Abstract
The chemical analysis of 129 groundwater samples in the Kadkan area, Khorasan-e-Razavi Province, NE of Iran was evaluated to determine the hydrochemical processes, assessment of groundwater quality for irrigation purposes, corrosiveness, and scaling potential of the groundwater. Accordingly, the suitability of groundwater for irrigation was evaluated based on the sodium adsorption ratio, residual sodium carbonate, sodium percent, salinity hazard, and US Salinity Laboratory hazard diagram. Based on the electrical conductivity and sodium adsorption ratio, the dominant classes are C3–S1, C3–S2, C2–S1, and C4–S2. According to the Wilcox plot, about 50 % of the samples fall in the “Excellent to Good” and “Good to Permissible” classes. Besides, the Langelier saturation index, Ryznar stability index (RSI), Larson–Skold index, and Puckorius scaling index were evaluated for assessing the corrosiveness and scaling potential of the groundwater. Corrosiveness and scaling indices stated that the majority of samples are classified into “Aggressive” and “Very Aggressive” category. In addition, chloride and sulfate interfere in 90 % of the samples. Assessment of hydrochemical characteristics indicates Na-Mg-Cl as the predominant hydrochemical type. Spatial distribution of hydrochemical parameters indicates that hydrochemical processes are influenced by geology and hydrogeology of Kadkan aquifer. The Gibbs plots gave an indication that groundwater chemistry in this area may have acquired the chemistry mainly from evaporation and mineral precipitation. Grouping the samples based on Q-mode hierarchical cluster analysis helped to more separation of similar samples. The R-mode HCA grouped analyzed parameters into two groups based on similarity of hydrochemical characteristics. As a result, the samples collected in northern and southern parts of the study area show the best quality (i.e., lowest salinity) for some purposes such as irrigation and drinking.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Throughout the world, sustainable socioeconomic development of every community depends on the sustainability of available water resources. Water of adequate quantity and quality is required to meet growing household, industrial, and agricultural needs (Azaza et al. 2011). Groundwater has become the major source of water supply for domestic, industrial, and agricultural sectors of many countries. Water shortages have become an increasingly serious problem in Iran, especially in the arid and semi-arid regions of Iran (Jalali 2006). Iran is located in a semi-arid area with an average annual precipitation less than one third of that of the world (Baghvand et al. 2010). Continued population growth in Iran is rapidly depleting groundwater supplies in some areas. Groundwater is not only the major source of drinking water and agriculture in Iran, but also supplies more than 50 % of public water utilities and accounts for almost all the water supply to rural households (Mahdavi 2007), and therefore, its contamination has been recognized as one of the most serious problems in Iran (Jalali 2005, 2007). The quality of groundwater is as important as its quantity, owing to the suitability of water for various purposes. Variation in groundwater quality in an area is a function of physical and chemical parameters that are greatly influenced by geological formations and anthropogenic activities (Subramani et al. 2005; Chin 2006). The chemical composition of groundwater is controlled by many factors that include composition of precipitation, geological structure and mineralogy of the watersheds and aquifers, and geological processes within the aquifer (Andre et al. 2005). Among the sources of contamination, agriculture has both direct and indirect effects on groundwater chemistry (Bohlke 2002; Jalali and Kolahchi 2008; Kvítek et al. 2009). Despite the importance of groundwater in Iran, quality assessment of groundwater has received little attention, and efforts to use hydrochemistry data to solve particular problems are even less or nonexistent. Therefore, hydrochemical analysis of the groundwater has become a high priority concern.
The enhanced anthropogenic pressure together with improved standards of living has substantially increased the demand for water. Intense agricultural and urban development has placed a high demand on groundwater resources, especially in the Kadkan region, Khorasan-e-Raavi Province, NE Iran (Fig. 1), and these resources are now at greater risk of contamination. The increasing exploitation due to farming frequently causes deterioration in water quality. Therefore, variations in natural and human activities reflect spatial variations in the hydrochemical parameters of the groundwater. The importance of water quality in human health has also recently attracted a great deal of interest (Pazand et al. 2012). The evaluation and management of groundwater resources require an understanding of hydrogeological and hydrochemical properties of the aquifer (Umar et al. 2001). The importance of the groundwater in the area should not be underestimated because they are sources of water resource for drinking and agricultural purposes, not only for the people living in this area, but also for those who live in the surrounding areas.
Location and geological map of the study area (modified after Vaezi-Pour et al. 1991)
Statistic techniques can be used to obtain relationships between parameters and sampling sites to identify the important factors and sources influencing groundwater quality and to suggest useful tools for both management of water resources and monitoring of groundwater quality (Singh et al. 2005; Papatheodorou et al. 2006; Shrestha and Kazama 2007; Omo-Irabor et al. 2008; Kvítek et al. 2009; Ravikumar and Somashekar 2012). In this paper, hydrochemical investigation and different multivariate statistical techniques, such as spatial analysis, and R and Q-mode hierarchical cluster analysis (HCA), were used to identify groundwater quality, groundwater geochemistry, and its suitability for irrigation purposes in Kadkan plain.
