Abstract
This study examined and compared arsenic (As) and other element [copper (Cu), iron (Fe), potassium (K), manganese (Mn), rubidium (Rb), and zinc (Zn)] distribution, as well as their interactions in historically irrigated and non-irrigated semi-arid agricultural soils of the Southern High Plains (SHP) in the USA, using findings from a portable X-ray fluorescence spectrometer. The portable X-ray fluorescence (PXRF) findings revealed that the concentrations of As (5.1 vs. 3.1 mg kg−1), Cu (7.2 vs. 6.2), Fe (7398 vs. 5677), K (8638 vs. 7061), Mn (80 vs. 68), Rb (36 vs. 27), and Zn (19.3 vs. 13.8) were higher in the historically irrigated field compared to the non-irrigated and, when examined within depths, were consistently and significantly higher (P < 0.001) in the subsurface soils of the non-irrigated field, a trend not evident in the irrigated field. The strengths of the correlation among elements were higher in the non-irrigated field compared to the irrigated (average R of 0.60 vs. 0.54), suggesting a possible external input of these pollutants in the historically irrigated field which could have altered the natural background concentration and association among them. Furthermore, findings from principal component analysis (PCA) indicated that As, Mn, Rb, Fe, Zn, and K were more closely associated in the non-irrigated field and likely of lithogenic origin. The observed differences in elemental concentration and interaction between the irrigated and non-irrigated soils support the hypothesis that water from the local aquifer could be a potential source of pollutant addition to these semi-arid alkaline soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Southern High Plains (SHP) in the USA, a region characterized by semi-arid climatic condition (Peel et al. 2007), is currently threatened by numerous environmental challenges such as wind erosion, soil salinization, and drought (Mehta et al. 2000; Stout 2001; Allen et al. 2005). The water scarcity problem is further compounded by the rapid depletion of the main water source in the SHP, the Ogallala Aquifer. Recent studies have also shown that the declining groundwater could be associated with a steady decline in water quality as evidenced from the gradual increase in the level of salts over the last decade (Young et al. 2015). Scanlon et al. (2005) also documented the widespread presence of arsenic (As) in the groundwater from the Ogallala Aquifer, reporting that 36 % of the wells tested in the SHP exceeded the drinking water maximum contaminant limit (MCL) of 10 μg L−1. The sources of As in the Ogallala Aquifer were found to be most likely geologic rather than anthropogenic (Scanlon et al. 2005). Conversely, studies have suggested that As presence in the Ogallala Aquifer resulted from soil contamination due to pesticides, herbicides, and defoliant-containing As (Hudak 2000; Welch et al. 2000; Wakelyn et al. 2004). The presence of As in agricultural areas is of human health concern because it could eventually reach the human food chain through plant uptake and the subsequent consumption of plant materials by animals or directly by humans (Rahman et al. 2008; Peralta-Videa et al. 2009; Chang et al. 2014). It could also reach the groundwater through leaching (Anawar et al. 2004), thereby increasing the risk of contamination, or it can be transported to other locations by soil and water erosion (Drahota et al. 2006). Johnson and Hiltbold (1969) reported that ∼50 to 70 % of As applied through pesticides and defoliants (containing 24 % As) was recovered from the soil. In the plant system, Marcus-Wyner and Rains (1982) reported that As applied through pesticides and defoliants mostly accumulated in the cotton seed compared to other parts of the crop; increasing the risk of human exposure to As through the food chain because of the subsequent consumption of the seed by livestock. Additionally, the application of certain types of phosphate fertilizers such as triple superphosphate or monoammonium phosphate, which often contain traces of As in high amounts, could over time lead to As introduction into the soil, eventually reaching the plants (Davenport and Peryea 1991; Creger and Peryea 1994). Moreover, some studies reported that animal manures, mainly from pigs and chickens, commonly applied to agricultural soils, are also potential sources of As, because of the organoarsenic compounds used in animal feed additives (Nicholson et al. 1999; Arai et al. 2003; Nziguheba and Smolders 2008; Yao et al. 2009).
