Abstract
Pig manure (PM) is widely used as an organic fertilizer to increase yields of crops. Excessive application of compost containing relatively great concentrations of copper (Cu) and zinc (Zn) can change soil quality. To clarify the effects of different rates of application and to determine the optimal rate of fertilization, PM containing 1,115 mg Cu kg−1, dry mass (dm) and 1,497 mg Zn kg−1, dm was applied to alkaline soil at rates of 0, 11, 22, 44, 88, and 222 g PM kg−1, dm. Phospholipid fatty acids (PLFAs) were used to assess soil microbial community composition. Application of PM resulted in greater concentrations of total nitrogen (TN), NH4 +-N, NO3 −-N, total carbon (TC), soil organic matter (SOM) but lesser pH values. Soils with application rates of 88–222 g PM kg−1, dm had concentrations of total and EDTA-extractable Cu and Zn significantly greater than those in soil without PM, and concentrations of T-Cu and T-Zn in these amended soils exceeded maximum limits set by standards in china. Except in the soil with a rate of 11 g PM kg−1, dm, total bacterial and fungal PLFAs were directly proportional to rate of application of PM. Biomasses of bacteria and fungi were significantly greater in soils with application rates of 44–222 g PM kg−1, dm than in the soil without PM. SOM, TC and EDTA-Zn had the most direct influence on soil microbial communities. To improve fertility of soils and maintain quality of soil, rate of application should be 22–44 g PM kg−1 dm, soil containing Cu and Zn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to recent rapid growth in intensive and large-scale farming in China, over 3 billion t of animal manures, among which pig manure (PM) accounts for a large proportion, are produced annually (Duan et al. 2012; Cao et al. 2013). PM applied to agricultural land, especially vegetable plots, is accepted as a good fertilizer for crops and land application of PM is a good disposal of organic waste (Shi et al. 2011; Xiong et al. 2010). Multiple studies have been conducted to evaluate effects of PM on soil properties. PM significantly increased nutrient content, pH, available water and decreased availability of heavy metals (Baker et al. 2011). Organic carbon, available phosphorus and available potassium of surface soil were significantly greater in the soils amended with medium (60 t ha−1, dm) or greater rates (120 t ha−1, dm) of PM (Lee et al. 2006). pH, electrical conductivity (EC), total carbon (TC) and total nitrogen (TN) were greater in soils to which PM had been applied over a long time at rates of 53.2 or 159.6 t fresh weight ha−1 year−1 than those amended with chemical fertilizers (Asada et al. 2012).
Although agricultural field application of PM is considered to be environment-friendly disposal of waste, accumulation of metals by excessive application of PM to soil is still an environmental problem (Duan et al. 2012). Furthermore, accumulated heavy metals in soil can migrate to surface and ground waters, and thus negatively affecting water quality and human health (Fan et al. 2013; Asada et al. 2012). Greater concentrations of copper (Cu) (from 699 to 988 mg kg−1) and zinc (Zn) (from 560 to 1514 mg kg−1) in PM are of particular concern because these metals are components of pig feed additives that are commonly or excessively used to stimulate growth and prevent disease of pigs (Jacela et al. 2012; Bhattacharyya et al. 2008; Meng et al. 2013; Shi et al. 2011). After application of the PM for 17 years, concentrations of these metals increased about 17.0- and 18.9-fold, respectively, compared with those of the control (Wu et al. 2012). Concentrations in soils were directly proportional to the rate of application of PM (Lee et al. 2006). These concentrations of metals (Cu >60 mg kg−1; Zn >90 mg kg−1) could adversely affect plants, humans and microbial ecosystems in soils (Baker et al. 2011; Lichtfouse 2009).
Due to widespread application of PM and the serious environmental pollution of Cu and Zn in agriculture soils, to improve fertilities of soils while minimizing adverse effects of metals, it was important to determine a safe rate of application of PM. Previous studies have focused on effects of applications of metal-contaminated animal manures on accumulation of metals in soils, plants and animals (Thu et al. 2013; Rees et al. 2013; Shi et al. 2011; Li et al. 2009), but few researches on their effects on microorganisms in soils has been conducted. Determining the potential effects of metals on the microbial community is important to maintain cycling of nutrients within soil ecosystems. Although effects of Cu and Zn on soil microorganisms has been reported by Azarbad et al. (2013) and application of PM significantly decreased availability of metals (Al Chami et al. 2013), little information was available on effects of different rates of application of PM containing Cu and Zn on structures of microbial communities in soils.
