Abstract
Soil samples at two depths were collected and analyzed to determine the concentrations of 16 polycyclic aromatic hydrocarbons (PAHs), organic carbon, and soil pH. The Σ16PAHs were 0.13 to 3.92 mg kg−1 at depth 1 and 0.21 to 50.32 mg kg−1at depth 2. The averages of the PAH compounds indicate that the area is contaminated with oil, and this pollution was greater at depth 2. Interpolation maps showed that the southern region, especially at depth 2, has been contaminated more by anthropogenic activity. The diagnostic ratios indicate several sources of pollution of the agricultural soil. A comparison of average PAHs and standard values revealed that higher molecular weight compounds in the topsoil (InP and BghiP) and subsoil (BaA, BkF, BaP, DBA, and BghiP) exceed standard values for farmland. The pH interpolation map for both depths showed that most of the area has alkaline soil from long-term irrigation with untreated urban wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are distributed across diverse environmental matrices, including soil (He et al. 2009; Fernández et al. 2000; Krauss et al. 2000; Gocht et al. 2007). Industrialization has also resulted in widespread pollution of soil (Telysheva et al. 2011). In addition, soil contamination by organic matter such as aromatic compounds (Wong et al. 2002; Ma et al. 2005) has increased its toxicity, mutagenicity, carcinogenicity, and teratogenicity (Gao et al. 2010). Soil consists of an inorganic phase and a biotic phase that includes bacteria, plants, and other animals. These two phases form an ecological balance that can destroy the soil structure if the balance changes. Oil and its compounds contaminate the environment as by-products of transportation, combustion of fossil fuels, biomass burning, runoff, incineration, and by accident. One source of oil pollution is an oil refinery which can pollute the surrounding area by leakages from equipment and pipelines and by runoff. Other sources of pollution include industrial wastewater and urban surface runoff. Sixteen polycyclic aromatic hydrocarbons are selected which are recognized as priority pollutants by the US Environmental Protection Agency (USEPA) and as representative of the group of these compounds (Hung et al. 2011; Gonul and Kucuksezgin 2012; Qiao et al. 2006; Magi et al. 2002; Hao et al. 2007; Yan et al. 2004). PAHs disperse into the environment by atmospheric transport or by waterways (Chen et al. 2012). These pollutants are toxic to humans and animals and could result in death and genetic mutation. Agricultural land must be protected from PAHs to assure its sustainability (Huang et al. 2007). PAHs might not be easily remediated and therefore would persist over a long period, causing a long-term risk of transport to environment such as groundwater (Fengpeng et al. 2009). They could also inhibit biological activity in soil, an important factor in soil fertility and nutrient cycling (Jensen 1999). The productive agricultural land in the south of the city of Tehran is primarily used to grow grain and vegetables. The area has been polluted by the freshwater and irrigated with untreated urban wastewater. The freshwater is usually taken from the aqueduct which is running under the oil refinery area. The aqueduct has been also polluted by the oil refinery tanks leakages during the last two decade (Falsafi et al. 2007). Tehran city is the main origin of untreated wastewater which is mixed with urban runoffs. According to the reports and studies both the underground water and untreated wastewater in this area contain HCs (Hani et al. 2010).
The present study determined the concentration and distribution of PAHs pollution and identified possible sources of oil pollution in soil around Tehran oil refinery. The study also examined the effect of irrigation of this agricultural land with untreated urban wastewater.
Materials and methods
Study area
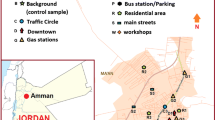
The area under study is located at 35° 49′ to 35° 57′ N longitude and 51° 43′ to 51° 46′ E latitude in a semi-arid region south of the city of Tehran. It comprises an area of about 20 km2. The average annual precipitation is 200 mm, maximum temperature is 42 °C in summer, and the minimum temperature is −4 °C in winter. Figure 1 shows the spatial position of the study area in Tehran Province.
