Abstract
The aim was to investigate the toxicity of selected metal oxide nanoparticles (MO-NPs) on the brine shrimp Artemia salina, by evaluating mortality and behavioural and biochemical responses. Larvae were exposed to tin(IV) oxide (stannic oxide (SnO2)), cerium(IV) oxide (CeO2) and iron(II, III) oxide (Fe3O4) NPs for 48 h in seawater, with MO-NP suspensions from 0.01 to 1.0 mg/mL. Mortality and behavioural responses (swimming speed alteration) and enzymatic activities of cholinesterase, glutathione-S-transferase and catalase were evaluated. Although the MO-NPs did not induce any mortality of the larvae, they caused changes in behavioural and biochemical responses. Swimming speed significantly decreased in larvae exposed to CeO2 NPs. Cholinesterase and glutathione-S-transferase activities were significantly inhibited in larvae exposed to SnO2 NPs, whereas cholinesterase activity significantly increased after CeO2 NP and Fe3O4 NP exposure. Catalase activity significantly increased in larvae exposed to Fe3O4 NPs. In conclusion, swimming alteration and cholinesterase activity represent valid endpoints for MO-NP exposure, while glutathione-S-transferase and catalase activities appear to be NP-specific.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoparticles (NPs) are currently used in many commercial applications and in a wide range of fields, such as technology, industry and medicine. In this regard, metal oxide NPs (MO-NPs), such as tin(IV) oxide (stannic oxide (SnO2)), cerium(IV) oxide (CeO2) and iron(II, III) oxide (Fe3O4) NPs, are of special interest due to their exceptional physicochemical properties, which make them highly suitable and desirable for many consumer products and industrial technologies (Montes et al. 2012). Indeed, SnO2 NPs have a wide range of applications, such as for gas sensors (Barsan et al. 1999), as photo-catalysts and in rechargeable lithium batteries (Idota et al. 1997). CeO2 NPs are used in a variety of consumer, pharmaceutical and agricultural products, as well as being used in the automotive industry as a catalyst, to improve oxidation of fossil fuels (Cornelis et al. 2011). The magnetic Fe3O4 NPs have raised great interest in the field of information storage, as well as in areas of biomedicine and magnetic sensing (Hui et al. 2008).
The MO-NPs inevitably enter into the aquatic ecosystems (Tso et al. 2010). Along with the emerging use of NPs in several commercial products and their consequent release into aquatic ecosystems, growing concerns have arisen about their fate, behaviour and potential toxicity (Klaine et al. 2008).
Marine ecosystems are extremely important, as these ultimately receive the run-off and wastewaters from domestic and industrial sources (Baun et al. 2008). Studies have been performed on marine organisms exposed to MO-NPs. Thus, the exposure of the Mediterranean mussel Mytilus galloprovincialis to titanium dioxide and silicon oxide NPs results in alterations in haemocyte immune parameters, oxidative stress and lysosomal stability in the digestive glands (Canesi et al. 2010a, b), besides inducing responses also at the cellular and subcellular level in mussel gills (D’Agata et al. 2013).
Likewise, Galloway and colleagues (2010) reported that the exposure of the polychaete Arenicola marina to titanium dioxide NPs resulted in a significant decrease in lysosomal stability and in DNA damage in coelomocytes. Exposure of the sea urchin Paracentrotus lividus to SnO2, CeO2 and Fe3O4 NPs by force feeding has toxic effects on the innate immune system (Falugi et al. 2012), while their exposure to titanium dioxide induces morphological, behavioural and enzymatic alterations (Gambardella et al. 2013). Zinc oxide NPs show toxicity towards marine diatoms (Peng et al. 2011) and the brine shrimp Artemia salina (Ates et al. 2013a, b).
A. salina is widely used as a nutritious live food source for the larvae of a variety of marine organisms (Radhika Rajasree et al. 2010). It is characterised by the common features of short life cycle, high adaptability to adverse environmental conditions, small body size, high offspring production and ease of culture (Nunes et al. 2006). A. salina is also included as one of the test species by the US Environmental Protection Agency (EPA 2002) for acute toxicity testing (Nunes et al. 2006; Kokkali et al. 2011). All of these features make A. salina one of the most valuable organisms available for ecotoxicological tests (Persoone and Wells 1987; Garaventa et al. 2010). Thus, the toxicities of several chemical compounds, including pesticides and antifouling biocides, have been tested using A. salina over the last few decades (Sorgeloos et al. 1978; Barahona and Sanchez-Fortun 1999; Koutsaftis and Aoyama 2007; Venkateswara Rao et al. 2007; Garaventa et al. 2010). Recently, ecotoxicological tests were performed using the A. salina life cycle to assess the hazard of MO-NPs, to determine the potential effects these might have on the marine environment (Radhika Rajasree et al. 2010; Ates et al. 2013a, b).