Materials and methods
Study area
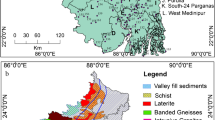
The investigated area is located in the NE Iran and is part of the Kadkan basin that lies between longitudes 58° 35′ 20″ to 59° 18′ 50″ E and latitudes 35° 27′ 30″ to 35° 49′ 10″ N with area occupies of about 1,490 km2 (Fig. 1). The area has a temperate to cold climate and the air temperature is highest in July (21.3 °C) and lowest in January (7.3 °C) with an annual average of 14.3 °C. The climate of the study area is semiarid and its average annual rainfall is about 220 mm, wherein 80 % of it falls during the spring and winter seasons. Groundwater is an important water resource for drinking, agricultural, and industrial uses in the study area. Low precipitation and overexploitation of groundwater resources in recent years have caused an extensive groundwater level decline in this plain prohibiting further development of the aquifer. The topography of the study area is the mixture of rugged mountains with flat plains. Most of the study area is formed by recent alluvium that consists of gravel fans and terraces (Fig. 1). Late Cretaceous tectonized ophiolitic melange (harzburgite, peridotite, diabase, serpentinite, splitic massive lavas, and pelagic limestone placed north to northeast of Kadkan plain). In the north and west of the basin, Tertiary volcanic rocks, including andesite (Eocene age), associated with limestone and conglomerate (Paleocene age), are distributed. Sedimentary units and clastic facies, consisting of conglomerate, sandstone, and marl with east–west trend, are located in the south of the study area during the Cenozoic period (Figs. 1; Vaezi-Pour et al. 1991).
According to groundwater level, the depth to water table ranges from 10 to 177 m (Fig. 2). The groundwater level map indicates that the deeper water table conditions are in the SSW of the study area, whereas the lowest water table conditions are close to the NNE of the study area.
Sampling and analysis
Groundwater samples were collected from 129 regional deep wells (4 dug wells and 98 bore wells), spring (11), and qanat (16) within the study area from 2011 to 2012. The selected wells, springs, and qanat are used for agricultural and domestic purposes. The minimum and maximum groundwater levels were 10 to 177 m during the sampling period. The samples were collected after 10 min of pumping and stored in polyethylene bottles. Immediately after sampling, pH, electrical conductivity (EC), and total dissolved solids (TDS) were measured in the field using a multi-parameter portable meter (HATCH, Germany). Water samples collected in the field were analyzed in the laboratory for cations (Ca2+, Mg2+, Na+, and K+) and anions (HCO3 −, CO3 2−, SO4 2−, and Cl−) using the standard methods. Calcium (Ca2+) and magnesium (Mg2+) were determined titrimetrically using standard EDTA. Chloride (Cl−) was determined by the standard AgNO3 titration method. Carbonate (CO3 2−) and bicarbonate (HCO3 −) were determined by titration with HCl. Sodium (Na+) and potassium (K+) were measured by flame photometry, and sulfate (SO4 2−) by spectrophotometric turbidimetry (Rowell 1994; Table 1). In this study, SPSS 17 software used for statistical analysis, AquaChem 3.7 program for hydrochemical assessment, Arc GIS 9.3 for spatial distribution maps of hydrochemical parameter, and PHREEQC code for the determination of saturation indices.
Results and discussion
Groundwater chemistry
The chemical composition of groundwater is controlled by geochemical processes during the water–mineral interaction (Appelo and Postma 1996). The water quality analyses included all major anions, cations, EC, TDS, and pH. Statistical summary of the physical and chemical parameters of Kadkan groundwater samples is presented in Table 1. The overall groundwater EC and pH values of the study area are ranging from 479.2 to 9,065 μS/cm and 6.5 to 8.9, respectively. Total dissolved solids in the study area vary between 314.7 and 4636.3 mg/l. In the study area, the Na+ and K+ concentrations in groundwater range from 6.9 to 1,402.4 and 0 to 184 mg/l, respectively. During the water/rock interaction and rock weathering, Mg2+, HCO3 −, Ca2+, SO4 2−, and Cl− can be added to the groundwater. The concentrations of Mg2+ and HCO3 − ions found in the groundwater samples are ranged from 2.7 to 173.7 and 67.1 to 347.6 mg/l, respectively. The concentrations of Ca2+ range from 12 to 216.8 mg/l, which is presumably derived from calcium rich minerals like feldspars, pyroxenes, amphiboles, and carbonate minerals. The concentrations of Cl− and SO4 2− increase from the recharge to discharge area and change from 10.6 to 2517 and 29 to 922 mg/l, respectively. Probably groundwater flow throughout evaporitic rocks has caused the increment of the chloride and sulfate concentration. The abundance of the major ions in groundwater is in following order: Na+ > Ca2+ > Mg2+ > K+ and Cl− > SO4 2− > HCO3 − > CO3 2.
Spatial variation in groundwater chemistry
Spatial distribution of hydrochemical parameters in the study area is shown in Fig. 3. As shown, the TDS, EC, chloride, sulfate, sodium, and calcium amount increase from the east to the west (Fig. 3a–f), following the decreasing trend of water table and far from the recharge zone (Fig. 2), which affects a replenishing water rate, and consequently, a water salt load. Carbonate, bicarbonate, and magnesium concentrations are higher in the northern part of the Kadkan aquifer (Fig. 3g–i). The absence of dolomitic and limestone units in this part of the aquifer indicates the influence of the presence of tectonized ophiolitic melange (harzburgite, peridotite, and pelagic limestone) and leaching of these ions from this unit (Fig. 1). Potassium amount in groundwater samples is low and do not show a certain distribution (Fig. 3j).