Measuring soil elemental concentrations often requires soil collection and extraction in the lab with harsh chemicals and measurement of the elements in the extractant using inductively coupled plasma (ICP)-optical emission spectroscopy (OES), atomic absorption spectrometry (AAS), or ICP-mass spectrometer (MS). Recently, proximal sensing tools such as the portable X-ray fluorescence (PXRF) spectrometer have been applied to contaminant quantification (Ran et al. 2014), soil chemical property predictions (Sharma et al. 2014, 2015), horizon differentiation (Weindorf et al. 2012a, b), and integrated with geographic information system (GIS) data for contaminant mapping (Weindorf et al. 2013) in various landscapes. Accordingly, the PXRF can be deployed in the field to rapidly determine elemental concentrations to help advance survey efforts and identify fields or areas within fields that have high pollutant concentrations. PXRF applications, however, have certain limitations, including moisture content, sensitivity of certain elements, and heterogeneity within the sample (Weindorf et al. 2014). With proper accounting of these limitations (see Kalnicky and Singhvi 2001; Kilbride et al. 2006), the benefits of rapid in situ characterization of targeted elements make PXRF a timely and valuable tool in agronomic and environmental soil science applications.
With the growing contaminants associated with water from the declining aquifer, environmental quality assessment is of utmost importance in the SHP. With agriculture being a major activity in this region and thus, a significant water user, it is almost certain that the cumulative effect of the degrading water quality of the aquifer will be more evident in agricultural fields receiving irrigation water from groundwater sources. To date, however, no studies have compared contaminant levels between frequently irrigated and historically non-irrigated fields, which could help in identifying and tracing contaminant sources in the SHP region. Thus, the goal of this study was to intensively survey two cotton (Gossypium hirsutum) fields under contrasting irrigation management (pivot irrigated vs. non-irrigated) on a fine sandy loam within the SHP. We hypothesized that elemental concentrations as well as their interactions could vary between continuously irrigated cotton fields and the historically non-irrigated fields within similar geological and geographical boundaries in this semi-arid region. Thus, the main objective of the study was to evaluate and compare the elemental concentration and interactions between historically irrigated and non-irrigated semi-arid agricultural soils of the SHP using findings from a PXRF spectrometer.
Materials and methods
Study area
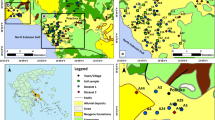
The study area is located in Welch, TX, USA (Fig. 1), approximately 75 km southwest of Lubbock, an important economic city in the SHP. The area lies roughly within longitude 102.11°W and 102.15°W and latitude 32.9°N and 32.95°N. It is characterized as a semi-arid climate with a 30-year average annual precipitation of approximately 470 mm and a mean annual temperature of ∼16 °C (NCDC 2015). The dominant soil series of the study area are Amarillo (fine-loamy, mixed, superactive, thermic Aridic Paleustalfs) and Patricia (fine-loamy, mixed, superactive, thermic Aridic Paleustalfs) (Soil Survey Staff 1999; WSS 2015). The SHP produces about 40 % of US cotton; thus, the land use in Welch is mostly irrigated agriculture, with cotton being the main cultivated crop (Scanlon et al. 2009). The primary irrigation water source for agricultural purposes is the Ogallala Aquifer.
Sample collection
Historically irrigated and non-irrigated fields were selected for this study. Each field was approximately 45 ha in size. Soil samples were collected from ∼0–5 cm (surface) and ∼5–10 cm (subsurface) depths for each georeferenced point in each field (Fig. 2). The irrigation history of each field was obtained from the respective owner. The irrigated field was confirmed to be under irrigation for the past 20 years or more. On the other hand, the non-irrigated field (dryland) has not been under irrigation for about 40 years. The coordinates of each sampled point were located using Garmin global positioning system (GPS) receiver (eTrex Summit HC, Garmin, China). A total of 140 soils samples (70 samples each from surface and subsurface) were collected from the irrigated field. The samples were collected at approximately 100 m intervals covering the entire field. Similarly, 102 samples (51 samples from each soil depth) were collected from the non-irrigated field. A total of 242 soil samples were collected and prepared for laboratory analyses. Sampling was conducted only once as the aim of the study was to evaluate the resultant cumulative effect of many years (>20) of irrigation using water from the local aquifer, on the concentration of elements in the receiving soil. Additionally, a water sample was collected from the sprinkler irrigation system located in one of the fields that utilizes water drawn from the Ogallala Aquifer. The sample was collected in a clean plastic bottle, rinsed three times with the irrigation water and then transported to the laboratory and stored at 4 °C until analysis.