Behavioural and biochemical responses represent valid sensitive biomarkers for environmental monitoring, as has been proposed for pesticides and insecticides (Jemec et al. 2007; Venkateswara Rao et al. 2007; Garaventa et al. 2010). Behavioural responses are sensitive endpoints to assess the impact of such contaminants at concentrations far below the lethal effects (Amiard-Triquet 2009). Some studies have looked for correlations between behavioural and biochemical responses (e.g., enzyme inhibition) in marine organisms exposed to NPs, although no specific links have been found between enzyme activities and behavioural impairment to date (Buffet et al. 2011). Locomotion is used as a stress indicator in ecotoxicological studies, and it represents a sensitive measure of toxic stress for a wide range of environmental contaminants (Little and Finger 1990). With aquatic organisms, swimming represents a behavioural response determinant that can be directly affected by physiological status, as has been demonstrated for A. salina exposed to toxic compounds (Venkateswara Rao et al. 2007; Garaventa et al. 2010; Alyuruk et al. 2013).
Biochemical biomarkers of damage and defence have been recently proposed for the evaluation of potential toxic effects of MO-NPs on marine organisms (Canesi et al. 2010a; Buffet et al. 2011; Falugi et al. 2012; Gambardella et al. 2013). Cholinesterase is an enzyme associated with the cholinergic nervous system, and it acts as a modulator of acetylcholine reception at neuromuscular sites. Recently, cholinesterase was shown to be a toxicological biomarker of damage (Jemec et al. 2007). NPs might bind to cholinesterase and impair its activity after entering the body (Wang et al. 2009), as has been reported for sea urchin and mussel exposed to MO-NPs (Canesi et al. 2010a; Falugi et al. 2012; Gambardella et al. 2013). Glutathione-S-transferase (GST) and catalase are biomarkers of defence that can be activated in the presence of MO-NPs, such as with zinc, silicon and titanium oxide NPs (Canesi et al. 2010a; Buffet et al. 2011). GST belongs to the class of phase II detoxifying enzymes, and the induction of its activity is an indication of the activation of detoxification processes (Jemec et al. 2007). Catalase is an antioxidant that catalyses the decomposition of hydrogen peroxide derived from the formation of reactive oxygen species (ROS), such as superoxide or hydroxyl radicals (Jemec et al. 2008a). Changes in GST and catalase activities can occur in marine organisms exposed to NPs, which depend upon the NP type and concentration (Canesi et al. 2010a; Buffet et al. 2011).
To date, no studies on biochemical and behavioural responses are available for A. salina exposure to NPs. Therefore, the main aim of the present study was to investigate the toxicity of the selected MO-NPs of SnO2, CeO2 and Fe3O4 on A. salina larvae through the evaluation of mortality and behavioural and biochemical responses. Mortality and alterations in swimming and enzyme activities of cholinesterase, GST and catalase were used as the endpoints to assess potential adverse effects of these MO-NPs in A. salina and to identify the most sensitive of these endpoints.
Materials and methods
Nanoparticles
The MO-NPs of SnO2, CeO2 and Fe3O4 were provided by Nanostructured and Amorphous Materials, Inc (Houston, USA), as at least 98 % pure. According to the manufacturer information, the particle sizes were determined using transmission electron microscopy as 61 nm, 50 to 105 nm, and 20 to 30 nm, respectively. Details of the behaviour, size and chemical characterisation of these MO-NPs in seawater were obtained using an environmental scanning electron microscope (ESEM) equipped with energy-dispersive spectroscopy (EDS; Quanta FEI 250, Gambardella et al. 2014). These analyses confirmed the chemical composition of NPs and the size of their aggregates, which were in the range of 1–2 μm for SnO2 and CeO2, and ~10 μm for Fe3O4.
The MO-NP suspensions were prepared according to Canesi et al. (2010a). Briefly, these were freshly prepared in 0.22 μm filtered natural seawater as stock concentrations of 1 mg/mL each, which were sonicated for 15 min at 100 W using a 50 % on/off cycle. The suspensions were kept in an ice bath during the preparation.
Model organism
Commercially available dehydrated cysts of Artemia sp. were used for the experiments. Instar I stage larvae were obtained as described by Garaventa et al. (2010). The newly hatched nauplii were separated from nonhatched cysts based on their phototaxis and then transferred by a Pasteur pipette into beakers containing the filtered natural seawater.
Acute and behavioural responses
Acute toxicity test
The toxicity test was performed by adding 10–15 larvae to each well of a 24-well polystyrene plate that contained 1 mL of filtered natural seawater with suspensions of different concentrations of the MO-NPs (0.01, 0.1, 1.0 mg/mL). The plates were stored at 20 °C for 48 h with a 16:8-h light/dark cycle. All of the tests were performed in triplicate. After 48 h, the larvae were collected from the wells, rinsed and transferred into new plates with clean filtered natural seawater to remove the MO-NPs. The numbers of dead larvae were counted under a stereomicroscope. Larvae that were completely motionless were counted as dead, and the percentages of mortality compared to the control were calculated.
Swimming speed alteration test
Swimming speed alteration tests were performed according to Garaventa et al. (2010). Briefly, after 48 h of exposure of the A. salina larvae to the MO-NPs, the swimming was recorded using a swimming behaviour recorder that consisted of a video camera with a macro-objective that was used to record the swimming paths of the samples of larvae. The apparatus was inside a black box (60 × 60 × 100 cm) to exclude external sources of light, and the recording chamber was monitored under infrared light. The A. salina larvae were dark-adapted for 2 min before the video recording (time fixed by preliminary tests, to reach steady speeds and a uniform spatial distribution). The swimming behaviour was digitally recorded for about 3 s at 25 frames/s, and the images were analysed using advanced image processing software to reconstruct the individual paths/ tracks, with the measurement of the mean swimming speeds (mm/s) for each A. salina larva in each sample (10–15 larvae/sample). The data are referred to as the swimming inhibition, following normalisation to the mean swimming speed of the controls (S, mean swimming speed), where
Microscopic analysis
After recording the swimming speed alterations, the larvae were observed under a Leica DMRB microscope (Leica, Switzerland). Images were acquired using a DFC420C Leica CCD camera and the Leica software (Leica Application Suite V3). The resulting images were stored and visualised in the Leica software programme, using the TIFF image format.
