Abstract
For 2 years, a baseline investigation was carried out to collect reference information of the present environmental status in the Fehmarn Belt and adjacent area. The temporal and spatial variability of phytoplankton was monitored by a combination of monitoring buoys, pigment analysis and fast screening microscopy. The overall phytoplankton succession in the Fehmarn Belt area was found to be influenced primarily by the seasonal changes, where various diatoms dominated the spring and autumn blooms and flagellates like Chrysochromulina sp., Dictyocha speculum and various dinoflagellates were occasionally abundant in late spring and summer. The phytoplankton groups were remarkably uniform horizontally in the investigation area while large differences in both biomasses and composition of individual phytoplankton groups were seen vertically in the water column, especially in the summer periods, in which the two-layer exchange flow between the North Sea and the Baltic Sea is showing a particularly strong stratification in the Fehmarn Belt. The chlorophyll a concentrations ranged continuously from 1 to 3 μg/L at the three permanent buoy stations during the 2 years of monitoring, except for the spring and autumn blooms where chlorophyll a increased up to 18 μg/L in the spring of 2010 and up to 8 μg/L in the autumn of 2009. Recurrent blooms of filamentous cyanobacteria are common during the summer period in the Baltic Sea and adjacent areas, but excessive blooms of cyanobacteria did not occur in 2009 and 2010 in the Fehmarn Belt area. The combination of the HPLC pigment analysis method and monitoring buoys continuously measuring fluorescence at selected stations with fast screening of samples in the microscope proved advantageous for obtaining information on both the phytoplankton succession and dynamic and, at the same time, getting information on duration and intensity of the blooms as well as specific information on the dominant species present both temporally and spatially in the large Fehmarn Belt area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuarine and coastal areas are very dynamic and challenging when it comes to determining status and trends in water quality and ecological conditions. Freshwater runoff interacts with the exchange of marine water, winds and currents, leading to complex circulation and mixing patterns. Furthermore, the chemical and biological characteristics and responses of such ecosystems to environmental changes and perturbations are strongly influenced by seasonal forcing.
The Fehmarn Belt, in the south-eastern Baltic Sea between Denmark and Germany, is an example of such an ecosystem located in a transitional area between the Baltic Sea and the North Sea, and the estuary is characterized by horizontal variations and vertical gradients in salinity-driven water level differences (Jakobsen et al. 2010). In the upper layer, water from the Baltic rivers flows into the area towards the North Sea while high saline water from the North Sea flows through the Fehmarn Belt along the sea bed into the Baltic Sea.
In 2008, it was decided to construct a fixed link between Denmark and Germany in the Fehmarn Belt, and the process of identifying, describing and assessing the environmental impacts of the construction project was commenced. For this purpose, it was necessary to carry out a 2-year baseline investigation to collect reference information of the present status and trends of the environmental status in the area. The baseline investigation was needed to be able to predict the environmental impact of the construction work, sediment spill, introduction of new surfaces such as bridge pillars and alterations of hydrodynamic conditions. For this purpose, the existing large-scale environmental condition had to be investigated by a comprehensive monitoring programme, the baseline investigation, of the major marine flora and fauna components. The coastal areas of the Fehmarn Belt and the adjacent sea and lagoons are important breeding and feeding grounds for fish populations and a large numbers of migrating birds. Fish and benthic invertebrates depend on plankton for food during their larval phases, and some species, e.g. mussels, continue to consume plankton in their entire lives. Phytoplankton supports the food webs and plays a central role in the carbon, nutrient and oxygen cycling in estuaries and is an important ecological indicator. This paper presents the baseline investigation of the phytoplankton communities in the Fehmarn Belt area. Given the dynamic nature of estuarine ecosystems, it was necessary to use efficient and cost-effective means for detecting the natural fluctuations and blooms of phytoplankton with sensitive and high temporal and spatial resolution methods. The microscopy method traditionally used for identifying phytoplankton species and estimating biomasses is a time-consuming and generally imprecise method (Schlüter et al. 2000; Higgins et al. 2011) and does not allow for high-frequency data acquisition. Furthermore, the quantitative results obtained by microscopy, where only a limited number of cells are counted and size-determined in a small subsample, can provide results that are severely flawed (Schlüter et al. 2000). Coefficients of variation on the volumes of the individual algal groups of up to 50 % have been demonstrated (Wilhelm et al. 1991).
In recent years, pigment analysis by high-performance liquid chromatography (HPLC) has proven suitable for determining large-scale phytoplankton dynamics (e.g. Barlow et al. 2007; Wright et al. 2010; Schlüter et al. 2011). Pigment analysis is a fast, very sensitive and objective method for determining the phytoplankton composition and estimating the biomass of the different algal groups. By applying the CHEMTAX program, the biomass of the individual phytoplankton groups can be calculated as chlorophyll a (Chl a) units (Mackey et al. 1996; Schlüter et al. 2000; Higgins et al. 2011), and Chl a has been found to be a more precise and dynamic biomass parameter than the carbon biomass derived from microscopic countings (Schlüter and Møhlenberg 2003). However, the pigment method only provides results on the overall class level. In the baseline investigation, it was desirable to obtain specific information on the phytoplankton species present, i.e. determining the species constituting the spring bloom, the potential toxic species of a late summer bloom and also to obtain knowledge on the overall dominating species present throughout the year as basic food for the higher trophic levels in the Fehmarn Belt estuary. In order to obtain this information, the traditional microscopy method was used as well but only for identifying the most important species present throughout the year by fast screening of the samples in the microscope. During the 2 years of baseline investigation, samples for HPLC analyses and microscopy screening were taken on monthly monitoring cruises. Furthermore, we combined these methods with continuous in vivo fluorescence measurements obtained by automated measuring buoys moored in the baseline area. The in vivo fluorescence measurements were included to obtain information on the development of the phytoplankton populations in between the in situ samplings, thus achieving the needed information on the temporal and spatial variability of phytoplankton populations at species level in the large investigation area of at Fehmarn Belt.
