Abstract
The environment is witnessing a downgrade caused by the amelioration of the industrial and agricultural sectors, namely, soil and sediment compartments. For those reasons, a comparative study was done between soil cores and sediments taken from two locations in the Qaraaoun reservoir, Lebanon. The soil cores were partitioned into several layers. Each layer was analyzed for several physicochemical parameters, such as functional groups, particle size distribution, ζ-potential, texture, pH, electric conductivity, total dissolved solids, organic matter, cation exchange capacity, active and total calcareous, available sodium and potassium, and metal content (cadmium, copper, and lead). The metal content of each site was linked to soil composition and characteristics. The two sites showed distinguishable characteristics for features such as organic matter, pH, mineral fraction, calcareous, and metal content. The samples taken toward the south site (Q1), though contain lower organic matter than the other but are more calcareous, showed higher metal content in comparison to the other site (Q2) (average metal content of Q1 > Q2; for Cd 3.8 > 1.8 mg/kg, Cu 28.6 > 21.9 mg/kg, Pb 26.7 > 19 mg/kg). However, the metal content in this study did not correlate as much to the organic matter; rather, it was influenced by the location of the samples with respect to the dam, the reservoir’s hydrodynamics, the calcareous nature of the soil, and the variation of the industrial and agricultural influence on each site.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soils are considered as settling compartments for metals that are discharged due to variable agricultural and industrial activities (Cottenic and Verloo 1984). Lebanon, for example, is witnessing an evolution in the agricultural and industrial sectors in the recent decades (MoE/LEDO (Ministry of Environment/Lebanese Environment and Development Observatory (2001). The industrial sector is showing a 20 % gross domestic production (GDP) in 2013, with a 5-year average of 19 %, while the agricultural one has a 5 % GDP in 2013 and a 5-year average as well (Matabadal 2013). This evolution is unfortunately accompanied with environmental degradation and, consequently, health hazards (Van der Gaag et al. 1991), especially in cases where effluent quality is not monitored, as is the case in the Qaraaoun area located in the Bekaa Valley (Fig. 1), containing one of the most viable water bodies in Lebanon, i.e., the Litani River. The Qaraaoun dam is the biggest in Lebanon, and it was built in 1959 to collect and store up to 2.2 million cubic meters of water (Shaban and Nassif 2007). The development of the industrial and agricultural sectors in that water-rich area is worsening the environmental compartments (Buccolieri et al. 2006), especially soil, sediment, and water (USAID 2005a), since effluents and discharges from those sectors are being released into the Litani River, or its tributaries, without preliminary treatment in many cases (Abi-Ghanem et al. 2009). In addition, agricultural runoffs in the Qaraaoun watershed will eventually reach the Qaraaoun reservoir along with pollutants and contaminants (such as heavy metals). However, heavy metal assessment in soils is a triggering case, because it depends on many parameters, such as the composition of soil’s organic matter (dissolved and particulate), mineral content, pH, biogeochemical fractionation, speciation, calcareous content, cation exchange capacity (CEC), redox state of the soil, water content, texture, and background levels of metals (Eriksson 1988; Mclean and Bledsoe 1992; Sukreeyapongse et al. 2002; Zaranyika and Chirinda 2011). Those factors are determining factors for the immobilization or leaching of heavy metals; hence, the changing of environmental conditions can alter those factors that in turn have an influence on the pollution level and bioavailability of metals (Soares et al. 1999; Demirbas et al. 2005). Heavy metal pollution assessment in soil and sediment samples and their leachability to underlying groundwater have been reported countless times, because they are nonbiodegradable and can cause serious environmental and health problems (Tokalioglu et al. 2003; Bentum et al. 2011; Kumar et al. 2011; Hafizur Rahman et al. 2012; Yisa et al. 2012; Shafie et al. 2013). For those reasons per se and because the Qaraaoun region is witnessing an exponential population growth and proliferation in the industrial and agricultural sectors, this study was conducted. A comparative study was done between two sites in the Qaraaoun dam vicinity.
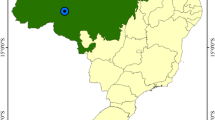
Sampling location. a Lebanon map. b Qaraaoun reservoir. 1 Q1 represents the first sampling site, and the highlighted area points to the urban and industrial nature of the site’s proximity; 2 Q2 represents the sampling site toward the north of the reservoir, and the highlighted area presents the agricultural nature of the site’s proximity
The aims of this study are to link the metal content to different physicochemical characteristics of soil and sediment samples, to have an insight about the spatial variation of those characteristics at locations influenced by reservoir hydrodynamics, on one hand, and industrial and agricultural sectors, on the other, and lastly to indicate the possibility of trace metal transfer at soil-water interface (whether surface or ground).
Materials and methods
Sampling site
The studied site is the Qaraaoun reservoir, located between Mount Lebanon and Anti-Lebanon Mount ranges, since it is an indispensable water body for the agricultural sector in the Bekaa Valley. A soil core and a surface sediment sample was taken from each site (cores are named Q1 and Q2, and sediments are named S1 and S2), both lying on the east side of the reservoir (Q1 33° 33′ 57.66″ N, 35° 42′ 26.87″ E and 858 m altitude; Q2 33° 35′ 0.53″ N, 35° 42′ 37.08″ E and 857 m altitude) (Fig. 1). The two cores were taken via a motorized core drill (Carottage à Percussion) on March 2013. Cores were taken on land at nearly 20 m away from the shoreline. The 50 cm cores were partitioned into six layers, 5 cm for the two upper most layers, and 10 cm for the deeper four. The sediment samples were taken from the surface of the reservoir bed that is submerged in approximately 30 cm water (a few meters away from the shoreline) by a polyethylene shovel. The sediment samples represent fine particles that settle on the reservoir bed. The soil samples were collected in polyethylene bags and stored at 4 °C till further analyses were performed, while sediments were stored at −80 °C to be freeze-dried by Alpha 1–4 LD plus freeze drier.
