 20 days after sowing. The seeds of spinach (variety Selection-1) were treated with Bavistin @ 2 g kg−1 seed in order to avoid fungal infection. Ten healthy seeds of spinach were sown by making 2–3 cm deep holes equidistantly in the soil before being covered. After germination, all seedlings were allowed to grow for 10 days, after which they were thinned out to leave five healthy plants in each pot.
Harvesting, processing, and analysis of plant and soil samples
After 60 days of sowing, the above-ground portions (leaf) and roots of the spinach plants were harvested separately, washed with distilled water, and air-dried. Roots were washed thoroughly with tap water to remove adhering soil particles, followed by washing with dilute hydrochloric acid (HCl) and then distilled water in sequence. The air-dried leaf and root samples were then oven-dried at 65 °C until their weights remained constant. Oven-dried plant parts (leaves and roots) were ground in a Willey mill and passed through a 2 mm sieve. Homogenized tissue samples were digested in a di-acid mixture containing nitric acid (HNO3) and perchloric acid (HClO4) (in the ratio 9:4 v/v) on a hot plate at 150–175 °C for about 2 h until a clear liquid was obtained.
Soil samples were collected separately from each pot for soil analysis after the harvest of the crop. The pH and electrical conductivity (EC) of post-harvest soil samples were measured in soil:water solution of 1:2 ratio, whereas soil organic carbon was estimated by following a wet oxidation method (Walkley and Black 1934). Soil samples were digested in the di-acid mixture (HNO3/HClO4 at 9:4 ratio) for total Cd content and plant-available soil Cd was extracted by the DTPA extractant (Lindsay and Norvell 1978). The concentrations of cadmium in digested samples (plant and soil) and in DTPA extractant were determined using an inductively coupled plasma-optical emission spectrophotometer (Perkin Elmer Optima DV 2100).
Statistical analysis
Two-way analysis of variance technique was carried out on each parameter in the experiment as applicable to factorial completely randomized design (CRD) (Gomez and Gomez 1984). To determine the significance of the difference between means of two treatments, the least significant difference was estimated at 5 % probability level and Duncan’s multiple range tests were used for comparing the means.
Results and discussion
Influence of biochar on soil pH, EC, and organic carbon content
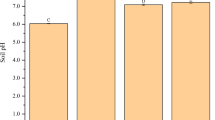
Biochar application resulted in increase in soil pH from 8.12 to 8.19, EC from 0.19 to 0.21 dSm−1, and organic carbon content from 0.43 to 0.70 % (Table 3). The increase in pH by 0.04 and 0.09 units was observed at 2.5 and 5 g kg−1 soil application of biochar. Further increasing levels of cadmium in the biochar amended soil significantly increased the soil pH (8.29) and EC (0.28 dSm−1). Several investigations also showed that soil pH, EC, and plant-available nutrients were increased as a result of biochar application (Amonette and Joseph 2009; Warnock et al. 2007; Nigussie et al. 2012). The presence of carbonates of alkali and alkaline earth metals, sesquioxides, silica, and plant nutrients, particularly N, P, K, and S, in ash residues of biochar might be the reason for increase in soil pH and EC (Clarholm 1994; Mahmood et al. 2003). Application of biochar at 2.5 and 5 g kg−1 soil resulted in 34.9 and 60.5 % increase in soil organic carbon (SOC) content, respectively, over the control (0 g kg−1 soil). The results further showed that the magnitude of increase in SOC in post-harvest soil due to biochar treatments was almost equal to the amount of carbon added through biochar. This shows that carbon mineralization from biochar during the crop growth period was almost negligible due to its wider C:N ratio (56:1). This may be due to recalcitrant nature of most of the carbon present in biochar (Fierer et al. 2001).
DTPA-extractable cadmium and transfer coefficient for cadmium in a soil-plant system
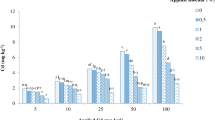
Significant reduction in DTPA-extractable cadmium was observed with increasing levels of biochar application (Fig. 1). The DTPA-extractable cadmium averaged over all cadmium levels ranged from 1.93 (at 0 g biochar kg−1 soil) to 1.13 mg kg−1 (at 5 g biochar kg−1 soil). Application of biochar at 2.5 and 5 g kg−1 decreased the extractability of cadmium by 27.2 and 41.1 %, respectively. As intended, increasing levels of cadmium application in soil significantly increased DTPA-extractable cadmium. The highest value (3.92 mg kg−1) for DTPA-extractable cadmium was observed at the highest level of Cd (10 mg kg−1) in the absence of biochar application. Namgay et al. (2010) showed that heavy metal mobility in soil is greatly reduced as a result of biochar application. Biochar application might have improved the overall sorption capacity of soil and hence, reduced Cd mobility in soil.