Soil sample collection and preparation
A total of 166 soil samples were collected in September 2012 at depths of 0–30 cm (depth 1; topsoil) and 30–60 cm (depth 2; subsoil) using systematic random sampling. The region was divided into zones I and II and was gridded using ArcGIS 9.3 (ESRI, 2009) using the same number of pixels (317 × 317 m). The center of each square was chosen as the sampling point (39 sampling points from zone I and 44 from zone II). Figure 1 shows the locations of the 83 points chosen. Sampling was performed at each point at both depths 1 and 2. Each sampling point represents an average of 10 ha. After determining the point positions, the soil samples were collected and were pooled in an aluminum sheet and packed in a polyethylene bag. These samples were immediately transferred to the laboratory and air-dried at 25 °C for pH and organic carbon (OC) analysis and freeze-dried was used for PAH analysis. They were then grinded and sieved through no. 10 mesh for pH analysis, no. 30 mesh to test for organic carbon (Ryan et al. 2007), and no. 230 mesh to test for PAHs (MOOPAM 1999). The soil pH was determined using a pH meter (PB-11 Sartorius), and the Walky-Blanks method was used to determine the amount of OC in the soil (Ryan et al. 2007). According to the method, 0.5 g of each grinded sample weighted and poured into a conical flask. Potassium dichromate and concentrated H2SO4 were used for digesting the samples. The solution was then mixed thoroughly and allowed it to stand for 30 min in a water bath to cool down. Finally, solution was titrated by ferrous ammonium sulfate solution.
Chemicals and extraction
Dichloromethane (HPLC grade, ≥99.8 %), anhydrous sodium sulfate (HPLC grade, ≥99 %), and silica gel were obtained from Chem-Lab (Belgium). n-Hexane of purity 99 % was purchased from Merck (Germany). The 16 PAH standards classified as priority pollutants by the USEPA and internal standards were obtained from Chem-Lab (Belgium).
Soxhlet extraction was used to extract the aromatic compounds from the soil samples. First, 3.5 g of soil sieved through no. 230 mesh was weighed and mixed with 4 g of sodium sulfate. Then, the sample was transferred to the soxhlet system for extraction using 75 ml n-hexane and dichloromethane solvent (1:1 v/v) for 8 h. The extract was concentrated to reach a total volume of 5 ml using a rotary evaporator. Then, it was fractionated in a clean-up column containing silica gel, sodium sulfate, and glass wool. The column was initially washed with 10 ml of n-hexane and dichloromethane (1:1 v/v), and the extracts were eluted using 40 ml of n-hexane/dichloromethane (9:1 v/v). The aromatic fractions were again concentrated to reach a total volume of 1.0 ml using a rotary evaporator and prepared for injection.
Quantification
An Agilent 7890A GC-FID and a splitless injector were used to determine the PAH concentrations. Separation was done in a fused silica capillary column (HP-5, 30 m, 0.32 mm ID, 0.25-μm film thickness). High-purity (99.999 %) nitrogen was used as the carrier gas at a flow rate of 1 ml min−1. The initial temperature of 60 °C was held for 3 min and increased to 295 °C at a rate of 3 °C min−1 and held for 10 min. The flame ionization detector was employed at 330 °C, and the initial injection temperature was 250 °C. Calibration graphs were constructed by plotting the peak area against the injected concentration every 3 days; in all cases, a linear relationship at r 2 > 0.99 was obtained. The limit of detection (LOD; 3 S m−1) was calculated for each analysis as described by Shahbazi et al. (2012). The limit of quantification (LOQ; 3 LOD) was calculated based on the method detection limit (MDL) (Maliszewska-Kordybach et al. 2009). Spike recoveries were 75 to 108 % (average of 95 %). Blanks were run under the same conditions after running 15 samples to monitor for possible contamination; the results for them were below detection limits.
Interpolation
Inverse distance weighting (IDW) was applied to interpolate the PAH data. Unknown points were estimated using the distances between known points because the nearest points have more value than the others. IDW can be summarized as follows:
where S is the estimation of the unsampled point, n is the number of points, d i is the distance between the known points, S i is the value at point i, and p is the optimum power.