Materials and methods
Sampling in the investigation area
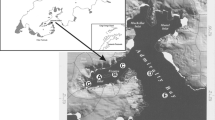
In order to obtain detailed information on the phytoplankton communities for the baseline investigation in the Fehmarn Belt and surrounding areas assumed to be affected by the building work of the fixed link, the investigation area was composed of 12 phytoplankton sampling stations (Fig. 1). Moored buoys were positioned at several stations (Fig. 1), where MS01, MS02 and MS03 were monitoring buoys equipped with automated water quality monitors (Aquaguard, recording time series of different parameters, such as salinity, temperature, turbidity, fluorescence of Chl a and oxygen). Chl a fluorescence was recorded at three depths, surface, 15 m and bottom. MS01 and MS02 were located in the alignment area close to the plankton sampling stations H033 and H037, respectively, and station MS03 was located towards the Baltic Sea (Fig. 1). Chl a fluorescence data collection started in March 2009.
For 22 months starting in February 2009, water samples were collected monthly at the 12 plankton sampling stations (Fig. 1) by the vessel M/S JHC Miljø equipped with a Seabird CTD (dual SBE911) collecting samples at the surface and at 15 m depth. Within 20 min after sampling, samples were filtered onto 25-mm Whatman GF/F filters at a vacuum of approximately 25 kPa and immediately frozen in liquid nitrogen and stored until analysis. Another set of subsamples was fixed in Lugol’s iodine solution and stored in a dark and cool place. All samples were brought to the laboratory for analysis.
Pigment analyses
Filters for pigment analysis by HPLC were extracted with 95 % acetone containing vitamin E as internal standard, sonicated on ice and extracted at 4 °C for 20 h. The filters and cell debris were filtered from the extracts using disposable syringes and 0.2-μm Teflon syringe filters directly into HPLC vials, and the vials were placed in the cooling rack of the HPLC together with a parallel set of vials with the injection buffer (90:10, 28 mM aqueous Tetrabutyl ammonium acetate (TbAA), pH 6.5:methanol). The samples were analyzed by a Shimadzu LC-10ADVP (HPLC) composed of a pump (LC-10ADVP), a photodiode array detector (SPD-M10A VP), a SCL-10ADVP System controller with Lab Solution software, a temperature-controlled auto sampler (set at 4 °C), a column oven (CTO-10ASVP) and a degasser. The samples and buffer were injected using the auto-injector by programming it to make a ‘sandwich’ injection of buffer and sample in the ratio 5:2 and analyzing according to the van Heukelem and Thomas (2001) method slightly modified to local conditions. The column was an Eclipse XDB C8, 4.6 mm × 150 mm (Agilent Technologies). Solvent A: (70:30) methanol: 28 mM aqueous TbAA, pH 6.4; solvent B: 100 % methanol. The time programme was 0 min: 95 % A, 5 % B; 2 min: 95 % A, 5 % B; 27 min: 5 % A, 95 % B; 30 min: 95 % A, 5 % B; 31 min: 100 % A, 0 % B; 34 min: 100 % A, 0 % B; 35 min: 5 % A, 95 % B; 41 min: stop. The flow rate was 1.1 mL min−1, and the temperature of the column oven was set at 60 °C. The HPLC was calibrated with pigment standards from DHI, Lab Products, Denmark. Peak identities were routinely confirmed by on-line photodiode array. A QA threshold procedure, application of limit of quantitation (LOQ) and limit of detection (LOD), was applied to the pigment data as described by Hooker et al. (2005) to reduce the uncertainty of pigments found either in low concentrations or not detected at all, causing false positives or false negatives, which frequently occurs when pigments are quantified closed to the detection limit (Schlüter et al. 2011).
Microscopic screening of samples
The Lugol-fixed samples were concentrated by settling 50 mL in sedimentation chambers for 24 h, after which, the samples were screened rapidly in an inverted microscope for determining the most abundant algal species present. Using this microscopy method, some of the smallest algal cells could, however, not be identified to species level and were grouped according to class or genus.
CHEMTAX calculations
The biomass in units of Chl a of the individual phytoplankton groups detected by the pigments was calculated by CHEMTAX (Mackey et al. 1996). Information on the dominant algal groups present determined by microscopy and the diagnostic pigments determined by HPLC pigment analysis was used to determine which phytoplankton groups to load into CHEMTAX using the relevant pigment ratios from Schlüter et al. (2000, 2004). Calculation of the pigment ratios: prasinoxanthin/Chl b and lutein/Chl b (Schlüter and Møhlenberg 2003) helped in determining whether prasinophytes without prasinoxanthin and chlorophytes were present in the samples as these phytoplankton species are often too small to be properly identified by the inverted microscopy method. The data set was separated into two depths and four seasons during each of the 2 years of sampling prior to the CHEMTAX calculations. The CHEMTAX program version 1.95 was used to construct 60 ratio matrices from the initial ratios for each of the four data sets, and 10 % (n = 6) of the ratios creating the lowest residual root mean square was averaged and run repeatedly until the ratios became stable as described in Higgins et al. (2011).