Transfer coefficient for Cd, which is the ratio of Cd concentration in plants to that in soil, was 4.36 in the absence of biochar but reduced significantly to 2.81 with 5 g biochar kg−1 soil application (Fig. 1). This indicates that biochar application reduced cadmium uptake by the above-ground biomass of spinach. This reduced uptake of cadmium by plants is also evident in its decreased extractability (by DTPA) from soil due to biochar application. Increasing levels of cadmium increased the transfer coefficient values for cadmium significantly. Various studies have shown that the mobility of metals in soils and their uptake by plants can be reduced by the addition of biochar (Namgay et al. 2010; Fellet et al. 2014; Park et al. 2011). The application of biochar reduces the soil solution concentration of heavy metals (such as DTPA-extractable Cd in this study) by making stable organic matter complexes with heavy metals, by surface sorption of heavy metals onto different functional groups of biochar (Beesley et al. 2011; Lu et al. 2012), the exchange of heavy metals with cations associated with biochar, such as Ca2+, Mg2+ (Lu et al. 2012), K+, and Na+ (Uchimiya et al. 2011), or due to physical adsorption (Lu et al. 2012). Another mechanism for the immobilization of heavy metals might be an increase in the soil pH, leading to an increase in the sorption capacity of functional groups of variable charges or precipitation of pollutants (Cao et al. 2009). All these mechanisms together might have resulted in the reduction in Cd mobility, Cd concentrations in spinach leaf and root as well as in the transfer coefficients in biochar amended soils.
Impact of biochar on dry matter yield of spinach
The main observable effect of cadmium on spinach leaf and root was the significant decrease in dry matter yields over the control (Fig. 2). The per cent decrease in dry matter yield of spinach leaf at 5 and 10 mg Cd kg−1 soil averaged over all biochar levels were 9.84 and 18.29 %, respectively, over control value. However, application of biochar (at 2.5 and 5 g kg−1 soil) significantly increased the dry matter yield of spinach leaf. The per cent increase in dry matter yield at 2.5 and 5 g biochar kg−1 soil application was 5.07 and 15.02 %, respectively, over the control. The highest dry matter yield of 7.90 g pot−1 was observed when biochar was applied at the rate of 5 g kg−1 soil in the absence of cadmium. Similar trends were also observed in spinach roots as a result of biochar and cadmium application. Biochar at 2.5 and 5 g kg−1 soil application significantly increased the dry matter yield of spinach roots in comparison to the control by 14.0 and 24.0 %, respectively.
One of the most interesting and potentially important properties of biochar is its effect on crop yield. There are also reports from around the world, indicating yield improvement ranging from 30 to 100 % in soybean, maize, carrots, and beans as a result of biochar application (Yamato et al. 2006; Rondon et al. 2007; Kimetu et al. 2008). It was clear from our experiment that biochar application significantly increased the dry matter yield of spinach leaf and root. Enhanced soil physical properties and a conducive chemical (pH) and biological environment (organic carbon content) along with increased nutrient availability in the biochar-amended soil might have contributed towards the observed increase in spinach yield (Blackwell et al. 2009).
On the other hand, the dry matter yield of spinach leaf and root was significantly reduced with increase in cadmium levels probably due to the toxic effects of Cd on several plant physiological processes (Krupa et al. 1987). Our results were similar to those results reported by Namgay et al. (2010) where cadmium applied at 50 mg Cd kg−1 soil resulted in the reduction of dry matter yield of tomato by 27 %. However, such toxic effects of cadmium on dry matter yield were considerably reduced as a result of biochar application.
Effect of biochar on cadmium content and uptake in leaf and root of spinach
Cadmium content and its uptake in leaf and root of spinach crop were significantly increased with increasing levels of cadmium (Table 4). The average cadmium content of spinach leaf and root ranged from 0.20 to 55.57 mg kg−1 and 1.47 to 84.80 mg kg−1, respectively. In general, cadmium content was higher in root than in leaf. Haghiri (1973) also reported that cadmium content in wheat and soybean leaves was significantly influenced by cadmium applied in the soil. Thus, application of Cd in soil significantly increased the cadmium uptake in the plant biomass.