Soil and water characterization and PXRF analysis
The collected soil samples were transported in plastic bags to the laboratory for analyses. Soil samples were air dried, ground, and passed through a 2-mm sieve. From each field, 25 samples were randomly selected following the zigzag sampling pattern to represent the entire field. A portion of each selected soil sample was characterized for soil pH, EC, and texture. Soil pH1:2 and EC1:2 were determined on a 1:2 soil/water ratio (Thomas 1996; Rhoades 1996). Soil texture was determined using the hydrometer method (Gee and Bauder 1986), where clay was determined after 1440 min via a model 152H soil hydrometer. Sand fractions were determined via separation by a 53-μm sieve.
A portion of each collected soil sample was saved for PXRF analysis. The analysis was based on United States Environmental Protection Agency (USEPA) method 6200 (USEPA 2007). The PXRF spectrometer used was Delta Premium DP-6000 PXRF (Olympus, Waltham, MA, USA). The instrument generates X-rays at a power level of 10–40 kV using the Rh X-ray tube. The instrument has two scanning modes: Soil Mode and Geochem Mode. For the purpose of this study, Soil Mode was used because it offers lower detection limits. Before scanning, the instrument was calibrated with “316” alloy clip fitted over the aperture following manufacturer’s recommendation. The elements that can be detected in the Soil Mode are P, S, Cl, Ti, V, Cr, Co, Ni, Se, Sr, Zr, Mo, Ag, Cd, Sn, Sb, Ba, Hg, Pb, As, K, Ca, Mn, Fe, Cu, Zn, and Rb. However, for this study only As, K, Ca, Mn, Fe, Cu, Zn, and Rb were examined because the rest were not detected in the studied soils by the instrument. Three-beam sequential scanning was used to detect the targeted elements. Each beam was set to scan for 30 s for a total scan time of 90 s per sample. Each soil sample was scanned in triplicate and the average value of elemental concentration reported. The PXRF unit was repositioned between scans to reduce errors resulting from sample heterogeneity. The instrument was recalibrated after every 30 scans. Relative percentage differences between duplicate samples were generally less than 10. In addition to the elemental concentration data, the limit of detection (LOD) (three times the standard error) for each element was also obtained and compiled. The manufacturer’s instrument detection limits are 1–3, 40–60, 25–40, 10, 10, 5–7, 3–5, and 1–3 mg kg−1 for As, K, Ca, Mn, Fe, Cu, Zn, and Rb, respectively (Olympus 2011).
The collected water sample was analyzed for As using the inductively coupled plasma-optical emission spectroscopy (ICP-OES) (iCAP 7400, Thermo Scientific, Waltham, MA). The analysis was conducted in replicates of three, following recommended quality control procedures.
Spatial analyses
Using PXRF data, GIS maps were produced showing the spatial distribution of As within the irrigated and the non-irrigated fields. The interpolation maps were produced using ArcGIS 10.2 (ESRI, The Redlands, CA), via kriging interpolation function in the spatial analyst tool (Krige 1966). Five semi-variogram model types were applied to generate the interpolation maps. However, only the best fitted model, i.e., the model with the lowest average standard error value, was used to generate the interpolation maps. The symbology and classification of As concentrations within the generated maps were set to five “equal interval” classes. Doing so, the visual interpretation and analysis will be easier and more convenient.
Statistical analyses
Statistical analyses were performed using XL Stat (version 2014.6.04) and Statistical Analysis Software (SAS 9.3, SAS Institute, Cary, NC). The correlation among elements was determined using linear regression analysis under the modeling data function in XL Stat. Principle component analysis (PCA), used in evaluating potential sources of As and the other elements quantified, was performed using PROC FACTOR in SAS. Within-pair comparison of mean concentration of elements between surface and subsurface depths in each field was evaluated using PROC TTEST.
