Abstract
Concentrations of Cu, Zn, Cd, Pb, Ni, Co, Fe, Mn, and Hg were measured successively in water, sediments, and six macroalgal species belonging to three algal classes during 3 years (2008–2010) from Abu Qir Bay, Alexandria, Egypt: Chlorophyceae (Enteromorpha compressa, Ulva fasciata), Phaeophyceae (Padina boryana), and Rhodophyceae (Jania rubens, Hypnea musciformis, Pterocladia capillacea). The study aimed to assess the bioaccumulation potential of the seaweeds, as well as to evaluate the extent of heavy metal contamination in the selected study site. Metals were analyzed using atomic absorption spectrophotometry coupled with MH-10 hydride system. The obtained data showed that the highest mean concentrations of Cu, Zn, Fe, and Mn were recorded in E. compressa; Cd, Ni, and Hg exhibited their highest mean concentrations in P. boryana, while Pb and Co were found in J. rubens. Abundance of the heavy metals in the algal species was as follow: Fe > Mn > Zn > Pb > Ni > Co > Cu > Cd > Hg. E. compressa showed the maximum metal pollution index (MPI) which was 11.55. Bioconcentration factor (BCF) for the metals in algae was relatively high with a maximum value for Mn. The Tomlinson pollution load index (PLI) values for the recorded algal species were low, which ranged between 1.00 in P. boryana and 2.72 in E. compressa. Enrichment factors for sediments were low fluctuating between 0.43 for Hg to 2.33 for Mn. Accordingly, the green alga E. compressa, brown alga P. boryana, and red alga J. rubens can be nominated as bioindicators. Based on MPI and PLI indices, Abu Qir Bay in the present study is considered as low-contaminated area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic releases of heavy metals into the marine environment have become increasingly frequent in recent decades, causing public concern for the health of nearshore ecosystems (Evans and Edwards 2011). In particular, coastal ecosystems located near the large metropolitan areas experience constant exposure to industrial effluents, urban and residential wastes, and recreational pollution, all of which add heavy metals to marine ecosystem (Kimbrough et al. 2008). Currently, the usefulness of bioindicator species to monitor the extent of the contamination in the marine environment is now well established (Warnau and Bustamante 2007). Seaweeds play an important role in the nutrient dynamics of coastal systems and reflect changes in water quality efficiently. Hence, any change in the nature of the dynamics (like increased inputs of contaminants such as heavy metals) will likely be reflected by these marine macroalgae (Żbikowski et al. 2007). They have proven their effectiveness as biomonitors of heavy metal contamination. However, evaluation of contamination in an area solely by conventional water or sediment analysis is often cumbersome and misleading (Topcuoǧlu et al. 2003), owing to either very low (due to frequent tidal fluxes of the contaminant) or high concentrations (due to absence of any tidal flux) (Chaudhuri et al. 2007). In this respect, biomonitors like seaweeds are efficient in reporting measurable bioavailable concentrations of the contaminants. Also, they have the advantages to accumulate contaminants without being severely affected or killed by levels encountered in the environment and sessile, they have long life span (Bryan 1971), they are representative of the study area, and they are abundant throughout most of the world’s rocky coasts (Rajfur et al. 2010; Anastasakis et al. 2011). In fact, they can be considered as the key role to establish geographical and temporal variations in the bioavailability of contaminants through measurements of those contaminants in its whole body or specific tissues (Rainbow 1995).
Chlorophyta (green algae) and Ochrophyta (brown algae) are useful for this type of study as they show a clear tendency to accumulate heavy metals (Chaudhuri et al. 2007; Jothinayagi and Anbazhagan 2009). However, many articles promote the use of Enteromorpha and Ulva for monitoring the heavy metal profile of coastal seawater (Fityanos et al. 1999; Żbikowski et al. 2007), whereas red algae are recently used for this purpose (Abdallah 2010; Laib and Leghouchi 2011).
Abu Qir Bay is located along the southeastern coast of Alexandria and is considered as hot spot area. It is subjected to several land-based sources, like freshwater from the Rosetta mouth of the Nile River, loaded by nutrients; Lake Edku effluent carried with heavy metals, pesticides, humic acids, and nutrients; and El-Tabia Pumping Station which pours industrial and domestic wastes. The bay is also exposed to oil pollution from fishing boats, the activities of gas production liquefying and export field, in addition to the activities of Abu Qir Fertilizers Company and Abu Qir Electrical Power Station, which make the area vulnerable to contaminants (El-Sherif and Mikhail 2003; Shams El-Din and Dorgham 2007; Shobier et al. 2011). However, few studies were carried on seaweeds and the heavy metal loads in them from this particular area, which cannot be considered monitoring studies as many of them were short term (EL-Deeb and Aboul-Naga 2002; Mohamed and Khaled 2005; Khairy and Omar 2008).
The present study aims to monitor heavy metal loads in different seaweeds and ambient medium (water and sediments) of Abu Qir Bay and to assess the bioaccumulation potential of the seaweeds with respect to Cu, Zn, Cd, Pb, Ni, Co, Fe, Mn, and Hg, as well as to evalute the extent of heavy metal contamination in the selected study site which will serve as a baseline for future studies in this area.
Material and methods
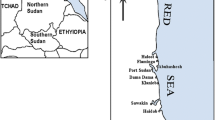
Three trips were carried out successively (2008–2010) during summer (August) at the beach of the touristic site “Bardiss” located at the extremely western head of Abu Qir Bay at longitudes 30° 07′ E and latitudes 31° 33′ N (Fig. 1). The bay is a hot spot area, and the sampling site is subjected properly to discharge of sewage water, without secondary and tertiary treatment, discharge of many inorganic and organic pollutants from El Temsah Company for shipbuilding, in addition to fishing activities, as well as industrial wastewater discharged to the bay through “Tabia Pumping Station” and the agricultural effluents from Lake Edku. This area is affected by the eastward wind-induced current (El-Tawil and Khalil 1983). However, this location is an exposed site consisting of successive substrate of rocks (El-Sarraf 1995) and is considered as highly diversified area, which harbored a large number of microalgal species (Aleem 1993).