Sample preparation
The soil samples where disaggregated gently and air-dried at ambient room temperature. Soil samples were sieved at 2 mm to remove particles coarser than sand; only sand, silt, and clay fractions of the soil are of interest. Soil samples were further oven-dried (105 °C for 1 day) before experimentation took place. The sediment samples, however, were fine and inferior to 2 mm; they were freeze-dried to remove water content. Soil and sediment preparation were in accordance with the Organization for Economic Cooperation and Development (OECD 2004).
Physical and chemical parameters
The soil samples were analyzed for several features. Spectral analysis for mineral content of the dried soil and sediment samples was determined by Fourier transform infrared (FT-IR-6300 from JASCO). The samples were homogenized with KBr and compressed into 0.7-cm holding disks and then subjected to infrared radiation (IR). The size of the samples’ particles was determined by Partica Laser Scattering Particle Size Distribution Analyzer LA-950 V2 (from HORIBA). In addition, ζ-potential (ζ-potential in mV) of the colloidal particles of the samples was determined by Zeta Meter 4.0 equipped with electrodes, electrophoretic cells, a microscope, a direct video imaging assembly, a pattern generator, and a monitor. ζ-Potential was performed to determine the stability of the colloidal systems in each sample.
Other physicochemical characterizations, namely, pH, pHKCl, electric conductivity (EC), total dissolved solids (TDS), organic matter (OM), cation exchange capacity (CEC), active and total calcareous (CaCO3), texture, and available sodium and potassium content, in duplicates for each (n = 2), were performed (Table 1). Lastly, sediment and soil samples were sieved by a 64-μm mesh and later acid digested for total metal content.
Metal content
Metal content of the soil and sediment samples were quantified after acid digestion was performed (according to NF ISO 11466 1995). A few milliliters of H2O2 were added to 3 g of each dried sample before the aqua regia solution (1:3 of HNO3/HCl) was added (Hseu et al. 2002). The samples were left to incubate in flasks connected to reflux condensers for 16 h with few millimeters of H2O2, 21 ml of 12 M HCl, and 7 ml of 15.8 M HNO3. The samples were heated at 50 °C and kept for 30 min (Shafie et al. 2013) and then heated till 120 °C was reached and kept for 2 h. The samples were left to cool, filtered by a 0.45 μm filter, transferred to a 50 ml flask, and made up to the line mark with 0.5 M HNO3. The samples were then stored at 4 °C until copper (Cu), cadmium (Cd), and lead (Pb) quantifications were performed by atomic absorption spectroscopy (AAS Rayleigh WFX-210) equipped with a WF-10A auto sampler and flame and graphite furnace modes.
Statistical analysis
Statistical analyses were performed by SPSS 17.0. ANOVA test (P < 0.05) was done to determine the significant influence of the site location, the deepness of the soil layer, and both, for the different physicochemical characteristic and metal content. ANOVA test, in case significant difference was found, was followed by Duncan test (P < 0.05) to partition different soil layers according to their physicochemical characteristics. Eta square was done to indicate whether the difference of the physicochemical parameter values is due to different locations, depths, or the combination of both.
Results
Elemental and physical composition
Even though the two cores were taken from the east side of the Qaraaoun reservoir, at approximately 2 km apart (Fig. 1), they showed peculiar characteristics in several parameters (distinctive functional groups, particle ζ-potential and size). The FT-IR results of the soil and sediment samples taken from Q1 and Q2 showed similar functional groups, such as O–H (as alcohol), S–H (mercaptan), C=O (carbonyl group in acid chloride compound), O–H (in carboxylic acid), S=O (sulfoxide), C–H (aromatic), and C–Br (alkyl bromide) at wave numbers 3,436; 2,513; 1,798; 1,428; 1,031; 795; and 500 cm−1, respectively. Sediment and soil samples toward the north (i.e., Q2) exclusively showed N–H (primary amine) and C=O (carbonyl group in anhydride) at 1,630 and 1,875 cm−1, respectively, while the latter site (Q1) showed C–H (alkane) at 2,981 cm−1. Even though some functional groups were common in both locations (Fig. 2), however, the transmittance of those functional groups was dissimilar in the soil layers of the same and different locations (Table 2). It is worth mentioning that transmittance % is inversely proportional to the functional group detection. Alcohol and aromatic containing compounds were detected in higher amounts in Q2, while acid chloride and mercaptan containing compounds were more prominent in Q1. The presence, absence, noticeable, or negligible content is very dependent on the state of soil and its aspects (Somasundaran 2006).