Descriptive and inferential statistical analyses for mean, range, standard error, Mann-Whitney, principal component analysis (PCA), and Spearman correlation were applied to determine possible correlations among variables. Outliers were eliminated for parameters that were not normal. R software (version 3.0.1) was used to perform statistical analysis (R Core Team 2011).
Results and discussion
PAH residues
The average pattern, minimum, and maximum values for individual PAHs, ∑4 rings, ∑5+6 rings, lower molecular weight (LMW), higher molecular weight (HMW), soil organic carbon, and pH were determined for sampling depths 1 and 2. As shown in Table 1, the PAH concentrations at depth 1 ranged from 0.001 mg/kg for Fl and Flu to 1.253 mg/kg for BaA. At depth 2, they ranged from 0.001 mg/kg for Chr to 6.52 mg/kg for Acy.
The mean values indicated that concentrations of these compounds were much higher at both depths than for results from other studies in different regions (Chung et al. 2007; Zhang et al. 2013; Maliszewska-Kordybach et al. 2009). The averages indicated that the concentration at depth 2 was higher than at depth 1 especially for LMWPAHs, indicating that these compounds are more soluble than HMWPAHs and have penetrated the subsoil after more than 30 years of irrigation with wastewater. The low concentration of LMW compounds at depth 1 may relate to the migration of them or increased volatility of these compounds, which react with sunlight at the surface. Table 1 also shows the standard values of PAHs for agricultural farmland and environmental conservation in Iran. A comparison of the average concentrations and their standard values for farmland indicates that InP and BghiP at depth 1 and BaA, BkF, BaP, DBA, and BghiP at depth 2 exceeded standards. It is known that HMW PAHs are major carcinogenic PAHs (Maliszewska-Kordybach et al. 2009) and are dangerous for field crops. It is notable that BaP, which is one of the most dangerous PAH (Ma et al. 2005) in the environment, was found in concentrations of about 3 mg kg−1 in some samples. A comparison of average PAH with standard values for underground water indicates that Na and An at depth 1 and Na, Phe, An, and Flu at depth 2 exceeded standard values. It appears that there is more risk at depth 2 in farmland and underground water than at depth 1. A comparison for standard values for environmental conservation and the average PAHs indicate no risk at either depth. It appears that irrigation with untreated urban wastewater and urban runoff has increased the PAH concentration in this land. Other researchers (Zhang et al. 2012; Tao et al. 2004) have reported similar increases in PAH concentrations in the soil of land irrigated with untreated urban wastewater. Total PAH concentrations were higher at depth 2 than at depth 1. Some researchers like Mikkonen et al. (2012) also found that the concentration of ΣPAHs increased as the depth increased, whereas Ma et al. (2005) and Zhang et al. (2013) concluded that PAH concentration in soil decreased at subsoil. Table 1 shows the average concentration of PAH compounds in two different agricultural regions (Hong Kong and Beijing). A comparison of mean PAHs for the present study with these results show extreme levels of pollution by PAH compounds in farmland near the Tehran oil refinery. Figure 2 is a bar plot (mean ± SE) for PAH compounds, ∑PAHs, ∑4 rings, and 5+6 rings. The figure and the Mann-Whitney test (not reported) confirm that there is a significant difference between Na, Acy, Ace, Fl, Ph, Flu, Pyr, BaA, InP, and total PAH between depths 1 and 2. The average soil OC decreased at depth 2, and there is a significant difference between OC and average pH between depths 1 and 2. Figure 2b, c are bar plots (mean ± SE) of PAH compounds at both depths in zone I and zone II, respectively. It can be seen that all compounds except InP, DBA, and BghiP appear in higher concentrations at depth 2 in zone I than in zone II. In zone II, all compounds except Chr, BbF, and InP show significant differences in concentration between depths 1 and 2, which reveals the role of wastewater irrigation and plowing for entry of these compounds into the subsoil. The highest PAH values were found at depth 2 in zone II. The results showed that OC in zone I was higher at depth 1 and that there was a significant difference between depths. In zone II, there was no difference between depths. Depth 1 in zone I showed uniform pH; however, pH in zone II decreased at depth 2. Soil pH in this area, as in most places in Iran, showed a high calcium carbonate content in the alkaline range. Irrigation with untreated urban wastewater could increase soil pH. The soil pH values appeared to be high for most field crops and may affect their yield because phosphorus, zinc and manganese become less available at high pH. Tables 2 and 3 have shown correlation coefficient among