Water samples were collected into 1-l capacity plastic bottles from the coastal area (>30-cm subsurface) for measurements of heavy metals with three replicas each year. The metals in seawater samples were preconcentrated using APDC and MIBK extraction procedure according to standard methods (Brewer et al. 1969; APHA 1989). Total Hg in water samples was directly measured after oxidation by KMnO4 followed by reduction with NaBH4.
About 100 g of sediments was collected from the superficial layer, then transferred to the laboratory, air-dried at room temperature (25–28 °C) for 2 days, and stored in plastic bags until analysis. Three replicas for each sample were prepared for measurement. About 2 g of dry sediments was heated in water bath at 90 °C with 4 ml HNO3 and 2 ml H2SO4 for 1 h, then allowed to cool; the volume was adjusted to 25 ml using deionized water (Adeloju et al. 1994).
Macroalgal species were collected from the same area of water sample collection at 0.50–1.00-m depth. Healthy specimens of different available seaweeds were handpicked whole, from their bases, scraping the substrata on which they were adhered. This is important for two reasons: for the taxonomic determination of the specimens and for measuring them. The specimens were separated in the field, then kept at 4 °C in icebox (Hardisson et al. 1998). Quick rinsing of the algae for removing of epiphytes, salts, and most impurities with tap water was carried out in laboratory throughout the same day of sampling. Microscopic identification of the investigated algae was carried out according to Riedel (1970) and Aleem (1993). A total of 18 algal samples were collected during 3 years (2008–2010) successively at the depths 0.5–1 m, representing three algal classes. Chlorophyceae was represented by the order Ulvales comprising one family: Ulvaceae, represented by two species, Enteromorpha compressa (Linnaeus) Nees and Ulva fasciata Delile. The class Phaeophyceae was represented by order Dictyotales comprising the family Dictyotaceae represented only by the species Padina boryana Thivy, whereas the third class Rhodophyceae was represented by three orders (Corallinales, Gigartinales, and Gelidiales) comprising one family each (Corallinaceae, Hypneaceae, Pterocladiaceae), with one species each, Jania rubens (Linnaeus) J. V. lamouroux, Hypnea musciformis (Wulfen) J. V. lamouroux, and Pterocladia capillacea (S. G. Gemlin) Bornet, respectively. The six species were represented at the study area yearly.
For measurement of heavy metals in algae, the samples were oven-dried at 37 °C for 2 days to a constant weight and homogenized manually into powder. From each sample, two replica subsamples were prepared and digested in Teflon vessels with 4 ml HNO3 and 2 ml HClO4 for 12 h at room temperature and heated subsequently at 100 °C for 2 h. After cooling, solutions were then made up to 25 ml with double-distilled water (Schuhmacher and Domingo 1996).
The analyses of metals in all samples were performed by PerkinElmer (AAnalyst 100) atomic absorption spectrophotometer. Hg concentration was measured by cold vapor technique using Mercury Hydride System MHS-10 while all the other metals were determined by flame technique. The detection limits of the studied metals were the following: Cu (1.5 ppb), Zn (0.85 ppb), Cd (0.18 ppb), Pb (9.7 ppb), Ni (2.06 ppb), Co (8.8 ppb), Fe (4.6 ppb), Mn (0.84 ppb), and Hg (0.26 ppb).
Accuracy and precision were verified by using reference materials for sediments (SD-M-2/IM) and biota (MA-A-2/TM) provided by the International Atomic Agency (IAEA). Analytical results of the quality control samples indicated a satisfactory performance of heavy metal determination within the range of certified values with 90.4–97.5 (for sediments) and 95–111 % (for biota) recovery for the metals studied.
Calculations
To compare the total metal content in algal species, sediment, and water in the study area, the metal pollution index (MPI) was used (Usero et al. 2005).
MPI = (M1 × M2 × M3 × … × Mn)1/n, where M is the concentration of metal and n is the metal number.
Bioconcentration factors were calculated as a ratio of the metal concentration in the macroalgae to the metal concentration in seawater, i.e., the soluble fraction (Black and Mitchell 1952). Bioconcentration is the accumulation of the contaminant by aquatic organisms through non-dietary uptake routes, e.g., from the soluble phase (Zauke et al. 1998).
To assess the health of the coastal bay with respect to the metals studied, the Tomlinson pollution load index (PLI) was used (Tomlinson et al. 1980; Angula 1996). PLI is used as an index of bioavailability of contaminants for organisms in coastal waters. It standardizes the data using the quotients obtained by dividing each concentration by a baseline concentration of the contaminant, i.e., the lowest concentration found during the study or reported in the literature. It is calculated using the following equation:
where CF = C metal/C baseline.
PLI values of ≥100 indicate a polluted site and those of ≥50 require intense monitoring of the site. A PLI value of <50 indicates that no drastic rectification measures are needed (Tomlinson et al. 1980). The advantage of using PLI is that it does not show any dependence on the year of sampling or on species, but it does detect an annual variation in metal concentrations. It has been widely used for the MUSSEL WATCH Program (Angula 1996) and is being used successfully for seaweed species in the study carried out for the first time by Chaudhuri et al. (2007).
To assess the enrichment or depletion of heavy metals in sediment sample, heavy metal concentrations can be normalized to an element which is conservative with respect to chemical weathering and which has no significant anthropogenic source. Generally, Al or Fe is selected as a normalizing parameter because they are lithophilic elements originating from the soil (Kontas 2008). In the present study, Fe was chosen as a normalizing parameter. According to Ergin et al. (1991), the enrichment factor (EF) is defined as the following:
where (Me/Fe)sample is the metal to Fe ratio in the sample of interest and (Me/Fe)Background is the natural background value of the metal to Fe ratio.
EF < 1 indicates no enrichment, EF < 3 indicates minor enrichment, EF = 3–5 is moderate enrichment, EF = 5–10 is moderatel to severe enrichment, EF = 10–25 is severe enrichment, EF = 25–50 is very severe enrichment, and EF > 50 is extremely severe enrichment (Birth 2003).