Microbes are sensitive to changes in the physical–chemical environment of soils, and the microbial community is considered as a useful indicator to monitor soil fertility and toxic effects of metals. Suitable concentrations of Cu and Zn can accelerate the growth of microorganisms in soils, but excessive concentrations of Cu and Zn can adversely affect microorganisms in soils (Mackie et al. 2013; Azarbad et al. 2013). Understanding stresses caused by application of PM containing Cu and Zn on microorganisms in soils can provide insight into nutrient availability and the combined effects of excessive Cu, Zn, organic matter and nutrients (Duarte et al. 2008). Thus, it was deemed important to use newly available non-culture-based techniques to study as great a proportion of the microbial community as possible. For this purpose, a community-level approach for characterizing (profiling) soil phospholipid fatty acids (PLFAs) of microorganisms, which has been found to be more discriminatory than other methods (Hinojosa et al. 2010), was developed and utilized. This approach enabled sensitive and culture-independent analyses of the structures of whole communities of microbes in soils, which provides a more effective assessment of the effects of both metals and PM on multiple aspects of communities of microbes in soils (Chodak et al. 2013; Hammesfahr et al. 2008; Azarbad et al. 2013).
In the current study, the profile of relative concentrations of PLFAs was applied to evaluate the effects of different rates of application of the PM on soil quality and microbial community structure. The aims were to (1) investigate effects of the application of PM containing high concentrations of Cu and Zn on soil properties; (2) determine whether different application of PM containing high concentrations of Cu and Zn might change the microbial community and identify their influencing factors; and (3) recommend the optimal application rate of PM. The results of this study can help us develop environmentally sound management strategies for the land application rate of PM containing Cu and Zn.
Materials and methods
PM was collected from an intensive pig farm in a suburb of Beijing. The studied soil was sampled from the topsoil (0–15 cm in depth) of clean forest near the farm. The PM and soil were air dried, sieved through a 1.0-mm mesh and thoroughly homogenized. Approximately 50 g dried PM and soils were set aside for the quantification of chemical parameters (pH, SOM, TN and TC). The soil was slightly alkaline (pH = 8.3), while the PM was almost neutral (pH = 6.7) (Table 1). The content of soil organic matter (SOM) of PM is greater (94 g kg−1) than that of the soil (16 g kg−1). The average concentration of TN and TC were 13- to 16-fold greater in PM than in the soil. The remaining PM was mixed thoroughly with the soil at the rate of 11, 22, 44, 88 or 222 g PM kg−1 soil, equivalent to 25, 50, 100, 200 or 500 t ha−1, dm, respectively. An 8-kg sample of each mixture was weighed and placed into a cylindrical plastic container 20 cm in diameter and 20 cm in height. A control treatment in which no PM was added to the soil in a container was also set up. Four replicates were performed for each mixture (treatment) and the control. The five treatments and the control were identified as P1, P2, P3, P4, P5 and CK. Ten seeds of mustard (Brassica sinensis L.) were planted in the soil of each container. A water content of approximately 60 % was maintained in all soils by adding water every week during the growth of B. sinensis L in a green house. After 60 days, soil outside the rhizosphere was sampled and B. sinensis L. was harvested. Each sampled soil was mixed gently and divided into two fractions, one of which was immediately sieved through a 2-mm mesh and stored without drying at 4 °C for microbial community characterization, while the other fraction was air dried and passed through a 2-mm sieve for chemical analyses.
The pH was determined with a Crison GLP 21 pH meter, using water ratios of 1:2.5 (w/v) for the soil and 1:5 (w/v) for the PM. Before sampling at the end of the pot experiment, the redox potential (Eh) in the soil was measured by inserting a platinum electrode (H800, USA) to a soil depth of 20 cm. TN was determined by the semi-micro Kjeldahl method. Ammonium nitrogen (NH4 +-N) and nitrate nitrogen (NO3 −-N) were measured by the colorimetric method (Bremner 1965). TC was measured by dry combustion with an elemental analyzer (Vario EL III, Germany). SOM was determined by the oil bath–K2Cr2O7 titration method. DOC was analyzed using a standard low-temperature Dohrmann DC-80 total organic C analyzer (Xertex/Dohrmann, Santa Clara, CA, USA) (Cook and Allan 1992). All soil and manure samples were digested by HNO3 and HF (USEPA method 3052) for the analysis of total Cu (T-Cu) and total Zn (T-Zn). Each soil sample was also extracted with a 0.05 mol l−1 EDTA aqueous solution at pH 7 at a ratio of 1:10 (w/v) for 1 h to determine the EDTA-extractable Cu (EDTA-Cu) and EDTA-extractable Zn (EDTA-Zn) levels (Wakelin et al. 2010). Concentrations of Cu and Zn in solution were quantified by inductively coupled plasma-mass spectrometry (ICP-MS) (Agilent 7500, USA). A reference soil (GSS-1) was used as a control for analysis quality. The Cu and Zn recovery rates were 97 % and 102 %, respectively.
