Abstract
Toxicological evaluation of Clarias gariepinus from bitumen-polluted River Oluwa, Nigeria, was carried out in furtherance of studies on the environmental impacts of the bitumen exploration in Ondo State, Nigeria. Samples were taken from three different (two polluted and one as control) sites. The effect of changes in monthly seasonal flow rate was assessed for the sites of study. Blood plasma clinical–chemical parameters (BCCPs) and histological changes/lesions in various organs were evaluated as markers of pollution in the fish blood using standard methods. The result of the physicochemical properties of water from the sampling points revealed some of the values conforming to approved standards while others showed deviation. Significant differences were found in the blood and histological endpoints between the control and the polluted sites as well as between the two seasons evaluated across the sites. The public health implications of consuming this fish are fully discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pollution of fresh water sources with varieties of pollutants is a global issue of concern to environmentalists (Honggang et al. 2010; Dahunsi et al. 2012, 2014). Also, the presence of toxicants in aquatic environment affects the water quality parameters thus leading to changes in the blood and histological indices of inhabiting fish (Kavitha et al. 2010; Dahunsi et al. 2011).
Fish contains high-quality protein and other essential amino acids useful in human and animal diets. They are considered one of the most susceptible aquatic organisms to toxic substances in water, since they are mostly found near the apex of the food chain (Prusty et al. 2011). Therefore, the safety level of freshwater fish should be of high priority (Keskin et al. 2007; Dahunsi et al. 2012).
Biological monitoring techniques like hematological and biochemical variables have become veritable tools in the assessment of environmental quality, water pollution, and the general health status of aquatic organisms especially fish (Pimpão et al. 2007; Olufayo, 2009; Li et al. 2011a). As reported in several studies, blood plasma clinical–chemical parameters (BCCPs) are markers of organ function or abnormality such as liver and kidney dysfunctions, bone disorders, and metabolic malfunctioning among others. These conditions are however triggered by factors such as infectious diseases, genetic defects, and environmental stressors including starvation, dehydration, and exposure to pollutants (Richards and Proszkowiec-Weglarz, 2007; Sonne et al. 2008, 2010, 2012). Other biochemical indices like glucose, protein, and enzymes are frequently used as indicators of the general state of health and early warning in fish under stress (Osman et al. 2010).
Many studies have documented changes in blood parameters resulting from exposures to certain environmental conditions and/or the presence of contaminants (Li et al. 2010, 2011b; Vasylkiv et al. 2010, 2011). Hematological variables such as hemoglobin (Hb), hematocrit (Ht), red blood cell (RBC) count, white blood cell (WBC) count, and others have widely been used to assess stress induced by environmental pollutants (Kreutz et al. 2011; Saravanan et al. 2011).
Fish exposed to pollutants in water also exhibits several histological anomalies which include disruption of hepatic architecture, leading to massive intrahepatic hemorrhage and death in a few hours; cytosolic vacuolation; single-cell necrosis; fibrosis; apoptosis; and tumor promotion (Lezcano et al. 2012).
The African sharptooth catfish (Clarias gariepinus) constitutes the major fauna population of River Oluwa and is usually available throughout the year in varying population. They are native and territorial in this environment, hence their suitability for pollution monitoring. Prior to this research, the toxicological impact of bitumen pollution on the fish species from this river was yet to be ascertained. Few studies have tried to establish the link between bitumen exploration and the pollution of River Oluwa and also to link it with adverse environmental health (Olajire et al. 2007; Ayandiran et al. 2014). There is however a need to assess the toxicological mechanism and the resultant effect of the river’s pollution on the inhabiting fish species which serves as a potent source of food and livelihood for the host communities. The aim of this study is therefore to evaluate the effects of bitumen pollution on the blood and histological parameters of the fish in relation to their seasonal distribution.
Materials and methods
Collection site description
Ondo State constitutes an economically significant part of south-western Nigeria and has one of the largest fresh and coastal water areas in the country. It is located in the coordinates 6° 35′ 19 N, 4° 50′ 3 E and altitude 61 m. This is where bitumen was first spotted in Nigeria in 1910 and two bitumen observatory wells were dug in the state in the 1960s during the early explorative activity of Nigerian natural bitumen. The seepage of the bitumen is brought about by naturally flowing out over large stretches of the river especially during the dry season when temperature is above 37 °C during which it occurs as a free flowing liquid and via exploration. Oluwa is a major river of economic, agricultural, and environmental significance flowing through many communities within the state, and it receives continuous seepage from bitumen exploration besides other activities (washing, farming, etc.) being carried out along its tributaries.
Collection of fish samples
Adult sizes of catfish between 4 and 6 months old (weight 0.7 ± 0.2 kg; length 23 ± 2 cm) were collected from two polluted sections (designated A and B) of River Oluwa with different levels of pollution activities and located widely apart from each other in terms of flow direction. One of these sites (B) receives more bitumen seepage than the other (A). Samples were also collected from the unpolluted portion of the river with neither bitumen pollution nor heavy domestic activities and were used as control. Fishing was done during late night with the help of professional local fishermen. Gill nets about 12.192-m long and 1.828-m wide with a cork line at the top rope and metal line with the ground rope made locally of nylon were used for fishing. Fishing was done monthly starting from the month of July, 2011, to February, 2012. A total of 432 fish samples were collected over this period. Samples were transported to the Ecotoxicology and Fisheries Laboratory, LAUTECH, in well-aerated containers into which ice cubes were added to lower the temperature of the fish before the commencement of further studies.