Statistical analysis
The correlation coefficient between the concentrations of the heavy metals in algal species was calculated using Minitab 14, and the relationship in all pairs of algal species, water, and sediments was calculated to evaluate statistical significance during 2008–2010 using Excel 2007. A probability of 0.05 was considered significant.
Results
Heavy metals in macroalgae
The accumulation of heavy metals in most studied species showed their maximum concentrations during 2010. The phaeophyte P. boryana recorded the highest concentrations of Cd (8.29 ppm), Zn (46.46 ppm), Ni (19.08 ppm), and Hg (757.50 ppb) while Pb and Co were recorded in the rhodophyte J. rubens during 2010 (26.50 and 11.36 ppm, respectively). The chlorophyte E. compressa showed the highest concentrations of Cu (6.10 ppm) and Fe (658.23 ppm) during 2009 and Mn (116.50 ppm) during 2010 (Fig. 2).
The highest average concentrations of metals during the study period (2008–2010) showed that P. boryana recorded the highest average values of Cd (4.28 ppm), Ni (13.39 ppm), and Hg (273.00 ppb), and E. compressa recorded the maximum average of Cu (4.60 ppm), Zn (31.10 ppm), Fe (430.34 ppm), and Mn (76.84 ppm), while Pb and Co showed their highest average values in J. rubens (22.41 and 8.52 ppm, respectively) (Table 1). The lowest average concentrations of all metals were found in U. fasciata except that of Hg, which was found in J. rubens. The concentrations of these metals showed more or less variations than those recorded in previous studies (Table 1).
The chlorophyte U. fasciata showed the lowest MPI (3.16), whereas E. compressa represented the maximum MPI (11.55) (Table 2).
The Tomlinson PLI values for the recorded algal species were low and fluctuated between 1.00 in P. boryana and 2.72 in E. compressa (Table 2).
The bioconcentration factor for the metals in algae (bioconcentration factor (BCF) × 103) was relatively high for all metals (>1,000) except that for Hg and differed in order of metals during the three successive years (2008–2010), with maximum value of Mn, whereas E. compressa, P. boryana, and J. rubens showed the maximum BCF values of most measured metals (Table 3).
The abundance of the heavy metals in the algal species was as follows:
Heavy metals in water
Co, Pb, and Ni showed no detected concentrations during the study period (2008–2010), whereas the other studied metals exhibited their lowest values during 2009, i.e., Cu (0.672 ppb), Zn (2.963 ppb), Fe (8.89 ppb), Mn (0.208 ppb), and Hg (0.20 ppb), while the highest values of Cu (1.24 ppb), Zn (11.67 ppb), and Mn (0.35 ppb) were recorded during 2008 and Cd (0.328 ppb), Fe (15.138 ppb), and Hg (0.48 ppb) were during 2010 (Fig. 3).
Apart from Co, Pb, and Ni, Mn showed the minimum average concentration in water, while Fe showed the maximum concentration (Table 4). The concentrations of these metals in water were lower than those recorded in the previous studies (Table 4), and they recorded low MPI value (1.11) (Table 2).
Heavy metals in sediments
All measured metals in the sediments recorded the lowest concentrations during 2010, i.e., Cu (0.95 ppm), Zn (4.45 ppm), Cd (0.94 ppm), Pb (1.47 ppm), Ni (2.06 ppm), Co (0.13 ppm), Fe (204.05 ppm), and Mn (46.61 ppm), except Hg which recorded its lowest concentration (17.57 ppb) during 2009, whereas Zn, Cd, Pb, Ni, Co, Fe, and Mn recorded their highest values during 2009 (11.29, 1.89, 22.05, 8.20, 6.44, 284.31, and 57.61 ppm, respectively), but Cu recorded its highest value (4.66 ppm) during 2008 and Hg (23.40 ppb) during 2010 (Fig. 4). The average concentrations of these metals were lower than those recorded in the previous studies (Table 5) and they recorded MPI (5.27) (Table 2). EF values were low fluctuating between 0.43 for Hg and 2.33 for Mn (Table 6).
Statistical analysis
The correlation coefficient between the concentrations of heavy metals in algal species from Abu Qir Bay during 2008–2010 revealed that some metal contents were influenced by the concentrations of other ones in the algal species at confidence limit 95 %, (n = 18 and r ≥ 0.47) (Table 7). On the other hand, the relationship between metals in all pairs of algal species, water, and sediments was calculated at confidence limit 95 %. The obtained data showed a strong direct correlation for some metals whereas others showed a reverse correlation (Table 8).
Discussion
Algae are among the organisms mostly used as bioindicators for heavy metal pollution (Rainbow 1995; Topcuoǧlu et al. 2003; Akcali and Kucuksezgin 2011). They are able to bind only free metal ions; the concentrations of which depend on the nature of the suspended particulate matter (Volterra and Conti 2000). This, in turn, comprises both organic and inorganic complexes, which permit the evaluation of the biologically available levels of contaminants in the ecosystem or the effects of contaminants on living organisms (Akcali and Kucuksezgin 2011). Thus, the change in heavy metal contents in these organisms specifically and temporally reflected the changes in the environment especially that the number of algae used in this study are representative to the study area since they are recorded during the 3 years (2008–2010). In contrast to the previous studies in this area which were either short-term (El-Sarraf 1995; EL-Deeb and Aboul-Naga 2002; Mohamed and Khaled 2005; Abdallah and Abdallah 2008; El-Nemr et al. 2012) or long-term study (Abdallah 2010), not all the recorded algal species were represented periodically. In fact, the average concentrations of all measured metals in the present study were comparable to those of the previous studies, except the recorded value of Zn in Eastern Harbor (El-Sarraf 1995); the concentrations of Fe recorded in E. compressa from El Mex Bay (Abdallah and Abdallah 2008) and Suez Bay (EL-Moselhy, Kh. M et al. 2006) and the concentration of Hg (EL-Naggar and Al-Amoudi 1989; Mohamed and Khaled 2005) were excessively high.