Briefly, lipids were extracted from soil in chloroform/methanol/citrate buffer (1:2:0.8 v/v/v) and separated into neutral lipids, glycolipids and phospholipids using a pre-packed silica column. The phospholipids were subjected to a mild alkaline methanolysis, and the fatty acid methyl esters were identified by gas chromatography (flame ionization detector) based on the relative retention times of the fatty acids; methyl nonadecanoate (19:0) was used as an internal standard. The PLFAs were designated by their total number of carbon atoms, total number of double bonds and the positions of the double bonds. The prefixes ‘a’, ‘I’, ‘cy’ and ‘Me’ indicate anteiso, iso, cyclopropyl and methyl branching, respectively. Non-specific branching was designated by ‘br’, whereas cis and trans configurations were indicated by ‘c’ and ‘t’, respectively. The total microbial biomass was estimated as the sum of all extracted PLFAs. The sum of the PLFAs considered to be predominantly of bacterial origin (i15:0, a15:0, 15:0, i16:0, 16:1ω9; 16:1ω7t, i17:0, a17:0, 17:0, cy17:0, 18:1ω7 and cy19:0) was used as an index of the bacterial biomass, and the quantity of 18:2ω6,9 was used as an indicator of fungal biomass (Fernández-Calviño et al. 2010). The PLFAs i14:0, a15:0, i16:0 and 10Me18:0 are predominantly found in gram-positive (G+) bacteria, while the PLFAs cy17:0, cy19:0, 16:1ω7c and 18:1ω7 characterize gram-negative (G−) bacteria (Zhang et al. 2012).
Statistical analyses were conducted in Microsoft Excel and SPSS 16.0 (SPSS, Chicago, IL, USA). Multiple comparisons of the mean values of the soil properties, total PLFAs, fungal PLFAs and bacterial PLFAs were performed by one-way ANOVA with a specified probability of Type I error (α) of 0.05. Correlation analyses (Pearson's correlation) of the chemical and microbial properties of soils amended with different application rates of Cu- and Zn-rich PM were performed. Principal component analysis (PCA) was employed to compare the structures of the microbial communities based on profiles of PLFAs at different rates of application of PM.
Results and discussion
Changes in soil properties after the application of PM
Properties of the soil and PM before experiment are given in Table 1. Soil was slightly alkaline (pH = 8.3), while the PM was almost neutral (pH = 6.7). However, the PM had significantly greater SOM (94 g kg−1) than the soil (SOM = 16 g kg−1). After the experiment, the mean concentrations of pH, TN and TC in CK (Table 2) were similar to those at the beginning of the experiment (Table 1). Concentrations of TN (1.0–3.5 g kg−1), NH4 +-N (6.6–19 mg kg−1) and NO3-N (29–86 mg kg−1) in all PM amended soils, except for in P1,were significantly greater than those in (p < 0.05) CK, which exhibited the following concentrations: TN = 0.9 g kg−1; NH4 +-N = 5.5 mg kg−1; NO3 −-N = 25 mg kg−1. Concentrations of TN, NH4 +-N and NO3 −-N were 3- to 4-fold greater in P5 than in CK. SOM concentrations in P3, P4 and P5 were 20, 31 and 49 g kg−1, respectively, significantly greater than that in CK (12 g kg−1). The SOM concentration in P5 was 4-fold greater than that in CK. In comparison to CK, the concentration of TC in P1 was not significantly greater, but in P2–P5, the concentrations of TC ranged from 17 to 42 g kg−1 were significantly greater than that in CK (15 g kg−1). The concentration of TC was approximately 3-fold greater in P5 than in CK. The concentration of DOC in P5 (369 mg kg−1) was three times greater than that in CK (126 mg kg−1), but there were no significant differences in concentrations of DOC between CK and P1–P4 (158–168 mg kg−1). No significant difference in Eh was observed among treatments (Table 2). Addition of PM resulted in a decreasing trend of pH values in P3, P4 and P5 (8.1, 8.1 and 7.8, respectively). These values were significantly lesser than that in CK, which were 8.4. Results of previous studies have indicated lesser pH (Huang et al. 2007) and greater concentrations of TC, TN and SOM in soils amended with PM (Lee et al. 2006; Angers et al. 2010). Fertility of soil was significantly improved when amended with PM.