The particle size distribution of the samples indicates the range of the particles (from 0.19 to 1531.91 μm in this case). The minimum and maximum particle sizes, in addition to the size limit of 50 % of the particle population (i.e., 50 % of particle population is below that limit), are shown in Table 3. Almost all samples were found to have sizes as low as 1 μm; however, the maximum range of the size varied from sample to other, where the lowest two layers of Q1 (30–40 and 40–50 cm) and uppermost Q2 layer (0–5 cm) recorded size of 1.53 and 1.3 mm, respectively. The size of the soil reflects the texture and OM content, which are both suitable indicators of metal sorption ability. By comparing the size limit of 50 % of soil population, the first 30-cm deep layer of Q1 is finer than Q2, while the latter depths, in addition to S1, are coarser than Q2.
The stability of the colloidal particles, as indicated by ζ-potential (Fig. 3), ranged from −53 to −62 mV, except for S1, that had the least negative value (−32.3 mV). The variation of ζ-potential of Q1 and Q2 was at variance, indicating different soil stability and colloidal behavior. In general, the high negative values of the samples indicate relatively higher stability of the colloidal systems, which can be in the form of OM that is slightly susceptible to association-dissociation mechanisms (Greenwood and Kendall 1999).
Soil and sediment physicochemical analysis
The dominant textures in all samples were clay and clay loam. Clay content as a function of depth showed a general increasing trend, especially in the top 30-cm layer of both cores (Table 4). A high content of sand was only seen in the bottom 20 cm layer of Q1 (sandy clay loam). Silt content, however, in Q2 showed a stable content in the soil layers. The pH values in both soil cores and sediment samples were basic in nature (according to Baize and Girard 1995); however, the value was stable and significantly the same throughout the layers of Q1, while it showed a slight variation through the layers of Q2. In both cases, the pH value of the sediment was significantly different than the soil in the same location. As for pHKCl, which indicates the pH variation in case of salt or fertilizer residue addition, they shifted the pH values of both cores to comparable values between Q1 and Q2. The greatest variation of pH-pHKCl was in the 40–50 cm layer of Q2 (18.4 %), and the same layer had the lowest EC and TDS values (461 μS/cm and 49 mg/L, respectively), while the other samples had fluctuating values, with lower values for the sample lying toward the north of the Qaraaoun reservoir (i.e., Q2). Most samples had moderate EC values, except for the superficial layers of Q2 and S1 that had high EC values (EC >1 mS/cm), while 40–50 cm layer of Q2 has a weak EC value (according to NF ISO 11265 1995). The variation of pH in the different samples is attributable to location (76.6 %), while pHKCl, EC, and TDS are rather affected majorly by the factors of location and depth combined (42.87, 46.07, and 42.47 %, respectively) (Table 7).
Q1 showed a decreasing value for the OM content in the soil layers (from 5.5 to 2.8 %) (Table 5). There is a significant difference in those layers (0–5, 5–20, 20–30, and 30–50 cm) with a poor OM content in the 20–50 cm layers; However, Q2 shows a rise and fall in OM, yet all are OM-rich soils (according to NF X31-109 1993). The variation of OM is due to the different nature of each location; this is proven by eta square test that indicates that location is 70 % responsible for OM variation between the samples; the same is said about active and total calcareous (97.8 and 95.9 %, respectively), while CEC variation is due to the combined effect of location and depth (Table 7). All CEC values lie in the moderate range (12–20 mEq), except for the 20–30-cm layer and sediment of Q1, which have elevated values of CEC (as stated by Baize and Girard 1992). The 0–20 and 20–50 cm layers of Q1 showed significantly different behavior regarding CEC and active calcareous content. As for calcareous, Q2 had very weak CaCO3 content, and only the surface layers (0–20 cm) contained a few percentage of active CaCO3. In general, the surface layers had higher calcareous content than the bottom ones (Table 5). Q1 samples, however, are very calcareous in nature (total calcareous >45 % according to standard NF X31-106 2002).
Trace elements and cation content
The cationic and total metal content varied in an element-dependent manner throughout the soil depth. Potassium and sodium content in Q1 and Q2, respectively, were the highest in the sediment (50.15 and 39 mg/L) followed by the superficial soil layer (36 and 35.2 mg/L) (Table 6). Sodium was found in higher concentrations in the top layers of Q1; thus, 72 % of Na+ variation is location-dependent (Table 7). As for potassium, it is mostly influenced by the factor of depth (42.68 %), and it was found in higher concentrations in the top 20 cm of Q1 and in the other layers in Q2. Regarding the metal content, the sediment samples had generally the lowest values (22.1 and 17.7 mg/kg Cu, 2.5 and 1.6 mg/kg Cd, and 24 and 16.4 mg/kg Pb in Q1 and Q2, respectively). Pb in the 5–10-cm layer in both locations (Q1 30.5 mg/kg and Q2 24 mg/kg), Cu in the 30–40-cm layer of Q1 (33 mg/kg), Cd in the 40–50-cm layer of Q1 (4.6 mg/kg), Cd in the 0–5-cm layer in Q2 (2 mg/kg), and Cu in the 10–20-cm layer of Q2 (24.2 mg/kg) had the highest concentrations (Table 6). Nonetheless, the distribution of Cu, Cd, and Pb, according to eta square analysis (Table 7), was majorly dependent on the location (72, 86, and 71 %, respectively), with significantly higher content in Q1.