Chemical analysis
To determine the chemical analysis, about 150 mL of the MO-NP suspensions at 1 mg/mL containing the hatched A. salina larvae were exposed for the 48-h toxicity testing. Three replicates were prepared for each MO-NP concentration, including controls with no MO-NP addition. At the end of the exposure, larvae were sampled and thoroughly washed with deionized water through 80 μm plankton net. About 0.1 mg of dry larvae was weighed, digested in Teflon vessels and analysed for metal (Sn, Ce, Fe) concentration by inductively coupled plasma-mass spectrometry (ICP-MS), as described in Ates et al. (2013a). The results were then normalised to the controls.
Biochemical responses
Chemicals
The following chemicals used for the determination of the enzyme activities were obtained from Sigma (Germany): dibasic and monobasic potassium phosphate, hydrogen peroxide (30 %), 1-chloro-2,4-dinitrobenzene, l-glutathione (reduced form), 5,5′ dithiobis-2-nitrobenzoic acid, sodium hydrogen carbonate and acetylthiocholine chloride. BCA Protein Assay Reagent A and BCA Protein Assay Reagent B were purchased from Pierce (USA). All chemicals were of the highest commercially available grades.
Determination of enzyme activities
To determine the enzymatic activities, about 200 larvae of A. salina were exposed to 50 mL of the MO-NP suspensions at serial concentrations (0.01, 0.1, 1.0 mg/mL) for the 48-h toxicity testing, as described by Radhika Rajasree et al. (2010). Three replicates were prepared for each MO-NP concentration, including controls with no MO-NP addition. After exposure, the larvae were placed into filtered natural seawater, rinsed four times in 50 mM of potassium phosphate buffer (pH 7.0) and homogenised for 3 min in 0.5 mL of homogenisation buffer (50 mM of phosphate buffer, pH 7.0), using a glass-glass Elvehjem-Potter homogeniser. The homogenates were centrifuged for 15 min at 15,000 × g at 4 °C, as described by Jemec et al. (2007). The supernatants were then stored at −80 °C until all of the tests were performed (within 1 week). The enzyme activities were all measured on the same day, and each experiment was repeated twice. No food was provided to the A. salina larvae during this exposure to the MO-NPs.
The cholinesterase activity was determined according to the method of Ellman et al. (1961), using a VIS microplate reader (Anthos, UK), as described by Mancini et al. (2004). The reaction mixture was prepared in 100 mM of potassium phosphate buffer (pH 7), containing 1 mM acetylthiocholine chloride and 0.5 mM 5,5′ dithiobis-2-nitrobenzoic acid, as final concentrations. The protein supernatants (100 μL) were added to start the reactions, which were followed spectrophotometrically at 405 nm at 25 °C for 1 h. In blank reactions, the protein supernatant was replaced by 50 mM of phosphate buffer (pH 7.0). Cholinesterase activity is expressed in nmoles of hydrolysed acetylcholine chloride/min/mg protein (extinction coefficient, ε 405 = 13.600 M−1 cm−1).
The GST activity was determined according to the method described by Habig et al. (1974), using a VIS microplate reader (Anthos, UK). Here, 1-chloro-2,4-dinitrobenzene was dissolved in ethanol to 50 mM, which was afterwards diluted in 100 mM of potassium phosphate buffer (pH 6.5) to the final concentration of 4 mM. This solution was used to prepare a reaction mixture containing 1 mM 1-chloro-2,4-dinitrobenzene and 1 mM reduced glutathione. Then, 50 μL of protein supernatant was added to start the reactions. The final concentration of ethanol in the reaction mixture was 2 %; at this concentration, the activity of GST was not inhibited (not shown). Two blank reactions in which the protein supernatant was replaced by 50 mM of phosphate buffer (pH 7.0) were followed, and the mean rate of the absorbance change was subtracted from the measurements for the samples containing the protein supernatant. The reaction was followed spectrophotometrically at 340 nm at 25 °C for 20 min. The GST activity was expressed in nmoles of conjugated reduced GSH/min/mg protein (extinction coefficient, ε 340 = 9.600 M−1 cm−1).
The catalase activity was determined according to Aebi (1984), Jamnik and Raspor (2003) and Jemec et al. (2008b). Here, 50 μL of protein supernatant was combined with 950 μL of hydrogen peroxide solution (10.8 mM) prepared in 50 mM of potassium phosphate buffer (pH 7.0). The final concentration of hydrogen peroxide was 10 mM. The reaction was followed spectrophotometrically for 2 min at 25 °C and 240 nm using a Shimadzu UV-2101PC spectrophotometer (Japan). The catalase activity is expressed as μmoles of degraded hydrogen peroxide/min/mg protein (extinction coefficient, ε 240 = 43.6 M−1 cm−1).