Results
The mean surface salinity in the Fehmarn Belt area varied between 8 and 20 PSU in the spring and the autumn and between 8 and 17 PSU during the summer. From spring to early autumn, a pycnocline was located at 13–15 m, separating surface water from the more saline bottom water. In the late autumn and in the winter, most often, the water column was completely mixed.
Fluorescence measurements
The water quality monitors recorded fluorescence quenching (i.e. the reduction in the fluorescence quantum yield often observed during daylight hours (Sackmann et al. 2008; Kinkade et al. 1999)), which caused a daily systematic oscillation in the in situ Chl a concentrations. Neglecting the quenched fluorescence data, the monitoring buoys showed that, in between blooms in 2009, the overall Chl a concentrations in the surface waters were between 1 to 3 μg Chl a/L (Fig. 2, station MS01, surface) with some minor peak values of shorter duration, indicating drifting macro-algae or minor pulses of algae flushed into the area (e.g. March 2009 in Fig. 2). In the autumn of 2009, only a minor increase in Chl a can be discerned in October at station MS01 (Fig. 2) whereas a major increase in Chl a concentrations was detected in November and December at station MS02 and particularly at station MS03 where a bloom was formed, causing an increase in the Chl a concentration to almost 8 μg Chl a/L for a period of 2 weeks (Fig. 3). An intrusion of less saline water from the Baltic Sea caused the sudden decline in Chl a values on 8 December 2009 in Fig. 3 and terminated the bloom. During the winter of 2009/2010, the fluorescence measurements at the measuring buoys were around 1–2 μg Chl a/L (results not shown). In the beginning of March 2010, the phytoplankton spring bloom developed and reached almost 18 μg Chl a/L in mid-March at both 1 and 15 m depth (station MS03, Fig. 4). In between blooms, the Chl a concentrations were generally in the same range as in 2009, though the summer 2010 values at station MS01 were slightly lower (stations MS01 and MS02, 2010, in Fig. 5 and station MS01, 2009, in Fig. 2). In the autumn of 2010, the Chl a concentrations increased to approximately 4–5 μg Chl a/L in October and November (results not shown). Occasionally, the water quality monitors recorded a subsurface bloom, e.g. in July 2009 when values of up to 8 μg Chl a/L were detected at 15 m whereas the Chl a concentration at the surface was only 1–2 μg/L (data not shown).
Phytoplankton groups detected by pigments
The pigment analyses showed the composition of the phytoplankton groups at the monthly monitoring cruises: the diagnostic pigments for dinoflagellates (peridinin), cryptophytes (alloxanthin) and prasinophytes (prasinoxanthin as well as other pigments present in prasinophytes and/or chlorophytes such as chlorophyll b, lutein, violaxanthin and neoxanthin) were present in most samples throughout the year. Occasionally, zeaxanthin concentrations were high, and pigment ratios calculated according to Schlüter and Møhlenberg (2003) showed the presence of cyanobacteria, e.g. Synechococcus sp. Furthermore, during the summer months, varying amounts of canthaxanthin, 4-keto-myxoxanthophyll, echinenone and myxoxanthophyll measured in the samples indicated that chain-forming cyanobacteria, e.g. Nodularia spumigena and Aphanizomenon sp. (Schlüter et al. 2004) were present as well. Fucoxanthin was continually present in a relatively large amount, indicating that diatoms and possibly also other chromophyte algal classes were abundant in the Fehmarn Belt area. The occurrence of chlorophyll c 3 as well as 19′-butanoyloxyfucoxanthin (19′-but), 19′-hexanoyloxyfucoxanthin (19′-hex) and 4-keto-19′- hexanoyloxyfucoxanthin (4-k-hex) in almost all samples indicated the presence of haptophytes and/or chrysophytes. Occasionally (March and April 2009), a gyroxanthin-diester was detected in the samples, indicating occurrence of dinoflagellates type 3 (Zapata et al. 2012).
Quantification of phytoplankton groups and dominant algae
The fast screening of all samples in microscope for identifying dominant species present confirmed the presence of the groups identified by pigment analysis though the smallest algal cells could rarely be identified by the applied microscopic method. The results of microscopy and pigment analyses were used for setting up the different ratio matrices for the CHEMTAX program. Accordingly, diatoms, peridinin-containing dinoflagellates, cryptophytes, including the autotrophic ciliate Mesodinium rubrum (=Myrionecta rubra) containing cryptophycean symbionts, prasinophytes type 3 (Higgins et al. 2011) and prasinophytes without prasinoxanthin (prasinophytes type 1, Higgins et al. 2011) including chlorophytes as well as euglenophytes were included in all CHEMTAX calculations. Furthermore, two types of cyanobacteria were included: synechococcus-like cyanobacteria, identified by zeaxanthin only, and the chain-forming cyanobacteria, where the pigment/Chl a ratios of 4-keto-myxoxanthophyll, echinenone, myxoxanthophyll and zeaxanthin were loaded into the ratio matrices of CHEMTAX (Schlüter et al. 2004). A group of haptophytes with chrysochromulina-like pigments including 4-k-hex (Zapata et al. 2004) and a group of chrysophytes, possibly also containing pelagophytes although not detected in the microscope, were loaded into CHEMTAX as well. In the spring of 2009, when gyroxanthin-diester was present in the samples, an extra group of dinoflagellates with gyroxanthin-diester and 19′-acyloxyfucoxanthins was loaded as well and subsequently added to the dinoflagellate group. The pigment/Chl a-ratios used for setting up the ratio matrices were mainly from species present in coastal areas (Schlüter et al. 2000, 2004; Higgins et al. 2011).
