Abstract
Hongze Lake, located in the east route of the South-to-North Water Diversion Project (SNWDP), is a potential drinking water source for the residents along this water diversion project. Based on a monthly sampling at 11 stations in three regions of Hongze Lake, the spatiotemporal distribution pattern of cyanobacteria community was comprehensively investigated from March 2011 to February 2013. A total of 23 cyanobacterial species which belong to 16 genera were identified, and Microcystis was the most predominant cyanobacterial genus mainly composed of Microcystis wesenbergii in Hongze Lake. The cyanobacterial abundance ranged from 0 to 2.6 × 107 cells/L, and the average cyanobacteria abundance of Northern region was significantly higher than those of Western region and Eastern region in the 2-year study. The total cyanobacteria abundance and the Microcystis abundance both took on a similar seasonal regularity in the three regions. The results of correlation analysis indicated that Microcystis abundance was correlated with water temperature, chemical oxygen demand (COD)Mn, nitrate (NO3-N), and total nitrogen (TN)/total phosphorus (TP) mass ratio, among which water temperature had the highest correlation coefficient. In summer, cyanobacteria blooms may take place under suitable environmental conditions at some special areas in Hongze Lake, especially where the concurrence of slow water exchange and steady wind direction exists.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, eutrophication has become a worldwide problem in many freshwater lakes (Sánchez-Carrillo et al. 2007; Liu et al. 2009). A common consequence of eutrophication in freshwater lakes is the increased occurrence of cyanobacterial population and even harmful algal blooms (HABs) (Paerl et al. 2001). The cyanobacterial blooms cause problems of surface scum and bad odor, and some cyanobacterial species even produce toxins that can threaten aquatic and human life (Wu et al. 2010). Cyanobacterial blooms in freshwater lakes are increasingly frequent and serious in China, such as the intensive cyanobacterial blooms in Chaohu Lake, Dianchi Lake, and Taihu Lake, where the genus Microcystis usually dominates the cyanobacterial population in summer and autumn (Liu et al. 2006; Chen et al. 2009; Lin et al. 2011; Zhang et al. 2011; Tian et al. 2012).
Up to now, factors identified as contributing toward the increasingly harmful cyanobacterial blooms have included excess nutrient elements, increased aquaculture production, and organic pollution (Ramsdell et al. 2005; Heisler et al. 2008). Besides these, many environmental factors have been found to be relevant to cyanobacterial population variation. It has been concluded that an increase of water temperature in warm seasons could play an important role in the proliferation of cyanobacterial blooms (Peperzak 2003; Paul 2008). In addition to that, different cyanobacterial species often show preferences for specific nutrient elements, for which reason, the effect of phosphate and nitrate concentration on growth and toxicity of cyanobacteria varies among species, and in many cases, the influences of the nutrient composition on harmful algal blooms are more complicated (Vezie et al. 2002; Vargo et al. 2004). There is a consensus that harmful cyanobacterial blooms are usually not caused by a single specific environmental factor but rather multiple factors occurring consecutively or simultaneously (Heisler et al. 2008; O’Neil et al. 2012).
Hongze Lake, the fourth largest freshwater lake in China, is a typical large shallow lake covering an area of 1,805 km2 (Huang et al. 2010). It is located in the east route of the South-to-North Water Diversion Project (SNWDP), used as the water channel and water resource regulating lake, which make them potential drinking water sources for the residents along this water diversion project. Consequently, it is necessary to confirm the possibility of the occurrence of cyanobacterial blooms during the implementation of this project owing to the fact that HABs would affect the water quality seriously. Studies of the spatiotemporal distribution pattern of cyanobacterial community and its relationship with environmental factors provide an effective way to understand the formation and development of cyanobacterial blooms (Webber et al. 2005; Domingues et al. 2008; Tian et al. 2012), due to the sensitivity of the phytoplankton species to changes in the water environment (Kümmerlin 1998). However, few investigations of the spatial and temporal distribution of cyanobacterial community in Hongze Lake have been conducted to date, and the intensity and frequency of cyanobacterial blooms have been also far less than Taihu Lake (Huang et al. 2010). Moreover, what changes will take place to the cyanobacteria community and the ambient aquatic environment are indeterminate after the SNWDP finally begins to work, so it is necessary to obtain the detailed data before the completion of the project in March 2013.
Therefore, the aims of this study were to (i) monitor the cyanobacteria population over long-term during the implementation of the water diversion project from March 2011 to February 2013, (ii) compare the spatiotemporal distribution pattern among different sampling sites, and (iii) analyze the relationship between cyanobacterial species and various environmental factors, which could provide a basis for studying the dynamic of the cyanobacterial community, determining the occurrence possibility of cyanobacterial blooms and protecting the aquatic environment in Hongze Lake.
Materials and methods
Study area
Hongze Lake (33° 06′–33° 40′ N, 118° 10′–118° 52′ E) mainly has its water supply from Huaihe River and mostly discharges into the Yangtze River through Sanhe River and Gaoyou Lake. The average water depth is 1.77 m, with the deepest part being 4.37 m. (Wang and Dou 1998; Ruan et al. 2008). Hongze Lake is a large water-carrying lake; hence, the water levels usually undergo large annual and seasonal changes (Wang and Chen 1999). The average annual rainfall is 926.7 mm, and besides, the annual rainfall shows a same distribution pattern that plum rain and autumn rain contributed nearly 65.4 % of the total rainfall and the annual rainfall varied greatly among different years (Chu 2001).
