Abstract
In this study, the chemical speciation of heavy metals and their distribution in surface sediments of Gowatr bay, southeast Iran, are investigated. Modified Bureau Commune de Reference of the European Commission (BCR) sequential extraction technique was applied to assess Cu, Pb, Zn, Mn, Ni, Co, Cr, V, and Fe in the four fractions of five surface sediment samples. Calculated contamination factors (C i f) indicate considerable to very high degree of contamination for Cu and Cr, and very high degree for Zn and Ni. Maximum contamination degree (C d) also suggests serious anthropogenic pollution at two sites. The dominance of average concentration of Cu, Pb, Zn, and Mn in non-residual fractions indicates higher ecological risk within Gowatr bay. Conversely, Ni, Co, Cr, Fe, and V mainly exist in residual phase and hence pose no immediate ecological risk. Calculated individual contamination factors (ICFs) indicate the highest risk of Cu, Pb, Zn, and Mn at two investigated sites. Global contamination factor (GCF) reveals that Pasabandar harbor is highly impacted by metal pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries and bays are two important environments for many persistent pollutants to accumulate in organisms and bottom sediments. Since the Industrial Revolution, a tremendous amount of toxic pollutants has been discarded into coastal environment, and hence, harbor sediments have become large sinks of heavy metals (Fukue et al. 1999; Turner 2000; Billon et al. 2002; Fan et al. 2002; S. Wang et al. 2010). Therefore, bay sediments are ecologically important components of the marine environment and have been contaminated by inorganic and organic materials (Uluturhan 2010).
Heavy metals because of their toxic effects on life in aquatic system, high enrichment factor, and slow removal rate are generally considered as serious inorganic pollutants (Alloway and Ayres 1997; Fu and Wang 2011). Occurrence of elevated levels of trace metals especially in sediments is a good indicator of man-induced pollution, while high level of heavy metals is often attributed to anthropogenic influences (Bloom and Ayling 1977; Shriadah 1999). Industrial development has brought the risk of heavy metal contamination in coastal zones (Esen et al. 2010). In addition, coastal traffic especially in and close to harbors and repair of marine vessels are also suspected to be responsible for elevated concentration in the upper reaches of harbors (Okoro et al. 2012).
Surficial sediment geochemistry is helpful in assessing pollution accumulation and mobilization of trace elements in the aquatic environment (Holm 1988; Geetha et al. 2008; Chibunda et al. 2010; Ahmad et al. 2010). Sediments, depending on environmental conditions, can act as either a sink or a source for trace metals in aquatic environments (Salomons and Förstner 1984; Qiu et al. 2011). During sediment transport, trace metals undergo numerous changes in their speciation due to dissolution, precipitation, sorption, and complexation phenomena which affect their behavior and bioavailability. The accumulation of metals from the overlying water to the sediment is dependent on a number of additional environmental factors such as pH, Eh, ionic strength, anthropogenic input, type and concentration of organic and inorganic ligands, and the available surface area for adsorption (Davies et al. 1991). Sediments integrate contaminants over time and are in constant flux with the overlying water column. Analysis of heavy metals in the sediments permits detection of pollutants that are present in low concentrations in the water column (Davies et al. 1991). Furthermore, their distribution in coastal sediments provides a record of the spatial history of pollution in a particular region or ecosystem.
Total metal content usually does not provide sufficient information about mobility, bioavailability, and toxicity of metals (Yuan et al. 2004). It is very imperative to study different forms of heavy metal mobility and bioavailability rather than the total concentration in order to obtain an indication of the bioavailability of metals. Heavy metal properties depend not only on their total concentration but also on the physicochemical form they occur (Davidson et al. 1994), generally described as “speciation” (Ure et al. 1993). The speciation of metals in sediments is therefore a critical factor in assessing the potential environmental impacts. For this reason, classical and modified sequential extraction procedures are commonly applied because they provide information on the fractionation of metals in the different lattices of the solid sample (Marguı et al. 2004; Nádaská et al. 2009). The aim of this study is the identification of surficial distribution, bioavailability, and fractionation of heavy metals in the surface sediment samples taken from Gowatr bay.