Results and discussion
Soil characteristics
Selected soil characteristics of the irrigated and non-irrigated fields are listed in Table 1. Soil pH1:2 ranged from 7.1 to 7.8 in the irrigated field with a mean of 7.5 and from 7.4 to 8.2 in the non-irrigated with a mean of 7.8, indicating the slightly to moderately alkaline nature of the soils of this area. Generally, the clay content was similar between the two fields, ranging from 1 to 8 % (mean of 3 %) in the irrigated field and from 0 to 6 % (mean of 4 %) in the non-irrigated. On average, the sand content was lower by about 5 % and the silt content higher by about 6 % in the irrigated field. Soil EC1:2 showed wide variation between the irrigated and non-irrigated fields. Soil EC1:2 ranged from 0.25 to 1.35 dS m−1 (mean of 0.50 dS m−1) in the irrigated and from 0.02 to 0.08 dS m−1 (mean of 0.05 dS m−1) in the non-irrigated fields. It is possible that the quality of irrigation water is contributing to the relatively higher EC value in the irrigated field, as recent studies have shown that water from the Ogallala Aquifer has shown increased concentration of Na, Cl−, K, HCO3 −, and elevated SAR and EC levels over the last decade or more (Young et al. 2015). Inorganic fertilizer input could also be responsible for the elevated soil EC. Overall, the findings revealed no substantial contrast in the aforementioned soil properties between the fields.
Distribution of As and other elements
Soil As ranged from 2.0 to 5.1 mg kg−1 (mean = 3.1 mg kg−1) and from 2.9 to 8.5 mg kg−1 (mean = 5.1 mg kg−1), for non-irrigated and irrigated, respectively (Table 2). The higher As concentration observed in the irrigated field may be attributed to the quality of irrigation water used in the field and possibly to differential applications of defoliants that historically contained As. This hypothesis is supported by the As concentration of 58.7 μg L−1 detected in the collected irrigation water sample, which exceeded the USEPA drinking water standard of 10 μg L−1 (USEPA 2006). The As detected in the water sample was within the range of 0.2 to 164 μg L−1 reported by Scanlon et al. (2009) in water samples collected from the Ogallala Aquifer. In the SHP, cotton requires approximately 400–450 mm of water during the growing season. On average, irrigation contributes about 206 mm of the total water required by cotton (Howell 2001; Howell et al. 2004; Snowden et al. 2013). Thus, using the As concentration of 58.7 μg L−1 detected in the water sample, irrigation water could add about 0.05 mg As kg−1 soil to the irrigated field per year, which could amount to approximately 1.0 mg kg−1 As over 20 years. The added As also could be subjected to leaching and runoff, as well as plant uptake and removal with harvest and these would need to be accounted for in a complete As budget. In general, As concentration in these soils (Table 2) exceeded the USEPA screening levels of 0.67 mg kg−1 (USEPA 2015) for residential areas. However, As concentrations were lower than the 20 mg kg−1 cleanup levels for As in soil for residential/unrestricted use for the state of Texas (TCEQ 2014). The relatively high As concentrations found in the study area may be attributed to various sources: natural abundance, groundwater (Ogallala Aquifer), and the use of agricultural chemicals. Kriging interpolation was performed and maps generated for each field and for each depth (Figs. 3 and 4). Different kriging models were applied, however, the exponential model was chosen for analysis and display since it generated the lowest average standard error. The interpolation maps also showed a difference in As distribution between the two fields. Analysis of the digital elevation model (not shown) and interpolation maps for the surface soils of both fields (Figs. 3a and 4a) revealed that both fields are mostly flat (<0.6 %). However, As concentration increased in the eastern part of the irrigated field, which has a lower elevation compared to the western part. This pattern was not observed in the non-irrigated field. This finding may support the idea that contaminants in irrigation water move following the slope of the field; thus, As attached to finer particles such as clay may be transported by water movement downslope.