Site and sampling
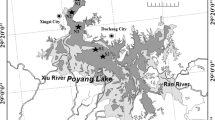
Sampling was conducted monthly from March 2011 to February 2013 at 11 sampling stations, which belong to three different lake regions respectively: Northern region (N1–N4 station), Western region (W1–W3 station), and Eastern region (E1–E4 station) (Fig. 1). Water samples were collected with a Ruttner water sampler (Hydro-Bios, Germany, 1,000 mL) at a depth of 0.5 m below water surface. One-liter water sample was preserved using 1 % Lugol’s iodine solution for identification and counting of cyanobacterial species.
Analysis methods
The physical and chemical parameters such as water temperature (WT), transparency (Trans), dissolved oxygen (DO), and pH were determined using on-site instruments. Total nitrogen (TN), total phosphorus (TP), ammonium (NH4-N), nitrate (NO3-N), nitrite (NO2-N), and orthophosphate (PO4-P) were analyzed according to the Chinese national standards for water quality (NEPAC 2002). Chemical oxygen demand (COD) was determined by acidic potassium permanganate method (NEPAC 2002). The water sample preserved using Lugol’s iodine solution was concentrated to 10 mL after sedimentation for 48 h, and then 0.1-mL concentrated water sample was counted under × 400 magnification with a compound microscope (CX31, OLYMPUS, Japan). Cyanobacteria species were identified according to phytoplankton morphology (Hu and Wei 2006).
Statistical analysis
The CANOCO v.4.5 computer program was used to perform all of the multivariate and ordination analyses (ter-Braak and Šmilauer 2002). Detrended correspondence analysis (DCA) was performed to select the appropriate model (whether linear or unimodal ordination methods were suitable for the dataset in this study) according to the length of first gradient. Finally, redundancy analysis (RDA), which indicated numerical analyses assuming linear species distributions, was applied to find out the relationship between the environmental variables and cyanobacterial species data. Before the analysis was performed, all the data above were log(x + 1) transformed except for pH variables. A Monte Carlo permutation test was used to determine the environmental variables best related to the cyanobacteria dynamics (Lepš and Šmilauer 2003). Pearson correlation analysis between environmental variables and cyanobacterial species was also carried out using the SPSS 18.0 software.
Results and discussion
Environmental parameters
The averages and ranges of nutrient concentrations (TN, TP, NH4-N, NO3-N, NO2-N, PO4-P, and TN/TP mass ratio), COD, and physical parameters (WD, Trans, WT, pH, and DO) of the sampling sites are shown in Table 1. Among all the environmental parameters, the physical parameters such as WD, Trans, and pH showed relatively slight variation than WT, DO, and other nutrient parameters like NH4-N and PO4-P.
From March 2011 to February 2013, water temperature exhibited an obvious seasonal pattern that the average temperature of summer and autumn was much higher than that of spring and winter (Fig. 2a). The maximum temperature value occurred in August 2011, while the minimum value occurred in January 2013. COD exhibited a similar variation trend as temperature except for a few months, with its range from 2.5 mg/L in January 2013 to 6.9 mg/L in August 2011 (Fig. 2a). The average of the water depth in Hongze Lake was 2.30 m, and water depths in summer were much lower than those in other seasons. Water transparency fluctuated within a narrow range with its mean 0.33 m, and the values of water transparency were less than 0.5 m in most months (Fig. 2b). The concentrations of TN and TP were both dynamic during the monitoring period, but they exhibited totally opposite seasonal trends. Generally, the maximum concentration of TP peaked in summer months when the concentration of TN was often at a relatively low level (Fig. 2c).
Composition of cyanobacterial community
A total of 23 cyanobacterial species, which belong to 16 genera, were microscopically identified in Hongze Lake from March 2011 to February 2013 (Table 2). At all the sampling sites, there were much more types of cyanobacterial species observed in summer and autumn than in winter and spring. The dominate cyanobacterial genera in Hongze Lake were Microcystis, Merismopedia, and Pseudanabaena, of which Microcystis wesenbergii, Merismopedia punctata, and Pseudanabaena limnetica were species that occurred most frequently. The average proportion of the genus Microcystis to total cyanobacteria community was 27.99 %, followed by Merismopedia 17.43 %, Pseudanabaena 11.80 %, Chroococcus 9.68 %, Oscillatoria 9.53 %, Phormidium 5.58 %, Pleurocapsa 4.93 %, and the others 22.59 %.
The spatiotemporal distribution pattern of cyanobacteria community
Hongze Lake is the fourth largest freshwater lake located in Huai’an City and Suqian City in China, with the multiple functions of irrigation, aquaculture, and merchant shipping (Chu 2001; Huang et al. 2010; Wang et al. 2010). Among the four regulating lakes that lie in the east route of the SNWDP, Hongze Lake is of great importance due to its large capacity in water storage and transportation. Unlike other large lakes such as Taihu Lake and Chaohu Lake in China, the cyanobacteria community structure and succession in Hongze Lake were rarely investigated to date. Wang et al. (2010) reported the cyanobacteria abundance in Hongze Lake from May to October in 2009 and calculated the mean value in the study period (1.35 × 106 cells/L), but the seasonal succession was not revealed.