Hydrochemical facies
To know the hydrochemical regime of the study area, the analytical values obtained from the groundwater samples are plotted on Piper’s trilinear diagram (Piper 1944). Plotting the water samples on Piper’s trilinear diagram (Fig. 4) reveal the analogies, dissimilarities, and different types of waters in the study area. The concept of hydrochemical facies was developed in order to understand and identify the water composition in different classes. It clearly explains the variations or domination of cation and anion concentrations. The values obtained from the groundwater sample analysis and their plot on the Piper’s trilinear diagram reveal that the dominant cation is Na+ and the anion is Cl−. According to Todd and Mays (2005), the results of AquaChem program and position of samples on Pipre diagram show that 30 different water types were found in the groundwater samples (Fig. 5 and Table 2). Na-Mg-Cl and Na-Cl are predominate forms accounting 39.53 and 10 % of samples, respectively (Table 2). Thus, based on the evolution of water types from the recharge zone to discharge zone (bicarbonate → sulfate → chloride), this evolution can be observed in the study area. Figure 5 shows the spatial distribution of general water types in the study area based on the inverse distance weighting interpolation method. This figure indicates that sulfate type, and in some cases, bicarbonate type are found in the northern and southern margins of the Kadkan plain and chloride type in the central parts (Fig. 5). This is probably related to the aquifer recharging by geological formation in the northern and southern margins of this plain and low residence time of groundwater in these parts. Probably more residence time of groundwater or water–evaporitic minerals interaction in the central part of Kadkan plain can also be other reasons for the presence of chloride type in this part (Todd and Mays 2005; Merkel and Planer-Friedrich 2008).
Saturation indices
The saturation index (SI) values of minerals were calculated using the PHREEQC Software and the equation given below (Lloyd and Heathcote 1985) to evaluate the degree of equilibrium between water and the respective mineral and to understand the geochemical processes governing hydrochemistry of groundwater in the area.
where IAP is ion activity product of the solution; Ksp is the solubility product of the mineral or equilibrium constant of the reaction considered at temperature T. SI < 0 indicates that the groundwater is undersaturated with respect to a particular mineral which is present in the geological formation either in insufficient amount or the groundwater has a short residence time. SI > 0 reflects that the groundwater is supersaturated with respect to a particular mineral, i.e., incapable of dissolving more of the mineral, and groundwater discharging from an aquifer containing ample amount of the mineral with sufficient residence time to reach equilibrium. The saturation indices of specific mineral phases such as carbonate [dolomite, CaMg(CO3)2; calcite, CaCO3; and aragonite, CaCO3] and evaporate [gypsum, CaSO4. 2H2O; and anhydrite, CaSO4] minerals are presented in Fig. 6. Results indicated that all samples were undersaturated with respect to all mentioned phases, suggesting that these phases have influenced the chemical composition of the study area (Fig. 6).
Mechanisms controlling groundwater chemistry
The concentration of dissolved ions in groundwater samples is generally governed by lithology, nature of geochemical reactions, and solubility of interaction rocks. The functional sources of dissolved ions can be broadly assessed by plotting the samples, according to the variation in the ratio of Na/(Na+Ca) and Cl/(Cl+HCO3) as a function of TDS (Gibbs 1970). A Gibbs plot of data indicates that groundwater chemistry in this area may have acquired its characteristics mainly from evaporation and mineral precipitation (Fig. 7). Precipitation and rock-weathered materials derived from the underlying rocks control the groundwater chemistry of water samples. Evaporation greatly increases concentrations of ions formed by chemical weathering, leading to higher salinity.
Mechanisms governing groundwater chemistry (after Gibbs 1970)
Irrigation water quality parameters
The most important characteristics of irrigation water in determining its quality and suitability are: (a) salinity hazard or total concentration of soluble salt as measured by EC and (b) sodium hazard or relative proportion of sodium to other principal cations as expressed by sodium adsorption ratio (SAR). In the past, the sodium hazard has been expressed as percent sodium of total cations. (c) Bicarbonate hazard or bicarbonate (HCO3 −) concentration as related to the concentration of calcium plus magnesium, and (d) boron hazard or concentration of boron or other element that may be toxic (Ravikumar and Somashekar 2012).
Salinity of water is inferred from the EC values, the latter being the measure of sodium and total salt content of the water. Based on the conductivity values, the groundwater samples have been classified into various classes (Handa 1969) and are given in Table 3. It is found that samples are falling under “Medium” to “Extensively High” salinity classes indicating that majority of the water samples are of permissible quality to not suitable for irrigation. In determining the suitability of groundwater for domestic and industrial purposes, hardness is an important criterion as it is involved in making the water hard. Water hardness has no known adverse effects; however, it causes more consumption of detergents at the time of cleaning and some evidence indicates its role in heart disease (Schroeder 1960). Hence, classification of groundwater of the study area based on hardness (Sawyer and McCarthy 1967) has been carried and is presented in Table 3. Approximately most of the samples fall under “Hard” and “Very Hard” class.