The concentrations of the substrates used for all of the enzymes tested were saturated and ensured linear changes in the absorbance with time and concentration of protein. The protein concentrations were determined using BCA™ Protein Assay kits, as a modification of the bicinchoninic acid protein assay (Pierce, Rockford, USA).
Statistical analysis
The experiments were carried out in triplicate for all of the treatments. All of the data are expressed as means ± standard error (SE) of the triplicates. The lethal concentration (LC20; concentration of suspended MO-NPs that resulted in 20 % death in the exposed organisms after 48 h), the effective concentration for swimming speed alteration (EC20; concentration of suspended MO-NPs that resulted in 20 % swimming speed alteration effect in the exposed organisms after 48 h) and the related 95 % confidence limits were calculated using the Probit method.
Significant differences between controls and treated samples were determined using one-way ANOVA followed by the Bonferroni nonparametric post hoc tests, where p < 0.05 is considered to be significantly different.
Results
Acute toxicity and behavioural responses
The results of the mortality and swimming speed alterations are reported in Fig. 1. Less than 20 % mortality was observed in the larvae exposed to all of the concentrations of the MO-NPs, including the highest, and therefore it was not possible to calculate the LC20 for any of the MO-NPs. Although there were low percentages of swimming alteration (all <50 %, Fig. 1), it is important to note that the swimming speed increased with 0.01 mg/mL SnO2 NPs and decreased for all of the other concentrations of the selected MO-NPs. The swimming was significantly inhibited in larvae exposed to 0.1 and 1.0 mg/mL CeO2 NPs (p < 0.05), where it was also possible to calculate the EC20 (EC20 = 0.08 mg/mL [confidence limits 0.07–0.09]).
Mortality (filled bars) and swimming speed alteration (open bars) of A. salina larvae after 48 h of exposure to increasing concentrations (as indicated) of SnO2, CeO2 and Fe3O4 NPs, relative to the controls. Data are the means ± SE (n = 3) of two experiments, analysed according to ANOVA followed by the Bonferroni post hoc tests. *p < 0.05
Microscopy observations and chemical analyses
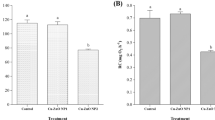
The microscopy observations showed that aggregation of the MO-NPs occurred after 48 h of exposure in all of the suspensions in the filtered natural seawater, as already reported in a previous study (Gambardella et al. 2014). In addition, larvae accumulated the aggregates of MO-NPs (Fig. 2), and the ingested MO-NPs appeared to be compressed inside the larval gut (Fig. 3). Ce and Fe levels accumulated within 48 h in larvae exposed to CeO2 and Fe3O4 NPs were statistically different from the controls.
Biochemical responses
The cholinesterase, GST and catalase activities measured in the A. salina larvae after 48 h of exposure to the MO-NPs are shown in Fig. 4. The cholinesterase activities in the larvae exposed to SnO2 NPs significantly decreased as compared to the control, whereas there was a significant increase with CeO2 NPs and Fe3O4 NPs (p < 0.05). A dose-dependent effect was observed in the cholinesterase activity of the larvae exposed to SnO2 and CeO2 NPs.
The GST activities decreased in the samples exposed to the MO-NPs. A significant dose-dependent response was observed with the exposure to SnO2 NPs (p < 0.05).
The catalase activities gradually decreased in the samples exposed to SnO2 NPs, while there were no changes in the catalase activities at any of the concentrations of CeO2 NPs. The catalase activities were significantly stimulated at all of the concentrations of Fe3O4 NPs (p < 0.05).
Discussion
A. salina exposure to these MO-NPs up to a concentration of 1.0 mg/mL did not induce any significant lethal effects. Microscopy observations and chemical analyses revealed that the larvae ingested the MO-NPs into the gut, although this accumulation did not result in significant mortality after 48 h. Brine shrimps are nonselective filter feeders, and they can readily ingest particles of up to 50 μm in diameter (Hund-Rinke and Simon 2006). When suspended in the seawater, these MO-NPs formed agglomerates that ranged from 400 nm up to several μm in diameter (Falugi et al. 2012; Gambardella et al. 2014), and the A. salina larvae were able to ingest them. Several studies have confirmed the accumulation of NPs inside the gut of A. salina larvae and their inability to eliminate these ingested particles (Ates et al. 2013a, b). However, this accumulation does not induce mortality after 24 h of exposure (Cornejo-Garrido et al. 2011), although mortality can be seen after prolonged exposures (e.g., 96 h of exposure; Gaiser et al. 2011). These data are in line with the results in the present study, as no significant mortality was observed for any of the MO-NP types and concentrations after the 48-h exposure, as compared to the controls. However, we found behavioural and biochemical responses, which confirm that significant changes occur after the exposure to these MO-NPs.
Behaviour is a determinant that results from molecular, physiological and ecological aspects of toxicology (Little et al. 1990; Xia et al. 2013); therefore, behaviour can provide insights into various levels of biological organisation (Scott and Sloman 2004). Swimming decreases were seen for these larvae exposed to all of the NPs, with the exception of the lowest concentration of SnO2 NPs, although significant swimming inhibition was only seen for the larvae exposed to CeO2 NPs. To date, little is known about the toxicity of CeO2 NPs. Gaiser et al. (2011) reported on inhibition of moulting and growth in Daphnia magna exposed to 0.01 mg/mL CeO2 NPs. These authors attributed the toxicity of these MO-NPs to a direct physical interaction between the CeO2 NPs and the daphnids, through impeding their movement and/or affecting their feeding, which resulted in their reduced swimming ability. In the present study, the size of the CeO2 NPs was greater than the sizes of the other two MO-NPs used; therefore, CeO2 NPs tended to aggregate and accumulate more rapidly (Fig. 2) and interfere with movement, thus causing significant behavioural responses, as described by Gaiser et al. (2011).