The seasonal variations of the total cyanobacteria abundance in Hongze Lake are shown in Fig. 3. The interannual variation of the cyanobacteria abundance ranged over seven orders of magnitude from the undetectable level to 2.6 × 107 cells/L at station N4 in August 2011. The maximum total cyanobacteria abundance of three lake regions all appeared in August 2011, with the value of 14.27 × 106 cells/L in Northern region, 6.41 × 106 cells/L in Western region, and 6.50 × 106 cells/L in Eastern region. During the 2-year study, the average cyanobacteria abundance of Northern region (1.96 × 106 cells/L) was significantly higher than that of Western region (1.22 × 106 cells/L) and Eastern region (0.91 × 106 cells/L). In addition, three lake regions took on a similar seasonal regularity that the total cyanobacteria abundance in summer and autumn was much higher than that in winter and spring, and the cyanobacteria abundance in summer 2011 was higher than that in 2012. From the trend of mean annual variation, it is evident that the increase of cyanobacteria abundance usually took place in April or May when the water temperature began to rise gradually, and then the cyanobacteria abundance displayed a sharp increase in July. The relatively high cyanobacteria abundance often lasts 3 or 4 months until the end of November in each year, followed by the continuous decrease in December, and finally the lowest cyanobacteria abundance was observed in January or February.
The seasonal variations of cyanobacteria community composition and the abundance of dominated cyanobacterial genera during the sampling sites are shown in Fig. 4. Generally, the cyanobacterial genera in Hongze Lake all exhibited a similar seasonal trend like the total cyanobacteria abundance. The increase of cell density began in late spring, and the peak occurred in summer months (July and August) followed by the continuous declination of cell density from the end of October.
The proliferation of cyanobacteria species occurs in freshwater ecosystems at all latitudes and leads to increasing concerns for scientists and water environment managers (Nicolas et al. 2007; Wilhelm et al. 2011). In the current study, the community structure and the succession pattern of the cyanophyta in Hongze Lake were investigated for 2 years. From March 2011 to February 2013, the composition of cyanobacteria community experienced temporal variation that there were more types of cyanobacteria species observed in summer and autumn than in winter and spring, which Tian et al. (2012) also discovered in their study concerning the variation of cyanobacteria in Nansi Lake. Owing to the fact that Hongze Lake covers a wide range of water area, it was artificially divided into three regions (Northern region, Western region, and Eastern region) based on the difference in water hydraulics characteristic and some other water environmental factors. Therefore, it was necessary to find out whether significant differences exist among the three lake regions at the cyanobacteria community structure and abundance. As expected, the average cyanobacteria abundance of Northern region was much higher than that of Western region and Eastern region. However, the cyanobacteria species observed at the same sampling time in three lake regions were almost the same, and similar seasonal distribution pattern was exhibited in these three regions. According to the research performed previously, the abundance and species richness of phytoplankton usually get influenced by water velocity, fluctuating water level, and age of water (Crayton and Sommerfield 1979; Sharma et al. 2007; Yang et al. 2011). The mobility of water body in Northern region was much slower than that in the other two regions (Ye et al. 2011), in favor of nutrient accumulation and the maintaining of stable water column, which may be the major physical environmental factor that resulted in the cyanobacteria abundance difference in three lake regions.
In the Northern region, Microcystis was the most predominant cyanobacterial genus that its cell density was far higher than that in the other two lake regions, and the maximum abundance occurred in August in 2011 with the value of 1.03 × 107 cells/L. This situation was a little different in Western region and Eastern region; unlike Microcystis being the only predominant genus, Merismopedia was the second dominated genus in several months, with a relatively high cell density than the other genera as well as Microcystis. It is a kind of ubiquitous species that could be found in rivers, lakes, and estuaries. Merismopedia has a strong adaptability for different habitat environments, and generally it was the dominant species in low-alkalinity freshwater lakes in summer (Brettum 1989; Blomqvist 2001; Qin et al. 2007; Tian et al. 2012). In addition to that, Pseudanabaena also acted as one dominated genus in months such as October 2012 and September 2011 in Western region.
As the most predominant cyanobacterial genus in Hongze Lake, Microcystis was mainly composed of M. wesenbergii, accounting for nearly 90 % of the total Microcystis abundance. There were similarities between the spatiotemporal distribution patterns of total cyanobacteria community and M. wesenbergii (Fig. 5). The cell density of M. wesenbergii usually had a rapid increase from July and then lasted for 3 months until October when the cell density declined to a low level. Besides, in the Northern region, the abundance was significantly higher than that in the other two lake regions, with the maximum value appeared in August 2011.
Microcystis was reported as the dominant genus in many eutrophic lakes at home and abroad (Rinta-Kanto et al. 2005; Mitsuhiro 2007; Tan et al. 2009; Liu et al. 2011). A sharp increase of Microcystis abundance began in July, and the peak occurred in August followed by the continuous declination of abundance that started from October. However, the seasonal distribution of dominant cyanobacteria genus in different lakes displayed dissimilar regularities. Yamamoto and Nakahara (2009) reported that the population density variation of Microcystis in Hirosawa-no-ike Pond in Japan increased rapidly from late June and lasted about 3 months, which is similar to the Microcystis variation in Hongze Lake. In Lake Chaohu, Microcystis was predominant in warm seasons, and large algal biomass increased quickly from April to June (Deng et al. 2008). Similar to that, the Microcystis abundance in Taihu Lake often experienced vigorous increase from April, with a relatively high value until November (Kong et al. 2009). The Microcystis abundance and the duration time in Hongze Lake were significantly less than those in lakes where harmful cyanobacterial blooms occurred more frequently, such as Taihu Lake and Dianchi Lake in China (Kong et al. 2009; Sheng et al. 2012).