Study area
Gowatr bay (a part of the Oman sea) in southeast Iran (25° 01′–25° 14′ N, 61° 20′–61° 40′ E), is a semi-closed water body with the average depth of 8.5 m and total surface area of 350 km2 (Fig. 1), with 30.6 % of it falling in the Iranian territory (western sector) and the rest to Pakistan. Three rivers including Kajou, Nahang, and Bahoukalat discharge into the Iranian sector of Gowatr bay. The bay is a natural reserve with unique ecological values. Aveccinia marina, the sole mangrove species in Iran, together with two halophile herbaceous species occupies more than 400.000 ha of Gowatr area. Mangrove muddy flats have a high capacity to retain heavy metals from tidal water, freshwater rivers, and storm water runoff, and they often act as sinks for heavy metals (Tam and Wong 1995).
Geologically, the study area is a part of coastal Makran and dates from late Cretaceous to Holocene (Farhoudi and Karig 1977). The basement is mostly intertidal deposits, alluvial terraces, and alluvial fan deposits including sandstone and conglomerate with shell fragments, and greenish gray gypsiferous and fossiliferous marls. Makran ophiolite upstream of the study area is an important geogenic source supplying heavy metals to Gowatr bay through Bahoukalat river. There are also several shipbuilding, plants along with shrimp, fish, and various fowl breeding farms in Gowatr bay coast that can act as potential anthropogenic sources.
Materials and methods
Sampling, sample treatment, and analysis
In September 2012, sediment samples were collected from 16 sites along with a local background sample (N27) in the Iranian sector of Gowatr bay (Fig. 1). Intertidal and inside water sediments were sampled using clean plastic spoon and Van veen grab, respectively, and then immediately transferred to polyethylene plastic bags and refrigerated. In each station, subsamples were mixed to obtain a composite representative sample. Preliminary treatment of the samples was carried out at the geology lab of Iranian National Institute for Oceanography and Atmospheric Science (INIOAS)–Chabahar research station, and after being transferred to the geochemistry lab at Shiraz University, the samples were air dried and sieved and the size smaller than 63 μm was retained and kept in clean polythene bags for further analysis.
Heavy metal concentrations in sediment samples were determined using inductively coupled plasma–mass spectrometry (ICP-MS, Agilent 7500, Agilent, USA), at the ACME Analytical Laboratories following Aqua Regia Digestion. Total organic matter content was estimated by measuring loss on ignition weight using a high-temperature furnace (Carbolite, Sheffield, UK) at 550 °C for 4 h (Heiri et al. 2001) at geochemistry lab, Shiraz university. pH was measured in situ using a portable pH meter (WTW pH 330i, Germany). The results of heavy metal analysis, pH, and TOC are presented in Table 1.
Sequential extraction technique
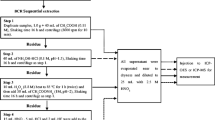
It has been demonstrated that metal speciation and solubility have a profound effect on the mobility, bioavailability, and toxicity of metals (Griscom et al. 2000; Yap et al. 2002; Amiard et al. 2007). The most widely used procedures among a long list of procedures using various extraction reagents and experimental conditions to investigate the distribution of heavy metals in sediments and soils, the five-step Tessier et al. (1979) and the six-step Kersten and Förstner (1986) extraction methods, are more favored. Considering the many schemes and diversity of existing procedures and the lack of uniformity in the different protocols used by the various authors, the European countries (EC) Standards, Measurement and Testing Programme launched a project to harmonize measurements of the extractable trace metal content in soils and sediments and also to improve original (three-stage) Bureau Commune de Reference of the European Commission (BCR) procedure (Quevauviller et al. 1994). It harmonized differential extraction schemes for sediment analysis. Modification of three-stage BCR sequential extraction procedure including changes to the concentration of the reagent and pH of the second step, which has resulted in better precision for the extraction of reducible metals, was carried out (Rauret et al. 2000). Also, the addition of a fourth step, an aqua regia digest on the solid residue remaining after step 3, has provided the opportunity for quality assurance by comparison of the sum of the four BCR steps to an independent aqua regia analysis on a second portion of sample (Rauret et al. 2000).