Changes in concentrations of Cu and Zn in the PM amended soils
To achieve maximum crop productivity, farmers have often applied excessive amounts of PM containing large amounts of metals to soils (Atafar et al. 2010). Consequently, accumulation of metals in soils is of concern (Duan et al. 2012). Concentrations of T-Cu and T-Zn were directly proportional to rates of application of PM to soils (Table 2). Concentrations of T-Cu ranged from 63 mg kg−1 dm, in CK to 177 mg kg−1 dm in P5, while those of T-Zn ranged from 78 mg kg−1 dm in CK to 426 mg kg−1 dm in P5. Concentrations of T-Cu and T-Zn in the treatments with greater rates of application of PM (P4 and P5) were not only significantly greater than those in CK, but also greater than those in treatments with lesser rates of application of PM (P1, P2 and P3) (p < 0.05) (Table 2). Concentrations of T-Cu in P4 and P5 were approximately 2- to 3-fold greater than that in CK, while those of T-Zn in P4 and P5 were 4- to 5-fold greater than that in CK. However, concentrations of both T-Cu and T-Zn were less than 1-fold greater with lesser rates of application of PM (P1, P2 and P3). Since only some forms of metals in the amended soils are bioavailable (Krishnamurti et al. 2013), effects could be less than predicted. In the current study, EDTA was used to identify the portion of metals in soil that were most readily bioavailable. Greater concentrations of EDTA-Cu and EDTA-Zn were consistent with those of T-Cu and T-Zn (Table 2). Concentrations of both EDTA-Cu and EDTA-Zn in P3–P5 were significantly greater than those in CK. Concentrations of EDTA-Cu and EDTA-Zn in the treatments with greater rates of application of PM (P4 and P5) were not only approximately 4- to 16-fold greater than those in CK, but also significantly greater than those in treatments with lesser rates of application of PM (P1, P2 and P3). This result indicated that the greater rates of application of PM (P4 and P5) resulted in greater amounts of mobile Cu and Zn, especially mobile Zn in the amended soils, and would cause great leaching of Cu and Zn, which might have significantly adverse effects on environments and ecosystems (Park et al. 2010). These results are consistent with those of a previous study in which addition of organic fertilizer was found to modify bioavailability of metals in soils (Al Chami et al. 2013). Application of 120 t ha−1, dm (between P2 and P3 in this study) containing 96 mg Cu kg−1 dm and 133 mg Zn kg−1 dm would provide moderate amounts of bio-available Cu and Zn.
Effects of PM on soil microbial communities
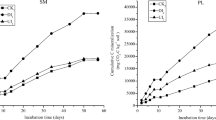
Application of PM can improve quality of soil for growing crops by increasing availability of nutrients, such as N, P and K, and increasing the content of organic matter and by changing the composition of the microbial community (Baker et al. 2011). Since total PLFAs represent an estimate of the microbial biomass in soils, great application rates of PM containing high concentrations of Cu and Zn in P4 and P5 significantly increased the total microbial biomass in the amended soils (Fig. 1). The greatest total PLFAs for bacteria and fungi were observed in P5. The combined effects of organic matter and heavy metal to microbial growth were shown in P1. Although application of PM in P1 can provide some soil nutrients, due to toxicities of Cu and Zn in the manure the net effect was lesser growth of the microbial community. Amounts of both bacterial and fungal PLFAs were significantly greater in P3, P4 and P5 than in CK (p < 0.05) and total PLFAs in P5 was approximately 2-fold greater than that in CK. The ratio of fungal to bacterial PLFAs in P5 was significantly greater than those in CK and P1–P3, but not significantly different from that in P4 (Fig. 1). Soil ecosystems with greater Fungi/Bacteria ratios indicate stimulation of fertilization to microbe, and a soil system that is more sustainable and stable (de Vries et al. 2006). Mineralization of residues from plants or animals by fungi is far outweighing bacteria, which can provide more nutrients to soil. Mineralization of residues from plants or animals by fungi is far outweighing bacteria, which can support more nutrition to soil.