However, biochar application at both the levels (2.5 and 5 g kg−1 soil) significantly reduced the leaf and root cadmium content of spinach over control values in a concentration-dependent manner. Application of biochar at 5 g kg−1 soil reduced the Cd content of spinach leaf significantly from 27.2 to 20.5 mg kg−1 and 55.6 to 20.5 mg kg−1 in 5 and 10 mg kg−1 Cd-spiked soils, respectively. Similarly, the root Cd contents were reduced from 45.1 to 30.0 mg kg−1 and 84.5 to 54.3 mg kg−1 in 5 and 10 mg kg−1 Cd-spiked soils, respectively (Table 4). The per cent decrease in cadmium content of spinach leaf averaged over all the cadmium levels was 12.16 and 25.50 % at 2.5 and 5 mg kg−1 soil application of biochar, respectively, over control values. Similarly, at 2.5 and 5 g kg−1 soil application of biochar, the per cent decrease in spinach root cadmium content was 21.75 and 35.06 %, respectively. The results further showed that biochar application at both the levels of 2.5 and 5 g kg−1 soil significantly reduced cadmium uptake as compared to the soil without any biochar. However, there was no significant difference observed in the reduction in Cd uptake between the two biochar levels.
Recent studies also showed that the addition of biochar to soil has led to definite increase in cation exchange capacity (CEC), pH, and improvement in other soil properties (Lehmann et al. 2003; Liang et al. 2006; Solomon et al. 2007). Therefore, biochar application in our study might have improved the overall sorption capacity of the soil, resulting in reduced heavy metal mobility in the soil and ultimately, lower cadmium content in spinach leaf and root. Namgay et al. (2010) also showed that biochar application decreased the concentration of Cd in maize shoots, which can be attributed to the formation of stable metal-organic complexes. It is evident from our results that significant difference is observed in spinach leaf and root Cd concentrations but not Cd uptake by spinach leaf and root for biochar application at 2.5 and 5 g kg−1 soil. Cadmium uptake is influenced by both DMY and Cd concentration, two very different factors as yield is significantly increased whereas Cd concentration is significantly decreased between biochar treatments. Hence, uptake, which is the product of these two parameters, is ultimately nullified. Therefore, there was no significant difference observed between the two biochar levels with respect to Cd uptake.
Conclusions
In the recent past, biochar has gained global importance for its role in carbon sequestration potential in soil. Therefore, application of carbon-rich amendments like biochar in heavy metal-contaminated sites provides better options for managing contaminated soil as well as for enhancing carbon sequestration in the degraded soil. From the present investigation, it can be observed that application of 5 g kg−1 soil of pigeon pea biochar increased dry matter yield of spinach by 15 %. Cadmium mobility in soil and its uptake by spinach leaf and root was considerably reduced in biochar-amended sandy loam soil spiked/artificially contaminated with cadmium. Moreover, it is evident that the DTPA-extractable cadmium in soil and transfer coefficient values (from soil to plant system) for cadmium was reduced in biochar-amended soil. This indicates that biochar reduced the mobility of the toxic trace element, cadmium, in soil. Soil pH and organic carbon content generally play major roles in heavy metal mobility in soil. In our study also, it was clear that increase in pH and OC played substantial roles in reducing the mobility of Cd in soil and its subsequent uptake by spinach crops through organic matter-trace metal complexes or adsorption processes. In conclusion, our study has demonstrated that pigeon pea biochar had the intrinsic potential to increase spinach yield and reduce cadmium mobility in sandy loam soil contaminated with cadmium.
References
Amonette, J. E., & Joseph, S. (2009). Characteristics of biochar: microchemical properties. In J. Lehmann & S. Joseph (Eds.), Biochar for environmental management science and technology (pp. 33–52). London: Earthscan.
Asai, H., Samson, B. K., Stephan, H. M., Songyikhangsuthor, K., Homma, K., Kiyono, Y., Inoue, Y., Shiraiwa, T., & Horie, T. (2009). Biochar amendment techniques for upland rice production in Northern Laos 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Research, 111, 81–84.
Beesley, L., Moreno-Jiménez, E., Gómez-Eyles, J. L., Harris, E., Robinson, B., & Sizmur, T. (2011). A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environmental Pollution, 159, 3269–3282.
Blackwell, P., Riethmuller, G., & Collins, M. (2009). Biochar application to soil. In J. Lehmann & S. Joseph (Eds.), Biochar for environmental management: science and technology (pp. 207–226). London: Earthscan.
Brendova, K., Tlustos, P., & Szakova, J. (2015). Biochar immobilizes cadmium and zinc and improves phytoextraction potential of willow plants on extremely contaminated soil. Plant, Soil and Environment, 61, 303–308.