For the purpose of diagnosis and classification, the total concentration of soluble salts (salinity hazard) in irrigation water can be expressed in terms of specific conductance. Classification of groundwater based on salinity hazard is presented in Table 3. It is found that the majority of the samples were “Doubtful” to “Unsuitable” for irrigation purposes. Another important factor in water quality assessment is the sodium concentration. Sodium concentration is important when evaluating the suitability of groundwater for irrigation Gupta et al. (2009). High concentrations of Na+ are undesirable in water because Na+ is adsorbed onto the soil cation exchange sites, causing soil aggregates to disperse, reducing its permeability (Jalali 2010). The SAR, which indicates the effect of relative cation concentrations on Na+ accumulation in the soil, is used for evaluating the sodicity of irrigation water. SAR is computed as
where all ionic concentrations are expressed in milliequivalents per liter (meq/l). The classification of groundwater samples from the study area with respect to SAR (Todd 1959) is represented in Table 3. Almost the majority of the SAR value of the samples is found to be less than 10 and is classified as “Excellent” for irrigation. On the basis of US Salinity Laboratory hazard diagram (Fig. 8) plotted by correlating the sodium absorption ratio and electrical conductivity (after Richards 1954), water classes of more than half of the water samples are (57.37 %) C3–S1, C3–S2, C2–S1, and C4–S2, indicating “Medium” to “High” salinity/“Low” to “Medium” sodium type, and 42.63 % of the samples are C3–S3, C4–S3, and C4–S4 classes, indicating “High” to “Very High” salinity/“Medium” to “Very High” sodium type.
US salinity hazard diagram samples (after Richards 1954)
Sodium content in irrigation waters involves exchange reactions with Ca2+ and Mg2+ of clay particles of the soil, which in turn leads to the reduction of soil permeability, internal drainage, and air circulation. Sodium content in natural waters is expressed in terms of percent sodium (%Na). %Na is calculated using the following formula:
where the quantities of Ca2+, Mg2+, Na+, and K+ are expressed in meq/l. The classification of groundwater samples with respect to percent sodium is shown in Table 3. As shown, the majority of the groundwater samples (61 % of the samples) is “Doubtful” for irrigation. A perusal of Wilcox’s (1995) diagram shows that out of 129 samples, 15 samples are “Excellent to Good,” 17 samples belong “Good to Permissible,” 34 samples fall in the “Permissible to Doubtful” class, 22 samples are “Doubtful to Unsuitable,” and 41 samples belong to “Unsuitable” classes (Fig. 9).
Percent sodium vs. EC plot (after Wilcox 1995)
The excess sum of CO3 2− and HCO3 − content in groundwater over the sum of calcium and magnesium content influences the suitability of water for irrigation. This is expressed as residual sodium carbonate (RSC). A high value of RSC in irrigation water leads to an increase in the adsorption of sodium by soils (Eaton 1950). RSC is calculated using the following equation.
where all ionic concentrations are expressed in meq/l. According to the US Department of Agriculture (USDA 1954), water having more than 2.5 mEq/l of RSC is not suitable for irrigation purposes. Groundwater of the study area is classified on the basis of RSC and the results are presented in Table 3. Accordingly, the majority of the samples (94.5 %) belong to “Good” category (Table 3). The negative RSC values indicate that dissolved CO3 2− and HCO3 − ions were less than that of Ca2+ and Mg2+ ions.
Corrosiveness and scaling indices
Langelier saturation index (LSI)
Langelier saturation index (Langelier 1936) is a system for estimating or predicting the amount or degree of problems with lime scale, caused in a particular water supply as it determines the corrosive or incrusting ability of a water sample. In other words, it can be used to predict the tendencies of water to precipitate or dissolve calcium carbonate, the main parameter for determining the corrosivity of water. According to Langelier (1936), the corrosive action of water is principally due to an excess of free CO2 and its interaction with calcium and magnesium carbonates. In the presence of carbon dioxide, these salts are held in solution as bicarbonates and for any given concentration of calcium and magnesium, there is a corresponding concentration of carbon dioxide to prevent the decomposition of these bicarbonates back into carbonates. Corrosion is accelerated by low pH values, so that in water of low alkalinity and high free carbon dioxide, the attack is much more rapid as compared to water, which is high in alkalinity and low in carbon dioxide content.
The LSI value usually lies between −3 and +3. The positive value of LSI indicates that the water is over or super saturated, depositing CaCO3 on the surface of metal and corrosion rates will be negligible. A negative index indicates that the water is under-saturated dissolving CaCO3 and will be considered as corrosive. The value close to zero states that the water is at saturation (equilibrium). In other words, positive LSI values indicate that the heavy deposition of calcium carbonate (e.g., calcite) resulting in the formation of sludge and thin scales, accounted with respect to the dissolution of calcium salt contents as carbonates due to precipitation; while negative LSI values accounts for slight corrosive nature in water with a tendency to dissolve CaCO3, as a result of low alkalinity and high free CO2 content (Sasidhar and Vijay Kumar 2008; Sivasankar and Ramachandramoorthy 2009; Ravikumar and Somashekar 2012). It can be interpreted as the pH change required making water in equilibrium. Langelier saturation index is calculated as the difference between measured pH and pH at saturation (pHs) of water with respect to calcium carbonate (i.e., calcite; Kumar et al. 2009) using the Eq. (5).