The relationship between cyanobacteria community variation and environmental factors
RDA based on cyanobacterial species data and environmental variables was carried out to explore the potential correlation between them. The observed variance of cyanobacteria abundance that can be explained by the axis is shown in Table 3, with the eigenvalues of the axis 1 and axis 2 listed as well. According to the results of RDA ordination (Fig. 6), the axis 1 and axis 2 explained 26.5 % of the cumulative percentage variance of cyanobacteria abundance, while all the environmental variables in the RDA analysis interpreted 29.3 % of the total variation of cyanobacteria abundance. A large proportion of variance (86.9) in the cyanobacteria-environment relationship was explained by the two ordination axes.
Environmental factors such as WT (−0.6141), DO (0.5531), NO3-N (0.4717), and CODMn (−0.4554) were strongly correlated with axis 1, while Trans (0.2133) and TN (0.2035) were most related with axis 2. During our study period, the cyanobacterial community in Hongze Lake experienced successive and substantial changes, which were closely related to many environmental factors including WT, DO, TN, Trans, COD, etc. Based on the results of RDA, there was clear evidence that water temperature played a key role in the succession of the cyanobacterial community in Hongze Lake, and the abundance variation of dominated cyanobacterial genera like Microcystis and Merismopedia was mainly correlated with environmental factors—WT, DO, NO3-N, and CODMn. More interpretation concerning the correlation between and cyanobacterial community and environmental factors in this ordination diagram can be obtained in Lepš and Šmilauer (2003).
The results of Pearson correlation analysis for species data and environmental variables are shown in Table 4. In terms of the correlation coefficients, it is obvious that most abundance of cyanobactrial genera in Hongze Lake was strongly related to WT, NO3-N, and CODMn, which was in accordance with the RDA analysis. Microcystis and Chroococcus had an abundance variation negatively correlated with WD and DO. Besides, the abundance variation of Microcystis, Merismopedia, and Phormidium correlated negatively with the ratio of TN to TP.
It is comprehensively accepted that the seasonal variations of phytoplankton are related to a series of environmental factors in aquatic environments (Wu and Chou 1998), and the dominance of specific species or a particular phytoplankton community feature is the result of a combination effect of multiple environmental factors (Reynolds 1998). In Hongze Lake, the spatiotemporal distribution pattern of cyanobacteria community varied at three lake regions, due to the otherness of hydraulics conditions and water quality parameters.
In a general way, cyanobacteria species have higher optimal temperature for cell growth than other kinds of phytoplankton assemblages. Most commonly, cyanobacterial dominance takes place at higher water temperatures (>20 °C) (McQueen and Lean 1987). As water temperatures exceed 20 °C, the growth rates of eukaryotic phytoplankton usually stabilize or decrease, while those of many cyanobacteria species always increase, providing a competitive advantage (Mur et al. 1999; Peperzak 2003; Paerl and Huisman 2009). In addition to that, the rising water temperatures will change many physical features of the aquatic environment that may favor the cyanobacterial species: the increase in nutrient diffusion (Vogel 1996; Peperzak 2003), the decrease in surface water viscosity occurred with the buoyancy regulation mechanisms of some cyanobacterial species (Wagner and Adrian 2009; Paerl and Huisman 2009), and the intensified vertical density stratification that also enhance the cyanobacterial species’ competitive advantage. Harmful bloom-forming cyanobacterial genus such as Microcystis, with the buoyancy regulating ability mentioned above, has been considered to succeed at or above 25 °C, and temperatures below 22 °C are not in favor of a mass proliferation (Robarts and Zohary 1987). Consistent with this issue, Microcystis dominated in Hongze Lake during the warm months (from July to September) during the 2-year study (Fig. 5), with the water temperature ranging from 24.7 to 31.3 °C and the mean value 28.5 °C. Besides, there is clear evidence that Microcystis abundance was significantly correlated with water temperature in Hongze Lake (Fig. 7a), with the obvious increase which appeared after the water temperature exceeded 25 °C. In the current study, water temperature proved to have a strong and positive effect on the growth and proliferation of Microcystis and the whole cyanobacteria abundance (Fig. 6).
Of all of the potential environmental factors behind harmful cyanobacterial blooms, anthropogenic nutrient pollution has been given the most attention. Many pieces of research believed that the eutrophication associated with the increased discharged pollutants has stimulated the occurrences of harmful cyanobacterial blooms (Hallegraeff 1993; Burkholder 1998; Anderson et al. 2002; Glibert et al. 2005; Heisler et al. 2008). In freshwater ecosystems, the availability of phosphorus is often one important factor limiting cyanobacterial species (Schindler et al. 2008). Generally, the increase of phosphorus concentration is in favor of the dominance of cyanobacteria species within the whole phytoplankton community (Smith 1986; Downing et al. 2001). On the other hand, the low TN/TP mass ratio does good for cyanobacterial species no matter whether they have the nitrogen fixing ability (Smith 1983). In our study, TN and TP exhibited opposite temporal variation trend: The maximum TN value occurred in winter and the TN values in summer were at a relatively low level, while the temporal trend of TP was opposite in most cases (Fig. 2c). Consequently, the TN/TP mass ratio during the study period kept a high level (41.0) in winter, and the ratio decreased to 18.3 from July to September, when the cyanobacteria abundance was much higher than that in other investigation period. The TN/TP mass ratio exhibited a negative correlation with the Microcystis abundance in Hongze Lake (Fig. 7b); in-line with the results of the study carried out in Meiliang Bay of Taihu Lake, the Microcystis species tended to dominate at low TN/TP (<30) in warm temperatures (Liu et al. 2011). However, Teubner et al. (1999) suggested that in some cases, it is the time when the critical ratio is reached rather than the ratio itself which really determines the dominance of one specific species. A number of lakes around the world with low TN/TP mass ratios are dominated by non-Microcystis; hence, other environmental factors besides this ratio actually also contribute to the occurrence of harmful Microcystis blooms (Liu et al. 2011).