The CHEMTAX calculations showed that, horizontally in the investigation area, the phytoplankton group composition was relatively uniform, and generally, the most abundant species detected by the microscopy screenings were the same at all stations and at the two depths as indicated in Figs. 6, 7, 8, 9, 10 and 11. However, large differences were often seen in the total biomass when comparing the individual stations for each month (Figs. 6, 7, 8, 9, 10 and 11).
The 2009 spring bloom started in February with a dominance of diatoms (Fig. 6, only four stations sampled). In 2010, the spring bloom started 1 month later in March, and while Skeletonema costatum was dominating the spring bloom in 2009, various diatom species were constituting the spring bloom of 2010 (Fig. 9). In late spring and summer, the biomasses decreased, and phytoplankton were mostly dominated by smaller flagellates, e.g. Chrysochromulina sp. were common both years in the early summer as well as other unidentifiable flagellates, but larger species of diatoms and various species of dinoflagellates were also present (Figs. 6, 7, 9, and 10). Occasionally, the biomasses of some of the phytoplankton groups were higher at 15 m depth; in March 2009, chrysophytes were abundant in a subsurface bloom where the microscopic screenings showed that the species Dictyocha speculum was dominating (Fig. 6); in June 2009, haptophytes were detected by pigment analysis and Chrysochromulina sp. were identified in the microscope (Fig. 7) and a generally increased abundance of dinoflagellates (Ceratium sp., Gyrodinium sp. and Dinophysis sp.) was seen in July and August in both 2009 (Fig. 7) and 2010 at 15 m depth (Fig. 10). Furthermore, the toxic cyanobacteria N. spumigena as well as the non-toxic Aphanizomenon sp. became abundant in June 2009 at the easternmost station towards the Baltic Sea (Fig. 7). In July 2010, Aphanizomenon sp. developed in the surface, while in August 2010, N. spumigena became abundant in the surface waters (Fig. 10). Small Synechococcus sp.-like cells were often numerous at 15 m depth during the summer periods (Figs. 7 and 10).
In both years, diatoms such as Leptocylindrus minimus, Chaetoceros sp. and Cerataulina pelagica dominated the phytoplankton communities in autumn (Figs. 8 and 11). Other phytoplankton species identifiable in the microscope, i.e. the euglenophyte Eutreptiella sp., the autotrophic ciliate M. rubrum as well as different dinoflagellates, e.g. Gyrodinium sp., were abundant in the autumn of 2009 (Fig. 8) while different Ceratium species, e.g. Ceratium longipes, were present in the 2010 autumn samples (Fig. 11).
Fewer stations were sampled during the winter periods due to storm incidents in the sampling periods. The phytoplankton biomass was generally low, i.e. 1–2 μg Chl a/L and the HPLC measurements documented presence of all phytoplankton groups, but diatoms were the most abundant group (results not shown).
When the stations were pooled in groups of three representing four main areas: the Great Belt (sta. H111, 360, 361), the Fehmarn Belt (sta. H033, H036, H037), the Mecklenburg Bight (sta. 11, 12, 22) and Darss Sill area (sta. 46, DS1, H131) (Fig. 1) and averaged for each survey year, a high community similarity was confirmed (ANOVA, P = 0.38 and P = 0.15 for surface and P = 0.07 and P = 0.40 for 15 m for 2009 and 2010, respectively) (Fig. 12). However, the distribution showed some non-significant differences from the western (more saline) part to the eastern (less saline) part of the investigation area, where particularly diatoms and chrysophytes appeared to be more abundant in the western part of the area while chlorophytes, prasinophytes, cryptophytes and chain-forming cyanobacteria became increasingly important toward the eastern part of the investigation area (Fig. 12).
When considering the seasonal variation, the phytoplankton composition changed markedly from month to month (Figs. 6, 7, 8, 9, 10 and 11), and multidimensional scaling (MDS) analysis of the phytoplankton groups showed clustering of the monthly cruises for the surface samples with at least four clusters of monthly cruises (Fig. 13; only 2009 shown).
Discussion
Phytoplankton is irregularly distributed horizontally and vertically in estuaries due to the varying strength of physical forcing, nutrient loads and biological processes (Cloern 1996; Mouritsen and Richardson 2003; Gameiro et al. 2007). In the Fehmarn Belt area, water level, wind and density differences are the main driving forces for the exchange flow between the freshwater-influenced Baltic Sea and the marine North Sea (Jakobsen et al. 2010). Furthermore, nutrient enrichment from land is affecting the Baltic Sea and the Fehmarn Belt area (Andersen et al. 2011; Korpinen et al. 2012), and large variations in the phytoplankton populations, both spatially and temporally, were thus expected. Monitoring phytoplankton is challenging due to their short generation times and the large turnover of populations, and in order to achieve knowledge on the phytoplankton dynamics at group level as well as at species level in the whole investigation area, a combination of in situ and automated monitoring methods was chosen to achieve high resolution data with a limited sampling and analyzing strategy.