individual PAHs, OC, and pH value at two depths. The correlation coefficient for depth 1 showed that OC had a negative correlation with Fl and that soil pH had positive correlation with An. No correlation was found between HMW PAHs and OC at either depth, which may be because PAHs are not adsorbed by organic matter in soil in this area, where the human activity is very high. Ma et al. (2005) also reported any correlation between PAHs and OM in both depths. By contrast, Zhang et al. (2013) and Jones et al. (1989) reported that PAHs were strongly adsorbed onto the surface of OM particles. In the present study, OC showed no correlation with PAHs at either depths except for Fl at depth 1. Zhang et al. (2013) and Agarwal et al. (2009) reported significant correlations between OC and PAHs (especially with LMW PAHs) in agricultural surface soil. Results of the PCA showed that the percentage of variance explained by PC1 (first component) and PC2 (second component) was 31 and 11, respectively (Fig. 3a, b). At depth 2, Na, Chr, and InP showed minimum variance; PC2 was heavily weighted by OC and BbF. The percentage of variance explained by the first axis was 74 % and the second axis was 6 %. The bi-plots effectively show that petrogenic and pyrogenic hydrocarbons (HCs) contributed to pollution at both depths. Petrogenic HCs compounds are associated with petroleum sources and pyrogenic hydrocarbon compounds are associated with the combustion of petroleum, wood, coal, etc.
Effect of wastewater on soil
The bar plots of PAH compounds in areas irrigated with fresh water and irrigated with untreated urban wastewater are shown in Fig. 4a for depth 1 and in Fig. 4b for depth 2. As shown, irrigation with untreated urban wastewater influenced soil at depth 1 more than at depth 2; all compounds except BaA and DBA occurred in greater amounts in the area irrigated with wastewater. Figure 4a indicates that HMW PAHs such as BkF, BaP, InP, and BghiP increased in the area irrigated with wastewater. At depth 2, all compounds except BbF and InP showed higher concentrations in the area irrigated with freshwater. PAHs carried in wastewater that has a high concentration of particles are adsorbed onto the particles and are less mobile and remain in the surface soil. Freshwater carries fewer particles and organic matter and could increase mobility of the PAH compounds in the soil and the concentration at depth 2. It can be concluded that irrigation with untreated wastewater can increase the PAH concentrations, especially for higher molecular weight PAHs at depth 1. This affects the health of the field crops and humans who eat the crops. Although the soil in this area is alkaline, irrigation with untreated wastewater (7 < pH < 8) could increase soil pH in the long term. Mohammad Rusan et al. (2007) and Schipper et al. (1996) reported siimilar results for irrigation with untreated wastewater and increased pH in soil.
Spatial variability
Interpolation maps for TPAHs and HMW PAH at depths 1 and 2 are shown in Fig. 5a–d. These maps show that the TPAH concentrations increased moving from north to south in the study area at both depths. The northern part of the region experiences less irrigation with wastewater and showed lower concentrations of hydrocarbons. The southern part of the region receives more irrigation by wastewater, is located downslope of the oil storage tanks, and showed the highest concentration of PAHs. One source of PAHs in the southern part of the region is that the elevation slopes downward slightly from north to south. Although PAHs are not soluble, they could be migrated southward along with the agricultural runoffs during the autumn, winter and spring. The interpolation maps of the TPAHs at depth 2 showed that the TPAH concentrations increased as the depth of the soil increased and the southeastern portion of the region had the highest concentrations of these pollutants. Figure 5c, d shows the spatial variability of HMW PAHs in the study area. At depth 1, the HMW PAH concentration is more uniform than at depth 2, which was not uniform. The HMW PAH concentration increased moving from north to southeast. The TPAHs and HMW PAHs at depth 2 show a similar trends to the southeast, where the high concentrations were found. Maliszewska-Kordybach et al. (2009) stated that PAHs with high molecular weight are the main carcinogenic PAHs in the environment; thus, the southern part of the study area is at more risk in the environment and underground water resources. These compounds accumulate more by irrigation with untreated urban wastewater and leaching to the underground waters and then enter the food chain and increase the incidence of cancer and other problems (Anonymous 2008) Interpolation maps of OC (Fig. 5e, f) showed that higher OC values are found in the northern part of the area is at depth 1 but that depth 2 has a more uniform distribution.