Other elements of interest such as K, Ca, Mn, Cu, Zn, and Rb were also examined and the concentration ranges and means in the irrigated and non-irrigated fields summarized in Table 2. The concentrations of the majority of the other elements (Cu, Fe, K, Mn, Rb, and Zn) examined were also higher in the irrigated field compared to the non-irrigated. The mean elemental concentration at the surface and the corresponding subsurface for the irrigated and non-irrigated fields were examined (Table 3). Findings reveal that the mean concentration of elements between the surface and subsurface was not significantly different in the irrigated field. However, the mean concentration of As, Ca, Fe, Mn, Rb, and Zn was consistently and significantly (P < 0.001) higher at the subsurface soil of the non-irrigated field.
Elemental interaction and source apportionment
Correlations among elements within the historically irrigated and non-irrigated fields are summarized in Table 4. Notably, within the irrigated field, As mostly correlated with Fe (R = 0.64, P < 0.05), Rb (R = 0.67, P < 0.05), and Zn (R = 0.66, P < 0.05). These relationships were also observed within the non-irrigated field but to a lower strength (Table 4). Generally, the strength of the correlations among elements was higher in the non-irrigated field compared to the irrigated (average R of 0.60 vs. 0.54). These weaker correlations within the irrigated field could be attributed to possible external input of these pollutants which could have altered the natural background concentration and interactions among these elements. Similar findings have been reported by Udeigwe et al. (2015) in their examination of the elemental interactions between managed (irrigated) and non-managed (non-irrigated) soils of golf course facilities in the same region. Findings from the interactions between elements and soil constituents revealed significant positive relationships (R = 0.46–0.57, P < 0.05) between pH and each of Mn, Fe, Zn, and Rb in the non-irrigated field with none of these found in the irrigated field.
The PCA is a multivariate statistical analysis used to group dependent variables to a fewer number of unrelated master variables often called “components.” This tool has been applied in geochemistry to identify pollutants and distinguish natural or lithogenic sources from anthropogenic sources (Sena et al. 2002; Fox and Metla 2005; Micó et al. 2006; Eze et al. 2010). The PCA loading plots of As and the other elements for irrigated and non-irrigated fields are presented in Fig. 5. Since the critical eigenvalue of 1 was used, only the first two components with an eigenvalue >1 were retained and interpreted. The elements were grouped into two-component models accounting for approximately 75 % of the variation associated with the data in each of the fields. The findings indicated that As, Mn, Rb, Fe, Zn, and K were more closely associated in the non-irrigated field, suggesting that the concentration of these elements in the soil could be influenced by the same source, likely lithogenic. It is possible that anthropogenic influences could have altered the natural background concentration and chemistry of these elements in the irrigated field leading to less association among them. Likewise, these findings could be compared to those of Udeigwe et al. (2015) who reported differences in association among elements between irrigated (managed) and non-irrigated portions of golf courses in the semi-arid climates of the SHP. The findings from this study suggest potential differences in interactions among the elements examined between the historically irrigated and non-irrigated fields, partly supporting the hypothesis that irrigation water could be a source of pollutant input to these semi-arid agricultural soils of the SHP.
Conclusions
In an effort to probe environmental contamination in agricultural settings, elemental distribution and interactions among elements and soil constituents were examined in historically irrigated and non-irrigated agricultural soils of the SHP using findings from a PXRF spectrometer. Majority of the elements (Cu, Fe, K, Mn, Rb, and Zn) examined were higher in the irrigated field compared to the non-irrigated. When examined within depth, findings reveal that the mean concentrations of these elements were significantly higher at the subsurface soil of the non-irrigated field compared to the subsurface. The strength of the correlations among elements was higher in the non-irrigated field compared to the irrigated with the weaker correlations within the irrigated field attributed to possible external input of these pollutants which could have altered the natural background concentrations and association among these elements. Results from PCA also indicated that As, Mn, Rb, Fe, Zn, and K were more closely associated in the non-irrigated field, suggesting that the concentration of these elements in the soil could be influenced by the same source, likely lithogenic. Conclusively, findings show differences in elemental concentration, distribution, and interactions between historically groundwater irrigated field and non-irrigated cotton field in these semi-arid alkaline soils of the SHP, supporting the hypothesis that irrigation could be a source of pollutant addition to these semi-arid soils.
References
Allen, V. G., Brown, C. P., Kellison, R., Segarra, E., Wheeler, T., Dotray, P. A., Conkwright, J. C., Green, C. J., & Acosta-Martinez, V. (2005). Integrating cotton and beef production to reduce water withdrawal from the Ogallala Aquifer in the Southern High Plains. Agronomy Journal, 97, 556–567.