The first and second principal component axes (PC1 and PC2) explained 39.6 % and 14.7 % of the total variation in the structure of the microbial communities in the soils, respectively (Fig. 2a). Soils amended with greater amounts of PM (P4 and P5) were separated from those that contained less PM (P1, P2 and P3) along axis 1, while P1 was separated from the other treatments along axis 2. This result indicated that the structures of the microbial communities in soils with great application rates of PM such as P4 and P5 were different from those to which low rates of PM such as P1, P2 and P3 were added. Fatty acids of 18:2ω6,9 and 10Me18:0 were used as indicators of fungi and actinomycetes, respectively. Meanwhile, a15:0, i16:0, 16:0, 15:0, 17:0, 16:1ω7t, 18:0, 18:1ω8 and cy19:0 represented the bacterial-derived lipids (Frostegård and Bååth. 1996; Zelles. 1999). The plot of correlation coefficients for individual fatty acids sampled on day 60 (Fig. 2b) indicated that four PLFAs — 18:1ω8, cy19:0, 18:2ω6,9 and 18:0 — were the predominant PLFAs ordinated along axis 1, while seven PLFAs — 10Me18:0, a15:0, i16:0, 16:0, 15:0, 17:0 and 16:1ω7t — were the predominant PLFAs of axis 2. Addition of PM containing Cu and Zn to soils promoted bacteria and fungi to be the main microbial communities in the amended soils. Results of PCA suggested that several necessary communities of bacteria and fungi were sensitive to organic matter or metals after application of PM, and therefore, those PLFAs can be used as one indicator for evaluation of the soil fertilization.
Principal component analyses (PCAs) and correlation coefficients for individual fatty acids. The scores for each fatty acid used for PCA represented the amount of that fatty acid present in a sample expressed as a percentage of the total fatty acids in the sample. a PCA of PLFA profiles of soils sampled at day 60. PC1 accounted for 39.60 % of the variance in the data, and PC2 accounted for 14.68 % of the variance in the data. b Plot of correlation coefficients for individual fatty acids at day 60
Factors influencing soil microbial communities
Total PLFAs and bacterial PLFAs were significantly and positively correlated (Pearson's correlation) with soil properties such as T-Zn, TN, TC, SOM, NH4 +-N, EDTA-Cu, EDTA-Zn (Table 3). However, the correlation coefficients between fungal PLFAs and the soil properties were lower than those for total PLFAs or bacterial PLFAs. Of these soil properties, SOM and EDTA-Zn had the most direct influences on microbial communities because SOM, TC, EDTA-Zn were strongly associated with great total PLFAs, especially bacterial PLFAs, seen from their greater correlation coefficients (r ≥ 0.88). The greater amounts of SOM, TC and EDTA-Zn improved soil quality by increasing CEC and Zn availability, thus provided nutrients to stimulate growth of bacteria and fungi in soils and reduced toxicity of metals (Park et al. 2010). Results of a previous study demonstrated that although application of PM to soils improved growth of soil microbes, greater concentrations of Cu and Zn in soil had little additional effects on soil microbial communities (Anderson et al. 2008; Lee et al. 2006). Alternatively, it has been reported that potential accumulation of Cu and Zn in soils and subsequent toxicity to microbial communities might adversely affect fertilities of soils and long-term sustainability of yields of crops (Baker et al. 2011). Therefore, some studies are still needed to understand the mechanisms of the eco-toxicology of Cu and Zn and their long-term effects on communities of microbes in soils.
Recommendations for application of manure
Although in China it is commonly recognized that increasing rates of application of PM can be economically beneficial by increasing yields of crops, optimal rates of application of PM that allows for benefits while minimizing long-term toxic effects of metals was still unknown. Based on the results of the study reported upon here, amounts of total, bacterial and fungal PLFAs in soils were proportional to the amounts of PM added and significantly greater rates of application of PM in P4 and P5 provided nutrients and promoted growth of microorganisms in soils. Alternatively, although lesser rates of application of PM in P1 can provide some soil nutrients, the amount of nutrients were insufficient to stimulate growth of the microbial communities in soils, as indicated by the low amounts of total, bacterial and fungal PLFAs. The rates of application in P2–P5 were ideal for agricultural production and maintaining the microbial communities of the amended soils. PM significantly increased nutrient content and available water to stimulate plant growth (Baker et al. 2011). However, uptake of nutrient by crops ranges from only 6 % to 59 % of the applied N. Surplus nutrient moves beyond agricultural fields and pollutes water environments, leading to non-point source pollution (Huang et al. 2014).