In our study, the availability of NO3-N (nitrate) showed a strong negative correlation with Microcystis abundance (Fig. 6). According to the research of Jacoby et al. (2000), the dominance of Microcystis over other cyanobacterial species had a close relationship with low TN/TP mass ratio and low NO3-N availability. High NO3-N concentration favors the growth of eukaryotic phytoplankton (Berg et al. 2003), while low NO3-N favors some cyanobacteria (Jacoby et al. 2000). For instance, non-nitrogen-fixing cyanobacteria species like Microcystis aeruginosa prefer NH4-N over NO3-N as a N source; moreover, it could be outcompeted by other phytoplankton species in high NO3-N availability condition due to its low rate of NO3-N assimilation (Kappers 1984; Blomqvist et al. 1994). In Hongze Lake, the low level of NO3-N availability corresponded with relatively high Microcystis abundance to a great content (Fig. 7c). Except for the potential inorganic N and P, recent pieces of research have indicated that organic N and P may be important nutrient sources for cyanobacteria species as well. Much soluble N and P nutrient in aquatic environment was comprised of organic compounds (Seitzinger and Sanders 1997; Kolowith et al. 2001), and many cyanobacteria species could make use of various dissolved and particulate organic N and P nutrient (Glibert and Bronk 1994; Berman and Chava 1999; Davis et al. 2010). Consequently, this topic requires further studies concerning the nutrient form and ratios.
The harmful cyanobacteria blooms in Hongze Lake have not been as serious as those in Taihu Lake or other typical eutrophic lakes such as Dianchi Lake and Chaohu Lake in China (Huang et al. 2010). The most convincing explanation is probably the much more frequent water exchange owing to its water-carrying characteristic (Ye et al. 2011), because other environmental factors such as water temperature and nutrient concentration do not show significant differences (Deng et al. 2008; Liu et al. 2011; Sheng et al. 2012). Frequent water exchange suppresses the large-scale accumulation of cyanobacteria, and accordingly, the possibility of cyanobacterial bloom in Hongze Lake is relatively low. On the other hand, there have been small-scale cyanobacterial blooms that occurred in Hongze Lake in warm temperatures in the recent decade (Wang et al. 2010; Ye et al. 2011), and the sites where these cyanobacterial blooms took place all have features as follows: slow water exchange, low velocity of the wind that favor cyanobacteria migration and accumulation (Kong et al. 2009; Wu et al. 2010), relatively steady wind direction, and nutrient concentration or ratio beneficial for cyanobacterial blooms. The conditions listed above which are in favor for cyanobacteria blooms are appropriate for other typical lakes in China such as Taihu Lake, Dianchi Lake, and Chaohu Lake (Deng et al. 2008; Kong et al. 2009; Wu et al. 2010; Liu et al. 2011; Sheng et al. 2012). Overall, the occurrence of mass proliferation of cyanobacteria species, especially Microcystis, was associated with a stable water column, low NO3-N availability, low TN/TP mass ratio caused by increased TP concentration, and surface water temperature greater than 25 °C.
Conclusion
From March 2011 to February 2013, a total of 23 cyanobacterial species which belong to 16 genera were identified in Hongze Lake based on a monthly sampling at 11 stations in three lake regions. The average cyanobacteria abundance of Northern region (1.96 × 106 cells/L) was significantly higher than that of Western region (1.22 × 106 cells/L) and Eastern region (0.91 × 106 cells/L), and the three regions took on a similar seasonal regularity. Microcystis was the most predominant cyanobacterial genus, and it was mainly composed of M. wesenbergii. The results of RDA and Pearson correlation analysis indicated that water temperature, CODMn, NO3-N, and TN/TP mass ratio were correlated with Microcystis abundance, among which water temperature had the highest correlation coefficient. In summer, cyanobacterial blooms may take place under suitable environmental conditions at some special areas in Hongze Lake, especially where the concurrence of slow water exchange and steady wind direction exists.
References
Anderson, D. M., Glibert, P. M., & Burkholder, J. M. (2002). Harmful algal blooms and eutrophication: nutrient sources, composition and consequences. Estuaries, 25(4), 704–726.
Berg, G. M., Balode, M., Purina, I., Bekere, S., Béchemin, C., & Maestrini, S. Y. (2003). Plankton community composition in relation to availability and uptake of oxidized and reduced nitrogen. Aquatic Microbial Ecology, 30, 263–274.
Berman, T., & Chava, S. (1999). Algal growth on organic compounds as nitrogen sources. Journal of Plankton Research, 21(8), 1423–1437.
Blomqvist, P., Pettersson, A., & Hyenstrand, P. (1994). Ammonium-nitrogen: a key regulatory factor causing dominance of non-nitrogen-fixing cyanobacteria in aquatic systems. Archiv fuer Hydrobiologie, 132(2), 141–164.