Collection and preservation of fish blood
Blood samples were collected from the test organism (C. gariepinus) caught from the two sampling sites during the high-flow months (July–September) and low-flow months (December–February). This was done with the use of 5-mL sterilized syringes by direct puncture in the heart. Blood samples were then transferred into sterilized plastic tubes having ethylenediaminetetraacetic acid (EDTA) to prevent blood clotting. The container were then rotated gently so as to mix thoroughly with the EDTA and also not to damage the blood cells. In the laboratory, each blood sample was then separated into two sets and stored in a refrigerator at 4 °C. One set of preserved blood sample was used for hematological studies while the other was used for the estimation of various blood biochemical parameters. Fish were later dissected and organs such as the livers, gills, and muscle were removed and fixed in 4 % calcium–formol fixative at 4 °C.
Evaluation of Plasma biochemical parameters
The biochemical assays were carried out using the methods of Sonne et al. (2008, 2010, 2012, 2013). Indices analyzed include the following: albumin (Alb, g L−1), glucose (Glu, mmol L−1), total protein (TP, g L−1), calcium (Ca, mmol L−1), sodium (Na, mmol L−1), potassium (K, mmol L−1), chloride (Cl, mmol L−1), and HCO3 − (HC, mmol L−1). All analyses were routinely conducted at the laboratory using an automated spectrophotometric analyzer also containing ion-selective electrodes (ADVIA1800, Siemens), and assays were subjected to daily internal and quarterly external quality control.
Evaluation of hematological parameters
The analyses were carried out at the Medical Laboratory of the University Health Centre, LAUTECH, Ogbomoso, following standard methods (Ptashynski et al. 2002; Kubrak et al. 2013). Packed cell volume (PCV) was evaluated using hematocrit method and Hb concentration (using cyanmethemoglobin method) was analyzed within 2 h after collection. RBC and WBC were counted by Neubauer’s improved hemocytometer using Hyem’s and Turk’s solution as a diluting fluid, respectively. The values were then used in calculating mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC) according to the method of Dacie and Lewis (1975).
Histological analyses
Fish organs (gills, liver, and body muscle) were fixed in 4 % paraformaldehyde, prior to being embedded in paraffin. Paraffin sections (5-μm thick) were prepared and stained with hematoxylin and eosin (H & E) according to standard procedures (Isitor et al. 2012; Chen et al. 2013). All sections were examined under a light microscope.
Statistical analysis
All the results were subjected to statistical tests performed using SPSS Version 19. The t test (two sample assuming equal variances) at a p value less than or equal to 0.05 was used to conduct the test of significance.
Result
Water quality
The result of the physicochemical properties of water from the three sampling points of River Oluwa is shown in Table 1. As revealed, some of the values are in conformity with approved standards by some regulatory bodies while others show deviation from such standards thereby revealing the polluted nature of some of the samples.
Hematology
The result of hematological parameters of C. gariepinus from the three sites and for both high- and low-flow seasons is shown in Table 2. The parameters include white blood cell count (WBCc), red blood cell count (RBCc), PCV, Hb, MCV, MCHC, and MCH. All the hematological parameters investigated during the high- and low-flow months showed significant differences from the control values except for MCH for site A in the month of January, 2010.
Biochemistry
The biochemical analysis results revealed that the values recorded for glucose and potassium at site B during the high-flow season of July 2009 were not significantly different from the control values for the same month. The values obtained for all other parameters were significantly different (higher) than the control values. During the low-flow season, the values for potassium and HCO3 − were both not significantly different from control values in the months of December 2009 (site B) and February 2010 (site A), respectively, as shown in Table 3.
Histology
Body muscle
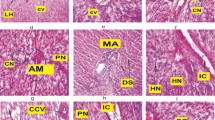
The various changes observed in the muscle are shown in Table 4. Figure 1a shows the normal structure of the body muscle of C. gariepinus from the unpolluted portion of River Oluwa (control) in comparison with those having histological alterations (Figs. 1b–m). Varying degrees of histological alterations were observed in the muscle during both low- and high-flow months which include atrophy of muscle bundles, necrosis, edema, vacuolar degeneration, and splitting of muscle fibers.
a A photomicrograph of normal muscle of Clarias gariepinus (control) from unpolluted local fish farm, H&E ×200. b Photomicrograph of muscle of Clarias gariepinus from Oluwa river at high flow season, July H&E ×200 (site A). A—atrophy of muscle bundle, B—splitting of muscle fibres. c Photomicrograph of muscle of Clarias gariepinus from Oluwa River at high flow season, July H&E ×200 (site B). A—atrophy of muscle bundle, B—splitting of muscle fibers, C—focal area of necrosis. d Photomicrograph of muscle of Clarias gariepinus from Oluwa River at high flow season, August H&E ×200 (site A). A—atrophy of muscle bundle, E—Vacuolar degeneration in muscle bundles. e Photomicrograph of muscle of Clarias gariepinus from Oluwa River at high flow season, August H&E ×200 (site B). A—atrophy of muscle bundle, B—splitting of muscle fibres, C—focal area of necrosis, E—vacuolar degeneration in muscle bundles. f Photomicrograph of muscle of Clarias gariepinus from Oluwa river at high flow season, September H&E ×200 (site A). A—atrophy of muscle bundle, D—edema between muscle bundles, E—vacuolar degeneration in muscle bundles. g Photomicrograph of muscle of Clarias gariepinus from Oluwa river at high flow season, September H&E ×200 (site B). A—atrophy of muscle bundle, C—focal area of necrosis, E—vacuolar degeneration in muscle bundles. h Photomicrograph of muscle of Clarias gariepinus from Oluwa River at low flow season, December H&E ×200 (site A). B—splitting of muscle fibres, C—focal area of necrosis, E—vacuolar degeneration in muscle bundles. i Photomicrograph of muscle of Clarias gariepinus from Oluwa River at low flow season, December H&E ×200 (site B). A—atrophy of muscle bundle, C—focal area of necrosis, E—vacuolar degeneration in muscle bundles. j Photomicrograph of muscle of Clarias gariepinus from Oluwa River at low flow season, January H&E ×200 (site A). A—atrophy of muscle bundle. C—focal area of necrosis. E—vacuolar degeneration in muscle bundles. k Photomicrograph of muscle of Clarias gariepinus from Oluwa River at low flow season, January H&E ×200 (site B). B—splitting of muscle fibres, C—focal area of necrosis, D—edema between muscle bundles, E—vacuolar degeneration in muscle bundles. l Photomicrograph of muscle of Clarias gariepinus from Oluwa River at low flow season, February H&E ×200 (site A). B—splitting of muscle fibers, E—vacuolar degeneration in muscle bundles. m Photomicrograph of muscle of Clarias gariepinus from Oluwa River at low flow season, February H&E ×200 (site B). C—focal area of necrosis, D—edema between muscle bundles, E—vacuolar degeneration in muscle bundles
Liver
The various changes observed in the liver are shown in Table 5. Figure 2a shows the normal histological structures of the liver of C. gariepinus from the unpolluted portion of River Oluwa (control) and those with varying degrees of histological alterations as shown in Figs. 2b–m. The most common lesions in the liver of the fish species under study were vacuolar degeneration in the hepatocytes, focal areas of necrosis, hypertrophy of hepatocytes, and loss of trabecular cell arrangement which were more prominent during the low-flow months.