However, OECD (2003) suggested that indicators are only useful for showing changes in the environment where time series data are available. In this trend, the heavy metals recorded in the present algal species during the 3 years can reveal the condition of the contaminants, where the maximum values of all studied metals were recorded during 2010, except those of Fe and Cu that were attained in 2009. Meanwhile, Fe and Mn were the most abundant metals in the studied algae, followed by Zn, Pb ≥ Ni, Co ≥ Cu, Cd, and Hg. Similar order of metals was recorded in the same species collected from Abu Qir Bay (Mohamed and Khaled 2005; Abdallah 2010; El-Nemr et al. 2012).
In this trend, Gunner et al. (1987) confirmed that Fe content is an essential element for biological activity, whereas Bhowmik et al. (2010) reported that Zn is required at low concentration for the metabolic activity of 300 of the body’s enzymes and is essential for cell division and synthesis of DNA and protein. For other species, previous studies in Egyptian waters or in other localities (EL-Naggar and Al-Amoudi 1989; El-Sarraf 1995; Denton et al. 2006; EL-Moselhy, Kh. M et al. 2006; Güven et al. 2007) recorded different patterns but with the priority to the essential elements Fe, Mn, Zn, and Cu. However, the high levels of some heavy metals in the algae reflect firstly the high bioavailability of the metals in the study area and secondly the capacity of the alga to take them up (Shriadah and Emara 1991; Mohamed and Khaled 2005). Many factors may influence bioavailability of metals in algae such as physicochemical parameters (Topcuoǧlu et al. 2003) and seasonal differences in time of collection (Tomlinson et al. 1980), and the stage of development and variation in growth and chemical composition of the algae may influence the pattern of metal accumulation (Carlson and Erlandsson 1991).
On the other hand, the concentrations of these metals were correlated with their concentrations in water and sediment, which can be considered as advantage in the use of these algae as pollution indicator (Haritonidis and Malea 1995). However, correlations and functional relationships must be established between abiotic and biotic levels of pollution exposure in order to make early and realistic environmental risk assessments (ERAs) (Torres et al. 2008). A positive correlation was established between some of these metals in algae, which can be explained by a common source of these metals or by synergistic interaction between them (Haritonidis and Malea 1995).
Many authors have set guidelines to indicate the degree of contamination in coastal area. Say et al. (1990) proposed three concentration ranges of zinc in Enteromorpha species as an indication of the degree of contamination within estuaries: 50 ppm for an uncontaminated area, 50–150 ppm for “moderate” contamination, and 150 ppm for “high” contamination. Stenner and Nickless (1975) and Phillips (1990) set a range for Cu concentrations in Enteromorpha species in uncontaminated sites (6–12 ppm) and contaminated sites (20–70 ppm). Riley and Skirrow (1965) set values for Co and Ni in the algae in unpolluted area (0.7 and 3 ppm, respectively). In this context, Zn and Cu concentrations in the algal species of the present study coincided with the values set for uncontaminated areas, while the concentrations of Co and Ni exceeded the values for unpolluted areas.
In fact, pollution indices like MPI and PLI were highly effective for comparison between species and between sites (Chaudhuri et al. 2007), since using raw values for direct comparison and setting guidelines can be problematic and error prone, as these values may be functions of several physicochemical variables such as salinity, pH, and water temperature (Philips 1976; Munda 1984). Unless compared under similar conditions, these values are of little use (Topcuoǧlu et al. 2003; Chaudhuri et al. 2007).
Accordingly, the range of MPI recorded in the same genera by Chaudhuri et al. (2007) (2.94–10.60) was comparable to the values of MPI in the present study (3.12–11.16). The results of some previous studies for the same species at the same area reported higher MPI (Mohamed and Khaled 2005; El-Nemr et al. 2012). On the other hand, the values of MPI in sediment were high compared with those of all the studied areas by El-Sikaily (2008) (1.13–2.75), while MPI in water was relatively low, but there was no available literature to compare with.
The Tomlinson PLI index was rarely applied for algae in the Egyptian Mediterranean waters. PLI values were low and have not exceeded 2.72, indicating that the study area has low contamination with Cu, Zn, Cd, Pb, Ni, Co, Fe, Mn, and Hg. The “low contamination” refers to the quality of the seawater, since macroalgae take up metals from seawater only from the soluble fraction (Chaudhuri et al. 2007). In this trend, they reported comparable low PLI values in the genera Enteromorpha (1.33–1.57) and Ulva (1.29–1.42) collected from different sites. The low PLI values are supported by the low concentrations of all the measured metals in water, which were lower than the previous studies and had never exceeded the concentration values of the minimal risk.
Mean BCF values are the second efficient index and may be calculated based on several determinations spanning over a considerable period of time (Leal et al. 1997). These values frequently exceed 1,000 (Moore 1991; El-Sayed and Dorgham 1994; EL-Deeb and Aboul-Naga 2002; El-Moselhy and Gabal 2004; EL-Moselhy, Kh. M et al. 2006) while the BCF values of Hg were usually lower than 1,000 (El-Sayed and Dorgham 1994). The extremely high concentration factors in this study support the suggestion that marine algae could be a principal partner in the recycling of metals in coastal waters beside their role as substantial metal buffers in the ecosystem (Higgins and Mackey 1978). The previous studies agreed with this concept concluding that algae are suitable indicators to monitor pollution and accumulation of heavy metals (El-Sayed and Dorgham 1994; Ahdy et al. 2006; EL-Moselhy et al. 2006; Chaudhuri et al. 2007; Güven et al. 2007). However, the green alga E. compressa, brown alga P. boryana, and red alga J. rubens recorded the highest bioconcentration factor for almost studied metals during 2008–2010, which emphasize their highest MPI values, nominating them as bioindicators. The high capacity of metal sequestration by macroalgae is directly related with hydroxyl, sulfate, and carboxyl groups of the cell wall polysaccharides (Steffens 1990; Tropin 1995). Alginic acids and fucoidans are pointed out as the main polysaccharides involved in the chelation of heavy metals by brown algae (Holan and Volesky 1994; Davis et al. 2003; Romera et al. 2007), and that can explain the high accumulation of some metals such as Cd and Mn in the brown alga P. boryana during 2010 while the concentration of these metals show no difference in water and sediment in all studied years. Laib and Leghouchi (2011) attributed the accumulation of metals in J. rubens to the presence of sulfated polysaccharides made of galactans (agar and carraghenates), which enhance the biosorption of metals (Romera et al. 2007). Also, Pawlik-Skowrońsk et al. (2007)) found that Cd appeared to be accumulated to higher concentrations in Fucus serratus especially from the less polluted sites than in other seaweeds.