Interpolation maps of PAHs (a, b), HMWPAHs (c, d), OC (e, f), and pH (g, h) at two sampled depth Fig. 5. Interpolation maps of PAHs (a, b), HMWPAHs (c, d), OC (e, f), and pH (g, h) at two sampled depth
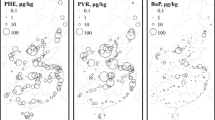
Source of PAH pollutants
PAH ratio pairs, such as Flu/Py, Ph/An, FIA/(FIA+Py), BaA/BaA+Chy (BaA/228), and IP/(IP+BghiP) have distinct chemical tracers for possible sources of PAH in environmental samples (Liu et al. 2008; Götz et al. 1998; Soclo et al. 2000; Kavouras et al. 2001; Yunker et al. 2002; Ma et al. 2005; Sicre et al. 1987). The ratios for Ph/An and Fl/Py are commonly used to distinguish between petrogenic and pyrogenic sources. Ph/An > 10, Chr/BaA < 1, LMW/HMW > 1, and Flu/Py <1 indicate that PAHs have petrogenic origins (Chen et al. 2012; Bouloubassi et al. 2012; Ma et al. 2005; He et al. 2009). The ratios for the 83 sampling points at depth 1 and depth 2 were calculated and then interpolated in Fig. 6. The interpolation maps show that there are both petrogenic and pyrogenic sources for the oil hydrocarbons in the study area. Flu/Py, Ph/An, and LMW/HMW showed pyrogenic characteristics, and Chr/BaA indicated that petrogenic sources were the main cause of pollution. BaP/BghiP (Fig. 6l, m) showed that emissions from vehicles in the area, except in the immediate vicinity of the roads, had no effect on the levels of oil hydrocarbons in the soil. It can be concluded that more than 30 years of runoff and irrigation by untreated urban wastewater onto agricultural land, oil refinery surface runoff after rainfall and leakage from storage tanks are the sources of PAHs pollution in agricultural soil. Although BaP, BghiP, and InP are usually emitted from automobiles (Rogge et al. 1993a, b; Ma et al. 2005) and the concentrations of these PAHs were higher than for the other PAHs, BaP/BghiP indicates that emissions from automobiles were not the source of hydrocarbons in the study area. HCs which are released from vehicles might be washed onto the sewage channels and finally are used as irrigation water in this region. This action can contribute to pyrogenic HCs in soil. On the other hand, leakages from storage tanks into the aqueduct might be originated from petrogenic source.
Conclusion
The samples at depths 1 and 2 in the study area show pollution with PAHs from petrogenic and pyrogenic sources. The level of all PAHs at depth 2 were higher (except Chr, BbF and InP) than their concentrations at depth 1. A comparison of the averages of these compounds with standard values shows that HMW PAHs carried more risk in agricultural land. Interpolation maps of the PAHs suggested that the southern parts of the study area are primarily contaminated by anthropogenic activity. These southern parts had the highest concentrations of PAHs and HMW PAHs from wastewater irrigation and water polluted with oil. If irrigation with untreated urban wastewater and polluted fresh water continues, this area will become more and more contaminated. The use of molecular ratios showed that there are two possible sources of PAH pollution in the study area. Wastewater and urban runoff had a major effect on HMW PAH content in the soil, especially at depth 1, which is important for crops. Leakages from storage tanks can also enter the LMW PAHs in the soil. PAH ratios indicated that different ratios produced different results. It is necessary to investigate the products affected by wastewater and oil refineries by introducing new ratios to determine the origins of PAHs in a particular site.