Brennan, A., Moreno Jimenez, E., Alburquerque, J. A., Knapp, C. W., & Switzer, C. (2014). Effects of biochar and activated carbon amendment on maize growth and the uptake and measured availability of polycyclic aromatic hydrocarbons (PAHs) and potentially toxic elements (PTEs). Environmental Pollution, 193, 79–87.
Cao, X. D., Ma, L. N., Gao, B., & Harris, W. (2009). Dairy-manure derived biochar effectively sorbs lead and atrazine. Environmental Science and Technology, 43, 3285–3291.
Clarholm, M. (1994). Granulated wood ash and a ‘N-free’ fertilizer to forest soil: effects on P availability. Forest Ecology and Management, 66, 127–136.
Clemente, R., & Bernal, M. P. (2006). Fractionation of heavy metals and distribution of organic carbon in two contaminated soils amended with humic acids. Chemosphere, 64, 1264–1273.
Downie, A., Crosky, A., & Munroe, P. (2009). Physical properties of biochar. In J. Lehmann & S. Joseph (Eds.), Biochar for environmental management: science and technology (pp. 13–32). London: Earthscan.
Fellet, G., Marmiroli, M., & Marchiol, L. (2014). Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Science of the Total Environment, 468–469, 598–608.
Fierer, N., Schimel, J. P., Cates, R. G., & Zou, J. (2001). Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biology and Biochemistry, 33, 1827–1839.
Glaser, B., & Birk, J. J. (2012). State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de Indio). Geochimica et Cosmochimica Acta, 82, 39–51.
Gomez, K. A., & Gomez, A. A. (1984). Statistical procedures for agricultural research. Singapore: Wiley.
Haghiri, F. (1973). Cadmium uptake by plants. Journal of Environmental Quality, 2, 93–96.
Hossain, M. K., Strezo, V., Cha, K. Y., & Nelson, P. F. (2010). Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere, 78, 1167–1171.
Houben, D., Evrard, L., & Sonnet, P. (2013). Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere, 92, 1450–1457.
Kimetu, J. M., Lehmann, J., Ngoze, J., Mugendi, S., Kinyangi, D. N., Riha, J., Verchot, S., Recha, L., & Pell, J. W. (2008). Reversibility of productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems, 45, 123–126.
Krupa, Z., Skorzynska, E., Maksymiec, W., & Baszyński, T. (1987). Effect of cadmium treatment on photosynthetic apparatus and its photochemical activities in greening radish seedlings. Photosynthetica, 21, 156–164.
Kumari, K. G. I. D., Moldrup, P., Paradelo, M., & de Jonge, L. W. (2014). Phenanthrene sorption on biochar-amended soils: application rate, aging and physicochemical properties of soil. Water, Air, and Soil Pollution, 225, 2105.
Kumpiene, J., Lagerkvist, A., & Maurice, C. (2008). Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–a review. Waste Management, 28, 215–225.
Laird, D. A., Fleming, P., Davis, D. D., Horton, R., Wang, B., & Karlen, D. L. (2010). Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma, 158, 443–449.
Lehmann, J. A. (2007). A handful of carbon. Nature, 447, 143–144.
Lehmann, J., Pereira, D. A., Silva, J. R. J., Steiner, C., Nehls, T., Zech, W., & Glaser, B. (2003). Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant and Soil, 249, 343–357.
Liang, B., Lehmann, J., Solomon, D., Kinyangi, J., Grossman, J., O’Neill, B., Skjemstad, J. O., Thies, J., Luizao, F. J., Peterson, J., & Neves, E. G. (2006). Black carbon increases cation exchange capacity in soils. Soil Science Society of America Journal, 70, 1719–1730.
Libra, J. A., Ro, K. S., Kammann, C., Funke, A., Berge, N. D., Neubauer, Y., Titirici, M. M., Fühner, C., Bens, O., Kern, J., & Emmerich, K. H. (2011). Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels, 2, 71–106.
Lindsay, W. L., & Norvell, W. A. (1978). Development of DTPA soil test for zinc, iron, manganese and copper. Soil Science Society of America Journal, 42, 421–428.
Lu, H., Zhang, Y. Y., Huang, X., Wang, S., & Qiu, R. (2012). Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Resources, 46, 854–862.
Lu, H., Li, Z., Fu, S., Mendez, A., Gasco, G., & Paz-Ferreiro, J. (2014). Can biochar and phytoextractors be jointly used for cadmium remediation? PloS One, 9, e95218.