It is of importance to adopt appropriate rates of application of PM to agricultural soils to provide nutrients and organic matter that increase yields of crops while minimizing risks posed by metals on long-term productivities of soils. In order to ensure agricultural production, the Chinese Soil Environmental Quality Standard-Class II (GB 15618-1995), indicating the maximum concentrations of various substances in soil in order to meet requirements for health of humans, suggests that the maximum allowable concentrations of Cu and Zn are 100 and 300 mg kg−1, respectively. Background concentrations of Cu and Zn in local soils are 18.7 and 57.5 mg/kg, respectively (Chen et al. 2004), while Chinese national soil background concentrations are 22.6 and 74.2 mg/kg, respectively (Wei et al. 1991). Before the application of PM to agricultural soils, the concentration of Cu (56 mg/kg) exceeded both local and national soil background concentrations, while the concentration of Zn (74 mg/kg) exceeded local background concentrations but was equal to the Chinese national soil background concentration. After the experiment, concentrations of Cu and Zn were greater than both local and national soil background concentrations. In the current study, concentrations of T-Cu (56 mg kg−1) and T-Zn (74 mg kg−1) in CK were within these limits. Thus, concentrations of both T-Cu and T-Zn in CK were considered safe for cultivation of plants. Concentrations of T-Cu and T-Zn in soils amended with the greatest rates of PM in P4 and P5 exceeded the maximum limits for soils in China Soil Environmental Quality Standard-Class II, and even those in P5 were approximately 2- to 1-fold greater than the standard. Bioavailability and mobility of Cu and Zn in the amended soils in P4 and P5 might have significantly adverse effects on microorganisms in soils (Park et al. 2010). Thus, greater rates of application of PM, such as P4 and P5, were not environmentally sustainable practice. Results of a previous study confirmed that concentrations of mobile Cu and Zn greater than 25 mg kg−1 (such as P3, P4 and P5 in the current study) will result in lesser yields of crops (Li et al. 1987). Consequently, potential for decreases in yields of crops should be given special attention, especially in China while a large population and minimal amounts of arable land, where PM is often applied to agricultural soils.
Combined with increasing activities of microbes in soils and soil quality as well as ecological risks of metals in soils, application of PM at rates of 50–100 t ha−1, dm) would be a good management practice to provide maximum long-term fertility of soils and yields of crops. This application rate is in agreement with the long-term land application rate of 40-60 t ha−1, dm of PM suggested by Lee et al. (2006), in terms of improvement of the soil physico-chemical and biological properties and crop yield. Since Cu and Zn are non-biodegradable and cumulative, necessary remediation is still needed for soils with long-term land applications of PM containing high Cu and Zn.
Conclusions
When added to soils, the concentrations of TN, NH4 +-N, NO3-N, SOM and TC in all PM amended soils increased compared with CK. Concentrations of T-Cu and T-Zn were directly proportional to rates of application of PM. Concentrations of T-Cu ranged from 63 in CK to 177 mg kg−1, dm in P5, while those of T-Zn ranged from 78 in CK to 426 mg kg−1, dm in P5. Concentrations of T-Cu and T-Zn in treatments with greater rates of application of PM (P4 and P5) exceeded maximum limits of Chinese Soil Environmental Quality Standard-Class II. In comparison to CK, total PLFAs for bacteria and fungi was greater in P2–P5, but less in P1, relative to that of control soils. The predominant PLFAs were 8:1ω8, cy19:0, 18:2ω6,9 and 18:0. SOM, TC and EDTA-Zn were key factors associated with great microbial biomass. Although high application rates of PM can improve soil fertility and microbial community, the great accumulation of Cu and Zn in soils can reduce the environmental safety. Therefore, application of PM at a rate of 22–44 g kg−1 (50–100 t ha−1) is suggested as environmentally sound rate of application of PM to agricultural land.
References
Al Chami, Z., Cavoski, I., Mondelli, D., & Miano, T. (2013). Effect of compost and manure amendments on zinc soil speciation, plant content, and translocation in an artificially contaminated soil. Environmental Science and Pollution Research, 20, 4766–4776.
Anderson, I. C., Parkin, P. I., & Campbell, C. D. (2008). DNA- and RNA-derived assessments of fungal community composition in soil amended with sewage sludge rich in cadmium, copper and zinc. Soil Biology and Biochemistry, 40, 2358–2365.
Angers, D. A., Chantigny, M. H., MacDonald, J. D., Rochette, P., & Cǒté, D. (2010). Differential retention of carbon, nitrogen and phosphorus in grassland soil profiles with long-term manure application. Nutrient Cycling in Agroecosystems, 86, 225–229.
Atafar, Z., Mesdaghinia, A., Nouri, J., Homaee, M., Yunesian, M., Ahmadimoghaddam, M., & Mahvi, A. H. (2010). Effect of fertilizer application on soil heavy metal concentration. Environmental Monitoring and Assessment, 160, 83–89.