a Photomicrograph of liver of Clarias gariepinus showing normal central vein and trabecular arrangements of cells, H&E ×200. b Photomicrograph of liver of Clarias gariepinus from Oluwa River at high flow season, July H&E ×200 (site A). B—dilation and thrombosis formation in central vein, C—fatty degeneration, F—focal area of necrosis. c Photomicrograph of liver of Clarias gariepinus from Oluwa River at high flow season, July H&E ×200 (site B). A—hypertrophy of hepatocytes, B—dilation and thrombosis formation in central vein, D—loss of trabecular arrangement of cells, F—focal area of necrosis. d Photomicrograph of liver of Clarias gariepinus from Oluwa river at high flow season, August H&E ×200 (site A). A—hypertrophy of hepatocytes, B—dilation and thrombosis formation in central vein, C—fatty degeneration, D—loss of trabecular arrangement of cells, F—focal area of necrosis. e Photomicrograph of liver of Clarias gariepinus from Oluwa river at high flow season, August H&E ×200 (site B). B—dilation and thrombosis formation in central vein, F—focal area of necrosis. f Photomicrograph of liver of Clarias gariepinus from Oluwa River at high flow season, September H&E ×200 (site A). A—hypertrophy of hepatocytes, B—dilation and thrombosis formation in central vein, D—loss of trabecular arrangement of cells, E—hepatocyte degeneration. g Photomicrograph of liver of Clarias gariepinus from Oluwa River at high flow season, September H&E ×200 (site B). D—Loss of trabecular arrangement of cells, E—hepatocyte degeneration, F—focal area of necrosis. h Photomicrograph of liver of Claris gariepinus from Oluwa River at low flow season, December H&E ×200 (site A). A—hypertrophy of hepatocytes, B—dilation and thrombosis formation in central vein, F—focal area of necrosis. i Photomicrograph of liver of Clarias gariepinus from Oluwa River at low flow season, December H&E ×200 (site B). A—hypertrophy of hepatocytes, C—fatty degeneration, E—hepatocyte degeneration, F—focal area of necrosis. j Photomicrograph of liver of Clarias gariepinus from Oluwa River at low flow season, January H&E ×200 (site A). A—hypertrophy of hepatocytes, B—dilation and thrombosis formation in central vein, D—loss of trabecular arrangement of cells, E—hepatocyte degeneration, F—focal area of necrosis. k Photomicrograph of liver of Clarias gariepinus from Oluwa river at low flow season, January H&E ×200 (site B). A—hypertrophy of hepatocytes, B—dilation and thrombosis formation in central vein, D—loss of trabecular arrangement of cells. E—hepatocyte degeneration, F—focal area of necrosis. l Photomicrograph of liver of Clarias gariepinus from Oluwa River at low flow season, February H&E ×200 (site A). A—hypertrophy of hepatocytes, C—fatty degeneration, D—loss of trabecular arrangement of cells, E—hepatocyte degeneration, F—focal area of necrosis. m Photomicrograph of liver of Clarias gariepinus from Oluwa River at low flow season, February H&E ×200 (site B). A—hypertrophy of hepatocytes, B—dilation and thrombosis formation in central vein, D—Loss of trabecular arrangement of cells, E—hepatocyte degeneration, F—focal area of necrosis
Gills
The various changes observed in the gills are as shown in Table 6. Figure 3a shows the normal histological structures of the gills of C. gariepinus from the unpolluted portion of River Oluwa (control) and those with varying degrees of histological alterations as shown in Figs. 3b–m. These changes include distortion of gill lamella, hyperplasia, edema in secondary lamella, necrosis, epithelial lifting, and hypertrophy of epithelial cell.