The monitoring buoys provided valuable information on the overall levels of Chl a concentrations, and in between the blooms, the Chl a concentrations were in the range of 1–3 μg/L at the three permanent buoy stations during the 2 years of monitoring (e.g. Figs. 2 and 4). These continuous fluorescence measurements on the fixed positions further facilitated distinction between patches of increased algal biomasses transported through the area and actual blooms building up inside the area. An example of a transitory increase was seen in March 2009 (Fig. 2). Such a Chl a biomass increase of very short duration is difficult to distinguish from a bloom when sampled by periodic monitoring cruises. The continuous measurements were also valuable for determining the duration and intensity of the blooms developing in the area. An example is the large spring bloom in March 2010 that lasted 1 month (Fig. 4). The monthly monitoring cruise took place on 8–11 March 2010 and coincided with a small decline of Chl a in the beginning of the bloom (Fig. 4). Consequently, the concentrations measured by the in situ samplings were only showing biomasses of approximately 5 μg Chl a/L in the surface waters and slightly higher at 15 m depth (Fig. 9) whereas the bloom recorded by the monitoring buoys continued the following days and reached a biomass of approximately 18 μg Chl a/L (Fig. 4).
The spatial variation of the phytoplankton groups measured by pigments showed that in each month, the composition of the phytoplankton groups was remarkably uniform horizontally in the investigation area (Figs. 6, 7, 8, 9, 10 and 11), and a high community similarity was confirmed statistically for each of the two layers sampled (Fig. 12). However, the distribution of phytoplankton groups shows some non-significant differences from the outermost western part to the outermost eastern part of the investigation area, where particularly diatoms, chrysophytes and dinoflagellates appeared more abundant in the Great Belt area, which is relatively more influenced by oceanic water from the North Sea, while prasinophytes/chlorophytes, cryptophytes and chain-forming cyanobacteria became increasingly important toward the freshwater-influenced Baltic Sea. Such a horizontal distribution pattern of phytoplankton has previously been described by Henriksen (2009) for two stations located in the Belt Sea and in the western Baltic Sea respectively just outside the present investigation area in 0–10 m depth integrated samples and microscopy enumerations as part of the Danish national monitoring programme. For the last three decades, dinoflagellates and diatoms have dominated the phytoplankton populations in the Kattegat and the Belt Sea area just north of the investigation area while, in the western Baltic Sea, east of the investigation area, apart from cyanobacteria, cryptophytes (including M. rubrum) and unidentified flagellates (possibly prasinophytes/chlorophytes) dominated the 0–10 m depth integrated samples (Henriksen 2009).
The largest variation in the phytoplankton populations was caused by the seasonal change, which created marked changes in the phytoplankton composition from month to month (Figs. 6, 7, 8, 9, 10 and 11). Multidimensional scaling analysis (MDS) confirmed that the temporal variation was more important than spatial variation in the Fehmarn Belt area (Fig. 13), which emphasizes the strong impact seasonality has on the development of phytoplankton. The impact of seasonal changes is also evident from the onset of the phytoplankton spring bloom that was delayed for a whole month in 2010 compared with 2009 (Figs. 6 and 9) due to a prolonged and cold winter period 2009–2010; a winter that had one of the most negative NAO (North Atlantic Oscillation) indexes measured during the almost 190-year records (Jung et al. 2011). The overall phytoplankton succession in the Fehmarn Belt is thus primarily influenced by the annual cycle in physical factors, i.e. light, temperature, water column stratification and mixing of the water column, which have the decisive effect on the development of the different groups influenced by other environmental parameters such as nutrient input and availability, grazing pressure, etc.
The pigment analyses revealed large differences of both biomasses and the composition of the groups vertically in the water column particularly in the summer periods (Figs. 7 and 10) where the mixing by wind is typically weak, and the two-layer exchange flow between the North Sea and the Baltic Sea, present most of the year, is showing a particularly strong stratification in the Fehmarn Belt (Jakobsen et al. 2010, and own unpublished results). Blooms of the dinoflagellates, e.g. Ceratium sp., Dinophysis sp. and Gyrodinium sp., were forming at the deepest sampling depth at the westernmost stations in July 2009 (Fig. 7). Motile phytoplankton organisms like dinoflagellates adjust their position in the water column by means of vertical migrations to benefit from the availability of nutrients in deep waters and light towards the surface (e.g. Blasco 1978; Figueroa et al. 1998; Brunet and Lizon 2003). Another subsurface bloom was the massive occurrence of D. speculum in March 2009 (Fig. 6). D. speculum was detected as a new subsurface bloom-forming species in the Kiel Bight area west of the investigation area in the 1980s (Jochem and Babenerd 1989) and has established as a bloom-forming species in this area (Wasmund et al. 2008). Large vertical differences in total biomasses of the individual groups were also seen in September and October 2010 when diatoms dominated at the deepest sampling depth whereas the phytoplankton community was more diverse at the surface and especially M. rubrum and Eutreptiella sp. were abundant (Fig. 11). M. rubrum and Eutreptiella sp. have also been shown to migrate in the water column when nutrients are depleted from surface waters (Villarino et al. 1995; Figueroa et al. 1998).