References
Agarwal, T., Khillare, P. S., Shridhar, V., & Ray, S. (2009). Pattern, sources and toxic potential of PAHs in the agricultural soils of Delhi, India. [Research Support, Non-U S Gov't]. Journal of Hazardous Materials, 163(2-3), 1033–1039.
Anonymous (2008). Investigation of petroleum hydrocarbons and heavy metals accumulation in agricultural soil and crops in south of Tehran city. Final Report, Alzahra university, (In Persion with English abstract), Tehran, Iran, 270P.
Bouloubassi, I., Roussiez, V., Azzoug, M., & Lorre, A. (2012). Sources, dispersal pathways and mass budget of sedimentary polycyclic aromatic hydrocarbons (PAH) in the NW Mediterranean margin, Gulf of Lions. Marine Chemistry, 142–144, 18–28.
Chen, H., Teng, Y., & Wang, J. (2012). Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Rizhao coastal area (China) using diagnostic ratios and factor analysis with nonnegative constraints. Science of the Total Environment, 414(0), 293–300. doi:10.1016/j.scitotenv.2011.10.057.
Chung, M. K., Hu, R., Cheung, K. C., & Wong, M. H. (2007). Pollutants in Hong Kong soils: polycyclic aromatic hydrocarbons. Chemosphere, 67, 464–473.
Fengpeng, H., Zhihuan, Z., Yunyang, W., Song, L., Liang, W., & Qingwei, B. (2009). Polycyclic aromatic hydrocarbons in soils of Beijing and Tianjin region: vertical distribution, correlation with TOC and transport mechanism. Journal of Environmental Sciences, 21(5), 675–685.
Fernández, P., Vilanova, R. M., Martínez, C., Appleby, P., & Grimalt, J. O. (2000). The historical record of atmospheric pyrolytic pollution over Europe registered in the sedimentary PAH from remote mountain lakes. Environmental Science & Technology, 34(10), 1906–1913.
Gao, Y., Wu, S. C., Yu, X. Z., & Wong, M. H. (2010). Dissipation gradients of phenanthrene and pyrene in the Rice rhizosphere. Chemosphere, 81, 1084–1090.
Gocht, T., Johannes, A. C., Epp, M., Jochmann, M., Blessing, M., Schmidt, T. C., & Grathwohl, P. (2007). Indications for pedogenic formation of perylene in a terrestrial soil profile: depth distribution and first results from stable carbon isotope ratios. Applied Geochemistry, 22(12), 2652–2663. doi:10.1016/j.apgeochem.2007.06.004.
Gonul, L. T., & Kucuksezgin, F. (2012). Aliphatic and polycyclic aromatic hydrocarbons in the surface sediments from the Eastern Aegean: assessment and source recognition of petroleum hydrocarbons. Environmental Science and Pollution Research, 19(1), 31–41. doi:10.1007/s11356-011-0524-2.
Götz, R., Bauer, O. H., Friesel, P., & Roch, K. (1998). Organic trace compounds in the water of the River Elbe near Hamburg part II. Chemosphere, 36(9), 2103–2118. doi:10.1016/S0045-6535(98)00009-5.
Hao, R., Wan, H.-F., Song, Y.-T., Jiang, H., & Peng, S.-L. (2007). Polycyclic aromatic hydrocarbons in agricultural soils of the southern subtropics, China. Pedosphere, 17(5), 673–680. doi:10.1016/S1002-0160(07)60081-2.
Hani, A., Pazira, E., Manshouri, M., Babaie Kafaky, S., & Ghahroudi Tali, M. (2010). Spatial distribution and mapping of risk elements pollution in agricultural soils of southern Tehran, Iran. Plant, Soil and Environment, 56(6), 288–296.
He, F., Zhang, Z., Wan, Y., Song, L., Wang, L., & Qingwei, B. (2009). Polycyclic aromatic hydrocarbons in soils of Beijing and Tianjin region: vertical distribution, correlation with TOC and transport mechanism. Journal of Environmental Sciences, 21(5), 675–685.
Huang, S. S., Liao, Q. L., Hua, M., Wu, X. M., Bi, K. S., Yan, C. Y., Chen, B., & Zhang, X. Y. (2007). Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere, 67(11), 2148–2155.