a A photomicrograph of normal gill of Clarias gariepinus from a local fish farm, H&E ×200. b Photomicrograph of gill of Clarias gariepinus from Oluwa River at high flow season, July H&E ×200 (site A). A—necrosis of gill lamella, C—degeneration of gill lamella. c Photomicrograph of gill of Clarias gariepinus from Oluwa River at high flow season, July H&E ×200 (site B). A—necrosis of gill lamella, C—degeneration of gill lamella, H—edema of gill filament. d Photomicrograph of gill of Clarias gariepinus from Oluwa River at high flow season, August H&E ×200 (site A). A—necrosis of gill lamella, B—hyperplasia, C—degeneration of gill lamella, E—hypertrophy of epithelial cells. e Photomicrograph of gill of Clarias gariepinus from Oluwa River at high flow season, August H&E ×200 (site B). A—necrosis of gill lamella, B—hyperplasia, D—edema of lamella. f Photomicrograph of gill of Clarias gariepinus from Oluwa River at high flow season, September H&E ×200 (site A). B—hyperplasia. g Photomicrograph of gill of Clarias gariepinus from Oluwa River at high flow season, September H&E ×200 (site B). A—necrosis of gill lamella, C—degeneration of gill lamella. h Photomicrograph of gill of Clarias gariepinus from Oluwa River at low flow season, December H&E ×200 (site A). A—necrosis of gill l amella, B hyperplasia, C—degeneration of gill lamella. i Photomicrograph of gill of Clarias gariepinus from Oluwa River at low flow season, December H&E ×200 (site B). C—degeneration of gill lamella, E—hypertrophy of epithelial cells, F—epithelial lifting, G—degeneration of gill lamella and filament. j Photomicrograph of gill of Clarias gariepinus from Oluwa River at low flow season, January H&E ×200 (site A). A—necrosis of gill lamella, C—degeneration of gill lamella, E—hypertrophy of epithelial cells, F—epithelial lifting. k Photomicrograph of gill of Clarias gariepinus from Oluwa River at low flow season, January H&E ×200 (site B). G—degeneration of gill lamella and filament, H—edema of gill filament, I—necrosis of gill filament. l Photomicrograph of gill of Clarias gariepinus from Oluwa River at low flow season, February H&E ×200 (site A). F—epithelial lifting, G—degeneration of gill lamella and filament, I—necrosis of gill filament. m Photomicrograph of gill of Clarias gariepinus from Oluwa River at low flow season, February H&E ×200 (site B). A—necrosis of gill lamella, B—hyperplasia, C—degeneration of gill lamella, D—edema of lamella, F—epithelial lifting, H—edema of gill filament
Discussion
There is an observed statistical difference (p < 0.05) in the mean values of temperature of the water from the three sites and it is highest in site B (25.38 ± 0.31). All temperature values fall within the acceptable temperature range (0–30 °C) for good surface water (Chapman, 1996). The mean pH values of water samples from all three sites of the river were found to be weakly acidic (Table 1) and are within the permissible limit of 6.5 to 9.5 by the Nigerian Industrial Standard (2007) and the World Health Organization (2011).
The significant decrease (p < 0.05) in values for total suspended solids (TSS) and total dissolved solids (TDS) of water from all the sites falls within the permissible limits of 500 mg L−1 for TSS and 1000 mg L−1 for TDS which are the standard limits of both the WHO and NIS for good water quality. The high BOD and COD values in sites A and B are indication of high organic and inorganic pollutant loads in the sites and are significantly different from the control value. The mean BOD values of the samples exceeded the permissible limit (3.0–6.0 mg L−1) of the European Union (EU) for good quality water that will adequately support fishes and other aquatic life forms since the BOD value for unpolluted waters is usually ≤2 mg L−1 while values for polluted ones can be as high as ≥10 mg L−1. The significantly high mean COD values in sites A and B also exceeded the permissible limit (≤20 mg L−1) for unpolluted surface water thus falling within the category of polluted waters (20–200 mg L−1) (Chapman, 1996). Lower DO values were recorded in sites A and B and are an indication that the river is depleted in oxygen, and this could be attributed to the polluted nature of the water by bitumen and other pollutants. Values obtained for all metals in both sites are very high and above the WHO and NIS permissible limits for surface waters and statistically different from the control value which in most cases falls within the standard limits. The high metal values could easily be attributed to bitumen pollution as well since bitumen has been reported to contain heavy metals (Yoon et al. 2009).
Hematological parameters such as erythrocyte count, hemoglobin concentration, and hematocrit values are known to vary in fish in response to different toxicants (Kavitha et al. 2010). Also, hematological investigations have proven to be valuable for fish biologists in the quick detection of changes in fish health since such changes usually precede behavioral and visible lesions (Kavitha et al. 2011). Decrease in the recorded values of PCV, Hb, and RBC showed that the fish species (C. gariepinus) under study became anemic due to the effect of the pollutants (bitumen and other environmental contaminants) in the water, and this agrees with previous submissions that the entry of toxicants into aquatic media may affect the water quality parameters, which in turn leads to changes in the hematological variables of fish, due to its close association with the external environment (Kavitha et al. 2010, 2011). Furthermore, the reduction in the count of RBC may be caused by stimulation of hemolyzing action of the RBC by the bitumen seeping into the river and may also be attributed to hemodilution resulting from impaired osmoregulation across the gill epithelium and which is similar to the report of Adeyemo (2005). Other toxicants had also been reported to have a similar reduction effect on the RBC of fishes (Kavitha et al. 2011). Reduction in the Hb content of the fish species may be an indication of decline in hemoglobin synthesis as well as the reduction in oxygen carrying capacity which could be as a result of interference of the bitumen seep with hemoglobin synthesis pathways. Atamanalp and Yanik (2003) observed similar result from fishes exposed to mancozeb. The observed increase in WBC may be due to stimulated lymphopoiesis and/or enhanced release of lymphocytes from lymphomyeloid tissue meant to be a defense mechanism of the fish against the water toxicants and the stress induced (Ates et al. 2008).