Lu, H., Li, Z., Fu, S., Mendez, A., Gasco, G., & Paz-Ferreiro, J. (2015a). Effect of biochar in cadmium availability and soil biological activity in an anthrosol following acid rain deposition and aging. Water, Air, and Soil Pollution, 226, 164–175.
Lu, H. P., Li, Z. A., Fu, S., Mendez, A., Gasco, G., & Paz-Ferreiro, J. (2015b). Combining phytoextraction and biochar addition improves soil biochemical properties in a soil polluted with Cd. Chemosphere, 119, 209–216.
Mahmood, S., Finlay, R. D., Fransson, A. M., & Wallander, H. (2003). Effects of hardened wood ash on microbial activity, plant growth and nutrient uptake by ectomycorrhiza spruce seedlings. FEMS Microbiology Ecology, 43, 121–131.
Mendez, A., Paz-Ferreiro, J., Araujo, F., & Gasco, G. (2014). Biochar from pyrolysis of deinking paper sludge and its use in the treatment of a nickel polluted soil. Journal of Analysis Applied Pyrology, 107, 46–52.
Mukherjee, A., Zimmerman, A. R., Hamdan, R., & Cooper, W. T. (2014). Physicochemical changes in pyrogenic organic matter (biochar) after 15 months of field aging. Solid Earth, 5, 693–704.
Mumme, J., Eckervogt, L., Pielert, J., Diakite, M., Rupp, F., & Kern, J. (2011). Hydrothermal carbonization of anaerobically digested maize silage. Bioresource Technology, 102, 9255–9260.
Namgay, T., Singh, B., & Singh, B.P. (2010). Plant availability of arsenic and cadmium as influenced by biochar application to Soil. Proceedings of 19th World Congress of Soil Science, Soil Solutions for a Changing World (Brisbane, Australia,1 – 6 August 2010), pp.78-81.
Nigussie, A., Kissi, E., Misganaw, M., & Ambaw, G. (2012). Effect of biochar application on soil properties and nutrient uptake of lettuces (Lactuca sativa) grown in chromium polluted soils. American-Eurasian Journal of Agricultural and Environmental Sciences, 12(3), 369–376.
Park, J. H., Choppala, G. H., Bolan, N. S., Chung, J. W., & Chuasavathi, T. (2011). Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant and Soil, 348, 439–451.
Paz-Ferreiro, J., Lu, H., Fu, S., Méndez, A., & Gascó, G. (2014). Use of phytoremediation and biochar to remediate heavy metal polluted soils: a review. Solid Earth, 5, 65–75.
Rondon, M. A., Lehmann, J., Ramirez, J., & Hurtado, M. (2007). Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biology and Fertility of Soils, 43, 699–708.
Solomon, D., Lehmann, J., Kinyangi, J., Amelung, W., Lobe, I., Pell, A., Riha, S., Ngoze, S., Verchot, L., Mbugua, D., Skjemstad, J., & Schafer, T. (2007). Long-term impacts of anthropogenic perturbations on dynamics and speciation of organic carbon in tropical forest and subtropical grassland ecosystems. Global Change Biology, 13, 511–530.
Uchimiya, M., Lima, I. M., Klasson, K. T., Chang, S., Wartelle, L. H., & Rodgers, J. E. (2010). Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. Journal of Agricultural Food Chemistry, 58, 5538–5544.
Uchimiya, M., Wartelle, L. H., Klasson, K. T., Fortier, C. A., & Lima, I. M. (2011). Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. Journal of Agriculture and Food Chemistry, 59, 2501–2510.
Walkley, A., & Black, I. A. (1934). An examination of the Degtijariff method for determining soil organic matter and a proposed modification the chromic acid titration method. Soil Science, 37, 29–38.
Warnock, D. D., Lehmann, J., Kuyper, T. W., & Rillig, M. C. (2007). Mycorrhizal responses to biochar in soil–concepts and mechanisms. Plant and Soil, 300, 9–20.
WHO. (1996). Guidelines for drinking water quality, Second Edition-Volume 2. Health criteria and other supporting information. World Health Organization (WHO) Geneva.
Yamato, M., Okimori, Y., Wibowo, I. F., Anshori, S., & Ogawa, M. (2006). Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Science and Plant Nutrition, 52, 489–495.
Acknowledgments
The author sincerely thanks Dr. Vidhya Iyer for English language editing during manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coumar, M.V., Parihar, R.S., Dwivedi, A.K. et al. Impact of pigeon pea biochar on cadmium mobility in soil and transfer rate to leafy vegetable spinach. Environ Monit Assess 188, 31 (2016). https://doi.org/10.1007/s10661-015-5028-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-5028-y