Discussion
Initial soil characterization
Both cores were taken from the east side of the Qaraaoun reservoir, and the resemblance of IR-detected functional groups present in the soil and sediment samples of each location indicates that the sediments in the reservoir bed are originated from soil erosion, especially Q2, in which the sediment and top soil have the same texture (Fig. 2 and Table 4) (Schmidt 2000), while S1 is rather influenced by the settling of the particulate matter that reach the bed’s periphery as a function of the wind direction and hydrodynamic action of the reservoir. Another indication of the results given by the samples functionality is the higher composition of mineral and organic fractions in Q1 and Q2, respectively. The mineralogical fraction in Q1 can be explained by the higher abundance of mercaptan and acid chlorides (Table 2), which reflect the presence of mineral salts (Takai and Asami 1962); the former is also found further in anaerobic conditions (Smil 2002), which is prominent in the 30–50-cm layer of Q1, indicating full water saturation at that depth during the wet season. On the other hand, the organic fraction of Q2 is explained by the dominance of aromatic, primary amine, alcoholic, and carboxylic functional groups (Mclean and Bledsoe 1992). The relatively higher particle sizes in 30–40 and 40–50 cm of Q1 and 0–5 cm of Q2 are correlated to the higher sand content of those soils (Tables 3 and 4). However, the fine particle distribution of the other layers gives a notion about higher particulate OM or clay content (Thien and Graveel 2003); however, an increase in either one (OM or clay content) indicates higher possible metal retention (Mclean and Bledsoe 1992; Carrillo-Gonzalez et al. 2006). In some cases, the ζ-potential is correlated to the clay content of the soil, as seen in Q1, because the clay content is dependent and inversely proportional to ζ-potential (Fig. 3 and Table 4) (Chesworth 2008). Higher clay content and lower (or more negative) ζ-potential indicate the presence of highly stable colloids and hence higher retention capacity of metals (Murray et al. 2004). According to ζ-potential values, most layers (0–5, 10–20, 20–30, and 30–40 cm of Q1 and 0–5, 5–10, 30–40, and 40–50 cm of Q2) showed good colloidal stability and S1 is of moderate stability (−32 mV), while the rest are of excellent stability (according to ranges set by Greenwood and Kendall 1999).
Soil and sediment analytical experimentation
Soil content (sand, silt, and clay) does not follow a steady behavior in the soil depth; rather, it depends on the geology and nature of the site (Bai et al. 2012). The clay content in Q2 showed a general increasing trend in the soil depth, while silt content did not have a wide range of variation; the same was observed by Hubert (2006); however, that was not the case of Q1. Hubert (2006) also stated that pH does not follow a certain trend in the soil depth; this is shown in the eta square values that statistically prove that pH variation is location-dependent (Table 7). The pH values of the soil samples lie between 7.5 and 8.3 and thus are considered calcareous, which is the case of many soils belonging to floodplain of humid regions (Foth 1990), such as the Qaraaoun reservoir. The basic pH value is an indication of high calcareous content in Q1 (Tables 4 and 5); the same pH variation range in the soil layers was indicated by Kassir et al. (2012) in a study done in North Lebanon of a clayey soil. The 40–50 cm layer of Q2 has the lowest EC, TDS, and pHKCl values (Table 4); the values are significantly different from values of other depths. This depth is very susceptible to pH variation (as seen by the difference in pH and pHKCl in Table 4); the low buffering capacity of that layer is due to a low mineralogical content, yet high OM content, and thus, higher available/exchangeable sites are present that enable higher proton exchange with the soil particles (Table 5) (Foth 1990). Furthermore, the higher pH values of Q1 create more negative charges in the soil matrix that are consequently able to immobilize metals and make them biologically unavailable (Zheng and Zhang 2011). The higher EC and TDS values of Q1 are due to higher mineralogical fraction of the site (Tables 2 and 4), since mineral input and weathering have a rising influence on river EC and TDS (Atekwana et al. 2004); also, the hydrodynamics of the water reservoir contribute to EC and TDS rise, where its location (Q1) is in favor of the wind direction and water flow originated from the reservoir mouth. In addition, the stratification of the soil, regarding EC and TDS (as proven by Duncan’s multiple range test in Table 4), indicates the variation of those solids as induced by the fluctuation in the water content of the soil layers (Foth 1990). Unlike Hubert (2006), the CEC values showed an increase neither with greater depths nor with increasing clay content; rather, it was generally correlated with OM (Mclean and Bledsoe 1992), and this is most prominent in the 40–50 cm layer of Q1 (Tables 4 and 5). Higher CEC values are found in cases where the particle size of the sample is lower (Tables 3 and 5). Although many authors reported a strong correlation between CEC, OM, and clay content (Callaway et al. 1998; Shriadah 1999; Horowitz et al. 2006; Du Laing et al. 2007), this was not visible in either site. OM in Q1 showed an undisturbed soil profile, while the fluctuation of OM in Q2 indicates plowing or other activities that cause the disturbance of the soil depths (Table 5); In fact, higher OM content in Q2 is due to the agricultural activity and littering process taking place in that location (see Fig. 1 for agricultural nature in the proximity of Q2). The relatively higher values of CaCO3 in Q1 are correlated to higher mineralogical fraction and alkalinity of the soils (Tables 2, 4, and 5) (USAID 2005b; Du Laing et al. 2009); in this case, total and active calcareous and pH are all dependent on location (76, 96, and 98 %, respectively) (Table 7). The carbonate fraction can serve as metal immobilizers (Adriano 2001), either by precipitation or chemisorption, in general (Papadopoulos and Rowell 1988), by creating a nucleating or adsorbing surface for Cu and Cd, in particular (Santillan-Medrano and Jurinak 1975; Cavallaro and McBride 1978; McBride and Bouldin 1984), or by Cu restraining via CaCO3 (Dudley et al. 1988, Dudley et al. 1991).