Hence, in this research, in order to access the mobility and bioavailability of heavy metals, the BCR four-step (modified BCR) sequential extraction scheme (including the residual fraction) was used (Guevara-Riba et al. 2004; Arain et al. 2008; Malferrari et al. 2009; Yan et al. 2010). All extractions were carried out for 16 h (overnight), at room temperature, using a mechanical shaker (Ratek MP4, Australia). The extract was then separated from the solid residue by centrifugation (KUBOTA 5100, Japan) for 20 min, at 3000 rpm, and the resultant supernatant liquid was transferred into a polyethylene volumetric flask. The residue was washed by adding 20 ml of deionized water, shaking for 15 min on the end-over-end shaker, and centrifuging for 20 min at 3000 rpm. Subsequently, the supernatant was decanted. The extractant solutions as listed in Table 2 were prepared from analytical grade reagents (Merck, Darmstadt, Germany). Extremely, heavy metals in each sediment fraction were carried to Zar Azma lab-Tehran, Iran, and determined using inductively coupled plasma–optical emission spectrometry (ICP-OES, PerkinElmer Optima 3300, USA).
Quality control
The quality assurance and control were monitored by using blanks and sample duplicates. To preclude uncertain contaminations, all laboratory equipment used was washed with phosphate-free soap, double rinsed with distilled water, and left in 10 % HNO3 for 24 h. Then, they were rinsed two times with double-distilled water and left semi-closed to dry at room temperature. The recovery of the sequential extraction procedure was accounted as follows:
The recovery rates for heavy metals ranged from 79.40 % for Pb at site N18 to 116.5 % for Cr at site N15 (Table 3).
Results and discussion
Geochemical maps of elements are used to display heavy metal surficial distribution (Fig. 2). It can be seen that the maximum concentration of Cu, Zn, and V, and Cr, Ni, Co, and Fe are related to Pasabandar and mangrove muddy flats, respectively. Also, Mn has the maximum concentration at site N10. Marine traffic, and ship yard activity at and close to Pasabandar harbor and mangrove jungle, along with transported weathered material from ophiolites, are apparently responsible for the observed elevated concentration of heavy metals in Gowatr bay (Moore et al. 2014).
Hakanson (1980) suggested a contamination factor (C i f) and the degree of contamination to describe the contamination of a given toxic substance in a basin given by
where, C i 0–1 is the mean content of the substance from at least five sample sites, and C i n is the reference values for the substance. The terminologies in Table 4 are used to describe the contamination factor and the degree of contamination. Heavy metal contamination factors for the sediment samples are indicated as boxplots in Fig. 3. The calculated contamination factor (C i f) values of heavy metals for surficial sediment samples of Gowatr bay are moderate for Pb, Mn, Co, V (except of site N24), and Fe, considerable to very high for Cu and Cr, and very high for Zn and Ni.
Figure 4 displays the contamination degree (C d) trend in Gowatr bay. C d values indicate very high degree of heavy metal contamination at all sites, especially at sites N11 and N18, suggesting serious anthropogenic pollution at these two sites.
Metal speciation in sediments
Since contamination degree values for samples N11, N18, N10, N12, and N15 were relatively high, they were selected for sequential extraction analysis. Fractionation patterns of elements in the bay sediments, based on sequential extraction procedure, are indicated as bar diagrams in Fig. 5.
Exchangeable fraction
It is widely accepted that exchangeable metal in sediments is labile, highly toxic, and the most bioavailable fraction. The results of this study also showed that the exchangeable fraction of Mn accounted for 52.62, 55.29, 67.37, 61.24, and 65.71 % of total Mn content in the sediments at sites N11, N18, N10, N12, and N15, respectively. Manganese concentration in the Earth’s crust and carbonatic rocks (950 and 620 mg/kg, respectively) is high (Huheey 1983; Batley 1989; Caplat et al. 2005). With respect to abundance of carbonate pearls and minerals in the bay, elevated percentage of exchangeable Mn is assumed to have derived from these minerals. Furthermore, Mn2+ ionic radius is similar to that of Ca2+ (0.91 and 1.08 Å, respectively), and with respect to similar charge, ionic potential of Mn2+ for substituting Ca2+ is high. Mn is generally weakly bound to the surface of carbonate minerals. Hence, Mn may easily be released via ionic exchange and dissociation processes (Tessier et al. 1979; Yuan et al. 2004). On the other hand, only a small amount of other heavy metals was extracted by acetic acid solution, and exchangeable Cu, Pb, Zn, Co, Ni, Cr, V, and Fe are negligible (Fig. 5).