Enzymatic activity alterations were observed in the larvae exposed to all of these MO-NPs. In particular, these alterations in cholinesterase activity were always significant, for all of the MO-NP exposures, which suggest that cholinesterase is the most sensitive of these biochemical biomarkers. Cholinesterase regulates cholinergic signalling by hydrolysing the neurotransmitter acetylcholine, which is involved in the motor control of somatic muscle (Taylor and Brown 1999). Here, the cholinesterase activity was inhibited in the A. salina larvae exposed to SnO2 NPs, while it was significantly increased by the exposure to CeO2 NPs and Fe3O4 NPs. It has been reported that cholinesterase inhibition can cause an overflow of acetylcholine at these receptor sites, which will affect the intracellular responses driven by both nicotinic and muscarinic receptors and the function of the innate immune system in marine invertebrates exposed to neurotoxic compounds and these MO-NPs (Falugi et al. 2011; Gambardella et al. 2013). Since A. salina has a well-developed neurotransmitter system and many cholinergic receptors (Varò et al. 2002), SnO2 NPs might cause alterations in the functions of the cholinergic neurotransmission system and the immune system, as has been reported for sea urchins exposed to the same MO-NPs (Falugi et al. 2012). However, further investigations on the molecules belonging to the cholinergic neurotransmitter system are required to support this hypothesis.
Considering further the increased cholinesterase activity after the exposure to the CeO2 NPs and Fe3O4 NPs, it is known that cholinesterase can function as a regulator of inflamed tissue (Falugi and Aluigi 2012) through its role in the support of apoptosis (Zhang et al. 2002; Zhu et al. 2008). Indeed, inflamed cells and tissues show greater cholinesterase expression and activity compared to healthy ones (de Oliveira et al. 2012; Falugi et al. 2012). Therefore, cholinesterase increases are correlated to inflammation, and here, the cholinesterase activity was significantly enhanced in the larvae exposed to CeO2 NPs and Fe3O4 NPs, which can be caused by inflammation. Inflammation is a normal response of the body to injury; when activated in moderation, this inflammation can stimulate the regeneration of healthy tissue (Buzea et al. 2007). Exposure of marine organisms to the MO-NPs might thus be associated to an inflammation response (Koehler et al. 2008). In addition, alterations in any behaviour coupled with changes in physiology can alter the population stability (Scott and Sloman 2004). In the present study, the significant swimming inhibition and the increases in cholinesterase activity after exposure to CeO2 NPs can be considered as indicators of inflammation.
The GST activity was significantly inhibited after exposure to SnO2 NPs. These MO-NPs thus altered the biomarkers of both defence (GST) and damage (cholinesterase). GST is responsible for the high-capacity metabolic inactivation of electrophilic compounds and toxic substrates, and its inhibition influences cellular stress responses and apoptosis (Yin et al. 2000).
GST and catalase are two indicators of oxidative stress, and in marine invertebrates exposed to NPs, changes in these parameters have been linked to population endpoints, as well as to mortality (Canesi et al. 2010a). Catalase is the main enzyme involved in hydrogen peroxide detoxification, and its alteration indicates induced ROS production (Jemec et al. 2008b).
The gradual decrease in catalase activity observed in the samples exposed to the SnO2 NPs might be correlated to the starting point of the hydroxyl radical production, as this inhibitory effect is not significant. ROS production might occur at higher concentrations of these MO-NPs, although this possibility was not examined in the present study. Hydroxyl radical production leads to the production of hydrogen peroxide (Halliwell and Gutteridge 1999), which results in rapid inactivation of catalase (Aebi 1984). Therefore, the larva exposure to SnO2 NPs induces defence responses as a result of a stress, through decreased GST and catalase activities.
The exposure to Fe3O4 NPs caused significant increases in catalase and cholinesterase and a gradual inhibition of GST activity. It might be suggested that the antioxidant mechanisms can prevent oxidative damage. This can be explained with the opposite trend of the defence biomarker activities. During exposure to Fe3O4 NPs, the larvae showed a stress response that was due to inflammation (an increase in cholinesterase), rather than ROS production.
Both of these biochemical biomarkers of defence (i.e., GST, catalase) showed decreased activities after acute exposure to the CeO2 NPs, although these were not statistically different from the control samples. Therefore, exposure to these MO-NPs results in a weak stress response. It is possible that the defence biomarker sensitivities towards this type of MO-NPs decrease at higher concentrations. Thus, GST and catalase do not represent significant biomarkers for concentrations of CeO2 NPs up to 1 mg/mL, as it has been reported in mussel gills exposed to oxide NPs (Canesi et al. 2010b).