For water, concentrations of metals were lower than those recorded in the previous studies. According to Waldichut (1974) Co concentration in water column in unpolluted area should not exceed 0.05 μg/l, whereas according to WQC (1972), the hazardous concentrations of Cu, Zn, Pb, Ni, Fe, and Mn which can have harmful effect on aquatic life were 50, 100, 50, 100, 300, and 100 ppb, respectively, whereas the lower limits of these metals for aquatic life protection should be 10, 20, 10, 20, 50, and 20 ppb, respectively, and 2.2 ppb for Cd.
For sediments, the concentrations of studied metals in the present investigation were comparable to those recorded in unpolluted areas (Sari and Catagay 2001; Svete et al. 2001; Camargo 2002; Santamaria-Fernandez et al. 2005). Comparing the metal concentration with the effect range low (ERL) and effect range medium (ERM) to evaluate the metal pollution status (Long et al. 1995), the concentrations of Cu, Zn, Cd, Ni, and Hg recorded during the 3 years of the current study and their total average had never exceeded the ERL, which indicates that there is no adverse effect on the benthic marine community in the study area. Based on the total metal concentration alone, it is difficult to distinguish between anthropogenic and natural sources (Angelidis and Aloupi 2001). Therefore, the EF was another tool used in the present study as an index for assessing the extent of the contamination in sediments (Vald’es et al. 2005; Chen et al. 2007; Khalil and Rifaat 2010). In the present study, EF values of Cu, Ni, and Hg were <1 indicating no enrichment, while the values of Zn, Cd, Pb, and Mn were <3 indicating minor enrichment (Birth 2003). These values were lower than those recorded in the previous studies at Abu Qir Bay (El-Sikaily 2008; Khalil and Rifaat 2010; Shobier et al. 2011). Accordingly, Abu Qir Bay in the present study can be considered less polluted than the precedent years and than other areas in the Egyptian waters.
However, in the present study, the usefulness of the metal pollution indices (BCF and PLI) for algae, MPI for algae, water, and sediment, and EF for sediment had more explicit picture about the study area, since all the previous studies in this area (El-Sarraf 1995; El-Nady 1996; Mohamed and Khaled 2005; El-Moselhy and Hamed 2006; Shakweer et al. 2006; El-Sikaily 2008; Abdallah 2010; EL-Naggar 2009; Khalil and Rifaat 2010; Shobier et al. 2011; El-Nemr et al. 2012) or in the Egyptian waters (Hamed and El-Moselhy 2000; El-Deeb and Abou El-Naga 2002; El-Moselhy and Gabal 2004; El-Moselhy and Abd El-Azim 2005; Abdallah 2007, 2008; Abdallah and Abdallah 2008; Abdel Ghani et al. 2010) had not used any of these indices, either their studies were based only on one pollution index or even more but using in this case one tool: biological such as algal species or clams or fishes or water either sediment.
Finally, it can be concluded that the green alga E. compressa, brown alga P. boryana, and red alga J. rubens recorded the highest bioconcentration factor for most studied heavy metals during 2008–2010, which emphasize their highest MPI values, nominating them as bioindicators. Based on MPI and PLI index, Abu Qir Bay in the present study is considered to be low-contaminated area. And, the values of EF were lower than those recorded in sediments comparing to those of the previous studies in Abu Qir Bay.
References
Abdallah, M. A. M. (2007). Chemical composition and trace element concentration of marine algae Enteromorpha spp as potential food source. In: Proceedings of the third Mediterranean symposium on marine vegetation (Marseilles, 27-29 March 2007). C. Pergent-Martini, S. El Asmi, C. Le Ravallec edits., RAC/SPA publ., Tunis: 225-228.
Abdallah, M. A. M., & Abdallah, A. M. A. (2008). Biomonitoring study of heavy metals in biota and sediments in the south eastern coast of Mediterranean Sea Egypt. Environmental Monitoring and Assessment, 146, 139–145.
Abdallah, M. A. M. (2008). Chemical composition, mineral content and heavy metals of some marine seaweeds from Alexandria coast. Egyptian Journal of Aquatic Research, 34(2), 84–94.
Abdallah, M. A. M. (2010). Heavy metal monitoring in marine seaweeds from the southeastern Mediterranean Sea off the Egyptian coast, 2006-2009. United Nations Environment Programme Mediterranean Action Plan, Regional Activity Centre for Specially Protected Areas. Proceedings of the 4th Mediterranean Symposium on marine vegetation, Yasmine-Hammamet, 2-4 December 2010, pp:11-16.
Abdel Ghani, S. A. H., El Naggar, M. F., El Zokm, G. M., Shakweer, L., & Okbah, M. A. (2010). Concentration level of some dissolved trace metals in Mediterranean coastal water NW-Egypt. Egyptian Journal of Aquatic Research, 36(4), 509–522.
Adeloju, S. B., Dhindsa, H. S., & Tandon, R. K. (1994). Evaluation of some wet decomposition methods for mercury determination in biological and environmental materials by cold vapour atomic absorption spectrometry. Analytical Chemistry Acta, 285, 359–364.
Ahdy, H. H., Mohammed, T. A., & Abdallah, A. M. A. (2006). Multi-elements content in some marine seaweeds from Egyptian Red Sea coast. Egyptian Journal of Aquatic Research, 32, 1–15. special issue.
Akcali, I., & Kucuksezgin, F. (2011). A biomonitoring study: heavy metals in macroalgae from eastern Aegean coastal areas. Marine Pollution Bulletin, 62, 637–645.