Higher increase in MCH and MCV values during the high-flow months compared to the low-flow months may be due to the reduction in cellular blood iron, resulting in reduced oxygen carrying capacity of the blood and eventually stimulating erythropoiesis (Wintrobe, 1978). It could also be due to swelling of red blood cells which is usually stress-related (Jastrzebska and Protasowickiz 2005). A reduced value of MCHC was recorded in both sites A and B during high-flow months compared to the low-flow months which signifies red blood cell swelling (Weppener et al. 1992a, 1992b). MCHC is usually not influenced by the blood volume or by the number of cells in the blood but can be interpreted incorrectly only when new cells, with a different hemoglobin concentration, are released into the blood circulation (Sobecka, 2001).
The increase in the values of MCV and MCH together with the decrease in the value of MCHC further confirmed the anemic condition in the fish species from polluted portions (sites A and B) of River Oluwa and that the anemia may be due to an increase in the rate of erythrocyte destruction in the hematopoietic organ of C. gariepinus, together with lysis of erythrocytes in the circulation and inhibition of erythropoiesis and hemosynthesis.
Measurement of plasma biochemical parameters is mostly used in clinical diagnosis of fish physiology to determine the general health status (Osman et al. 2010). Serum glucose is one of the most sensitive biochemical indices for analyzing the state of health of an organism, and its high concentration in the blood of fish indicates that such fish is under stress and it is intensively utilizing its energy reserves, i.e., glycogen in the liver and muscles, whereas a decrease in concentration indicates the exhaustion of energy of resources and, subsequently, the worsening of an organic status (Kavitha et al. 2011).
The above statement better supports the increased serum glucose level recorded in the sites A and B during the low-flow months and decrease in the values during high-flow months. Increased level of glucose during low-flow months reflects hyperglycemia, while decrease during high-flow months reflects hypoglycemia. Both conditions of different responses of serum glucose suggested the presence of ailment in the fish which may have been induced by the toxicants (bitumen and other contaminants). Hyperglycemic conditions develop either due to increased glycogenolysis or decrease in glucose utilization by the cell which means the inhibition of glycolysis (Bedii and Kenan, 2005). The different responses of glucose as related to different toxicants have been reported by previous researchers (Dahunsi et al. 2011; Saravanan et al. 2011; Sonne et al. 2008, 2010, 2012, 2013).
Furthermore, the plasma protein levels recorded in both polluted sites were lower than the control values during high- and low-flow seasons. The low level of plasma protein can be attributed to possible inhibition or decrease in the process of protein synthesis occurring in the body. Other possible causes may include nutritional imbalance, infectious disease, kidney damage, leakage, and edema. Similar observations have been made (Kavitha et al. 2011; Vani et al. 2011).
Significantly different levels of albumin was recorded during the low-flow season which could be a precursor of liver disease for the inhabiting fish since albumin is known to increase during liver disease and dehydration (Sonne et al. 2013). All values for potassium, calcium, and sodium were found to be statistically different from the control during both seasons. High levels of these elements may be detrimental to fish health since potassium increases during kidney disease while a high calcium concentration has been implicated in bone disease (Sonne et al. 2013). Sodium on the other hand has been indicated to be a useful tool in the measurement of stress, primarily due to the active movement across the gill structure depending on the concentration in the external medium.
The histological examination of the gill filaments of the fish (C. gariepinus) from sites A and B showed a varying degree of aberrations. Gills perform numerous functions which include respiration, excretion of nitrogenous waste products, and acid–base balance. Therefore, functional impairment of gills caused by pollutants leads to a significant damage to the health of fish, and fish gills are considered to be the most appropriate indication of water pollution levels (Carmargo and Martinez, 2007).
In this study, lesions like hypertrophy, hyperplasia of epithelial cells, epithelial lifting, destruction of gill lamella, necrosis, and degeneration of gill filaments and lamella were more prominent during low-flow months at site B than in high-flow months. This observation could possibly be attributed to the absence of dilution of water in the low-flow months since the river experienced low flows during this period as a result of increase in water evaporation rate, while mild aberrations recorded in the high-flow months could be due to dilution as a result of large volume of water in the river during flood. It could also be as a result of pollutants from higher human anthropogenic activities of in site B. The pathological changes observed in the gill of the test organisms may be a reaction to toxicant intake or an adaptive response to prevent the entry of the pollutant through the gill surface. The observed epithelial lifting and hyperplasia of lamella could be interpreted as a defense response of the fish as these alterations increase the distance across which water-borne irritants must diffuse to reach the blood stream. Observed alterations like hypertrophy and necrosis can adversely affect the gas exchange and ionic regulation (Simonato et al. 2008). Edema in gill filament and lamella could probably be due to increased capillary permeability (Olurin et al. 2006). The present results are in agreement with those observed in other fish species under the influence of different pollutants (Simonato et al. 2008; Fagbenro and Akinduyite, 2011).
The organ most associated with detoxification and biotransformation process is the liver, and it is also one of the organs most affected by contaminant in the water due to its function, position, and blood supply (Thophon et al. 2003). The liver of C. gariepinus from sites A and B of River Oluwa during high- and low-flow seasons showed pathological changes such as degeneration of hepatocytes, focal areas of necrosis, thrombosis formation in central veins, hypertrophy of hepatocytes, fatty degeneration, and loss of trabecular arrangement of cells. However, these changes may be attributed to direct toxic effects of pollutant on hepatocytes, since the liver is the site of detoxification of all types of toxins and chemicals (Soufy et al. 2007). The degeneration of hepatocytes might indicate an imbalance between the rate of synthesis of substances in the parenchymal cells and their release into the circulatory system (van Dyk et al. 2007). Oxygen deficiency as a result of gill degeneration is considered as the most common cause of cellular degeneration in the liver. Hypertrophy of hepatocytes observed in the liver of the test organisms were noticed in virtually all the livers investigated, and this could be as a result of fatty degeneration of accumulated lipid droplets leading to the collapse of blood sinusoids. Dilation and thrombosis formation observed in the blood vessel may be responsible for cellular degeneration and necrosis in the liver (van Dyk et al. 2007). The present results are in agreement with those observed by many authors who have studied the effect of different pollutants on fish liver (Ptashynski et al. 2002; Fanta et al. 2003; Renugadevi and Prabu 2010; Fagbenro and Akinduyite, 2011).