Our data so far show that the acute exposure of A. salina larvae to these different MO-NPs induces significant changes in their behaviour and enzyme activities. SnO2 NPs and CeO2 NPs appear to affect the larval behavioural and biochemical responses more so than Fe3O4 NPs. The sensitivities of these behavioural and biochemical endpoints appear to depend on the type of MO-NPs. Nevertheless, cholinesterase activity represents a sensitive and significant biomarker of damage for all of these selected MO-NPs. Conversely, biomarkers of defence appear to be MO-NP-specific, as already proposed for the exposure of freshwater crustaceans to chemicals and NPs (Jemec et al. 2008b; Canesi et al. 2010a; Buffet et al. 2011). GST is more sensitive to SnO2 NPs than the other MO-NPs, whereas Fe3O4 NPs induce higher catalase activities compared to the other MO-NPs, with a 1-fold increase with respect to the control at all of these concentrations.
Conclusions
The exposure of these A. salina larvae to the selected MO-NPs did not induce significant mortality, although the NPs accumulated in the gut. However, behavioural and biochemical changes occurred after the exposure. The swimming speed alterations and the cholinesterase activities represent valid endpoints for SnO2-NP exposure, whereas the other two enzyme activities appear to be MO-NP-specific.
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Alyuruk, H., Demir, G. K., & Cavas, L. (2013). A video tracking based improvement of acute toxicity test on Artemia salina. Marine and Freshwater Behaviour and Physiology. doi:10.1080/10236244.2013.814224.
Amiard-Triquet, C. (2009). Behavioral disturbances: the missing link between sub-organismal and supra-organismal responses to stress? Prospects based on aquatic research. Human and Ecological Risk Assessment, 15, 87–110.
Ates, M., Daniels, J., Arslan, Z., & Farah, O. I. (2013a). Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: assessment of nanoparticle aggregation, accumulation, and toxicity. Environmental Monitoring and Assessment, 185, 3339–3348.
Ates, M., Daniels, J., Arslan, Z., Farah, O. I., & Rivera, H. F. (2013b). Comparative evaluation of impact of Zn and ZnO nanoparticles on brine shrimp (Artemia salina) larvae: effects of particle size and solubility on toxicity. Environmental Science: Processes and Impacts, 15, 225.
Barahona, M. V., & Sanchez-Fortun, S. (1999). Toxicity of carbamates to the brine shrimp Artemia salina and the effect of atropine, BW284c51, iso-OMPA and 2-PAM on carbaryl toxicity. Environmental Pollution, 104, 469–476.
Barsan, N., Schweizer-Berberich, M., & Gopel, W. (1999). Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: a status report, Fresenius. Journal of Analytical Chemistry, 365, 287–304.
Baun, A., Hartmann, N. B., Grieger, K., & Kusk, K. O. (2008). Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology, 17, 387–395.
Buffet, P. E., Tankoua, O. F., Pan, J.-F., Berhanu, D., Herrenknecht, C., Poirier, L., et al. (2011). Behavioural and biochemical responses of two marine invertebrates Scrobicularia plana and Hediste diversicolor to copper oxide nanoparticles. Chemosphere, 84, 166–174.
Buzea, C., Blandino, P., & Robb, K. (2007). Nanomaterials and nanoparticles: sources and toxicity. Biointerphases, 2, MR17–MR172.
Canesi, L., Fabbri, R., Gallo, G., Vallotto, D., Marcomini, A., & Pojana, G. (2010a). Biomarkers in Mytilus galloprovincialis exposed to suspension of selected nanoparticles (nanocarbon black, C60 fullerene, nano-TiO2, nano-SiO2). Aquatic Toxicology, 100, 168–177.
Canesi, L., Ciacci, C., Vallotto, D., Gallo, G., Marcomini, A., & Pojana, G. (2010b). In-vitro effects of suspensions of selected nanoparticles (C60 fullerene, TiO2, SiO2) on Mytilus hemocytes. Aquatic Toxicology, 96, 151–158.
Cornejo-Garrido, H., Kibanova, D., Nieto-Camacho, A., Guzmàn, J., Ramirez-Apan, T., Fernandez-Lomelin, P., et al. (2011). Oxidative stress, cytotoxicity, and cell mortality induced by nano-sized lead in aqueous suspensions. Chemosphere, 84, 1329–1335.
Cornelis, G., Ryan, B., McLaughlin, M. J., Kirbi, J. K., Beak, D., & Chittleborough, D. (2011). Solubility and batch retention of CeO2 nanoparticles in soils. Environmental Science and Technology, 45, 2777–2782.
D’Agata, A., Fasulo, S., Dallas, L. J., Fisher, A. S., Maisano, M., Readman, J. W., et al. (2013). Enhanced toxicity of “bulk” titanium dioxide compared to “fresh” and “aged” nano-TiO2 in marine mussels (Mytilus galloprovincialis). Nanotoxicology, 8, 549–558.
de Oliveira, P., Gomes, A. Q., Pacheco, T. R., de Almeida, V. V., Saldanha, C., & Calado, A. (2012). Cell-specific regulation of cholinesterase expression under inflammatory conditions. Clinical Hemorheology and Microcirculation, 51, 129–137.
Ellman, G. L., Courtney, K. D., Andres, V., & Featherstone, R. M. (1961). A new and rapid colorimetric determination of cholinesterase activity. Biochemical Pharmacology, 7, 88–95.
EPA (2002). Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 5th ed. http://water.epa.gov/scitech/methods/cwa/wet/disk2_index.cfm. Accessed October 2002.