Aleem, A. A. (1993). The marine algae of Alexandria, Egypt.139 pp.
Anastasakis, K., Ross, A. B., & Jones, J. M. (2011). Pyrolysis behaviour of the main carbohydrates of brown macro-algae. Fuel, 90, 598–607.
Angelidis, M. O., & Aloupi, M. (2001). Geochemical study of coastal sediments influenced by river-transported pollution: southern Evoikos Gulf. Marine Pollution Bulletin, 40(1), 77–82.
Angula, E. (1996). The Tomlinson pollution index applied to heavy metal, Mussel-Watch data: a useful index to assess coastal pollution. Science of the Total Environment, 187, 19–56.
APHA. (1989). “AWWA and WPCF standard methods for the examination of water and wastewater” 17 th ed., APHA, Washington.
Birth, G. (2003). A scheme for assessing human impacts on coastal aquatic environments using sediments. In : Woodcoffe, C.D., Furnes, R.A. (Eds.). Coastal GIS, 2003. Wollongong University papers in Center for Maritime Policy, 14, Australia.
Bhowmik, D., Chiranjib, K. P., & Kumar, S. (2010). A potential medicinal importance of zinc in human health and chronic disease. Review article. International Journal of Pharmaceutical and Biomedical Sciences, 1(1), 5–11.
Black, W. A. P., & Mitchell, R. L. (1952). Trace elements in the common brown algae and in seawater. Journal of Marine Biology Assessment, UK, 30, 575–584.
Brewer, P. G., Spencer, D. W., & Smith, C. L. (1969). Determination of trace metals in seawater by atomic absorption spectroscopy. American Society for Testing Materials Tech. Publication, 443, 70–77.
Bryan, G. W. (1971). The effects of heavy metals (other than mercury) on marine and estuarine organisms. The Royal society, London, series B, 177:389 - 410.
Camargo, J. A. (2002). Contribution of Spanish-American silver mines (1570-1820) to the present high mercury concentrations in the global environment: a review. Chemosphere, 48, 51–57.
Carlson, L., & Erlandsson, B. (1991). Seasonal variation of radionuclides in Fucus vesiculosus L. from the oresund. Southern Sweden. Environmental Pollution, 73, 53–70.
Chaudhuri, A., Mitra, M., Havrilla, C., Waguespack, Y., & Schwarz, J. (2007). Heavy metal biomonitoring by seaweeds on the Delmarva, Peninsula, east coast of the USA. Botanica Marina, 50, 151–158.
Chen, C. W., Kao, C. M., Chen, C. F., & Dong, C. D. (2007). Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere, 66, 1431–1440.
Davis, T. A., Voleskya, B., & Mucci, A. (2003). A review of the biochemistry of heavy metal biosorption by brown algae. Water Research, 37, 4311–4330.
Denton, G. R. W.; Kelly, W. C.; Wood, H. R. & Wen, Y. (2006). Impact of metal enriched leachate from ordot dump on the heavy metal status of biotic and abiotic components of Pago Bay. Water and Environmental Research Institute (WERI) Technical Report No. 113, University of Guam, Mangilao, Guam, 63 pp.
EL-Deeb, M. K., & Aboul-Naga, W. M. (2002). Trace metals: Fe, Zn, Mn, Cu, Ni and Cr in macroalgae from Alexandria coast. Bulletin Facts Science, 42(1,2), 51–60. University of Alexandria.
El-Moselhy, Kh. M., & Gabal, M. N. (2004). Trace metals in water, sediments and marine organisms from the northern part of the Gulf of Suez, Red Sea. Journal of Marine Systems, 46, 39–46.
EL-Moselhy, Kh. M., & Abd El-Azim, H. (2005). Heavy metals content and grain size of sediments from Suez Bay, Red Sea, Egypt. Egyptian Journal of Aquatic Research, 31(2), 224–238.
EL-Moselhy, Kh. M.; Amer, A. M. & Shams El Din, N. G. (2006). Trace metals concentration in water, sediments and macroalgae species in the intertidal zone of Suez Bay, Red Sea, Egypt. International Journal Oce. and Oceano. 1 (3) in press.
EL-Moselhy, Kh. M., & Hamed, M. A. (2006). Impact of land-based activities on hydrographic conditions and levels of heavy metals in water and sediments along the Mediterranean coast of Egypt. Egyptian Journal of Aquatic Research, 32(2), 63–82.
El-Nady, F. E. (1996). Heavy metal pollution problems in the southeastern Mediterranean waters of Alexandria, Egypt. Proceeding of the 6th international conference on environmental protection is a must, NIOF, VEA, ISA and SFD, Alexandria, Egypt, 21-31 May: 364-381.
EL-Naggar, M. E. E. & Al-Amoudi, O. A. (1989). Heavy metal levels in several species of marine algae from the Red Sea of Saudi Arabia. J.K.A.U.: Science, 1:5-13.
EL-Naggar, M. F. (2009). Heavy metals accumulation in the biotic environment of the Bitter to the Mediterranean Sea, west of Alexandria. Egyptian Journal of Aquatic Research, 31(special issue), 120–129.
El-Nemr, A., Khaled, A., & El-Sikaily, A. (2012). Heavy metal contamination in the seaweeds of Abu-Qir, Egypt. Journal of Blue Biotechnology, 1(2), 273–287.
EL-Sarraf, W. M. (1995). Heavy metal content in some marine algae from Alexandria, Egypt. Bulletin Facts Science, 35(2), 475–484. Alexandria University.
EL-Sayed, M. A., & Dorgham, M. M. (1994). Trace metals in macroalgae from the Qatari coastal water. Journal of King Abdulaziz University-Science, 5, 13–24.
El-Sherif, M. Z., & Mikhail, S. K. (2003). Phytoplankton dynamics in the southwestern part of Abu-Qir Bay Egypt. Egyptian Journal of Aquatic Biology and Fisheries, 7(1), 219–239.
El-Sikaily, A. (2008). Assessment of some heavy metals pollution in the sediments along the Egyptian Mediterranean coast. Egyptian Journal of Aquatic Research, 34(3), 58–71.