Reducible fraction
Due to the large surface area, amorphous hydrous Fe-Mn oxides are important geochemical phases impacting the mobility and behavior of trace metals (Turner 2000). Large numbers of studies have been conducted to remove organic or inorganic pollutants by utilizing the strong adsorption capacity of hydrous Fe or Mn oxides (L. Wang et al. 1997; Choo and Kang 2003; Root et al. 2007). Due to their chemical characteristics, Fe and Mn extracted by hydroxylamine hydrochloride solution are regarded as amorphous hydrous Fe-Mn oxides in sediments or soils (Gomez Ariza et al. 2000). Our results showed that 37.65–55.11 % of Cu, 33.18–61.45 % of Pb, and 13.84–50.60 % of Zn (averagely 46.29 % of Cu, 42.36 % of Pb, and 23.05 % of Zn) occur as amorphous Fe-Mn oxides in sediments of Gowatr bay. Occurrence of Cu, Pb, and Zn in reducible fraction is considerable at site N11. Occurence of other heavy metals in this fraction is not significant. Cu and Pb adsorption on Fe-Mn oxy-hydroxides surface increases in alkaline pH (Kabata and Pendias 2001). Thus, alkaline pH of Gowatr bay (Table 1) is probably appropriate for Cu and Pb association in reducible fraction. In polluted sediments, Pb also mostly occurs in reducible and carbonate phases. This suggests that hydrous Fe-Mn oxides may play a major role in controlling the fate and transport of Pb and Cu in the sediments of Gowatr bay. Previous investigations demonstrated that reductive dissolution of iron oxy-hydroxides and subsequent release of adsorbed Pb are potential sources of Pb in porewater (Gallon et al. 2004). Several authors (Hong and Förstner 1984; Szefer et al. 1995; Li et al. 2001; Korfali and Davies 2004) have indicated that Zn is mainly associated with the reducible phase, mostly due to strong binding and formation constant of Zn with hydrous Fe-Mn oxides (Korfali and Davies 2004). Zn can be adsorbed on and precipitated with hydrous Fe-Mn oxides. According to Li et al. (2001), Zn also constitutes a multiple oxide like Franklinite through the following reaction:
Reducible fraction of sediment at site N11 plays a major role in Pb, Cu, and Zn speciation (Fig. 5). Thus, in anoxic condition, Pb, Cu, and Zn bound to hydrous Fe-Mn oxides may contribute a fraction to interstitial or above water in the bay. Finally, moderate percentage of V at sites N11 (25.97 %) and N18 (20.54 %) was found binding to hydrous Fe-Mn oxides. Other reducible heavy metals were negligible.
Oxidizable fraction
Organic matter and sulfides are important factors that control the mobility and bioavailability of heavy metals. Current opinions differ on the toxicity of heavy metals influenced by organic matters. For example, Höss et al. (2001) proposed that Cd complexation by organic matter, serving as food, might elevate the toxicity of Cd to nematode in sediment. In contrast, Besser et al. (2003) suggest that amendments of humus shifting the partitioning of Cd and Cu toward greater concentrations in sediments and lesser concentrations in pore waters reduce the toxicity of Cd and Cu. In the present study, relatively low percentage of metals was found bound to organic matter and sulfides, probably due to relatively low contents of organic matter (1.2–5.28 %) and sulfide (0.04–0.35 %) in the sediments (Table 1). Only moderate amounts of Zn (26.15 and 28.30 % at sites N18 and N10, respectively) and Cu (20.12 % at site N11) were found binding to oxidizable fraction. Maximum concentration of Cu, organic matter, and sulfide at site N11 is also the reason for high percentage of Cu in oxidizable fraction at this site. Even though the aforementioned is not true for Zn at sites N18 and N10, oxidizable fraction in adsorbing Zn is more efficient than reducible fraction. On the whole, low percentage of metals in oxidizable fraction (Fig. 5) indicates that organic matter and sulfide content are not the primary factor impacting the behavior of heavy metals in the study area.
Residual fraction
Residual phases of metals are generally much less toxic for organisms in aquatic environment. In Gowatr bay, dominant fractions of Fe (62.03–78.98 %), V (59.74–71.43 %), Cr (66.32–77.25 %), Ni (68.07–77.80 %), and Co (67.53–75.47 %) were found residing in residual fraction while Zn at sites N12 and N15, Pb at sites N10, N12, and N15, and Cu at site N15 were dominant (Fig. 5). Apparently, weathering of Makran ophiolites and oceanic crust supplies the above heavy metals.