Blomqvist, P. (2001). Phytoplankton responses to biomanipulated grazing pressure and nutrient additions-enclosure studies in unlimed and limed Lake Njupfatet, central Sweden. Environmental Pollution, 111(2), 333–348.
Brettum, P. (1989). Alger som indikator paÊ vannkvalitet i norske innsjùer. Planteplankton (pp. 1–111). Oslo, Norwegian: Norwegian Institute for Water Research (NIVA).
Burkholder, J. M. (1998). Implications of harmful microalgae and heterotrophic dinoflagellates in management of sustainable marine fisheries. Ecological Applications, 8, S37–S62.
Chen, J., Zhang, D. W., Xie, P., Wang, Q., & Ma, Z. (2009). Simultaneous determination of microcystin contaminations in various vertebrates (fish, turtle, duck and water bird) from a large eutrophic Chinese lake, Lake Taihu, with toxic Microcystis blooms. Science of the Total Environment, 407, 3317–3322.
Chu, E. G. (2001). Analysis on hydrological characteristic for Hongze Lake. Hydrology, 21, 56–59.
Crayton, W. M., & Sommerfield, M. R. (1979). Composition and abundance of phytoplankton in tributaries of the lower Colorado river, Grand Canyon region. Hydrobiologia, 66, 81–93.
Davis, T. W., Harke, M. J., Marcoval, M. A., Goleski, J., Orano-Dawson, C., Berry, D. L., & Gobler, C. J. (2010). Effects of nitrogenous compounds and phosphorus on the growth of toxic and non-toxic strains of Microcystis during bloom events. Aquatic Microbial Ecology, 61, 149–162.
Deng, D. G., Xie, P., Zhou, Q., Yang, H., Guo, L. G., & Geng, H. (2008). Field and experimental studies on the combined impacts of cyanobacterial blooms and small algae on crustacean zooplankton in a large, eutrophic, subtropical, Chinese lake. Limnology, 9, 1–11.
Domingues, R. B., Barbosa, A., & Galvao, H. (2008). Constraints on the use of phytoplankton as a biological quality element within the Water Framework Directive in Portuguese waters. Marine Pollution Bulletin, 56(8), 1389–1395.
Downing, J. A., Watson, S. B., & McCauley, E. (2001). Predicting cyanobacteria dominance in lakes. Canadian Journal of Fisheries and Aquatic Sciences, 58, 1905–1908.
Glibert, P. M., Anderson, D. A., Gentien, P., Graneli, E., & Sellner, K. G. (2005). The global, complex phenomena of harmful algal blooms. Oceanography, 18, 136–147.
Glibert, P. M., & Bronk, D. A. (1994). Release of dissolved organic nitrogen by marine diazotrophic cyanobacteria, Trichodesmium spp. Applied and Environmental Microbiology, 60, 3996–4000.
Hallegraeff, G. M. (1993). A review of harmful algal blooms and their apparent global increase. Phycologia, 32, 79–99.
Heisler, J. P., Gilbert, J., Burkholder, J., Anderson, D. M., Cochlan, W., Dennison, W. C., Dortch, Q., Gobler, C. J., Heil, C. A., Humphries, E., Lewitus, A., Magnien, R., Marshallm, H. G., Sellner, K., Stockwell, D. A., Stoecker, D. K., & Suddleson, M. (2008). Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae, 8, 3–13.
Hu, H. J., & Wei, Y. X. (2006). The freshwater algae of China: systematics, taxonomy and ecology. Beijing: Science Press.
Huang, L., Sun, K., Ban, J., & Bi, J. (2010). Public perception of blue-algae bloom risk in Hongze Lake of China. Environmental Management, 45(5), 1065–1075.
Jacoby, J. M., Collier, D. C., Welch, E. B., Hardy, F. J., & Crayton, M. (2000). Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Canadian Journal of Fisheries and Aquatic Sciences, 57, 231–240.
Kappers, F. I. (1984). On population dynamics of the cyanobacterium Microcystis aeruginosa. Ph.D. Dissertation, University of Amsterdam, Amsterdam, Netherlands.
Kolowith, L. C., Ingall, E. D., & Benner, R. (2001). Composition and cycling of marine organic phosphorus. Limnology and Oceanography, 46, 309–320.
Kong, F. X., Ma, R. H., Gao, J. F., & Wu, X. D. (2009). The theory and practice of prevention, forecast and warning on cyanobacteria bloom in Lake Taihu. Journal of Lake Sciences, 21(3), 314–328.
Kümmerlin, R. E. (1998). Taxonomical response of the phytoplankton community of Upper Lake Constance (Bodensee-Obersee) to eutrophication and re-oligotrophication. Advances in Limnology, 53, 109–117.
Lepš, J., & Šmilauer, P. (2003). Multivariate analysis of ecological data using CANOCO. Cambridge: Cambridge University Press.
Lin, S., Shen, J. Z., Liu, Y., Wu, X. Q., Liu, Q. G., & Li, R. H. (2011). Molecular evaluation on the distribution, diversity, and toxicity of Microcystis (Cyanobacteria) species from Lake Ulungur-a mesotrophic brackish desert lake in Xinjiang, China. Environmental Monitoring and Assessment, 175, 139–150.
Liu, W. C., Chen, W. B., & Kimura, N. (2009). Impact of phosphorus load reduction on water quality in a stratified reservoir-eutrophication modeling study. Environmental Monitoring and Assessment, 159, 393–406.