The saturation pH indicates the value attained when all forms of alkalinity are adjusted to the point where the water are just saturated with respect to calcium carbonate (Davil et al. 2009). It can be calculated as:
where pH = ‐ log[H +]
In the study area, the LSI values are given in Table 4 and illustrated through histograms (Fig. 10), showed that 58.92 % of the groundwater samples are supersaturated containing positive LSI index with a tendency to deposit CaCO3 and 41.08 % of the groundwater samples are under-saturated containing negative LSI index, accounts for its slight corrosive nature with a tendency to dissolve CaCO3 as a result of low alkalinity and high free CO2 content (Sivasankar and Ramachandramoorthy 2009).
Ryznar stability index (RSI)
Ryznar (1944) has designed an empirical method to determine stability index for predicting scaling tendencies of water. This index is often used in combination with LSI to improve the accuracy in predicting the scaling or corrosion tendencies of water towards mild steel under the assumption that if water is scale forming, corrosion rates will be negligible and waters that are not scale forming are considered corrosive (Kannan 1991; Ravikumar and Somashekar 2012). It can be calculated like LSI as follows:
The result indicates that 86.82 % of the samples are classified into “Aggressive” category and 12.40 % of the samples are classified into “Very Aggressive category.” Only one sample exhibits “No Scale” forming tendency (Table 4 and Fig. 11).
Larson–Skold index (L-S index)
Larson–Skold index (Larson and Skold 1958) is used to describe the corrosiveness of water. They found that alkalinity tended to reduce the corrosion rates of mild steel and postulated that it is a natural inhibitor that participates in formation of an inhibitor film. Chloride and sulfate were found to increase the corrosivity of water. This effect is explained by the interference of these anions in the formation of a natural inhibitor film. The L-S index is the ratio of meq/l of sulfate and chloride to the alkalinity in the form of bicarbonate and carbonate (Jevaprabha et al. 2006; Ravikumar and Somashekar 2012).
The value of L-S index below 0.8 indicates that chloride and sulfate do not interfere with natural inhibitor film formation while the value greater than 1.2 states the tendency towards high corrosion. The value of L-S index between 0.8 and 1.2 indicates that these ions may interfere with natural film formations (Rabbani et al. 2008). In the study area, chloride and sulfate interfere in 89.92 % of the samples, do not interfere with natural film formation in 7.75 % of the samples and may interfere with natural film formation in 2.33 % of the samples (Table 4 and Fig. 12).
Puckorius scaling index (PSI)
The Puckorius scaling index is used to account the buffering capacity and the maximum quantity of precipitation that can form in bringing water to equilibrium (Davil et al. 2009). The PSI index is calculated in a manner similar to the Ryznar stability index, Puckorius has used an equilibrium pH rather than the actual system pH to account for the buffering effects. Conveniently, the PSI uses the same numbering systems and general interpretation as does the RSI (Sargaonkar and Deshpande 2003; Ravikumar and Somashekar 2012).
where pHeq = 1.465 log10 [TA] + 4.54.
The result indicated that all samples (100 %) are classified into “Very Aggressive” category (Table 4 and Fig. 13).
Statistical screening of groundwater quality data correlation matrix
Table 5 gives the correlation coefficient matrix for the hydrochemical parameters. If the correlation coefficient (r) is greater than 0.7, two parameters are considered to be strongly correlated; whereas if the r value is between 0.5 and 0.7, it indicates a moderate correlation at a significance level p < 0.05 (Guey-Shin et al. 2011). Parameters having high degrees of correlations are EC and TDS (r = 0.99) because all of the dissolved components cause increased ionic concentration, as well as increased EC concentration. EC and TDS are highly related to Na+ (r = 0.96 and 0.97 respectively) and Cl− (r = 0.97) but moderately related to SO4 2− (r = 0.66 and 0.67, respectively), Ca2+ (r = 0.60), K+ (r = 0.61), and Mg2+ (r = 0.55 and 0.49, respectively). The results indicated that these ions involve various physical and chemical reactions: e.g., oxidation/reduction reactions and ion exchange in groundwater aquifers, which suggest that the same factor strongly affect them (Rao 2002; Rao 2003). Ca2+ and SO4 2− have a relatively high correlation (r = 0.69), revealing that the calcium ion in groundwater comes mainly from gypsum. Na+ and Cl− also have high correlation (r = 0.97).