Falugi, C., Rakonczay, Z., Thielecke, H., Guida, C., Aluigi, M.G. (2011). Cholinergic pesticides. In: Pesticides—the impacts of pesticides exposure, Agricultural and Biological Sciences. Ed. Stoytcheva M, ISBN: 978-953-307-531-0, Chapter 11: 221-242.
Falugi, C., Aluigi, M. G., Chiantore, M. C., Privitera, D., Ramoino, P., Gatti, M. A., et al. (2012). Toxicity of metal oxide nanoparticles in immune cells of the sea urchin. Marine Environmental Research, 76, 114–121.
Falugi, C., & Aluigi, M. G. (2012). Early appearance and possible functions of non-neuromuscular cholinesterase activities. Frontiers in Molecular Neuroscience, 5, 54.
Gaiser, B. K., Biswas, A., Rosenkaranz, P., Jepson, M. A., Lead, J., Stone, V., et al. (2011). Effects of silver and cerium dioxide micro- and nano-sized particles on Daphnia magna. Journal of Environmental Monitoring, 13, 1227.
Galloway, T., Lewis, C., Dolciotti, I., Johnston, B. D., Moger, J., & Regoli, F. (2010). Sublethal toxicity of nano-titanium dioxide and carbon nanotubes in a sediment dwelling marine polychaete. Environmental Pollution, 158, 1748–1755.
Gambardella, C., Aluigi, M. G., Ferrando, S., Gallus, L., Ramoino, P., Gatti, A. M., et al. (2013). Developmental abnormalities and changes in cholinesterase activity in sea urchin embryos and larvae from sperm exposed to engineered nanoparticles. Aquatic Toxicology, 130(131), 77–85.
Gambardella, C., Gallus, L., Gatti, M., Faimali, M., Carbone, S., Antisari Vittori, L., Falugi, C., Ferrando, S. (2014). Toxicity and transfer of metal oxide nanoparticles from microalgae to sea urchin larvae. Chemistry and Ecology. doi:10.1080/02757540.2013.873031.
Garaventa, F., Gambardella, C., Di Fino, A., Pittore, M., & Faimali, M. (2010). Swimming speed alteration of Artemia sp. and Brachionus plicatilis as a sub-lethal behavioural end-point for ecotoxicological surveys. Ecotoxicology, 19, 512–519.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249, 7130–7139.
Halliwell, B., & Gutteridge, J. M. C. (1999). Free radicals in biology and medicine. New York: Oxford University Press.
Hui, C., Shen, C., Yang, T., Bao, L., Tian, J., Ding, H., et al. (2008). Large-scale Fe3O4 nanoparticles soluble in water synthesized by a facile method. Journal of Physiology and Chemistry Part C, 12, 11336–11339.
Hund-Rinke, K., & Simon, M. (2006). Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environmental Science and Pollution Research, 13, 225–232.
Idota, Y., Kubota, T., Matsufuji, A., Maekawa, Y., & Miyasaka, T. (1997). Tin-based amorphous oxide: a high-capacity lithium-ion-storage material. Science, 276, 1395–1397.
Jamnik, P., & Raspor, P. (2003). Stress response of yeast Candida intermedia to Cr(VI). Journal of Biochemical and Molecular Toxicology, 17, 316–323.
Jemec, A., Tišler, T., Drobne, D., Sepčić, K., Fournier, D., & Trebše, P. (2007). Comparative toxicity of imidacloprid, of its commercial liquid formulation and of diazinon to a non-target arthropod, the microcrustacean Daphnia magna. Chemosphere, 68, 1408–1418.
Jemec, A., Drobne, D., Remškar, M., Sepčić, K., & Tišler, T. (2008a). Effects of ingested nano-sized titanium dioxide on terrestrial isopods (Porcellio scaber). Environmental Toxicology and Chemistry, 9, 1904–1914.
Jemec, A., Tišler, T., Drobne, D., Sepčić, K., Jamnik, P., & Roš, M. (2008b). Biochemical biomarkers in chronically metal-stressed daphnids. Comparative Biochemistry and Physiology Part C, 147, 61–68.
Klaine, S. J., Alvarez, P. J. J., Batley, G. E., Fernandes, T. F., Handy, R. D., Lyon, D. Y., et al. (2008). Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environmental Toxicology and Chemistry, 27, 1825–1851.
Koehler, A., Marx, U., Broeg, K., Bahns, S., & Bressling, J. (2008). Effects of nanoparticles in Mytilus edulis gills and hepatopancreas—a new threat to marine life? Marine Environmental Research, 66, 12–14.
Kokkali, V., Katramados, I., & Newman, J. D. (2011). Monitoring the effect of metal ions on the mobility of Artemia salina nauplii. Biosensors, 1, 45.
Koutsaftis, A., & Aoyama, I. (2007). Toxicity of four antifouling biocides and their mixtures on the brine shrimp Artemia salina. Science of the Total Environment, 387, 166–174.
Little, E. E., & Finger, S. E. (1990). Swimming behaviour as an indicator of sublethal toxicity in fish. Environmental Toxicology and Chemistry, 9, 13–19.
Little, E. E., Archeski, R. D., Flerov, B. A., & Kozlovskaya, V. I. (1990). Behavioral indicators of sublethal toxicity in rainbow trout. Archives of Environmental Contamination and Toxicology, 19, 380–385.