Azarbad, H., Niklińska, M., van Gestel, C. A., van Straalen, N. M., Röling, W. F., & Laskowski, R. (2013). Microbial community structure and functioning along metal pollution gradients. Environmental Toxicology and Chemistry, 32, 1992–2002.
Baker, L. R., White, P. M., & Pierzynski, G. M. (2011). Changes in microbial properties after manure, lime, and bentonite application to a heavy metal-contaminated mine waste. Applied Soil Ecology, 48, 1–10.
Bhattacharyya, P., Tripathy, S., Chakrabarti, K., Chakraborty, A., & Banik, P. (2008). Fractionation and bioavailability of metals and their impacts on microbial properties in sewage irrigated soil. Chemosphere, 72, 543–550.
Bremner, J. M. (1965). Methods of soil analysis. Madison, WI: American Society of Agronomy.
Cao, Y., Chang, Z., Wang, J., Ma, Y., & Fu, G. (2013). The fate of antagonistic microorganisms and antimicrobial substances during anaerobic digestion of pig and dairy manure. Bioresource Technology, 136, 664–671.
Chen, T. B., Zheng, Y. M., Chen, H., & Zheng, G. D. (2004). Background concentrations of soil heavy metals in Beijing. Environmental Sciences, 25, 117–112.
Chodak, M., Gołębiewski, M., Morawska-Płoskonka, J., Kuduk, K., & Niklińska, M. (2013). Diversity of microorganisms from forest soils differently polluted with heavy metals. Applied Soil Ecology, 64, 7–14.
Cook, B. D., & Allan, D. L. (1992). Dissolved organic carbon in old field soils: total amounts as a measure of available resources for soil mineralization. Soil Biology & Biochemistry, 24, 585–594.
de Vries, F. T., Hoffland, E., van Eekeren, N., Brussaard, L., & Bloem, J. (2006). Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biology and Biochemistry, 38, 2092–2103.
Duan, G., Zhang, H., Liu, Y., Jia, Y., Hu, Y., & Cheng, W. (2012). Long-term fertilization with pig-biogas residues results in heavy metal accumulation in paddy field and rice grains in Jiaxing of China. Soil Science and Plant Nutrition, 58, 637–646.
Duarte, S., Pascoal, C., Alves, A., Correia, A., & Cassio, F. (2008). Copper and zinc mixtures induce shifts in microbial communities and reduce leaf litter decomposition in streams. Freshwater Biology, 53, 91–101.
Fan, J., Ding, W., & Ziadi, N. (2013). Thirty-year manuring and fertilization effects on heavy metals in black soil and soil aggregates in northeastern China. Communications in Soil Science and Plant Analysis, 44, 1224–1241.
Fernández-Calviño, D., Martín, A., Arias-Estévez, M., Bååth, E., & Díaz-Raviña, M. (2010). Microbial community structure of vineyard soils with different pH and copper content. Applied Soil Ecology, 46, 276–282.
Frostegård, A., & Bååth, E. (1996). The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biology and Fertility of Soils, 22, 59–65.
Hammesfahr, U., Heuer, H., Manzke, B., Smalla, K., & Thiele-Bruhn, S. (2008). Impact of the antibiotic sulfadiazine and pig manure on the microbial community structure in agricultural soils. Soil Biology and Biochemistry, 40, 1583–1591.
Hinojosa, M. B., Garcia-Ruiz, R., & Carreira, J. A. (2010). Utilizing microbial community structure and function to evaluate the health of heavy metal polluted soils. Soil Heavy Metals, 19, 185–224.
Huang, Z. P., Xu, B., & Tu, D. Y. (2007). Organic substrate change in the swine manure-applied vegetable soils. Journal of Anhui Agricultural University, 34, 262–264.
Huang, D. F., Li, W. H., & Wang, L. M. (2014). Study on water and fertilizer managements and evaluation of nitrogen and phosphorus non-point source pollution from paddy field. Image Processing for Cinema, 4, 335.
Jacela, J. Y., DeRouchey, J. M., Tokach, M. D., Goodband, R. D., Nelssen, J. L., & Renter, D. G. (2012). Feed additives for swine: fact sheets—high dietary levels of copper and zinc for young pigs, and phytase. Journal of Swine Health and Production, 18, 87–91.
Krishnamurti, G. S., Subashchandrabose, S. R., Megharaj, M., & Naidu, R. (2013). Assessment of bioavailability of heavy metal pollutants using soil isolates of Chlorella sp. Environmental Science and Pollution Research, 1–7.