Whereas the flagellates take advantage of a stable water column, the diatoms bloom in the spring when the water column is well mixed and light and nutrients become available and in the autumn when the water column once again becomes well mixed (Wasmund and Uhlig 2003). Diatoms have been shown to be the predominant phytoplankton group in estuaries (Lemaire et al. 2002; Schlüter and Møhlenberg 2003) and were the most common group in the Fehmarn Belt. Although most abundant in spring and autumn, the diatoms also formed a large part of the phytoplankton population all year round and constituted 33 and 28 % of the total phytoplankton community biomass in the surface in 2009 and 2010, respectively, and a larger part at 15 m depth: 44 and 42 % in 2009 and 2010, respectively.
S. costatum dominated the spring bloom of 2009, and in 2010, also Chaetoceros sp., Thalassiosira sp. and Rhizosolenia sp. were abundant at both depths at the sampled stations (Figs. 6 and 9). These species are common in spring blooms in the nearby areas measured in the Danish national monitoring programme (Henriksen 2009) and in the HELCOM programme in the southern Baltic Sea (Wasmund et al. 1998). The 2009 diatom spring bloom was followed by a period in March 2009 with dominance of flagellates (e.g. the dinoflagellates Peridinium sp. and Gyrodinium sp.), the prymnesiophyte Chrysochromulina sp. and the abovementioned D. speculum in March 2009, while the autotrophic ciliate M. rubrum was abundant in the surface in April 2010 (Figs. 6, 7, 9 and 10). This pattern was common for the whole baseline area and has previously been described for the Baltic Sea area (Hadju et al. 2007 and references herein).
Surface blooms of cyanobacteria, e.g. Aphanizomenon sp. and N. spumigena, occur regularly in the Baltic Sea and adjacent areas in late summer when surface waters warm up and the water column becomes calm (Kononen 1992; Kahru et al. 1994; Schlüter et al. 2004). These cyanobacterial blooms have never been observed in the Kattegat north of the Great Belt area (Kahru et al. 1994; Wasmund 1997; Henriksen 2009). N. spumigena is toxic; it produces a hepatotoxin, nodularin, which poses a health risk to humans and animals (Kononen 1992; Rzymski et al. 2011). This toxic species has been shown to contain a diagnostic pigment, 4-ketomyxoxanthophyll, which is specific for N. spumigena in the Baltic Sea, and consequently, 4-ketomyxoxanthophyll can be used to detect the presence of N. spumigena in the Baltic Sea (Schlüter et al. 2004, 2008). 4-Ketomyxoxanthophyll was detected by the in situ samplings in the surface waters in June 2009 and in August 2010 by use of HPLC, and subsequently, N. spumigena was also detected by microscopy (Figs. 7 and 10). The non-toxic chain-forming cyanobacteria Aphanizomenon sp. was present as well, particularly at the easternmost stations in July 2010 (station DS1, Fig. 10). Blooms of the chain-forming cyanobacteria are due to their buoyancy primarily located in the surface and are patchier than those of other common bloom-forming algal groups, e.g. diatoms and dinoflagellates (Wasmund and Uhlig 2003). Actually, larger blooms of cyanobacteria can thus remain undetected by in situ samplings if the patches are not sampled at the station actually visited. In the present study, the chlorophyll fluorescence in the surface waters recorded by the measuring buoys provided the additional information needed to conclude that excessive blooms of cyanobacteria were not occurring during 2009 and 2010 in the investigation area. This agrees with the observations recorded by satellite of surface waters in the Baltic Sea proper where, in 2010, a cyanobacterial bloom of normal intensity, as defined by Hansson and Håkansson (2007), was restricted to the first 3 weeks of July and mostly affected the south-eastern parts of the Baltic Sea proper adjacent to the present investigation area whereas only minor surface blooms were detected in August (Hansson and Öberg 2010). Cyanobacterial blooms in the summer of 2009 were categorized as well below average in comparison with previous years (Hansson and Öberg 2009).
Conclusion
In large monitoring programmes, the HPLC pigment analytical method is ideally suited as an alternative to the cumbersome and time-consuming enumerations of algal cells and measurements for biovolume calculation when determining the spatial and temporal resolution of phytoplankton (Sarmento and Descy 2008), and in particular, it is also well suited for detecting pico- and nano-phytoplankton that are difficult to identify microscopically (Higgins et al. 2011; Schlüter et al. 2011).
The combination of the HPLC pigment analysis method and monitoring buoys continuously measuring fluorescence at selected stations with fast screening of samples in the microscope proved advantageous. The fast screening of samples in the microscope provided information on the dominant phytoplankton species present in the samples and, furthermore, aided the setting up of the pigment:Chl a ratios for CHEMTAX calculations of the biomass of the phytoplankton groups. This combination of methods was effective for providing the required information on both the phytoplankton succession and dynamic in the Fehmarn Belt area and, at the same time, getting information on duration and intensity of the blooms as well as specific information on the dominant species present both temporally and spatially in the large investigation area. Subsequently, the results of this survey were used for an environmental impact assessment in which the potential impact on the plankton communities of sediment spill, introduction of new surfaces and alterations of hydrodynamic conditions due to the construction of a fixed link between Denmark and Germany in the Fehmarn Belt was evaluated.