Mancini, I., Sicurelli, A., Guella, G., Turk, T., Maček, M., & Sepčić, K. (2004). Synthesis and bioactivity of linear oligomers related to polymeric alkylpyridinium metabolites from the Mediterranean sponge Reniera sarai. Organic and Biomolecular Chemistry, 2, 1368–1375.
Montes, M. O., Hanna, S. K., Lenihan, H. S., & Keller, A. A. (2012). Uptake, accumulation and biotransformation of metal oxide nanoparticles by a marine suspension-feeder. Journal of Hazardous Materials, 225–226, 139–145.
Nunes, B. S., Carvalho, F. D., Guilhermino, L. M., & Van Stappenn, G. (2006). Use of the genus Artemia in ecotoxicity testing. Environmental Pollution, 144, 453–462.
Peng, X., Palma, S., Fisher, N. S., & Wong, S. S. (2011). Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquatic Toxicology, 102, 186–198.
Persoone, G., & Wells, P. G. (1987). Artemia in aquatic toxicology: a review. In P. Sorgeloos, D. A. Bengtson, W. Decleir, & F. Jasper (Eds.), Artemia research and its application (Morphology, genetics, strain characterization, Vol. 1, pp. 259–275). Wetteren: Toxicology. Universa Press.
Radhika Rajasree, S. R., Ganesh Vumar, V., Stanley Abraham, L., & Indabakandan, D. (2010). Studies on the toxicological effects of engineered nanoparticles in environment—a review. International Journal of Applied Bioengineering, 4, 2.
Scott, G. R., & Sloman, K. A. (2004). The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquatic Toxicology, 68, 369–392.
Sorgeloos, P., Van Der Wielen, R., & Persoone, G. (1978). The use of Artemia nauplii for toxicity tests—a critical analysis. Ecotoxicology and Environmental Safety, 2, 249–255.
Taylor, P., & Brown, J. H. (1999). Acetylcholine. In G. J. Siegel, B. W. Agranoff, R. W. Albers, S. K. Fisher, & M. D. Uhler (Eds.), Basic neurochemistry: molecular, cellular, and medical aspects (pp. 213–242). Philadelphia: Lippincott-Raven.
Tso, C., Zhung, C., Shih, Y., Tseng, Y. M., Wu, S., & Doong, R. (2010). Stability of metal oxide nanoparticles in aqueous solutions. Water Science and Technology, 61, 127–133.
Varò, I., Navarro, J. C., Amat, F., & Guilhermino, L. (2002). Characterisation of cholinesterases and evaluation of the inhibitory potential of chlorpyrifos and dichlorvos to Artemia salina and Artemia parthenogenetica. Chemosphere, 48, 563–569.
Venkateswara Rao, J., Kavitha, P., Jakka, N. M., Sridhar, V., & Usman, P. K. (2007). Toxicity of organoposphates on morphology and locomotor behavior in brine shrimp, Artemia salina. Archives of Environmental Contamination and Toxicology, 53, 227–232.
Wang, Z., Zhao, J., Li, F., Gao, D., & Xing, B. (2009). Absorption and inhibition of acetylcholinesterase by different nanoparticles. Chemosphere, 77, 67–73.
Xia, J., Fu, S., Cao, Z., Peng, J., Peng, J., Dai, T., et al. (2013). Ecotoxicological effects of waterborne PFOS exposure on swimming performance and energy expenditure in juvenile goldfish (Carassius auratus). Journal of Environmental Science, 25, 1672–1679.
Yin, Z., Ivanov, V. N., Habelhah, H., Tew, K., & Ronai, Z. (2000). Glutathione S-transferase elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer Research, 60, 4053–4057.
Zhang, X. J., Yang, L., Zhao, Q., Caen, J. P., He, H. Y., Jin, Q. H., et al. (2002). Induction of acetyl-cholinesterase expression during apoptosis in various cell types. Cell Death and Differentiation, 9, 790–800.
Zhu, B. K., Wang, P., Zhang, X. D., Jiang, C. C., Li, H., Avery-Kiejda, K. A., et al. (2008). Activation of Jun N-terminal kinase is a mediator of vincristine-induced apoptosis of melanoma cells. Anti-Cancer Drugs, 19, 189–200.
Acknowledgments
Chiara Gambardella would like to thank Prof Carla Falugi for her kind suggestions, and POSDRU/89/1.5/S/63663-Sectorial Operational Programme for Human Resources Development through the project “Transnational network for integrated management of postdoctoral research in Science Communication. Institutional framing (postdoctoral school) and scholarship programme (CommScie).” The Slovenian authors gratefully acknowledge the Slovenian Research Agency, and Tina Mesarič acknowledges the Slovene Human Resources Development and Scholarship Fund for financial support. The experiments comply with the current European laws of bioethics. Dr. Christopher Berrie is acknowledged for editing and appraisal of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 501 kb)
Rights and permissions
About this article
Cite this article
Gambardella, C., Mesarič, T., Milivojević, T. et al. Effects of selected metal oxide nanoparticles on Artemia salina larvae: evaluation of mortality and behavioural and biochemical responses. Environ Monit Assess 186, 4249–4259 (2014). https://doi.org/10.1007/s10661-014-3695-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3695-8