Metals and cation variation in comparable cores and sediments
Total sodium and potassium are found in high amounts in natural soils since they are building structures of soil minerals; however, only a small fraction is available in the ionic form, and the rest is mostly adsorbed to clay minerals, OM, and silt (Chesworth 2008). In the undisturbed nature of Q1, the available potassium content decreases with depth (Table 6), as did OM (Table 5), this correlation is due to the nature of the weak interaction between K+ and OM, where higher OM is able to hold higher potassium cations (Abdel-Salam and Salem 2012), and this was the case of sediment samples (Tables 5 and 6). The saturation of colloidal complexes with exchangeable bases, such as sodium hydroxide, constructs the alkalinity of the soil, and thus, higher sodium concentrations were linked to the more basic site of Q1 (Tables 4 and 6) (Chesworth 2008).
The average values of cadmium, copper, and lead content of the soils of this study were compared to other studies done in the same area (Qaraaoun) and same country (North Lebanon) and later compared to guidelines and general metal content of soil (Table 8). Cd content is highly variable in the Qaraaoun watershed; it ranged from 0.01 to 11 mg/kg (Jurdi et al. 2011) and 0.02–5.16 mg/kg (USAID 2005a); nonetheless, the average Cd content in this study exceeded other averages done in the Qaraaoun area (Kanbar 2012) and North Lebanon (Kassir et al. 2012), and it was higher than the Canadian guidelines (CCME 2002; NGSO 2005) and proposed background concentrations by Martin and Whitfield (1983) (see Table 8 for background levels). Cu concentrations ranged in the same interval as in previous studies done in the Qaraaoun basin (Jurdi et al. 2011; Kanbar 2012), yet the average was the lowest in this study. Pb, on the other hand, had comparable values with nearby sites (Kanbar 2012), and the average lies between the average values of the other two studies done in that area (Jurdi et al. 2011; Kanbar 2012). However, copper and lead concentrations in this study did not exceed the permissible limits set by Martin and Whitfield (1983); CCME (2002) or NGSO (2005).
The variation of the metal concentrations in the Qaraaoun area is influenced by several factors, such as the physicochemical characteristics of the soil sample (Foth 1990; Mclean and Bledsoe 1992; Du Laing et al. 2007; Zheng and Zhang 2011), the fluctuation of the water content (Foth 1990), and time of sampling (wet or dry season) (USAID 2005a; Jurdi et al. 2011; Mohiuddin et al. 2011). On these notes, the higher values of cadmium may be explained by one of those factors. Cadmium content is thought to be originated from batteries and agricultural practices, copper from nearby smelting operation, while lead from smelting activities and automobile emissions (Jurdi et al. 2011). The proximity of the Qaraaoun is alive with agropastoral factories (Ablah and Chtaura), food and milk processing industries (Ferzol, Anjar, and Jdita), paper mills (Kab Elias and Qaa Al-Rim), rock cutting industries (Chtaura and Zahle), wastewater treatment plants (Mekse, Chtaura, Zahle, Qab Elias, and Zahle), and slaughterhouses (Zahle), all of which contribute to polluting the Litani River, its tributaries, and nearby soils, especially by heavy metals (USAID 2005a). Q1 higher metal content is thought to be originated from the particulate matter that comes from the effluents that are rejected in the Litani river course and from nearby smelting and rock-cutting factories that lie in the Qaraaoun watershed. Lead, however, was found more prominently in Q1 that is more influenced by industrial and urban activities (Fig. 1) (ELARD 2011). So, Pb in that area is more correlated to atmospheric (wet and dry) or direct effluents and domestic and diffuse industrial discharge (Abi-Ghanem et al. 2009). On another note, the fluctuation of the water level in the Qaraaoun reservoir is a key player in determining the metal behavior in those soils, since the alteration of the oxic/anoxic interface, redox reactions taking place, humidity, and exposure to the ambient environment can be factors in changing the metal fraction and speciation in that sample (Du Laing et al. 2009; Schulz-Zunkel and Krueger 2009). So, the increase in the water level during the wet season may come not only just as a metal transporter, but also as a sinker (Du Laing et al. 2007).
Unlike Murray et al. (2004) and Mclean and Bledsoe (1992) reported, metals in this study were not held at higher concentrations in sites having higher clay content, CEC, or OM; rather, metals are believed to be held by the mineral fraction, especially the carbonate fractions. In this case, the metals may be bound more strongly to their support and may not be as available as those held by OM; so, metal leaching may be a serious threat in the 0–10 and 40–50-cm layers of Q2, since these layers have, respectively, higher OM and lower negative ζ-potential, since deformation or degradation of these particles may alter the metals available for surface water or underground water.
Q1 and Q2 are located in the same watershed, yet Q2 covers a higher area and thus gets higher volumes of runoffs. Nonetheless, runoffs reaching Q1 are originated from industrial and urban sources, since the proximity of Qaraaoun Square (with relatively higher population) lies less than 1 km away from Q1, while those of Q2 are majorly agricultural (Fig. 1), therefore containing fertilizers, biocides, or other chemical products and residues used in agriculture (ELARD 2011).