Liu, X., Lu, X. H., & Chen, Y. W. (2011). The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu, China: an 11-year investigation. Harmful Algae, 10, 337–343.
Liu, Y. M., Chen, W., Li, D. H., Shen, Y. W., Li, G. B., & Liu, Y. D. (2006). First report of aphantoxins in China-waterblooms of toxigenic Aphanizomenon flos-aquae in Lake Dianchi. Ecotoxicology and Environmental Safety, 65, 84–92.
McQueen, D. J., & Lean, D. R. S. (1987). Influence of water temperature and nitrogen to phosphorus ratios on the dominance of blue-green algae in Lake St. George, Ontario. Canadian Journal of Fisheries and Aquatic Sciences, 44, 598–604.
Mitsuhiro, Y. (2007). Dynamics of microcystin-producing and non-microcystin producing Microcystis populations is correlated with nitrate concentration in a Japanese lake. FEMS Microbiology Letters, 266, 49–53.
Mur, L. R., Skulberg, O. M., Utkilen, H. (1999). Cyanobacteria in the environment. In I. Chorus & J. Bartram (Eds.), Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management (pp. 15–40). World Health Organization.
NEPAC (The National Environmental Protection Agency of China), (Ed.). (2002). Standard methods for the examination of water and wastewater. 4th edn (in Chinese). Beijing: Chinese Environmental Science Press.
Nicolas, C., Robert, G., Céline, B., & Arlette, C. (2007). Seasonal succession of cyanoprokaryotes in a hypereutrophic oligo-mesohaline lagoon from the South of France. Estuarine, Coastal and Shelf Science, 72, 591–602.
O’Neil, J. M., Davis, T. W., Burford, M. A., & Gobler, C. J. (2012). The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae, 14, 313–334.
Paerl, H. W., Fulton, R. S., Moisander, P. H., & Dyble, J. (2001). Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Scientific World Journal, 1, 76–113.
Paerl, H. W., & Huisman, J. (2009). Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology Report, 1, 27–37.
Paul, V. J. (2008). Global warming and cyanobacterial harmful algal blooms. In: Hudnell, K.H. (Ed.), Cyanobacterial harmful algal blooms: state of the science and research needs. Advances in Experimental Medicine and Biology, 619, 239–257.
Peperzak, L. (2003). Climate change and harmful algal blooms in the North Sea. Acta Oecologica, 24, 139–144.
Qin, X. B., Huang, P. Y., Liu, M. H., Ma, C. X., & Yu, H. X. (2007). Phytoplankton assemblage classification in Anbang river wetland in summer. South China Fisheries Science, 3(6), 1–7.
Ramsdell, J. S., Anderson, D. M., & Glibert, P. M. (Eds.). (2005). HARRNESS (Harmful algal research and response: a national environmental science strategy) 2005–2015. Washington DC: Ecological Society of America.
Reynolds, C. S. (1998). What factors influence the species composition of phytoplankton in lakes of different trophic status? Hydrobiologia, 369–370, 11–26.
Rinta-Kanto, J. M., Ouellette, A. J. A., Boyer, G. L., Twiss, M. R., Bridgeman, T. B., & Wilhelm, S. W. (2005). Quantification of toxic Microcystis spp. during the 2003 and 2004 blooms in western Lake Erie using quantitative real-time PCR. Environmental Science and Technology, 39, 4198–4205.
Robarts, R. D., & Zohary, T. (1987). Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. New Zealand Journal of Marine and Freshwater Research, 21, 391–399.
Ruan, R., Zhang, Y., & Zhou, Y. (2008). Change detection of wetland in Hongze Lake using a time series of remotely sensed imagery. Remote Sensing and Spatial Information Sciences, 37, 1545–1548.
Sánchez-Carrillo, S., Alatorre, L. C., Sánchez-Andrés, R., & Garatuza-Payán, J. (2007). Eutrophication and sedimentation patterns in complete exploitation of water resources scenarios: an example from northwestern semi-arid Mexico. Environmental Monitoring and Assessment, 132, 377–393.
Schindler, D. W., Hecky, R. E., Findlay, D. L., Stainton, M. P., Parker, B. R., Paterson, M., Beaty, K. G., Lyng, M., & Kasian, S. E. M. (2008). Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37 year whole ecosystem experiment. Proceedings of the National Academy of Sciences of the United States of America, 105, 11254–11258.
Seitzinger, S., & Sanders, R. (1997). Contribution of dissolved organic nitrogen from rivers to estuarine eutrophication. Marine Ecology Progress Series, 159, 1–12.
Sharma, A., Sharma, R. C., & Anthwal, A. (2007). Monitoring phytoplanktonic diversity in the hill stream Chandrabhaga of Garhwal Himalaya. Life Science Journal, 4, 80–84.
Sheng, H., Liu, H., Wang, C. Y., Guo, H. C., Liu, Y., & Yang, Y. H. (2012). Analysis of cyanobacteria bloom in the Waihai part of Dianchi Lake, China. Ecological Informatics, 10, 37–48.
Smith, V. H. (1983). Low nitrogen to phosphorus ratios favor dominance by bluegreen algae in lake phytoplankton. Science, 221, 669–671.
Smith, V. H. (1986). Light and nutrient effects on the relative biomass of blue-green algae in lake phytoplankton. Canadian Journal of Fisheries and Aquatic Sciences, 43, 148–153.