Anawar, H. M., Akai, J., & Sakugawa, H. (2004). Mobilization of arsenic from subsurface sediments by effect of bicarbonate ions in groundwater. Chemosphere, 54(6), 753–762.
Arai, Y., Lanzirotti, A., Sutton, S., Davis, J. A., & Sparks, D. L. (2003). Arsenic speciation and reactivity in poultry litter. Environmental Science and Technology, 37(18), 4083–4090.
Chang, C. Y., Yu, H. Y., Chen, J. J., Li, F. B., Zhang, H. H., & Liu, C. P. (2014). Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environmental Monitoring and Assessment, 186(3), 1547–1560.
Creger, T. L., & Peryea, F. J. (1994). Phosphate fertilizer enhances arsenic uptake by apricot liners grown in lead-arsenate-enriched soil. HortScience, 29(2), 88–92.
Davenport, J. R., & Peryea, F. J. (1991). Phosphate fertilizers influence leaching of lead and arsenic in a soil contaminated with lead arsenate. Water, Air, and Soil Pollution, 57(1), 101–110.
Drahota, P., Paces, T., Pertold, Z., Mihaljevic, M., & Skrivan, P. (2006). Weathering and erosion fluxes of arsenic in watershed mass budgets. Science of the Total Environment, 372(1), 306–316.
Eze, P. N., Udeigwe, T. K., & Stietiya, M. H. (2010). Distribution and potential source evaluation of heavy metals in prominent soils of Accra Plains, Ghana. Geoderma, 156(3), 357–362.
Fox, G. A., & Metla, R. (2005). Soil property analysis using principal components analysis, soil line, and regression models. Soil Science Society of America Journal, 69(6), 1782–1788.
Gee, G. W., & Bauder, J. W. (1986). Particle-size analysis. In A. Klute (Ed.), Methods of soil analysis, part 1-physical and mineralogical methods (pp. 383–411). Madison: Soil Science Society of America.
Howell, T. A. (2001). Enhancing water use efficiency in irrigated agriculture. Agronomy Journal, 93(2), 281–289.
Howell, T. A., Evett, S. R., Tolk, J. A., & Schneider, A. D. (2004). Evapotranspiration of full-, deficit-irrigated, and dryland cotton on the Northern Texas High Plains. Journal of Irrigation and Drainage Engineering, 130(4), 277–285.
Hudak, P. F. (2000). Distribution and sources of arsenic in the southern high plains aquifer, Texas, USA. Journal of Environmental Science & Health Part A, 35(6), 899–913.
Johnson, L. R., & Hiltbold, A. E. (1969). Arsenic content of soil and crops following use of methanearsonate herbicides. Soil Science Society of America Journal, 33(2), 279–282.
Kalnicky, D. J., & Singhvi, R. (2001). Field portable XRF analysis of environmental samples. Journal of Hazardous Materials, 83(1), 93–122.
Kilbride, C., Poole, J., & Hutchings, T. R. (2006). A comparison of Cu, Pb, As, Cd, Zn, Fe, Ni and Mn determined by acid extraction/ICP–OES and ex situ field portable X-ray fluorescence analyses. Environmental Pollution, 143(1), 16–23.
Krige, D. G. (1966). Two-dimensional weighted moving average trend surfaces for ore-evaluation. Journal of the South African Institute of Mining and Metallurgy, 66, 13–38.
Marcus-Wyner, L., & Rains, D. W. (1982). Uptake, accumulation, and translocation of arsenical compounds by cotton. Journal of Environmental Quality, 11(4), 715–719.
Mehta, S., Fryar, A. E., & Banner, J. L. (2000). Controls on the regional-scale salinization of the Ogallala aquifer, Southern High Plains, Texas, USA. Applied Geochemistry, 15, 849–864.
Micó, C., Recatalá, L., Peris, M., & Sánchez, J. (2006). Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere, 65(5), 863–872.
National Climatic Data Center (NCDC) (2015). Online data request for the period 1931-2014. http://www1.ncdc.noaa.gov/pub/orders/cdo/478790.csv. Accessed 24 February 2015.