Heavy metal content relative to other localities
Average total heavy metal concentrations (wt. mg/kg) and average percentages of heavy metals in each sediment fraction are compared with selected similar analyses in the world (Table 5). Average total concentrations of Cu, Ni, and Co of the sediment samples are greater when compare with those of other regions, while Pb content is lower. As well as average total Zn content of sediments is mostly similar to that from the coast of Kranji (Cuong and Obbard 2006). The predominance of Mn in non-residual fractions of the sediment samples obtained from the current study is similar to that from East China Sea (Yuan et al. 2004) and Daya bay (Gao et al. 2010). Cu, Pb, and Zn in both the study area and Manchar Lake of Pakistan (Arain et al. 2008), Cu and Zn in coast of Kranji, and Pb in East China Sea are mostly found in non-residual fractions of sediments, reflecting high mobility of these metals. The data indicate that fractional distribution of vanadium and chromium in East China Sea, and nickel and cobalt in Daya bay is mainly similar to that in the study area, suggesting the dominance of these heavy metals in residual fraction and hence their immobility.
Risk assessment
Individual and global contamination factor
The distribution of metal speciation associated with different geochemical fractions is a critical parameter to assess the potential mobility and bioavailability of heavy metals in sediments. The determination of contamination factor of heavy metals is an important aspect that indicates degree of risk of heavy metals to the environment in relation with its retention time. The individual contamination factors (ICFs) for various sampling sites were calculated from the result of the fractionation study by dividing the sum of the first three extractions (i.e., exchangeable, reducible, and oxidizable-organic forms) by the residual fraction for each site. The global contamination factor (GCF) for each site was calculated by summing the ICFs for heavy metals obtained for a site (Ikem et al. 2003). The ICF and GCF were computed for each station using the following equation:
The individual contamination factors for heavy metals in the surface sediments of the bay are indicated as bar diagrams in Fig. 6. The highest individual contamination factor for Cu, Pb, Zn, Ni, Co, V, Cr, and Fe occurs at site N11, while site N10 indicates the highest ICF for Mn. ICF reflects the risk of contamination of a water body by a pollutant (Ikem et al. 2003). Therefore, the highest risk of heavy metals except for Mn was computed at site N11 and for Mn at site N10. The average individual contamination factors in surface sediment of sampling stations range in the order of Ni (0.343) < Fe (0.383) < Cr (0.389) < Co (0.413) < V (0.494) < Zn (1.292) < Pb (1.802) < Cu (1.840) < Mn (2.909).
The global contamination factor analyzed from ICF values showed that site N11 is highly impacted by metal pollutants (Fig. 7). The tendency of trace metals to accumulate in sediments, contamination from each source tends to be localized in a hotspot near the input, and then disperses regionally in lower concentrations (Luoma et al. 2008). Hence, the results obtained in this investigation revealed that site N11 located at Pasabandar fishing-commercial harbor is a high potential risk to fauna and flora of the Gowatr bay.
Conclusion
The present study indicates that Pasabandar harbor in Gowatr bay is highly impacted by metal pollutants and the highest risk of Cu, Pb, and Zn is seen at this harbor. The results of modified BCR fractionation scheme revealed that Cu and Pb mainly occur in reducible fraction, while Mn mostly occurs in exchangeable fraction. Cu, Pb, Zn, and Mn dominance in non-residual phases poses a higher ecological risk within Gowatr bay. The results of this investigation also revealed that Fe-Mn oxy-hydroxides play a positive role in complexing Cu, Pb, and Zn in the surface sediment of Gowatr bay.
In the northwest Arabian Sea, upwelling occurs each summer by strong southwest monsoon winds. Upwelling areas generally display natural geochemical anomalies. In the process of upwelling, marine deep water rises to the surface, bringing with it dissolved heavy metals and eventually leads to heavy metal accumulation in marine organisms. On the other hand, continuous erosion of Makran ophiolites will also supply Ni, Co, Cr, Fe, and V to Gowatr bay. Thus, in time, geogenic processes together with anthropogenic activity will lead to elevated heavy metal accumulation in the study area. The analytical data of this study indicated that current strategies on Gowatr bay utilization especially in Pasabandar harbor should be reviewed due to poor environmental quality. Total heavy metal content in most sites investigated does not display extreme enrichment in the surface sediments and hence does not pose a serious threat to the local fauna and flora. However, it is recommended to reduce and regulate anthropogenic sources of pollution.