References
Andersen, J. H., Axe, P., Backer, H., Carstensen, J., Claussen, U., Fleming-Lehtinen, V., et al. (2011). Getting the measure of eutrophication in the Baltic Sea: towards improved assessment principles and methods. Biogeochemistry, 106, 137–156.
Barlow, R., Stuart, V., Lutz, V., Sessions, H., Sathyendranath, S., Platt, T., et al. (2007). Seasonal pigment patterns of surface phytoplankton in the subtropical southern hemisphere. Deep-Sea Research I, 54, 1687–1703.
Blasco, D. (1978). Migration of dinoflagellates off Baja California Coast. Marine Biology, 46, 41–47.
Brunet, C., & Lizon, F. (2003). Tidal and diel periodicities of size-fractionated phytoplankton pigment signatures at an offshore station in the southeastern English Channel. Estuarine, Coastal and Shelf Science, 56, 833–843.
Cloern, J. E. (1996). Phytoplankton bloom dynamics in coastal ecosystems: a review with some general lessons from sustained investigation of San Francisco Bay, California. Reviews of Geophysics, 34, 127–168.
Figueroa, F. L., Niell, F. X., Figueiras, F. G., & Villarino, M. L. (1998). Diel migration of phytoplankton and spectral light field in the Ría de Vigo (NW Spain). Marine Biology, 130, 491–499.
Gameiro, C., Cartaxana, P., & Brotas, V. (2007). Environmental drivers of phytoplankton distribution and composition in Tagus Estuary, Portugal. Estuarine, Coastal and Shelf Science, 75, 21–34.
Hadju, S., Höglander, H., & Larsson, U. (2007). Phytoplankton vertical distributions and composition in Baltic Sea cyanobacterial blooms. Harmful Algae, 6, 189–205.
Hansson, M., & Håkansson, B. (2007). The Baltic Algae Watch System—a remote sensing application for monitoring cyanobacterial blooms in the Baltic Sea. Journal of Applied Remote Sensing, 1(1), 011508. doi:10.1117/1.2834770.
Hansson, M., & Öberg, J. (2009). Cyanobacterial blooms in the Baltic Sea. HELCOM Baltic Sea Environment Fact Sheets. Online. 08.04.2014, http://helcom.fi/baltic-sea-trends/environment-fact-sheets.
Hansson, M., & Öberg, J. (2010). Cyanobacterial blooms in the Baltic Sea. HELCOM Baltic Sea Environment Fact Sheets. Online. 08.04.2014, http://helcom.fi/baltic-sea-trends/environment-fact-sheets.
Henriksen, P. (2009). Long-term changes in phytoplankton in the Kattegat, the Belt Sea, the Sound and the western Baltic Sea. Journal of Sea Research, 61, 114–123.
Higgins, W. H., Wright, S. W., & Schlüter, L. (2011). Quantitative interpretation of chemotaxonomic pigment data. In S. Roy, C. A. Llewellyn, E. S. Egeland, & G. Johnsen (Eds.), Phytoplankton pigments: characterization, chemotaxonomy and applications in oceanography (pp. 257–313). Cambridge: Cambridge University.
Hooker, S. B., van Heukelem, L., Thomas, C. S., Claustre, H., Ras, J., Schlüter, L., et al. (2005). The Second Sea-WiFS HPLC Analysis Round-Robin Experiment (SeaHARRE-2). NASA Techical Memorandum. 2005–212785, NASA Goddard Space Flight Center, Greenbelt, Maryland.
Jakobsen, F., Hansen, I. S., Hansen, N. O., & Østrup-Rasmussen, F. (2010). Flow resistance in the Great Belt, the biggest strait between the North Sea and the Baltic Sea. Estuarine, Coastal and Shelf Science, 87, 325–332.
Jochem, F., & Babenerd, B. (1989). Naked Dictyocha speculum—a new type of phytoplankton bloom in the Western Baltic. Marine Biology, 103, 373–379.
Jung, T., Vitart, F., Ferranti, L., & Morcrette, J. J. (2011). Origin and predictability of the extreme negative NAO winter of 2009/10. Geophysical Research Letters, 38, L07701. doi:10.1029/2011GL046786.
Kahru, M., Horstmann, U., & Rud, O. (1994). Satellite detection of increased cyanobacterial blooms in the Baltic Sea: natural fluctuation or ecosystem change? Ambio, 23, 469–472.
Kinkade, C. S., Marra, J., Dickey, T. D., Langdon, C., Sigurdson, D. E., & Welle, R. (1999). Diel bio-optical variability observed from moored sensors in the Arabian Sea. Deep-Sea Research, 46, 1813–1831.
Kononen, K. (1992). Dynamic of the toxic cyanobacterial blooms in the Baltic Sea. Finnish Marine Research, 261, 3–36.
Korpinen, S., Meski, L., Andersen, J. H., & Laamanen, M. (2012). Human pressures and their potential impact on the Baltic Sea ecosystem. Ecological Indicators, 15, 105–114.
Lemaire, E., Abril, G., De Wit, R., & Etcheber, H. (2002). Distribution of phytoplankton pigments in nine European estuaries and implications for an estuarine typology. Biogeochemistry, 59, 5–23.
Mackey, M. D., Mackey, D. J., Higgins, H. W., & Wright, S. W. (1996). CHEMTAX—a program for estimating class abundance from chemical markers: application to HPLC measurements of phytoplankton. Marine Ecology Progress Series, 144, 265–283.