Nicholson, F. A., Chambers, B. J., Williams, J. R., & Unwin, R. J. (1999). Heavy metal contents of livestock feeds and animal manures in England and Wales. Bioresource Technology, 70(1), 23–31.
Nziguheba, G., & Smolders, E. (2008). Inputs of trace elements in agricultural soils via phosphate fertilizers in European countries. Science of the Total Environment, 390(1), 53–57.
Olympus Innov-X. (2011). DELTA_Handheld XRF R and D configuration guide DS_103-09-11. http://www.gwm-engineering.fi/Delta_R.pdf. Accessed 30 March 2015.
Peel, M. C., Finlayson, B. L., & McMahon, T. A. (2007). Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences Discussions, 4(2), 439–473.
Peralta-Videa, J. R., Lopez, M. L., Narayan, M., Saupe, G., & Gardea-Torresdey, J. (2009). The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. International Journal of Biochemistry and Cell Biology, 41(8), 1665–1677.
Rahman, M. A., Hasegawa, H., Rahman, M. M., Miah, M. M., & Tasmin, A. (2008). Arsenic accumulation in rice (Oryza sativa L.): human exposure through food chain. Ecotoxicology and Environmental Safety, 69(2), 317–324.
Ran, J., Wang, D., Wang, C., Zhang, G., & Yao, L. (2014). Using portable X-ray fluorescence spectrometry and GIS to assess environmental risk and identify sources of trace metals in soils of peri-urban areas in the Yangtze Delta region, China. Environmental Science: Processes & Impacts, 16(8), 1870–1877.
Rhoades, J. D. (1996). Salinity: electrical conductivity and total dissolved solids. In D. L. Sparks, A. L. Page, P. Helmke, R. H. Loeppert, P. N. Soltanpour, M. A. Tabatabai, C. T. Johnston, & M. E. Sumner (Eds.), Methods of soil analysis. Part 3-chemical methods (pp. 417–435). Madison: Soil Science Society of America.
Scanlon, B. R., Nicot, J. P., Reedy, R. C., Tachovsky, J. A., Nance, S. H., Smyth, R. C., et al. (2005). Evaluation of arsenic contamination in Texas. The Univ. of Texas at Austin, Bureau of Economic Geology, final report prepared for Texas Commission on Environmental Quality, under umbrella contract no. 582-4-56385 and work order no. UT-08-5-70828, 177 p, 167.
Scanlon, B. R., Nicot, J. P., Reedy, R. C., Kurtzman, D., Mukherjee, A., & Nordstrom, D. K. (2009). Elevated naturally occurring arsenic in a semiarid oxidizing system, Southern High Plains aquifer, Texas, USA. Applied Geochemistry, 24(11), 2061–2071.
Sena, M. M., Frighetto, R. T. S., Valarini, P. J., Tokeshi, H., & Poppi, R. J. (2002). Discrimination of management effects on soil parameters by using principal component analysis: a multivariate analysis case study. Soil and Tillage Research, 67(2), 171–181.
Sharma, A., Weindorf, D. C., Man, T., Aldabaa, A. A. A., & Chakraborty, S. (2014). Characterizing soils via portable X-ray fluorescence spectrometer: 3. Soil reaction (pH). Geoderma, 232, 141–147.
Sharma, A., Weindorf, D. C., Wang, D., & Chakraborty, S. (2015). Characterizing soils via portable X-ray fluorescence spectrometer: 4. Cation exchange capacity (CEC). Geoderma, 239, 130–134.
Snowden, C., Ritchie, G., & Thompson, T. (2013). Water use efficiency and irrigation response of cotton cultivars on subsurface drip in west Texas. Journal of Cotton Science, 17, 1–9.
Soil Survey Staff (1999). Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. 2nd ed. USDA-NRCS. Washington, DC: United States Government Printing Office.
Stout, J. E. (2001). Dust and environment in the southern high plains of North America. Journal of Arid Environments, 47, 425–441.
Texas Commission on Environmental Quality (TCEQ) (2014). Tier 1 protective concentration levels (PCL) document and table (September 2014). http://www.tceq.state.tx.us/remediation/trrp/trrppcls.html. Accessed 24 March 2015.