References
Ahmad, M., Islam, S., Rahman, S., Haque, M., & Islam, M. (2010). Heavy metals in water, sediment and some fishes of Buriganga river, Bangladesh. International Journal of Environmental Research, 4(2), 321–332.
Alloway, B., & Ayres, D. C. (1997). Chemical principles of environmental pollution: CRC Press.
Amiard, J.-C., Geffard, A., Amiard-Triquet, C., & Crouzet, C. (2007). Relationship between the lability of sediment-bound metals (Cd, Cu, Zn) and their bioaccumulation in benthic invertebrates. Estuarine, Coastal and Shelf Science, 72(3), 511–521.
Arain, M. B., Kazi, T. G., Jamali, M. K., Jalbani, N., Afridi, H. I., & Baig, J. A. (2008). Speciation of heavy metals in sediment by conventional, ultrasound and microwave assisted single extraction methods: a comparison with modified sequential extraction procedure. Journal of Hazardous Materials, 154(1), 998–1006.
Batley, G. E. (1989). Trace Element Speciation Analytical Methods and Problems: CRC Press.
Besser, J. M., Brumbaugh, W. G., May, T. W., & Ingersoll, C. G. (2003). Effects of organic amendments on the toxicity and bioavailability of cadmium and copper in spiked formulated sediments. Environmental Toxicology and Chemistry, 22(4), 805–815.
Billon, G., Ouddane, B., Recourt, P., & Boughriet, A. (2002). Depth variability and some geochemical characteristics of Fe, Mn, Ca, Mg, Sr, S, P, Cd and Zn in anoxic sediments from Authie Bay (northern France). Estuarine, Coastal and Shelf Science, 55(2), 167–181.
Bloom, H., & Ayling, G. (1977). Heavy metals in the Derwent Estuary. Environmental Geology, 2(1), 3–22.
Caplat, C., Texier, H., Barillier, D., & Lelievre, C. (2005). Heavy metals mobility in harbour contaminated sediments: the case of Port-en-Bessin. Marine Pollution Bulletin, 50(5), 504–511.
Chibunda, R., Pereka, A., Phiri, E., & Tungaraza, C. (2010). Ecotoxicity of mercury contaminated sediment collected from mabubi river (geita district, Tanzania) to the early life stages of African catfish (Clarias gariepinus). International Journal of Environmental Research, 4(1), 49–56.
Choo, K.-H., & Kang, S.-K. (2003). Removal of residual organic matter from secondary effluent by iron oxides adsorption. Desalination, 154(2), 139–146.
Cuong, D. T., & Obbard, J. P. (2006). Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Applied Geochemistry, 21(8), 1335–1346.
Davidson, C. M., Thomas, R. P., McVey, S. E., Perala, R., Littlejohn, D., & Ure, A. M. (1994). Evaluation of a sequential extraction procedure for the speciation of heavy metals in sediments. Analytica Chimica Acta, 291(3), 277–286.
Davies, C. A., Tomlinson, K., & Stephenson, T. (1991). Heavy metals in river tees estuary sediments. Environmental Technology, 12(11), 961–972.
Esen, E., Kucuksezgin, F., & Uluturhan, E. (2010). Assessment of trace metal pollution in surface sediments of Nemrut Bay, Aegean Sea. Environmental Monitoring and Assessment, 160(1–4), 257–266.
Fan, W., Wang, W.-X., & Chen, J. (2002). Geochemistry of Cd, Cr, and Zn in highly contaminated sediments and its influences on assimilation by marine bivalves. Environmental Science and Technology, 36(23), 5164–5171.
Farhoudi, G., & Karig, D. (1977). Makran of Iran and Pakistan as an active arc system. Geology, 5(11), 664–668.
Fu, F., & Wang, Q. (2011). Removal of heavy metal ions from wastewaters: a review. Journal of Environmental Management, 92(3), 407–418.
Fukue, M., Nakamura, T., Kato, Y., & Yamasaki, S. (1999). Degree of pollution for marine sediments. Engineering Geology, 53(2), 131–137.
Gallon, C., Tessier, A., Gobeil, C., & Alfaro-De La Torre, M. C. (2004). Modeling diagenesis of lead in sediments of a Canadian Shield lake. Geochimica et Cosmochimica Acta, 68(17), 3531–3545.