Multivariate analysis (cluster analysis)
Cluster analysis is a multivariate pattern recognition technique that helps to identify natural groupings in data sets. Detailed explanation of the technique is given by Swan and Sandilands (1995) and by Davis (2002). Cluster analysis is a multivariate pattern recognition technique that helps to identify natural groupings in data sets. Detailed explanation of the technique is given by Swan and Sandilands (1995) and by Davis (2002). In the present study, both Q-mode and R-mode HCA were performed on the hydrochemical data. The R-mode HCA was done to classify the parameters into groups or facies based on their similarity with each other. In Q-mode HCA, comparisons based on multiple parameters from different samples are made and the samples grouped in clusters according to their “similarity” to each other, forming higher clusters step-by-step (Alberto et al. 2001; Guler and Thyne 2004). Different methods for calculating the similarity between samples/ clusters exist. Here, the relative similarities between the samples were quantified using the Euclidean distance. The Euclidean distance d xy between two points x and y in dimensional space is given by:
where j defines each of the parameters. Ward’s method was used to combine the clusters. This method is commonly applied in water chemistry investigations, e.g., by Farnham et al. (2002), Kuells et al. (2000), Alberto et al. (2001), Guler and Thyne (2004), and Thyne et al. (2004).
Grouping the variables based on two, three, and four clusters classifications are presented in Table 6. This table indicates the position and similarity degree of water samples by change in the number of clusters. In other words, this method helps to more separation of similar samples from other samples. Increasing the clusters leads to determination of more similar samples. So, classifying the water samples in 4 clusters presents the samples with more differentiation than 2 and 3 clustering states. Two groups include: (a) 30 samples (7, 12, 17, 28, 34, 47, 49, 58, 60–62, 67, 72, 74–76, 79, 82, 87, 98, 100, 105–108, 113, 115, 122, 125, and 126) and (b) 15 samples (19, 27, 32, 35, 38, 40, 53, 57, 96, 97, 104, 109, 114, 116, and 123) with the highest separation indicate the most similarity. These samples often have similar type and are located in the central parts of Kadkan aquifer. The majority of the samples in these groups have different features (less similarity) that reflect the effect of different factors.
In the R-mode HCA, the dendrogram reveals that the major portion of the examined physic-chemical components are grouped into two main groups (Fig. 14). The first group contains EC and TDS because all of the dissolved components cause increased ionic concentration. All other ions fall within the second group which represents similar features and natural backgrounds that were affected by similar hydrochemical processes.
Conclusion
The quality, scaling potential,l and corrosiveness of groundwater are evaluated in Kadkan area, northeastern Iran. Predominant hydrochemical facie is Na-Mg-Cl for the study area with Cl− and Na+ as the dominant anion and cation, respectively. According to the PHREEQC and Gibbs plots, evaporation and mineral precipitation predominantly regulate the groundwater chemistry in the study area. Spatial distribution of hydrochemical parameters indicates that hydrochemical processes are influenced by geology and hydrogeology of Kadkan aquifer. Most of the samples in study area fall in the “Suitable” and “Doubtful” range based on SAR and %Na values, respectively. According to EC and SAR, it is evident that more than half of the water samples (57.37 %) are grouped within C3–S1, C3–S2, C2–S1, and C4–S2, indicating “Medium” to “High” salinity/“Low” to “Medium” sodium type, and 42.63 % of the samples are C3–S3, C4–S3, and C4–S4 classes, indicating “High” to “Very High” salinity/“Medium” to “Very High” sodium type and need better drainage to overcome salinity problem. From Wilcox plot, it is observed that about 50 % of the samples from the study area fall in the “Excellent to Good” and “Good to Permissible” classes while remaining samples are “Permissible to Doubtful,” “Doubtful to Unsuitable,” and “Unsuitable” classes for irrigation purpose. Calcium carbonate scale potential indices such as the theoretical Langelier saturation index stated that more than half of the groundwater samples have the positive value of index indicating super saturation and low corrosiveness nature of water towards mild steel. The Ryznar stability index has underlined that 86 % of the samples are classified into “Aggressive” category and remaining samples are classified into “Very Aggressive” category (except for one sample). Based on the Larson–Skold index, chloride and sulfate interfere in 90 % of the samples. The Puckorius scaling index has classified all the samples into “Very Aggressive” category. Results indicate that the water samples collected at the northern and southern margines of Kadkan alluvial aquifer (e.g., samples 11, 14, 24, 26, 36, 43, 101, 103, 112, and 126) are in the best quality conditions to the future uses such as irrigation and drinking. The main reason for the better quality of these samples is that these samples are located near the aquifer recharging zones. Based on Q-mode and R-mode HCA, the hydrochemistry of groundwater in Kadkan aquifer is influenced by many factors, including the water types, degree of dissolution, and similarity of hydrochemical processes. The results indicate that combination of different methods, such as those used in this study, can make an accurate assessment of the quality of groundwater for irrigation.
References
Alberto, W. D., Del Pilar, D. M., Valeria, A. M., Fabiana, P. S., Cecilia, H. A., & De Los Angeles, B. M. (2001). Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality: a case study, suquia river basin (Cordoba-Argentina). Water Research, 35(12), 2881–2894.
Andre, L., Franceschi, M., Pouchan, P., & Atteia, O. (2005). Using geochemical data and modelling to enhance the understanding of groundwater flow in a regional deep aquifer, Aquitaine Basin, south-west of France. Journal of Hydrology, 305, 40–62.
Appelo, C. A. J., & Postma, D. (1996). Geochemistry, groundwater and pollution. Rotterdam: Balkema.