Conclusions
The two sites in the proximity of the Qaraaoun reservoir showed similar characteristics in functional groups, particle size distribution, ζ-potential, texture, and potassium content, yet they varied as a function of depth. Still, some parameters were significantly different between location, such as pH, OM, active and total calcareous, available sodium, Cd, Cu, and Pb. As indicated by the sample functionality, Q1 can be concluded as a soil rich in calcareous and minerals, while Q2 is rich in OM. The variation of particle size distribution, CEC, and OM in the soil layers of Q1 showed a natural stratification. Both samples have stable colloidal particles that do not associate-dissociate simply. Different ranges of pH, EC, OM, and TDS were found between Q1 and Q2. The metal content was significantly higher in Q1 samples. Several factors account for that high metal content; the first is the mineral fraction, and, to a larger extent, the carbonate content that is able to adsorb and immobilize metals, while organic bound metals may cause a leaching threat to water bodies. Another factor is the spatial distribution of the sites, in which the first (Q1), domestically and industrially active, is very much influenced by wind and river water flow that can transport metals from the watershed to the reservoir beds and consequently to the nearby soils during the wet season, while the latter (Q2) is majorly influenced by agricultural practices that occur in the vicinity of the site.
References
Abdel-Salam, M. A., & Salem, H. M. (2012). Interaction between potassium and organic manure application on growth of cowpea (Vigna unguiculata L.) and soil properties in newly reclaimed sandy soil. World Journal Agricultural Science, 8(2), 141–149.
Abi-Ghanem, C., Bermond, A., Besançon, S., Nakhlé, K., & Khalaf, G. (2009). Cd and Pb extractability with EDTA in sediments of three contrasted sites of the Lebanese coast. Lebanon Science Journal, 10(2), 33–48.
Adriano, D. C. (2001). Trace elements in terrestrial environments: biogeochemistry bioavailability and risks of metals. New York: Springer.
ASTM D 2974. (2007). Standard test methods for moisture, ash, and organic matter of peat and other organic soils, American Society for Testing and Materials. Philadelphia, PA, USA, 4p.
Atekwana, E. A., Atekwana, E. A., Rowe, R. S., Werkema, D. D., & Legall, F. D. (2004). The relationship of total dissolved solids measurements to bulk electrical conductivity in an aquifer contaminated with hydrocarbon. Journal of Applied Geophysics, 56(4), 281–294.
Bai, E., Boutton, T. W., Liu, F., Wu, X. B., Hallmark, C. T., & Archer, S. R. (2012). Spatial variation of soil δ13C and its relation to carbon input and soil texture in a subtropical lowland woodland. Soil Biology and Biochemistry, 44(1), 102–112.
Baize, D., & Girard, M. C. A. F. E. S. (1992). Rèfèrentiel pèdologique: principaux sols d’Europe. Paris: INRA Èditions.
Baize, D., Girard, M. C., & AFES (Association française pour l’etude du sol). (1995). Référentiel pédologique. Paris: INRA.
Bentum, J. K., Anang, M., Boado, K. O., Koranteng-Addo, E. J., & Owusu Antwi, E. (2011). Assessment of heavy metals pollution of sediments from Fosu lagoon in Ghana. Bio Chemistry Society Ethiopian, 25(2), 191–196.
Buccolieri, A., Buccolieri, G., Cardellicchio, N., Dell’Atti, A., Di Leo, A., & Maci, A. (2006). Heavy metals in marine sediments of Taranto gulf (Ionian Sea, Southern Italy). Marine Chemistry, 99(1–4), 227–235.
Callaway, J. C., Delaune, R. D., & Patrick, W. H. (1998). Heavy metal chronologies in selected coastal wetlands from Northern Europe. Marine Pollution Bulletin, 36(1), 82–96.
Carrillo-Gonzalez, R., Simunek, J., Sauvé, S., & Adriano, D. (2006). Mechanisms and pathways of trace element mobility in soils. Advances in Agronomy, 91, 111–178.
Cavallaro, N., & McBride, M. B. (1978). Copper and cadmium adsorption characteristics of selected acid and calcareous soils. Soil Science Society of America Journal, 42(4), 550–556.
CCME. Canadian Council of Ministers of the Environment. (2002). Canadian sediment quality guidelines for the protection of aquatic life (ISQGs) summary tables. Retrieved from www.ccme.ca/assets/pdf/sedqg_summary_table.pdf. Accessed Dec 2011.
Chesworth, W. (ed) (2008). Encyclopedia of soil science. Springer. The Netherlands.
Cottenic, A., & Verloo, M. (1984). Analytical diagnosis of soil pollution with heavy metals. Fresenius’ Journal of Analytical Chemistry, 317(3–4), 389–393.
Demirbas, A., Pehlivan, E., Gode, F., Altun, T., & Arslan, G. (2005). Adsorption of Cu (II), Zn (II), Ni (II), Pb (II), and Cd (II) from aqueous solution on Amberlite IR-120 synthetic resin. Journal of Colloid and Interface Science, 282(1), 20–25.
Du Laing, G., Vandecasteele, B., De Grauwe, P., Moors, W., Lesage, E., Meers, E., Tack, F. M., & Verloo, M. G. (2007). Factors affecting metal concentrations in the upper sediment layer of intertidal reedbeds along the river Scheldt. Journal of Environmental Monitoring, 9(5), 449–455.