Thomas, G. W. (1996). Soil pH and soil acidity. In D. L. Sparks, A. L. Page, P. Helmke, R. H. Loeppert, P. N. Soltanpour, M. A. Tabatabai, C. T. Johnston, & M. E. Sumner (Eds.), Methods of soil analysis. Part 3-chemical methods (pp. 475–488). Madison: Soil Science Society of America.
Udeigwe, T. K., Young, J., Kandakji, T., Weindorf, D. C., Mahmoud, M. A., & Stietiya, M. H. (2015). Elemental quantification, chemistry, and source apportionment in golf course facilities in semi-arid urban landscape using portable x-ray fluorescence spectrometer. Solid Earth Discussions, 7(1), 37–62.
United States Environmental Protection Agency (USEPA) (2006). Arsenic in drinking water. http://water.epa.gov/lawsregs/rulesregs/sdwa/arsenic/index.cfm. Accessed 24 March 2015.
United States Environmental Protection Agency (USEPA) (2007). Method 6200: field portable X-ray fluorescence spectrometry for the determination of elemental concentrations in soil and sediment. http://www.epa.gov/solidwaste/hazard/testmethods/sw846/pdfs/6200.pdf. Accessed 24 March 2015.
United States Environmental Protection Agency (USEPA) (2015). Regional screening levels, region 6: residential soil. http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/Generic_Tables/docs/master_sl_table_run_JAN2015.pdf. Accessed 24 March 2015.
Wakelyn, P.J., Adair, P.K., Wolf, S. (2004). Public and environmental issues. Proceedings of the 2004 Beltwide Cotton Conferences, National Cotton Council, Memphis, TN, pp. 275–302.
Web Soil Survey (WSS) (2015). http://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey. aspx. Accessed 24 March 2015.
Weindorf, D. C., Zhu, Y., Haggard, B., Lofton, J., Chakraborty, S., Bakr, N., et al. (2012a). Enhanced pedon horizonation using portable X-ray fluorescence spectrometry. Soil Science Society of America Journal, 76(2), 522–531.
Weindorf, D. C., Zhu, Y., McDaniel, P., Valerio, M., Lynn, L., Michaelson, G., et al. (2012b). Characterizing soils via portable x-ray fluorescence spectrometer: 2. Spodic and Albic horizons. Geoderma, 189, 268–277.
Weindorf, D. C., Paulette, L., & Man, T. (2013). In-situ assessment of metal contamination via portable X-ray fluorescence spectroscopy: Zlatna, Romania. Environmental Pollution, 182, 92–100.
Weindorf, D. C., Bakr, N., & Zhu, Y. (2014). Advances in portable X-ray fluorescence (PXRF) for environmental, pedological, and agronomic applications. Advances in Agronomy, 128, 1–45.
Welch, A. H., Westjohn, D. B., Helsel, D. R., & Wanty, R. B. (2000). Arsenic in ground water of the United States: occurrence and geochemistry. Groundwater, 38(4), 589–604.
Yao, L. X., Li, G. L., Dang, Z., He, Z. H., Zhou, C. M., & Yang, B. M. (2009). Arsenic uptake by two vegetables grown in two soils amended with As-bearing animal manures. Journal of Hazardous Materials, 164(2), 904–910.
Young, J., Udeigwe, T. K., Weindorf, D. C., Kandakji, T., Gautam, P., & Mahmoud, M. A. (2015). Evaluating management-induced soil salinization in golf courses in semi-arid landscapes. Solid Earth Discussions, 7, 91–114.
Acknowledgments
The authors thank Dr. David Weindorf for assisting with part of the soil sampling and providing the PXRF spectrometer; Dr. Abdalsamad Aldabaa for assisting in soil sampling and providing guidance on the use of the PXRF spectrometer; and Mr. Trent Smith for assisting in soil sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kandakji, T., Udeigwe, T.K., Dixon, R. et al. Groundwater-induced alterations in elemental concentration and interactions in semi-arid soils of the Southern High Plains, USA. Environ Monit Assess 187, 665 (2015). https://doi.org/10.1007/s10661-015-4863-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4863-1