Azaza, F., Ketata, M., Bouhlila, R., Gueddari, M., & Riberio, L. (2011). Hydrogeochemical characteristics and assessment of drinking water quality in Zeuss–Koutine aquifer, southeastern Tunisia. Environmental Monitoring and Assessment, 174, 283–98.
Baghvand, A., Nasrabadi, T., Bidhendi, N. G., Vosoogh, A., Karbassi, A., & Mehrdadi, N. (2010). Groundwater quality degradation of an aquifer in Iran central desert. Desalination, 260, 264–275.
Bohlke, J. K. (2002). Groundwater recharge and agricultural contamination. Hydrogeology Journal, 10, 153–179.
Chin, D. A. (2006). Water quality engineering in natural systems. Hoboken: Wiley.
Davil, M. F., Mahvi, A. H., Norouzi, M., Mazloomi, S., Amarluie, A., Tardast, A., & Karamitabar, Y. (2009). Survey of corrosion and scaling potential produced water from Ilam water treatment plant. World Applied Science Journal, 7, 01–06.
Davis, J. C. (2002). Statistics and data analysis in geology. New York: Wiley.
Eaton, E. M. (1950). Significance of carbonate in irrigation water. Soil Science, 69, 12–133.
Farnham, I. M., Stetzenbach, K. J., Singh, A. K., & Johannesson, K. H. (2002). Treatment of nondetects in multivariate analysis of groundwater geochemistry data. Chemometrics and Intelligent Laboratory Systems, 60, 265–281.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 17, 1088–1090.
Guey-Shin, S., Bai-You, C., Chi-Ting, C., Pei-Hsuan, Y., & Tsun-Kuo, C. (2011). Applying factor analysis combined with Kriging and information entropy theory for mapping and evaluating the stability of groundwater quality variation in Taiwan. International Journal of Environmental Research and Public Health, 8, 1084–1109.
Guler, C., & Thyne, G. D. (2004). Hydrologic and geologic factors controlling surface and groundwater chemistry in Indian wells-Owens valley area, Southeastern California, USA. Journal of Hydrology, 285, 177–198.
Gupta, S., Dandele, O. S., Verma, M. B., & Maithani, P. B. (2009). Geochemical assessment of groundwater around Macherla Karempudi area, Guntur district, Andhra Pradesh. Journal of the Geological Society of India, 73, 202–212.
Handa, B. K. (1969). Description and classification of media for hydro-geochemical investigations. Symposium on Ground Water Studies in Arid and Semiarid Regions, Roorkee.
Jalali, M. (2005). Major ion chemistry in the Bahar area, Hamedan, western Iran. Environmental Geology, 47, 763–772.
Jalali, M. (2006). Chemical characteristics of groundwater in parts of mountainous region, Alvand, Hamedan, Iran. Environmental Geology, 51, 433–446.
Jalali, M. (2007). Salinization of groundwater in arid and semi-arid zones: an example Tajarak, western Iran. Environmental Geolology, 52, 1133–1149.
Jalali, M., & Kolahchi, Z. (2008). Groundwater quality in an irrigated, agricultural area of northern Malayer, western Iran. Nutrient Cycling in Agroecosystems, 80, 95–105.
Jalali, M. (2010). Groundwater geochemistry in the Alisadr, Hamadan, Western Iran. Environmental Monitoring and Assessment, 166(1–4), 359–369.
Jevaprabha, C., Sathiyanarayanan, S., Muralidharam, S., & Venkatachari, G. (2006). Corrosion inhibition of iron in 0.5 mol L−1 H2SO4 by halide ions. Journal of the Brazilian Chemical Society, 17, 61–67.
Kannan, K. (1991). Fundamentals of environmental pollution. New Delhi: Chand and company limited.
Kuells, C., Adar, E. M., & Udluft, P. (2000). Resolving patterns of ground water flow by inverse hydrochemical modeling in Semi-Arid Kalahari Basin. Tracers Model Hydrogeology, 262, 447–451.
Kumar, H., Saini, V., Kumar, D., & Chaudhary, R. S. (2009). Influence of trisodium phosphate (TSP) anti-salant on the corrosion of carbon steel in cooling water systems. Indian Journal of Chemical Technology, 16, 401–410.
Kvítek, T., Zla’bek, P., Bystricky, V., Fucı’k, P., Lexa, M., Gergel, J., Nova’k, P., & Ondr, P. (2009). Changes of nitrate concentrations in surface waters influenced by land use in the crystalline complex of the Czech Republic. Physics and Chemistry of the Earth, 34(8–9), 541–551.
Langelier, W. F. (1936). The analytical control of anti-corrosion water treatment. American Water Works Association, 28, 1500–1521.
Larson, T. E., & Skold, R. V. (1958). Laboratory studies relating mineral water quality of water on corrosion of steel and cast iron. Corrosion, 14, 285–288.
Lloyd, J. W., & Heathcote, J. A. (1985). Natural inorganic hydrochemistry in relation to groundwater: an introduction. Oxford: Clarendon.
Mahdavi, M. (2007). Applied hydrology. Iran: Tehran University Publication (In Persian).