Hung, C. C., Gong, G. C., Ko, F. C., Lee, H. J., Chen, H. Y., Wu, J. M., Hsu, M. L., Peng, S. C., Nan, F. H., & Santschi, P. H. (2011). Polycyclic aromatic hydrocarbons in surface sediments of the East China Sea and their relationship with carbonaceous materials. Marine Pollution Bulletin, 63(5–12), 464–70.
Jensen, J. (1999). Fate and effects of linear alkylbenzene sulphonates (LAS) in the terrestrial environment. Science of the Total Environment, 226(6), 93–111.
Jones, KC., Grimmer, G., Jacob, J., & Johnston, A. E. (1989). Changes in the polynuclear aromatic hydrocarbon content of wheat grain and pasture grassland over the last century from one site in the U.K. Science of the Total Environment, 78, 117–30.
Kavouras, I. G., Koutrakis, P., Tsapakis, M., Lagoudaki, E., Stephanou, E. G., Von Baer, D., & Oyola, P. (2001). Source apportionment of urban particulate aliphatic and polynuclear aromatic hydrocarbons (PAHs) using multivariate methods. Environmental Science and Technology, 35(11), 2288–94.
Krauss, M., Wilcke, W., & Zech, W. (2000). Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in forest soils: depth distribution as indicator of different fate. Environmental Pollution, 110(1), 79–88.
Liu, Y., Chen, L., Jianfu, Z., Qinghui, H., Zhiliang, Z., & Hongwen, G. (2008). Distribution and sources of polycyclic aromatic hydrocarbons in surface sediments of rivers and an estuary in Shanghai, China. Environmental Pollution, 154(2), 298–305. doi:10.1016/j.envpol.2007.10.020.
Ma, L., Chu, S., Cheng, H., Wang, X., Liu, X., & Xu, X. (2005). Polycyclic aromatic hydrocarbons contamination in subsoil from outskirts of Beijing, People’s Republic of China. Geoderma, 129(3–4), 200–210. doi:10.1016/j.geoderma.2004.11.026.
Magi, E., Bianco, R., Ianni, C., & Di Carro, M. (2002). Distribution of polycyclic aromatic hydrocarbons in the sediments of the Adriatic Sea. Environmental Pollution, 91(1), 91–98. doi:10.1016/S0269-7491(01)00321-9.
Maliszewska-Kordybach, B., Smreczak, B., & Klimkowicz-Pawlas, A. (2009). Concentrations, sources, and spatial distribution of individual polycyclic aromatic hydrocarbons (PAHs) in agricultural soils in the Eastern part of the EU: Poland as a case study. Science of the Total Environment, 407(12), 3746–53.
Mikkonen, A., Hakala, K. P., Lappi, K., Kondo, E., Vaalama, A., & Suominen, L. (2012). Changes in hydrocarbon groups, soil ecotoxicity and microbiology along horizontal and vertical contamination gradients in an old landfarming field for oil refinery waste. Environmental Pollution, 162(0), 374–380. doi:10.1016/j.envpol.2011.12.012.
Mohammad Rusan, M. J., Hinnawi, S., & Rousan, L. (2007). Long term effect of wastewater irrigation of forage crops on soil and plant quality parameters. Desalination, 215(1–3), 143–152. doi:10.1016/j.desal.2006.10.032.
MOOPAM. (1999). Manual of Oceanographic Observation and Pollution Analysis. Regional organization for the protection of marine environment (ROPME, Kuwait):220P.
Qiao, M., Wang, C., Huang, S., Wang, D., & Wang, Z. (2006). Composition, sources, and potential toxicological significance of PAHs in the surface sediments of the Meiliang Bay, Taihu Lake, China. Environment International, 32(1), 28–33. doi:10.1016/j.envint.2005.04.005.
R Core Team. (2011). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 12 May 2011.
Rogge, W. F., Hildemann, L. M., Mazurek, M. A., Cass, G. R., & Simoneit, B. R. T. (1993a). Sources of fine organic aerosol: 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks. Environmental Science and Technology, 27, 636–651.