Tan, X., Kong, F. X., Zeng, Q. F., Cao, H. S., Qian, S. Q., & Zhang, M. (2009). Seasonal variation of Microcystis in Lake Taihu and its relationships with environmental factors. Journal of Environmental Sciences, 21, 892–899.
ter-Braak, C. J. F., & Šmilauer, P. (2002). CANOCO Reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination (version 4.5). New York: Microcomputer Power.
Teubner, K., Feyerabend, R., Henning, M., Nicklisch, A., Woitke, P., & Kohl, J. G. (1999). Alternative blooming of Aphanizomenon flos-aquae or Planktothrix agardhii induced by the timing of the critical nitrogen:phosphorus ratio in hypertrophic riverine lakes. Archiv fuer Hydrobiologie, Advances in Limnolology, 54, 325–344.
Tian, C., Pei, H. Y., Hu, W. R., & Xie, J. (2012). Variation of cyanobacteria with different environmental conditions in Nansi Lake, China. Journal of Environmental Sciences, 24(8), 1394–1402.
Vargo, G. A., Heil, C. A., Ault, D. N., Neely, M. B., Murasko, S., Havens, J., Lester, K. M., Dixon, L. K., Merkt, R., Walsh, J., Weisberg, R., & Steidinger, K. A. (2004). Four Karenia brevis blooms: a comparative analysis. In K. A. Steidinger, J. A. Landsberg, C. R. Tomas, & G. A. Vargo (Eds.), Harmful Algae 2002, Proceedings of the Xth International Conference on Harmful Algae (pp. 14–16). St. Petersburg: Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and the Intergovernmental Oceanographic Commission of UNESCO.
Vezie, C., Rapala, J., Vaitomaa, J., Seitsonen, J., & Sivonen, K. (2002). Effect of nitrogen and phosphorus on growth of toxic and nontoxic Microcystis strains and on intracellular microcystin concentrations. Microbial Ecology, 43, 443–454.
Vogel, S. (1996). Life in moving fluids: the physical biology of flow (p. 84). Princeton: Princeton University Press.
Wagner, C., & Adrian, R. (2009). Cyanobacteria dominance: quantifying the effects of climate change. Limnology and Oceanography, 54, 2460–2468.
Wang, Q., & Chen, J. (1999). Formation and evolution of Hongze Lake and the Huaihe River mouth along the lake. Journal of Lake Sciences, 11, 237–244.
Wang, S., & Dou, H. (1998). The annals of Chinese Lakes (pp. 268–278). Beijing: The Science Press.
Wang, Z. Q., Zhang, N. H., Zhang, Y., Liu, Z. K., & Zhou, W. H. (2010). Eutrophication assessment of water quality in Hongze Lake. Environmental Monitoring and Forewarning, 2(6), 33–37.
Webber, M., Edwards-Myers, E., Campbell, C., & Webber, D. (2005). Phytoplankton and zooplankton as indicators of water quality in Discovery Bay, Jamaica. Hydrobiologia, 545(1), 177–193.
Wilhelm, S. W., Farnsley, S. E., LeCleir, G. R., Layton, A. C., Satchwell, M. F., DeBruyn, J. M., Boyer, G. L., Zhu, G. W., & Paerl, H. W. (2011). The relationships between nutrients, cyanobacterial toxins and the microbial community in Taihu (Lake Tai), China. Harmful Alage, 10, 207–215.
Wu, J. T., & Chou, J. W. (1998). Dinoflagellate associations in Feitsui Reservoir, Taiwan. Botanical Bulletin of Academica Sinica, 39, 137–145.
Wu, X. D., Kong, F. X., Chen, Y. W., Qian, X., Zhang, L. J., Yu, Y., Zhang, M., & Xing, P. (2010). Horizontal distribution and transport processes of bloom-forming Microcystis in a large shallow lake (Taihu, China). Limnologica, 40, 8–15.
Yang, M., Bi, Y., Hu, J., Zhu, K., Zhou, G., & Hu, Z. (2011). Seasonal variation in functional phytoplankton groups in Xiangxi Bay, Three Gorges Reservoir. Chinese Journal of Oceanology and Limnology, 29(5), 1057–1064.
Ye, C., Li, C., Wang, B., Zhang, J., & Zhang, L. (2011). Study on building scheme for a healthy aquatic ecosystem of Lake Hongze. Journal of Lake Sciences, 23, 725–730.
Yamamoto, Y., & Nakahara, H. (2009). Seasonal variations in the morphology of bloom-forming cyanobacteria in a eutrophic pond. Limnology, 10, 185–193.
Zhang, H., Yu, Z. L., Huang, Q., Xiao, X., Wang, X., Zhang, F. Y., Wang, X. Q., Liu, Y. D., & Hu, C. X. (2011). Isolation, identification and characterization of phytoplankton-lytic bacterium CH-22 against Microcystis aeruginosa. Limnologica, 41, 70–77.
Acknowledgments
We acknowledge the financial support of the International Science and Technology Cooperation Program of China (2010DFA91150), International Cooperation Research of Shandong Province (2011176), Science and Technology Development Project of Shandong Province (2012GHZ30020), and Policy and Technology Research Center of South-to-North Diversion Project Office, State Council. The authors thank the staff in Hydrological Bureau of Huai’an City for the sampling implementation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, Y., Pei, H., Hu, W. et al. Spatiotemporal distribution pattern of cyanobacteria community and its relationship with the environmental factors in Hongze Lake, China. Environ Monit Assess 186, 6919–6933 (2014). https://doi.org/10.1007/s10661-014-3899-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3899-y