Gao, X., Arthur Chen, C.-T., Wang, G., Xue, Q., Tang, C., & Chen, S. (2010). Environmental status of Daya Bay surface sediments inferred from a sequential extraction technique. Estuarine, Coastal and Shelf Science, 86(3), 369–378.
Geetha, R., Chandramohanakumar, N., & Mathews, L. (2008). Geochemical reactivity of surficial and core sediment of a tropical mangrove ecosystem. International Journal of Environmental Research, 2(4), 329–342.
Gomez Ariza, J., Giraldez, I., Sanchez-Rodas, D., & Morales, E. (2000). Metal sequential extraction procedure optimized for heavily polluted and iron oxide rich sediments. Analytica Chimica Acta, 414(1), 151–164.
Griscom, S. B., Fisher, N. S., & Luoma, S. N. (2000). Geochemical influences on assimilation of sediment-bound metals in clams and mussels. Environmental Science and Technology, 34(1), 91–99.
Guevara-Riba, A., Sahuquillo, A., Rubio, R., & Rauret, G. (2004). Assessment of metal mobility in dredged harbour sediments from Barcelona, Spain. Science of the Total Environment, 321(1), 241–255.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14(8), 975–1001.
Heiri, O., Lotter, A. F., & Lemcke, G. (2001). Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. Journal of Paleolimnology, 25(1), 101–110.
Holm, N. G. (1988). Arsenic regeneration from estuarine sediments of the Bothnian Bay, Sweden. Chemical Geology, 68(1), 89–98.
Hong, Y., & Förstner, U. (1984). Chemical forms of some heavy metals in Huanghe River sediments (China) and comparison with data from Rhine River sediments (West Germany). Geophysical Journal of the Royal Astronomical Society, 3(1), 37–44.
Höss, S., Henschel, T., Haitzer, M., Traunspurger, W., & Steinberg, C. E. (2001). Toxicity of cadmium to Caenorhabditis elegans (Nematoda) in whole sediment and pore water—the ambiguous role of organic matter. Environmental Toxicology and Chemistry, 20(12), 2794–2801.
Huheey, J. E. (1983). Inorganic chemistry (3rd ed.). New York: Harper and Row.
Ikem, A., Egiebor, N., & Nyavor, K. (2003). Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern USA. Water, Air, and Soil Pollution, 149(1–4), 51–75.
Kabata, A., & Pendias, H. (2001). Trace elements in soils and plants. New York: CRC.
Kersten, M., & Förstner, U. (1986). Chemical fractionation of heavy metals in anoxic estuarine and coastal sediments. Water Science & Technology, 18(4–5), 121–130.
Korfali, S. I., & Davies, B. E. (2004). Speciation of metals in sediment and water in a river underlain by limestone: role of carbonate species for purification capacity of rivers. Advances in Environmental Research, 8(3), 599–612.
Li, X., Shen, Z., Wai, O. W., & Li, Y.-S. (2001). Chemical forms of Pb, Zn and Cu in the sediment profiles of the Pearl River Estuary. Marine Pollution Bulletin, 42(3), 215–223.
Luoma, S. N., Rainbow, P. S., & Luoma, S. (2008). Metal contamination in aquatic environments: science and lateral management: Cambridge University Press.
Malferrari, D., Brigatti, M. F., Laurora, A., & Pini, S. (2009). Heavy metals in sediments from canals for water supplying and drainage: mobilization and control strategies. Journal of Hazardous Materials, 161(2), 723–729.
Marguı, E., Salvadó, V., Queralt, I., & Hidalgo, M. (2004). Comparison of three-stage sequential extraction and toxicity characteristic leaching tests to evaluate metal mobility in mining wastes. Analytica Chimica Acta, 524(1), 151–159.
Moore, F., Nematollahi, M. J., Keshavarzi, B., & Hamzeh, M. A. (2014). Surficial and vertical distribution of heavy metals in marine and intertidal sediments in the Iranian sector of Gowatr bay. Journal of Khoramshahr Marine Science and Technology, In Press.
Nádaská, G., Polčová, K., & Lesný, J. (2009). Manganese fractionation analysis in specific soil and sediment samples. Nova Biotechnological, 9(3), 295–301.
Okoro, H. K., Fatoki, O. S., Adekola, F. A., Ximba, B. J., & Snyman, R. G. (2012). A review of sequential extraction procedures for heavy metals speciation in soil and sediments. Open Access Scientific Reports, 181(1)
Qiu, Y.-W., Lin, D., Liu, J.-Q., & Zeng, E. Y. (2011). Bioaccumulation of trace metals in farmed fish from South China and potential risk assessment. Ecotoxicology and Environmental Safety, 74(3), 284–293.