Rogge, W. F., Hildemann, L. M., Mazurek, M. A., Cass, G. R., & Simoneit, B. R. T. (1993b). Sources of fine organic aerosol: 3. Road dust, tire debris, and organometallic brake lining dust: roads as sources and sinks. Environmental Science and Technology, 27, 1892–1904.
Ryan, J., Estefan, G., & Rashid, A. (2007). Soil and plant analysis laboratory manual: ICARDA.
Schipper, L. A., Williamson, J. C., Kettles, H. A., & Speir, T. W. (1996). Impact of land-applied tertiary-treated effluent on soil biochemical properties. Journal of Environmental Quality, 25(5), 1073–1077.
Shahbazi, A., Bahramifar, N., & Smolders, E. (2012). Elevated concentrations of pesticides and PCBs in soils at the southern Caspian Sea (Iran) are related to land use. Soil and Sediment Contamination: An International Journal, 21(2), 160–175.
Sicre, M. A., Marty, J. C., Saliot, A., Aparicio, X., Grimalt, J., & Albaiges, J. (1987). Aliphatic and aromatic hydrocarbons in different sized aerosols over the Mediterranean Sea: occurrence and origin. Atmospheric Environment (1967), 21, 2247–2259. doi:10.1016/0004-6981(87)90356-8.
Soclo, H. H., Ph, G., & Ewald, M. (2000). Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: case studies in Cotonou (Benin) and Aquitaine (France) areas. Marine Pollution Bulletin, 40(5), 387–396. doi:10.1016/S0025-326X(99)00200-3.
Tao, S., Cui, Y. H., Xu, F. L., Li, B. G., Cao, J., Liu, W. X., Schmitt, G., Wang, W. J., Shen, W. R., Qing, B. P., & Sun, R. (2004). Polycyclic aromatic hydrocarbons (PAHs) in agricultural soil and vegetables from Tianjin. Science of the Total Environment, 320(1), 11–24. doi:10.1016/S0048-9697(03)00453-4.
Telysheva, G., Jashina, L., Lebedeva, G., Dizhbite, T., Solodovnik, V., Mutere, O., Grigiškis, S., Baškys, E., & Aikaite, J. (2011). Use of plants to remediate soil polluted with oil. Proceedings of the 8th International Scientific and Practical Conference Volume 1.
Wong, S. C., Li, X. D., Zhang, G., Qi, S. H., & Min, Y. S. (2002). Heavy metals in agricultural soils of the Pearl River Delta, South China. Environmental Pollution, 119(1), 33–44. doi:10.1016/S0269-7491(01)00325-6.
Yan, J., Wang, L., Fu, P. P., & Yu, H. (2004). Photomutagenicity of 16 polycyclic aromatic hydrocarbons from the US EPA priority pollutant list. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 557(1), 99–108. doi:10.1016/j.mrgentox.2003.10.004.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33(4), 489–515. doi:10.1016/S0146-6380(02)00002-5.
Zhang, J., Yang, J., Wang, R., Hou, H., Du, X., Fan, S., Liu, J., & Dai, J. (2013). Effects of pollution sources and soil properties on distribution of polycyclic aromatic hydrocarbons and risk assessment. Science of the Total Environment, 463–464
Zhang, J., Dai, J., Du, X., Li, F., Wang, W., & Wang, R. (2012). Distribution and sources of petroleum-hydrocarbon in soil profiles of the Hunpu wastewater-irrigated area, China’s northeast. Geoderma, 173–174(0), 215–223. doi:10.1016/j.geoderma.2011.12.004.
Acknowledgments
This study was supported by the Tehran Regional Water Company. The authors thank Dr. Salemi for his help with the GC, Dr. Mirbagheri from the GIS and Remote Scene Institute for his help with the GIS, and Ms. Refahati, manager of the lab, for her assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayat, J., Hashemi, S.H., Khoshbakht, K. et al. Monitoring of polycyclic aromatic hydrocarbons on agricultural lands surrounding Tehran oil refinery. Environ Monit Assess 187, 451 (2015). https://doi.org/10.1007/s10661-015-4646-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4646-8