The histological alteration in the muscle of the fish species under study could be a direct result of the heavy metal present in the heavy oil which the bitumen contained. The alterations like atrophy, splitting of muscle fibers, focal area of necrosis, vacuolar degeneration, and edema recorded in this study are in agreement with those observed by many investigators who earlier studied the effect of different pollutants on fish muscles (Das and Mukherjee 2000).
Conclusion
In conclusion, the current research has assessed the toxicological impact of bitumen runoff on the fish from River Oluwa, and it is evident that the fish health status is negatively affected by this pollution menace. Significant differences were found in the blood and histological endpoints between the control and the polluted (A and B) sites as well as between the two seasons evaluated across the sites. Farming and fishing are the major occupations of the populace whose lives depend on the river to a great extent. These polluted fishes could harbor pathogens that in turn cause serious diseases in the consumers, and thus, their consumption is discouraged. It is further advocated that qualitative measures be put in place to stop the indiscriminate discharge of bitumen runoff and other anthropogenic activities into this economically important river as the public health consequences are numerous and fatal. Further studies are ongoing as to determine the occurrence of parameters such as concentration of polycyclic aromatic hydrocarbons (PAHs) and low-volatile lipophilic substances in the fish tissues.
References
Adeyemo, O. K. (2005). Haematological and histopathological effects of cassava mill effluent in Clarias gariepinus. African Journal of Biomedical Research, 8, 179–83.
Atamanalp, M., & Yanik, T. (2003). Alterations in hematological parameters of rainbow trout (Oncorhynchus mykiss) exposed to mancozeb. Turkish Journal of Veterinary and Animal Science, 27, 1213–1217.
Ates, B., Orun, I., Talas, Z. S., Durmaz, G., & Yilmaz, I. (2008). Effects of sodium selenite on some bio- chemical and hematological parameters of rainbow trout (Oncorhynchus mykiss Walbaum, 1792) exposed to Pb2+ and Cu2+. Fish Physiology and Biochemistry, 34, 53–9.
Ayandiran, T. A., Ayandele, A. A., Dahunsi, S. O., & Ajala, O. O. (2014). Microbial assessment and prevalence of antibiotic resistance in polluted Oluwa River, Nigeria. Egyptian Journal of Aquatic Research, 40, 291–299.
Bedii, C., & Kenan, E. (2005). The effects of cadmium on levels of glucose in serum and glycogen reserves in the liver and muscle tissues of Cyprinus carpio (L., 1758). Turkish Journal of Veterinary and Animal Science, 29, 113–117.
Camargo, M. M., & Martinez, C. B. (2007). Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Ichthyology, 5, 327–336.
Chapman, D. (1996). Water quality assessment: a guide to the use of Biota, sediments and water in environmental monitoring (2nd ed.) London, UK: Chapman and Hall.
Chen, Q., Zhang, R., Lia, W., Niu, Y., Guo, H., Liuc, X., Hou, Y., & Zhao, L. (2013). The protective effect of grape seed procyanidin extract against cadmium-induced renal oxidative damage in mice. Environmental Toxicology and Pharmacology, 36, 759–768.
Dacie, J. V., & Lewis, S. M. (1975). Measurement and calculation of size of red cells, practical haematology (5th ed., pp. 40–43). London: Churchill Livingstone.
Dahunsi, S. O., Oranusi, S. U., & Ishola, R. O. (2011). Biochemical profile of Clarias gariepinus exposed to sub-lethal concentrations of chemical additives effluent. International Journal of Research in Environmental Science and Technology, 1(4), 52–58.
Dahunsi, S. O., Oranusi, S. U., & Ishola, R. O. (2012). Differential bioaccumulation of heavy metals in selected biomarkers of Clarias gariepinus (Burchell, 1822) exposed to chemical additives effluent. Journal of Research in Environmental Science and Toxicology, 1(5), 100–106.
Dahunsi, S. O., Owamah, H. I., Ayandiran, T. A., & Oranusi, U. S. (2014). Drinking water quality and public health of selected communities in South Western Nigeria. Water Quality Exposure and Health, 6, 143–153.
Das, B. K., & Mukherjee, S. C. (2000). Chronic toxic effect of quinalphos on some biochemical parameters in Labeo robita (Ham). Toxicology Letters, 114, 11–18.
Fagbenro. O., Akinduyite, I., (2011). Acute toxicity of aqueous Morinda lucida leaf extracts to Nile tilapia, Oreochromis niloticus (Linnaeus 1857). Proceedings of the Ninth International Symposium on Tilapia in Aquaculture. Shanghai, China. April 22nd-24th, 2011. 52–59
Fanta, E., Rios, F.S., Roma˜o, S., Vianna, A.C.C., Freiberger, S., (2003). Histopathology of the fish Corydoras paleatus contaminated with sublethal levels of organophosphorus in water and food. Ecotoxicology and Environmental Safety, 54, 119–130
Honggang, Z., Baoshan, C., Rang, X., & Hui, Z. (2010). Heavy metals in water, soils and plants in riparian wetlands in the Pearl River Estuary, South China. Proceedings in Environmental Science, 2, 1344–1354.
Isitor, G.N., Rao, S., Nayak, S.B., Sundaram, V., (2012). Autofluorescent vesicular structures in hematoxylin and eosin stained duodenal mucosa of the domestic cat. West Indies Veterinary Journal, 9
Jastrzebska, E. B., & Protasowickiz, M. (2005). Effects of cadmium and nickel exposure on hematological parameters of common carp, Cyprinus carpio L. Acta Ichthyology Piscat, 35(1), 29–38.