Merkel, B., & Planer-Friedrich, B. (2008). Groundwater geochemistry—a practical guide to modeling of natural and contaminated aquatic systems. Berlin: Springer.
Omo-Irabor, O. O., Olobaniyi, S. B., Oduyemi, K., & Akunna, J. (2008). Surface and groundwater water quality assessment using multivariate analytical methods: a case study of the Western Niger Delta, Nigeria. Physics and Chemistry of the Earth, 33(8–13), 666–673.
Papatheodorou, G., Demopouloua, G., & Lambrakis, N. (2006). A long-term study of temporal hydrochemical data in a shallow lake using multivariate statistical techniques. Ecological Modelling, 193, 759–776.
Pazand, K., Hezarkhani, A., Ghanbari, Y., & Aghavali, N. (2012). Geochemical and quality assessment of groundwater of Marand Basin, East Azarbaijan Province, northwestern Iran. Environmental Earth Sciences, 67, 1131–1143.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water analysis. Transactions American Geophysical Union, 25, 914–923.
Rabbani, D., Mirranzadeh, M. B., & Motlagh, A. A. (2008). Study for determination of industrial water corrosivity in Kashan Fajre Sepahan galvanizing mills during 2005–2006, Iran. Pakistan Journal of Biological Sciences, 11, 131–134.
Raghunath, H. M. (2003). Groundwater. New Delhi: New Age International (P) Ltd.
Rao, N. S. (2002). Geochemistry of groundwater in parts of Guntur district, Andhra Pradesh, India. Environmental Geology, 41, 552–562.
Rao, S. V. L. (2003). Cluster analysis of groundwater quality data of Venkatagiri Taluq, Nellore district, Andhra Pradesh. Journal of the Geological Society of India, 62, 447–454.
Ravikumar, P., & Somashekar, R. K. (2012). Assessment and modelling of groundwater quality data and evaluation of their corrosiveness and scaling potential using environmetric methods in Bangalore South Taluk, Karnataka State, India. Water Resources, 39, 446–473.
Richards, L. A. (1954). Diagnosis and improvement of saline and alkaline soils. Washington, DC: US Department of Agriculture.
Rowell, D. L. (1994). Soil science: methods and applications. New York: Longman and Scientific Technical.
Ryznar, J. W. (1944). A new index for determining amount of calcium carbonate scale formed by water. American Water Works Association, 36, 472–486.
Sargaonkar, A., & Deshpande, V. (2003). Development of an overall index of pollution for surface water based on a general classification scheme in Indian context. Environmental Monitoring and Assessment, 89, 43–67.
Sasidhar, P., & Vijay Kumar, S. B. (2008). Assessment of groundwater corrosiveness for unconfined aquifer system at Kalpakkam. Environmental Monitoring and Assessment, 145, 445–452.
Sawyer, G. N., & McCarthy, D. L. (1967). Chemistry of sanitary engineers. New York: McGraw Hill.
Schroeder, H. A. (1960). Relations between hardness of water and death rates from certain chronic and degenerative diseases in the United States. Journal of Chronic Disorders, 12, 586–591.
Shrestha, S., & Kazama, F. (2007). Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environmental Modelling & Software, 22, 464–475.
Singh, K. P., Malik, A., & Sinha, S. (2005). Water quality assessment and apportionment of pollution sources of Gomti River (India) using multivariate statistical techniques—a case study. Analytica Chimica Acta, 538, 355–374.
Sivasankar, V., & Ramachandramoorthy, T. (2009). An investigation on the pollution status of holy aquifers of Rameswaram, Tamil Nadu, India. Environmental Monitoring and Assessment, 156, 307–315.
Subramani, T., Elango, L., & Damodarasamy, S. R. (2005). Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environmental Geology, 47, 1099–1110.
Swan, A. R. H., & Sandilands, M. (1995). Introduction to geological data analysis. Oxford: Blackwell.
Thyne, G., Guler, C., & Poeter, E. (2004). Sequential analysis of hydrochemical data for watershed characterization. Groundwater, 42(5), 1–12.
Todd, D. K. (1959). Groundwater hydrology. New York: Wiley.
Todd, D. K., & Mays, L. W. (2005). Ground-water hydrology. New York: Wiley.
Umar, A., Umar, R., & Ahmad, M. S. (2001). Hdrogeological and hydrochemical framework of regional aquifer system in Kali-Ganga sub-basin India. Environmental Geology, 40(4–5), 602–661.
US Department of Agriculture (USDA). (1954). Diagnosis and improvement of saline and alkali soils. Washington, DC: US Department of Agriculture.
Vaezi-Pour, M. J., Alavi-Tehrani, N., Behrouzi, A., & Kholghi, M. H. (1991). Geological map of Torbat-e-Heydarieh 1:250,000. Iran: Geological Survey of Iran.
Wilcox, L. V. (1995). Classification and use of irrigation waters. Washington, DC: US Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esmaeili-Vardanjani, M., Rasa, I., Amiri, V. et al. Evaluation of groundwater quality and assessment of scaling potential and corrosiveness of water samples in Kadkan aquifer, Khorasan-e-Razavi Province, Iran. Environ Monit Assess 187, 53 (2015). https://doi.org/10.1007/s10661-014-4261-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4261-0