El-Tawil, B. A. H., & Khalil, A. N. (1983). Chemical constituents of some algal species from Abu-Qir Bay. Egyptian Journal Facts Marine Science, 3, 85–94.
Ergin, M., Saydam, C., Bastruk, O., Erdem, E., & Yoruk, R. (1991). Heavy metal concentrations in surface sediments from the two coastal inlets (Golden Horn Estuary and Izmit Bay) of the northeastern Sea of Marmara. Journal of Chemical Geology, 91, 269–285.
Evans, L. K., & Edwards, M. S. (2011). Bioaccumulation of copper and zinc by the giant kelp Macrocystis pyrifera. Algae, 26(3), 265–275.
Fityanos, K., Evgenidou, E., & Zachariadis, G. (1999). Use of macroalgae as biological indicators of heavy metal pollution in Thermaikos Gulf, Greece. Bulletin of Environmental Contamination and Toxicology, 62, 630–7.
Gunner, H. V.; Aysel, O.; Ozeisel, S. & Sukatar, A. (1987). Periodical variation of trace element accumulations in some algae found in the bay of Izmir. Review of International Oceanography of Mediterranean Tomes. LXXXV-LXXXVI: 52-55.
Güven, K.; Topcuoglu, S.; N. Balkis, N.; Ergul H. & Aksu, A. (2007). Heavy metals concentrations in marine algae from the Turkish coast of the Black Sea. Rapp. Comm. int. Mer Medit., 38, p.66.
Hamed, M. A. & El-Moselhy, Kh., M. (2000). Levels of some metals in the coastal water and sediments of the Red Sea, Egypt, A.M.S.E. vol.61 (1, 2):43-57.
Hardisson, A., Frı’as, I., Lozano, G., & Ba’ez, A. (1998). Mercury in algae of the Canary Islands littoral. Environment International, 24(8).
Haritonidis, S., & Malea, P. (1995). Seasonal and local variation of Cr, Ni, and Co concentrations in Ulva rigida C.Agardh and Enteromorpha linza (Linnaeus) from Thermaikos Gulf, Greece. Journal of Environmental Pollution, 89(1), 319–327.
Higgins, H. W., & Mackey, D. J. (1978). Role of Ecklonia radiate (C. Ag.) J. Agardh in determining trace metal availability in coastal waters. I. Total trace metals. Australian Journal of Marine and Fresh water Research, 38, 307–315.
Holan, Z. R., & Volesky, B. (1994). Biosorption of lead and nickel by biomass of marine algae. Biotechnology and Bioengineering, 43, 1001–1009.
Jothinayagi, N., & Anbazhagan, C. (2009). Heavy metal monitoring of Rameswaram Coast by some Sargassum species. American - European Journal of Scientific Research, 4(2), 73–80.
Khairy, H. M., & Omar, H. H. (2008). Effect of heavy metals on some metabolic activities of Jania rubens and Ulva lactuca from Eastern harbor and Abu-Qir bay of Alexandria Egypt. Egyptian Journal of Aquatic Research, 34(2), 114–129.
Khalil, M. K., & Rifaat, A. E. (2010). Enrichment of zinc, copper, lead and nickel in bottom sediments from three environmentally different regions off Alexandria. Egypt. Egyptian Journal of Aquatic Research, 36(3), 379–394.
Kimbrough, K. L.; Johnson, W. E.; Lauenstein, G. G.; Christensen, J. D. & Apeti, D. A. (2008). An assessment of two decades of contaminant monitoring in the Nation’s Coastal Zone. NOAA Technical Memorandum NOS NCCOS 74. National Oceanic and Atmospheric Centers for Coastal Ocean Science, Center for Coastal Monitoring and Assessment, Silver Spring, MD, 105 pp.
Kontas, A. (2008). Trace metals (Cu, Mn, Ni, Zn, Fe) contamination in marine sediment and zooplankton samples from Izmior bay. (Aegean Sea, Turkey). J. Water Air and Soil Pollution, 188, 323–333.
Laib, E. & Leghouchi, E. (2011). Cd, Cr, Cu, Pb, and Zn concentrations in Ulva lactuca, Codium fragile, Jania rubens, and Dictyota dichotoma from Rabta Bay, Jijel (Algeria) Environmental Monitoring and Assessment. DOI 10.1007/s10661-011-2072-2080.
Leal, M. C. F., Vasconcelos, M. T., Sousa-Pinto, I., & Cabral, J. P. S. (1997). Biomonitoring with benthic macroalgae and direct assay of heavy metals in seawater of the Oporto coast (Northwest Portugal). Marine Pollution Bulletin, 34(12), 1006–1015.
Long, E. R., MacDonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Journal of Environmental Management, 19, 81–97.
Mohamed, L. A., & Khaled, A. (2005). Comparative study of heavy metal distribution in some coastal seaweeds of Alexandria, Egypt. Chemistry and Ecology, 21(3), 181–189.
Moore, J. W. (1991). Inorganic contaminants of surface water: Research and monitoring priorities, Springer-Verlag New York Inc.
Munda, I. M. (1984). Salinity dependent accumulation of Zn, Co and Mn in Scytosiphon lomentaria (Lyngb.) Link and Enteromorpha intestinalis (L.) Link from the Adriatic Sea. Botanica Marina, 27, 371–376.
OECD, (2003). Environmental indicators, development, measurement and use, Reference Paper, OECD, Paris.
Pawlik-Skowrońsk, B., Pirsze, J., & Brown, M. T. (2007). Concentrations of phytochelatins and glutathione found in natural assemblage of seaweeds depend on species and metal concentrations of the habitat. Aquatic Toxicology, 83(3), 190–199.
Philips, D. J. H. (1976). The common mussel Mytilus edulis as an indicator of pollution by zinc, cadmium, lead and copper. I. Effects of environmental variables on uptake of metals. Marine Biology, 38, 59–69.
Phillips, D. J. H. (1990). Use of macroalgae and invertebrates as monitors of metal levels in estuaries and coastal waters. In R. W. Furness & P. S. Rainbow (Eds.), Heavy metals in the marine environment (pp. 81–99). Boca Raton: CRC Press.