Kavitha, C., Malarvizhi, A., Senthil, K. S., & Ramesh, M. (2010). Toxicological effects of arsenate exposure on haematological, biochemical and liver transaminase activity in an Indian major carp, Catla catla. Food and Chemical Toxicology, 48(28), 48–54.
Kavitha, C., Mathan, R., Satyanarayanan, S.K., (2011). Toxicity of Moringa oleifera seed extract on some haematological and biochemical profiles in a freshwater fish, Cyprinus carpio. Experimental and Toxicologic Pathology doi:10.1016/jetp.2011. 01.001
Keskin, Y., Raskaya, R., Ozyaral, O., Yurdun, T., Luleci, N. E., & Hayran, O. (2007). Cadmium, lead, mercury and copper in fish from the Marmara Sea, Turkey. Bulletin of Environmental Contamination and Toxicology, 78, 258–261.
Kreutz, L. C., Barcellos, L. J. G., de Faria Valle, S., de Oliveira Silva, T., Anziliero, D., dos Santos, E. D., Pivato, M., & Zanatta, R. (2011). Altered hematological and immunological parameters in silver catfish (Rhamdia quelen) following short term exposure to sublethal concentration of glyphosate. Fish and Shellfish Immunology, 30, 51–57.
Kubrak, O. I., Atamaniuk, T. M., Storey, K. B., & Lushchak, V. I. (2013). Goldfish can recover after short-term exposure to 2,4-dichlorophenoxyacetate: use of blood parameters as vital biomarkers. Comparative Biochemistry and Physiology Part C, 157, 259–265.
Lezcano, N., Sedan, D., Lucotti, I., Giannuzzi, L., Vittone, L., Andrinolo, D., & Mundiña-Weilenmann, C. (2012). Subchronic microcystin-LR exposure increased hepatic apoptosis and induced compensatory mechanisms in mice. Journal of Biochemistry and Molecular Toxicology, 26(4), 131–138.
Li, Z. H., Velisek, J., Zlabek, V., Grabic, R., Machova, J., Kolarova, J., & Randak, T. (2010). Hepatic antioxidant status and hematological parameters in rainbow trout, Oncorhynchus mykiss, after chronic exposure to carbamazepine. Chemico-Biological Interactions, 183, 98–104.
Li, Z. H., Velisek, J., Grabic, R., Li, P., Kolarova, J., & Randak, T. (2011a). Use of hematological and plasma biochemical parameters to assess the chronic effects of a fungicide propiconazole on a freshwater teleost. Chemosphere, 83, 572–578.
Li, Z. H., Velisek, J., Zlabek, V., Grabic, R., Machova, J., Kolarova, J., Ping, L., & Randak, T. (2011b). Chronic toxicity of verapamil on juvenile rainbow trout (Oncorhynchus mykiss): effects on morphological indices, hematological parameters and antioxidant responses. Journal of Hazardous Materials, 185, 870–880.
Nigerian Industrial Standards (2007). Nigerian Standards for drinking water quality. NIS 554: 2007, ICS 13.060.20. Approved by the Standard Organization of Nigeria (SON) Governing Council, pp 14–18
Olajire, A. A., Alade, A. O., Adeniyi, A. A., & Olabemiwo, O. M. J. (2007). Distribution of polycyclic aromatic hydrocarbons in surface soils and water from the vicinity of Agbabu bitumen field of South Western Nigeria. Environmental Science and Health Part A, 42, 1043.
Olufayo, M. O. (2009). Hematological characteristics of Clarias gariepinus (Burchell 1822) juveniles exposed to Derris elliptica root powder. African Journal of Food, Agriculture and Nutritional Science, 9(3), 920–33.
Olurin, K. B., & Aderibigbe, K. (2006). Length-weight relationship and condition factor of pond reared juvenile Oreochromis niloticus. World Journal of Zoology, 1(21), 82–85.
Osman, A. G. M., Koutb, M., & Sayed, A. E. D. H. (2010). Use of hematological parameters to assess the efficiency of quince (Cydonia oblonga Miller) leaf extract in alleviation of the effect of ultraviolet—a radiation on African catfish Clarias gariepinus (Burchell, 1822). Journal of Photochemistry, Photobiology B Biology, 99, 1–8.
Pimpão, C. T., Zampronio, A. R., & Silva de Assis, H. C. (2007). Effects of deltamethrin on hematological parameters and enzymatic activity in Ancistrus multispinis (Pisces, Teleostei). Pesticides Biochemistry and Physiology, 88, 122–127.
Prusty, A. K., Kohli, M. P. S., Sahu, N. P., Pal, A. K., Saharan, N., Mohapatra, S., & Gupta, S. K. (2011). Effect of short term exposure of fenvalerate on biochemical and haematological responses in Labeo rohita (Hamilton) fingerlings. Pesticides Biochemistry and Physiology, 100, 124–129.
Ptashynski, M. D., Pedlar, R. M., Evans, R. E., Baron, C. L., & Klaverkamp, J. F. (2002). Toxicology of rats chronically treated with an organophosphate. Ecotoxicology and Environmental Safety, 66, 447–450.
Renugadevi, J., & Prabu, S. M. (2010). Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Experimental Toxicology and Pathology, 62(2), 171–181.
Richards, M. P., & Proszkowiec-Weglarz, M. (2007). Mechanisms regulating feed intake, energy expenditure, and body weight in poultry. Poultry Science, 86, 1478–1490.