Du Laing, G., Rinklebe, J., Vandecasteele, B., Meers, E., & Tack, E. M. G. (2009). Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Science of the Total Environment, 407(13), 3972–3985.
Dudley, L. M., McLean, J. E., Sims, R. C., & Jurinak, J. J. (1988). Sorption of copper and cadmium from the water soluble fraction of an acid mine waste by two calcareous soils. Soil Science, 145(3), 207–214.
Dudley, L. M., McLean, J. E., Furst, T. H., & Jurinak, J. J. (1991). Sorption of cadmium and copper from an acid mine waste extract by two calcareous soils: column studies. Soil Science, 151(2), 121–135.
ELARD. (2011). Business plan for combating pollution of the Qaraoun Lake. Earth Link and Advanced Resources Development S.A.R.L. Beirut: UNDP.
EN ISO 10693 (2014) Qualité du sol—détermination de la teneur en carbonate—méthode volumétrique, AFNOR, 11p
Eriksson, J. E. (1988). The effects of clay, organic matter and time on adsorption and plant uptake of cadmium added to the soil. Water, Air, and Soil Pollution, 40, 359–373.
Foth, H. (1990). Fundamentals of soil science (8th ed.). New York: Wiley.
Greenwood, R., & Kendall, K. (1999). Selection of suitable dispersants for aqueous suspensions of zirconia and titania powders using acoustophoresis. Journal of the European Ceramic Society, 19, 479–488.
Hafizur Rahman, S., Khanam, D., Mehedi Adyel, T., Shahidul Islam, M., Aminul Ahsan, M., & Ahedul Akbor, M. (2012). Assessment of heavy metal contamination of agricultural soil around Dhaka export processing zone (DEPZ), Bangladesh: implication of seasonal variation and indices. Applied Sciences, 2, 584–601.
Horowitz, A. J., Elrick, K. A., & Hooper, R. P. (2006). The prediction of aquatic sediment-associated trace element concentrations using selected geochemical factors. Hydrological Processes, 3(4), 347–364.
Hseu, Z., Chen, Z., Tsai, C., Tsui, C., Cheng, S., Liu, C., & Lin, H. (2002). Digestion methods for total heavy metals in sediments and soils. Water, Air, and Soil Pollution, 141(1–4), 189–205.
Hubert, F. (2006). Modelisation des diffractogrammes de mineraux argileux en assemblages complexes dans deux sols de climat tempere. Implications mineralogique et pedologique. PhD thesis. Poitiers University, France.
Jurdi, M., Korfali, S., Karahagopian, N., Kreidieh, K. (2011). Wet season water quality survey of the Litani river basin project. American University of Beirut (AUB), Lebanon.
Kanbar, H.J., (2012). Trace-metal mobility in soil-water interface in an agricultural watershed and an industrial region in Lebanon. M.Sc thesis. Lebanese University, Hadat, Lebanon.
Kassir, L. N., Darwish, T., Shaban, A., Lartiges, B., & Ouaini, N. (2012). Mobility of selected trace elements in Mediterranean red soil amended with phosphogypsum: experimental study. Environmental Monitoring and Assessment, 184(7), 4397–4412.
Kumar, B., Kumar, S., Mishra, M., Singh, S. K., Prakash, D., Sharma, C. S., & Mukherjee, D. P. (2011). Geochemical fractionation of some heavy metals in soils in the vicinity of Sukinda mining area. Orissa Advanced Applications Science Research, 2(5), 263–272.
Martin, J., & Whitfield, M. (1983). The significance of the river input of chemical elements to the ocean. Trace Metals in Sea Water, 9, 265–296.
Matabadal, A. (2013). Country report Lebanon. Rabobank. Rabobank, The Netherlands.
McBride, M. B., & Bouldin, D. R. (1984). Long-term reactions of copper II in a contaminated calcareous soil. Soil Science Society of America Journal, 48(1), 59–59.
Mclean, J.E., Bledsoe, B.E. (1992). Ground water issue, behavior of metals in soils. EPA/540/S-92/018. Utah, USA.
MoE/LEDO (Ministry of Environment/ Lebanese Environment and Development Observatory. (2001). State of the environment report, Lebanon.
Mohiuddin, K. M., Ogawa, Y., Zakir, H. M., Otomo, K., & Shikazono, N. (2011). Heavy metals contamination in water and sediments of an urban river in a developing country. International Journal Environment Science Technology, 8(4), 723–736.
Murray, K. S., Rogers, D. T., & Kaufman, M. M. (2004). Heavy metals in an urban watershed. Journal of Environmental Quality, 33(1), 163–172.
NF ISO 10390 (2005) Qualité du sol—détermination du pH, AFNOR, 11p.
NF ISO 11265 (1995) Qualité du sol—détermination de la conductivité électrique spécifique, AFNOR, 7p.
NF ISO 11466 (1995) Qualité du sol —extraction des éléments en traces solubles dans l’eau regale, AFNOR, 10p.
NF X31-106 (2002) Qualité des sols—détermination du calcaire actif, AFNOR, 6p.
NF X31-107 (2003) Qualité du sol—détermination de la distribution granulométrique des particules du sol—méthode à la pipette, AFNOR, 20p.