Quevauviller, P., Rauret, G., Muntau, H., Ure, A., Rubio, R., López-Sánchez, J., et al. (1994). Evaluation of a sequential extraction procedure for the determination of extractable trace metal contents in sediments. Fresenius Journal of Analytical Chemistry, 349(12), 808–814.
Rauret, G., López-Sánchez, J.-F., Sahuquillo, A., Barahona, E., Lachica, M., Ure, A., et al. (2000). Application of a modified BCR sequential extraction (three-step) procedure for the determination of extractable trace metal contents in a sewage sludge amended soil reference material (CRM 483), complemented by a three-year stability study of acetic acid and EDTA extractable metal content. Journal of Environmental Monitoring, 2(3), 228–233.
Root, R. A., Dixit, S., Campbell, K. M., Jew, A. D., Hering, J. G., & O’Day, P. A. (2007). Arsenic sequestration by sorption processes in high-iron sediments. Geochimica et Cosmochimica Acta, 71(23), 5782–5803.
Salomons, W., & Förstner, U. (1984). Metals in the Hydrocycle: Springer-Verlag.
Shriadah, M. (1999). Heavy metals in mangrove sediments of the United Arab Emirates shoreline (Arabian Gulf). Water, Air, and Soil Pollution, 116(3–4), 523–534.
Szefer, P., Glasby, G., Pempkowiak, J., & Kaliszan, R. (1995). Extraction studies of heavy-metal pollutants in surficial sediments from the southern Baltic Sea off Poland. Chemical Geology, 120(1), 111–126.
Tam, N., & Wong, Y. (1995). Spatial and temporal variations of heavy metal contamination in sediments of a mangrove swamp in Hong Kong. Marine Pollution Bulletin, 31(4), 254–261.
Tessier, A., Campbell, P. G., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851.
Turner, A. (2000). Trace metal contamination in sediments from UK estuaries: an empirical evaluation of the role of hydrous iron and manganese oxides. Estuarine, Coastal and Shelf Science, 50(3), 355–371.
Uluturhan, E. (2010). Heavy metal concentrations in surface sediments from two regions (Saros and Gökova Gulfs) of the Eastern Aegean Sea. Environmental Monitoring and Assessment, 165(1–4), 675–684.
Ure, A., Quevauviller, P., Muntau, H., & Griepink, B. (1993). Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. International Journal of Environmental and Analytical Chemistry, 51(1–4), 135–151.
Wang, L., Chin, Y.-P., & Traina, S. J. (1997). Adsorption of (poly) maleic acid and an aquatic fulvic acid by geothite. Geochimica et Cosmochimica Acta, 61(24), 5313–5324.
Wang, S., Jia, Y., Wang, S., Wang, X., Wang, H., Zhao, Z., et al. (2010). Fractionation of heavy metals in shallow marine sediments from Jinzhou Bay, China. Journal of Environmental Sciences, 22(1), 23–31.
Yan, C., Li, Q., Zhang, X., & Li, G. (2010). Mobility and ecological risk assessment of heavy metals in surface sediments of Xiamen Bay and its adjacent areas, China. Environmental Earth Sciences, 60(7), 1469–1479.
Yap, C. K., Ismail, A., Tan, S. G., & Omar, H. (2002). Correlations between speciation of Cd, Cu, Pb and Zn in sediment and their concentrations in total soft tissue of green-lipped mussel Perna viridis from the west coast of Peninsular Malaysia. Environment International, 28(1–2), 117–126. doi:10.1016/S0160-4120(02)00015-6.
Yuan, C. G., Shi, J. B., He, B., Liu, J. F., Liang, L.-N., & Jiang, G.-B. (2004). Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environment International, 30(6), 769–783.
Acknowledgments
The authors would like to express their special thanks to agents of Iranian National Institute for Oceanography and Atmospheric Science (INIOAS)–Chabahar research station, and medical geology research center of Shiraz University for financial and experimental supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moore, F., Nematollahi, M.J. & Keshavarzi, B. Heavy metals fractionation in surface sediments of Gowatr bay-Iran. Environ Monit Assess 187, 4117 (2015). https://doi.org/10.1007/s10661-014-4117-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4117-7