Mouritsen, L. T., & Richardson, K. (2003). Vertical microscale patchiness in nano- and microplankton distributions in a stratified estuary. Journal of Plankton Research, 25(7), 783–797.
Rzymski, P., Poniedziałek, B., & Karczewski, J. (2011). Gastroenteritis and liver carcinogenesis induced by cyanobacterial toxins. Gastroenterologia Polska, 18(4), 159–162.
Sackmann, B. S., Perry, M. J., & Eriksen, C. C. (2008). Seaglider observations of variability in daytime fluorescence quenching of chlorophyll-a in Northeastern Pacific coastal waters. Biogeosciences Discussions, 5, 2839–2865.
Sarmento, H., & Descy, J. P. (2008). Use of marker pigments and functional groups for assessing the status of phytoplankton assemblages in lakes. Journal of Applied Phycology, 20, 1001–1011.
Schlüter, L., & Møhlenberg, F. (2003). Detecting presence of phytoplankton groups with non-specific pigment signatures. Journal of Applied Phycology, 15, 465–476.
Schlüter, L., Møhlenberg, F., Havskum, H., & Larsen, S. (2000). The use of phytoplankton pigments for identifying and quantifying phytoplankton groups in coastal areas; testing the influence of light and nutrients on pigment/chlorophyll a-ratios. Marine Ecology Progress Series, 192, 49–63.
Schlüter, L., Garde, K., & Kaas, H. (2004). 4-keto-myxoxanthophyll-like pigment is a diagnostic pigment for the toxic cyanobacteria Nodularia spumigena in the Baltic Sea. Marine Ecology Progress Series, 275, 69–78.
Schlüter, L., Lutnæs, B. F., Liaaen-Jensen, S., Garde, K., Kaas, H., Jameson, I., et al. (2008). Correlation of the content of hepatotoxin nodularin and glycosidic carotenoids, 4-ketomyxol-20-fucoside and novel 10-O-methyl-4-ketomyxol-20-fucoside, in 20 strains of the cyanobacterium Nodularia spumigena. Biochemical Systematics and Ecology, 36, 749–757.
Schlüter, L., Henriksen, P., Nielsen, T. G., & Jakobsen, H. H. (2011). Phytoplankton composition and biomass across the southern Indian Ocean. Deep-Sea Research I, 58, 546–556.
Van Heukelem, L., & Thomas, C. (2001). Computer assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. Journal of Chromatography, A, 910, 31–49.
Villarino, M. L., Figueiras, F. G., Jones, K. J., Alvarez-Salgado, X. A., Richard, J., & Edwards, A. (1995). Evidence of in situ diel vertical migration of a red-tide microplankton species in Ria de Vigo (NW Spain). Marine Biology, 123, 607–617.
Wasmund, N. (1997). Occurrence of cyanobacterial blooms in the Baltic Sea in relation to environmental conditions. Internationale Revue der gesamten Hydrobiologie, 82, 169–184.
Wasmund, N., & Uhlig, S. (2003). Phytoplankton trends in the Baltic Sea. ICES Journal of Marine Science, 60, 177–186.
Wasmund, N., Nausch, G., & Matthäus, W. (1998). Phytoplankton spring blooms in the southern Baltic Sea–Spatio-temporal development and long-term trends. Journal of Plankton Research, 20, 1099–1117.
Wasmund, N., Göbel, J., & Von Bodungen, B. (2008). 100-years-changes in the phytoplankton community of Kiel Bight (Baltic Sea). Journal of Marine Systems, 73, 300–322.
Wilhelm, C., Rudolph, I., & Renner, W. (1991). A quantitative method based on HPLC-aided pigment analysis to monitor structure and dynamics of the phytoplankton assemblage—a study from Lake Meerfelder (Eifel, Germany). Archiv für Hydrobiologie, 123, 21–35.
Wright, S. W., van den Enden, R. L., Pearce, I., Davidson, A. T., Scott, F. J., & Westwood, K. J. (2010). Phytoplankton community structure and stocks in the Southern Ocean (30–80°E) determined by CHEMTAX analysis of HPLC pigment signatures. Deep-Sea Research II, 57, 758–778.
Zapata, M., Jeffrey, S. W., Wright, S. W., Rodríguez, F., Garrido, J. L., & Clementson, L. (2004). Photosynthetic pigments in 37 species (65 strains) of Haptophyta, implications for oceanography and chemotaxonomy. Marine Ecology Progress Series, 270, 83–102.
Zapata, Z., Fraga, S., Rodríguez, F., & Garrido, J. L. (2012). Pigment-based chloroplast types in dinoflagellates. Marine Ecology Progress Series, 465, 33–52.
Acknowledgments
This work was carried out under contract to Femern A/S, and their consent to publish the results is gratefully acknowledged. We are grateful to Simon Wright, Australian Antarctic Division, CSIRO, for the CHEMTAX program ver. 1.95. M. Allerup is thanked for skilful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schlüter, L., Møhlenberg, F. & Kaas, H. Temporal and spatial variability of phytoplankton monitored by a combination of monitoring buoys, pigment analysis and fast screening microscopy in the Fehmarn Belt Estuary. Environ Monit Assess 186, 5167–5184 (2014). https://doi.org/10.1007/s10661-014-3767-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3767-9