NF X31-109 (1993) Qualité du sol—détermination du carbone organique par oxydation sulfochromique, AFNOR, 7p.
NGSO. The National Guidelines and Standards Office. (2005). The Canadian Environmental Quality Guidelines. www.ec.gc.ca/ceqg-rcqe/English/default.cfm. Accessed May 2005
OECD. Organization for Economic Cooperation and Development. (2004). Test No. 312: leaching in soil columns. OECD guidelines for the testing of chemicals, section 3, OECD Publishing.
P05-002A, Harron, W. R. A., Webster, G. R., & Cairns, R. R. (1983). Relationships between exchangeable sodium and sodium adsorption ratio in a solonetzic soil association. Canadian Journal of Soil Science, 63, 461–467.
Papadopoulos, P., & Rowell, D. L. (1988). The reactions of cadmium with calcium carbonate surfaces. European Journal of Soil Science, 39(1), 23–36.
Santillan-Medrano, J., & Jurinak, J. J. (1975). The chemistry of lead and cadmium in soil: solid phase formation. Soil Science Society of America Journal, 39(5), 851–856.
Schmidt, J. (Ed.). (2000). Soil erosion: application of physically based models. Freiberg: Springer Science & Business Media.
Schulz-Zunkel, C., & Krueger, F. (2009). Trace metal dynamics in floodplain soils of the river Elbe: a review. Journal of Environmental Quality, 38(4), 1349–1362.
Shaban, A., & Nassif, N. (2007). Pollution in Qaraaoun Lake, Central Lebanon. Journal of Environmental Hydrology, 15(11), 1–14.
Shafie, N. A., Aris, A. Z., Zakaria, M. P., Haris, H., Lim, W. Y., & Isa, N. M. (2013). Application of geoaccumulation index and enrichment factors on the assessment of heavy metal pollution in the sediments. Journal of Environmental Science and Health, Part A Environmental Science, 48, 182–190.
Shriadah, M. M. A. (1999). Heavy metals in mangrove sediments of the United Arab Emirates shoreline (Arabian Gulf). Water, Air, and Soil Pollution, 116(3–4), 523–534.
Smil, V. (2002). A handbook of industrial ecology. Part IV: industrial ecology at the national/regional level (p. 680). Cheltenham: Edward Elgar Publishing Limited.
Soares, H. M. V. M., Boaventura, R. A. R., Machado, A. A. S. C., & Esteves da Silva, J. C. G. (1999). Sediments as monitors of heavy metal contamination in the Ave River Basin (Portugal): multivariate analysis of data. Environmental Pollution, 105(3), 311–323.
Somasundaran, P. (Ed.). (2006). Encyclopedia of surface and colloid science, vol. 1 (2nd ed.). Florida: CRC Press.
Sukreeyapongse, O., Holm, P. E., Strobel, B. W., Panichsakpatana, S., Magid, J., & Hansen, H. C. (2002). pH-dependent release of cadmium, copper, and lead from natural and sludge-amended soils. Journal of Environmental Quality, 31(6), 1901–1909.
Takai, Y., & Asami, T. (1962). Formation of methyl mercaptan in paddy poils l. Journal Soil Science Plant Nutrition, 8(3), 40–44.
Thien, S. J., & Graveel, J. G. (2003). Laboratory manual for soil science, agricultural and environmental principles Preliminary. Iowa: Kendall/Hunt publishing company.
Tokalioglu, S., Kartal, S., & Birol, G. (2003). Comparison of three sequential extraction procedure for portioning of heavy metals in car park dust. Journal of Environmental Monitoring, 5, 468–476.
USAID. United States Agency for International Development. (2005a). Litani water quality management project (BAMAS). Technical survey report, summer conditions. Beirut: USAID.
USAID. United States Agency for International Development. (2005b). Litani water quality management project (BAMAS). Beirut: Final report. USAID.
Van der Gaag, M. A., Stortelder, P. B. M., Van Der Kooy, L. A., & Bruggeman, W. A. (1991). Setting environmental quality criteria for water and sediments in the Netherlands: a programmatic ecotoxicological approach. Europe Water Pollut Control, 1(3), 13–20.
Yisa, J., Jacob, J. O., & Onoyima, C. C. (2012). Assessment of toxic levels of some heavy metals in road deposited sediments in Suleja. Nigeria American Journal Chemistry, 2(2), 34–37.
Zaranyika, M. F., & Chirinda, T. (2011). Heavy metal speciation trends in mine slime dams: a case study of slime dams at a goldmine in Zimbabwe. Journal Environment Chemistry Ecotoxicoly, 3(5), 103–115.
Zheng, S., & Zhang, M. (2011). Effect of moisture regime on the redistribution of heavy metals in paddy soil. Journal of Environmental Sciences, 12(3), 434–443.
Acknowledgments
This research was funded by the research grant programs of the Lebanese University and the Lebanese Council for Scientific Research (CNRS). We also acknowledge the support of “Projets de Coopération Scientifique Inter-universitaire” (PCSI) programs in the “Agence Universitaire de la Francophonie” (AUF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanbar, H.J., Hanna, N., El Samrani, A.G. et al. Metal binding in soil cores and sediments in the vicinity of a dammed agricultural and industrial watershed. Environ Monit Assess 186, 8793–8806 (2014). https://doi.org/10.1007/s10661-014-4044-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-4044-7