Lee, C. H., Wu, M. Y., Asio, V. B., & Chen, Z. S. (2006). Using a soil quality index to assess the effects of applying swine manure compost on soil quality under a crop rotation system in Taiwan. Soil Science, 171, 210–222.
Li, G. C., Wang, Y. P., & Chang, J. M. (1987). Publication by the Environmental Protection Administration (EPA) of Taiwan. Taipei: Taiwan. Heavy metal concentrations in soils of Taiwan.
Lichtfouse, E. (2009). Organic farming, pest control and remediation of soil pollutant. Dijon, France: Springer.
Mackie, K., Müller, T., Zikeli, S., & Kandeler, E. (2013). Long-term copper application in an organic vineyard modifies spatial distribution of soil micro-organisms. Soil Biology and Biochemistry, 65, 245–253.
Meng, J., Wang, L., Liu, X., Wu, J., Brookes, P. C., & Xu, J. (2013). Physicochemical properties of biochar produced from aerobically composted swine manure and its potential use as an environmental amendment. Bioresource Technology, 142, 641–646.
Park, J. H., Lamb, D., Paneerselvam, P., Choppala, G., Bolan, N., & Chungd, J. W. (2010). Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. Journal of Hazardous Materials, 185, 549–574.
Rees, H., Chow, L., Zebarth, B., Xing, Z., Toner, P., & Lavoie, J. (2013). Impact of supplemental poultry manure application on potato yield and soil properties on a loam soil in north western New Brunswick. Canadian Journal of Soil Science, 94, 1–17.
Shi, J. C., Yu, X. L., Zhang, M. K., Lu, S. G., Wu, W. H., Wu, J. J., & Xu, J. M. (2011). Potential risks of copper, zinc, and cadmium pollution due to pig manure application in a soil–rice system under intensive farming: a case study of Nanhu, China. Journal of Environmental Quality, 40, 1695–1704.
Thu, T. D., Jusselme, D. M., Lata, J. C., Van Nguyen, B., & Jouquet, P. (2013). The earthworm species Metaphire posthuma modulates the effect of organic amendments (compost vs. vermicompost from buffalo manure) on soil microbial properties. A laboratory experiment. European Journal of Soil Biology, 59, 15–21.
Wakelin, S. A., Chu, G. X., Broos, K., Clarke, K. R., Liang, Y. C., & McLaughlin, M. J. (2010). Structural and functional response of soil microbiota to addition of plant substrate are moderated by soil Cu levels. Biology and Fertility of Soils, 46, 333–342.
Wei, F. S., Chen, J. S., Wu, Y. Y., & Zheng, C. J. (1991). Study on the background contents on 61 elements of soil in China. Environmental Sciences, 12, 12–20.
Wu, L. H., Tan, C. Y., Liu, L., Zhu, P., Peng, C., Luo, Y. M., & Christie, P. (2012). Cadmium bioavailability in surface soils receiving long-term applications of inorganic fertilizers and pig manure. Geoderma, 173, 224–230.
Xiong, X., Li, Y., Li, W., Lin, C., Han, W., & Yang, M. (2010). Copper content in animal manures and potential risk of soil copper pollution with animal manure use in agriculture. Resources, Conservation and Recycling, 54, 985–990.
Zelles, L. (1999). Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biology and Fertility of Soils, 29, 111–129.
Zhang, Q. C., Shamsi, I. H., Xu, D. T., Wang, G. H., Lin, X. Y., Jilani, G., Hussainc, N., & Chaudhry, A. N. (2012). Chemical fertilizer and organic manure inputs in soil exhibit a vice versa pattern of microbial community structure. Applied Soil Ecology, 57, 1–8.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (Nos. 41271502 and C031001), Strategic Priority Research Program of the Chinese Academy of Sciences, Grant No. XDB03030504, the Special Program for Basic Science and Technology under Grant No. 2013FY111100. Portions of the research were supported by a Discovery Grant from the National Science and Engineering Research Council of Canada (Project No. 6807. Prof. Giesy’s participation in the project was supported as an at-large Chair Professorship from the City University of Hong Kong and by an "Area of Excellence" Grant (AoE P-04/04) from the Hong Kong Grants Council, the Einstein Professor Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Luo, W., Jia, J. et al. Effects of pig manure containing copper and zinc on microbial community assessed via phospholipids in soils. Environ Monit Assess 186, 5297–5306 (2014). https://doi.org/10.1007/s10661-014-3778-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3778-6