Rainbow, P. S. (1995). Biomonitoring of heavy metal availability in the marine environment. Marine Pollution Bulletin, 31, 183–192.
Rajfur, M., Klos, A., & Waclawek, M. (2010). Sorption properties of algae Spirogyra sp. and their use for determination of heavy metal ions concentrations in surface water. Bioelectrochemistry, 80, 81–86.
Riedel, R. (1970). Fauna und Flora der Adria (p. 702). Hamburg und Berlin: Verlag Paul Parey.
Riley, J. P. & Skirrow, G. (1965). Chemical Oceanography, Academic press, London, vol. 1, II, 411 pp.
Romera, E., González, F., Ballester, A., Blázquez, M. L., & Munoz, J. A. (2007). Comparative study of biosorption of heavy metals using different types of algae. Bioresource Technology, 98, 3344–3353.
Santamaria-Fernandez, R., Cave, M. R., & Hill, S. J. (2005). Trace metal distribution in the Arosa estuary (N.W. Spain): the application of a recently developed sequential extraction procedure for metal partitioning. Analytical chemistry Acta, 557(1–2), 344–352.
Sari, E., & Catagay, M. N. (2001). Distribution of heavy metals in the surface sediments of the Gulf of Saros, NE Agean Sea. Environment International, 26, 169–173.
Say, P. J., Burrows, I. G., & Whitton, B. A. (1990). Enteromorpha as a monitor of heavy metals in estuaries. Hydrobiologia, 195, 119–126.
Schuhmacher, M., & Domingo, J. L. (1996). Concentrations of selected elements in oyster Crassostra angulata from the Spanish coast. Bulletin of Environmental Contamination and Toxicology, 56, 106–113.
Shakweer, L., Shiridah, M., Fahmi, M., & Fattah, L. A. (2006). Distribution and concentrations of trace elements along the Mediterranean coastal water of Egypt. Egyptian Journal of Aquatic Research, 32(2), 95–127.
Shams El-Din, N. G., & Dorgham, M. M. (2007). Phytoplankton community in Abu-Qir as a hot spot on the southeaster Mediterranean coast. Egyptian Journal of Aquatic Research, 33(1), 163–182.
Shobier, A. H., Abdel Ghani, S. A., & Shreadah, M. A. (2011). Distribution of total mercury in sediments of four semi-enclosed basins along the Mediterranean coast of Alexandria. Egyptian Journal of Aquatic Research, 37(1), 1–11.
Shriadah, M. A. & Emara, H. I. (1991). The distribution of chromium, copper, cadmium and lead in areas in multi-polluting factors of Alexandria. Proceeding of Symposium of Marine Chemistry in the Arab region, Suez, April, 39-50.
Steffens, J. C. (1990). The heavy metal-binding peptides of plants. Annual of Review Plant Physiology and Plant Molecular Biology, 41, 553–575.
Stenner, R. D., & Nickless, G. (1975). Heavy metal in organisms of the Atlantic coast of SW. Spain and Portugal. Marine Pollution Bulletin, 6, 89–92.
Svete, P., Milacic, R., & Pihlar, B. (2001). Partitioning of Zn, Pb, and Cd in river sediments from a lead and zinc mining area using the BCR three-step sequential extraction procedure. Journal of Environmental Monitoring, 3, 586–590.
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavy metal levels in estuaries and the formation of a pollution index. Helgol Meeresunters, 33, 566–575.
Topcuoǧlu, S., Güven, K. C., Balkıs, N., & Kirbașoǧlu, Ҫ. (2003). Heavy metal monitoring of marine algae from the Turkish Coast of the Black Sea, 1998–2000. Chemosphere, 52, 1683–1688.
Torres, M. A., Barros, M. P., Campos, S. C. G., Pinto, E., Rajamani, S., Sayre, R. T., & Colepicolo, P. (2008). Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicology and Environmental Safety, 71, 1–15.
Tropin, I. V. (1995). Distribution of metals in thalluses of red alga with special reference to their taxonomy and ecology. Oceanology, 35(1), 92–98.
Usero, J., Morillo, J., & Gracia, I. (2005). Heavy metal contamination in mollusks from the Atlantic coast of southern Spain. Chemosphere, 59, 1175–1181.
Vald’es, J., Vargas, G., Sifeddine, A., Ortlieb, L., & Guinez, M. (2005). Distribution and enrichment evaluation of heavy metals in Mejillones Bay (23-S), Northern Chile: geochemical and statistical approach. Marine Pollution Bulletin, 50, 1558–1568.
Volterra, L., & Conti, M. E. (2000). Algae as biomarkers, bioaccumulators and toxin producers. International Journal of Environment and Pollution, 13, 92–125.
Waldichut, M. (1974). Some biological concern in heavy metals pollution. In F. J. Vernberg & W. B. Vernerberg (Eds.), Pollution and Physiology of marine organisms (pp. 1–54). New York: Academic Press.
Warnau, M., & Bustamante, P. (2007). Editorial: Radiotracer techniques: a unique tool in marine ecotoxicological studies. Environmental Bioindicators, 2(4), 217–218.
WQC, (1972). A report of the committee on water quality criteria. NAS. Washington. DC. 593pp.
Zauke, G. P., Ritterhoff, J. & Rinderhagen, M. (1998). Concepts and applications in aquatic biomonitoring – internal review paper. Aquatic Ecology Group, ICBM, CvO Universitat, Oldenburg, Germany, pp. 38.
Żbikowski, R., Szefer, P., & Latała, A. (2007). Comparison of green algae Cladophora sp. and Enteromorpha sp. As potential biomonitors of chemical elements in the southern Baltic. Science of the Total Environment, 387, 320–332.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shams El-Din, N.G., Mohamedein, L.I. & El-Moselhy, K.M. Seaweeds as bioindicators of heavy metals off a hot spot area on the Egyptian Mediterranean Coast during 2008–2010. Environ Monit Assess 186, 5865–5881 (2014). https://doi.org/10.1007/s10661-014-3825-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3825-3