Saravanan, M., Karthika, S., Malarvizhi, A., & Ramesh, M. (2011). Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: hematological, biochemical, ionoregulatory and enzymological responses. Journal of Hazardous Materials, 195, 188–194.
Simonato, J. D., Guedes, C. L. B., & Martinez, C. B. R. (2008). Biochemical, physiological, and histological changes in the neotropical fish Prochilodus lineatus exposed to diesel oil. Ecotoxicology and Environmental Safety, 69, 112–120.
Sobecka, E. (2001). Changes in the iron level in the organs and tissues of wels catfish, Silurus glanis L. caused by nickel. Acta Ichthyology Piscat, 31(2), 127–43.
Sonne, C., Dietz, R., Kirkegaard, M., Letcher, R. J., Shahmiri, S., Andersen, S., Møller, P., Olsen, A. K., & Jensen, A. L. (2008). Effects of organohalogen pollutants on haematological and urine clinical–chemical parameters in Greenland sledge dogs (Canis familiaris). Ecotoxicology and Environmental Safety, 69, 381–390.
Sonne, C., Bustnes, J. O., Herzke, D., Jaspers, V. L. B., Covaci, A., Halley, D. J., Minagawa, M., Moum, T., Eulaers, I., Eens, M., Ims, R. A., Hanssen, S. A., Erikstad, K. E., Johnsen, T., Schnug, L., & Jensen, A. L. (2010). Relationships between organohalogen contaminants and blood plasma clinical–chemical parameters in chicks of three raptor species from Northern Norway. Ecotoxicology and Environmental Safety, 73, 7–17.
Sonne, C., Bustnes, J. O., Herzke, D., Jaspers, V. L. B., Covaci, A., Eulaers, I., Halley, D. J., Moum, T., Ballesteros, M., Eens, M., Ims, R. A., Hanssen, S. A., Erikstad, K. E., Johnsen, T. V., Rige´t, F. F., Jensen, A. L., & Kjelgaard-Hansen, M. (2012). Blood plasma clinical–chemical parameters as biomarker endpoints for organohalogen contaminant exposure in Norwegian raptor nestlings. Ecotoxicology and Environmental Safety, 80, 76–83.
Sonne, C., Rige´t, F. F., Leat, E. H. K., Bourgeon, S., Borg, K., Strøm, H., Hanssen, S. A., Gabrielsen, G. W., Petersen, A., Olafsdottir, K., Magnusdottir, E., Bustnes, J. O., Furness, R. W., & Kjelgaard-Hansen, M. (2013). Organohalogen contaminants and blood plasma clinical–chemical parameters in three colonies of North Atlantic Great skua (Stercorarius skua). Ecotoxicology and Environmental Safety, 92, 245–251.
Soufy, M. K., Soliman, E. M., El-manakhly, A. U., & Gafar, U. (2007). Some biochemical and pathological investigations on monosex tilapia following chronic exposure to carbofuran pesticide. Global Veterinary Journal, 1, 45–52.
Thophon, S., Kruatrachue, M., Upatham, E. S., Pokethitiyook, P., Sahaphong, S., & Jaritkhuan, S. (2003). Histopathological alterations of white seabass, Lates calcarifer, in acute and subchronic cadmium exposure. Environmental Pollution, 121, 307–320.
van Dyk, J. C., Pieterse, G. M., & van Vuren, J. H. J. (2007). Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicology and Environmental Safety, 66, 432–440.
Vani, T., Saharan, N., Mukherjee, S. C., Ranjan, R., Kumar, R., & Brahmchari, R. K. (2011). Deltamethrin induced alterations of hematological and biochemical parameters in fingerlings of Catla catla (Ham.) and their amelioration by dietary supplement of vitamin C. Pesticides Biochemistry and Physiology, 101, 16–20.
Vasylkiv, O. Y., Kubrak, O. I., Storey, K. B., & Lushchak, V. I. (2010). Cytotoxicity of chromium ions may be connected with induction of oxidative stress. Chemosphere, 80, 1044–1049.
Vasylkiv, O. Y., Kubrak, O. I., Storey, K. B., & Lushchak, V. I. (2011). Catalase activity as a potential vital biomarker of fish intoxication by the herbicide aminotriazole. Pesticides Biochemistry and Physiology, 101, 1–5.
Wepener, V., Van Vuren, J. H. J., & Du Preez, H. H. (1992a). The effect of iron and manganese at an acidic pH on the hematology of the banded tilapia (Tilapia sparrmanii, Smith). Bulletin of Environmental Contamination and Toxicology, 49, 613–619.
Wepener, V., Van Vuren, J. H., & Preez, H. H. (1992b). The effect of hexavalent chromium at different pH values on the haematology of Tilapia sparmani (Cichlidae). Comparative Biochemistry and Physiology, 101, 375–381.
Wintrobe, M. M. (1978). Clinical haematology (p. 488). London: Henry Kimpton.
World Health Organization (2011). Guidelines on drinking water quality, 4th Edition. NLM Classification: WA 675 (pp 307–433). Geneva, Switzerland: World Health Organization.
Yoon S, Batt SD, Lee W, Jeong SY, Baeg JO, Lee CW (2009). Separation and characterization of bitumen from Athabaca oil sand. Korean Journal of Chemical Engineering, 26, 64–71.
Acknowledgments
The authors appreciate the efforts of the faculty and staff of the Department of Pure and Applied Biology, LAUTECH, Nigeria, for making this work a success. Special thanks to our mentor, Professor Fawole OO for guiding us well.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ayandiran, T.A., Dahunsi, S.O. Toxicological assessment of fish (Clarias gariepinus) from bitumen-polluted River Oluwa, Nigeria. Environ Monit Assess 188, 71 (2016). https://doi.org/10.1007/s10661-015-5003-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-5003-7