Abstract
This study aims to assess the relative importance of natural and anthropogenic variables on the change of the red-crowned crane habitat in the Yellow River Nature Reserve, East China using multitempopral remote sensing and geographic information system. Satellite images were used to detect the change in potential crane habitat, from which suitable crane habitat was determined by excluding fragmented habitat. In this study, a principal component analysis (PCA) with seven variables (channel flow, rainfall, temperature, sediment discharge, number of oil wells, total length of roads, and area of settlements) and linear regression analyses of potential and suitable habitat against the retained principal components were applied to explore the influences of natural and anthropogenic factors on the change of the red-crowned crane habitat. The experimental results indicate that suitable habitat decreased by 5,935 ha despite an increase of 1,409 ha in potential habitat from 1992 to 2008. The area of crane habitat changed caused by natural drivers such as progressive succession, retrogressive succession, and physical fragmentation is almost the same as that caused by anthropogenic forces such as land use change and behavioral fragmentation. The PCA and regression analyses revealed that natural factors (e.g., channel flow, rainfall, temperature, and sediment discharge) play an important role in the crane potential habitat change and human disturbances (e.g., oil wells, roads, and settlements) jointly explain 51.8 % of the variations in suitable habitat area, higher than 48.2 % contributed by natural factors. Thus, it is vital to reduce anthropogenic influences within the reserve in order to reverse the decline in the suitable crane habitat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Land use changes constitute one of the most important driving forces of biodiversity loss (Sala et al. 2000), usually resulting from habitat destruction and habitat fragmentation. Habitat destruction is the most significant cause in the decline and extinction of populations and species of animals and plants (Prugh et al. 2008). Habitat fragmentation is considered the leading cause of species extinction (Pimm and Raven 2000) and the most serious threat to biological diversity (Wilcox and Murphy 1985).
The red-crowned crane (Grus japonensis) is the second rarest species of crane, with a total population of 2,750 in the wild (Bird Life International 2012). A migratory population of about 1,550 bird breeds in southeastern Russia, northeastern China, and Mongolia (Wang 2008). Red-crowned cranes prefer to nest in a marsh with relatively shallow water and in areas with standing dead vegetation (Smirenski 1988). The main factors affecting the selection of wetlands as a habitat by the crane are water, food, shelters, and disturbance (Xiao et al. 2001). The first three factors are related to the physical needs of the crane, whereas the last factor is associated with human activities. The key threat of the red-crowned crane is the loss and degradation of wetlands in its breeding and wintering grounds (Harris 1997), which is responsible for a declined crane population.
Existing studies on the habitat selection of the red-crowned crane have been carried out so far via field observations (Ma et al. 1999; Shu et al. 2006; Wu and Zou 2011). Restricted by human resources and funding, such observations usually last 3–6 years. With global climate change, economic development, wetland restoration, and other human activities, a crane habitat has inevitably been changed, which in turn alters how the crane favors a habitat and the spatial distribution of the crane population (Li et al. 2012; Jiang et al. 2012).
The red-crowned crane is prone to environmental changes induced by natural and anthropogenic factors, such as climate change, hydrologic change, marsh degradation, shoreline erosion (Halpern 1992), and reclamation of wetlands for other uses (Lee et al. 2007). The net effect of such changes is crane habitat loss that can be efficiently detected from satellite imagery (Gottschalk et al. 2005; Liu et al. 2010). In particular, integration of multitemporal remotely sensed data with geographic information system (GIS) provides a powerful tool for analyzing spatial–temporal patterns of the habitat change (Nagendra 2001). Analysis of the change between crane habitat and other covers is conducive to revealing the drivers of habitat change. Analysis of habitat fragmentation, shrinkage, and loss helps understand the complex interactions between natural environmental changes and anthropogenic activities (Forman 1997).
The Yellow River Delta National Nature Reserve (YRDNR), established in 1992 by the Chinese government, has been listed in the world and Chinese biodiversity conservation and wetland protection directory. Found in the reserve are 283 bird species, 9 of which (including red-crowned crane) have been given the highest priority of protection. This reserve has the most suitable settings for waterfowls because it encompasses a large number of tidal flats and marshes, abundant wetland vegetation, and freshwater organisms. However, over the last three decades, a large part of the natural wetlands has been transformed to other uses, such as aquacultural ponds and agricultural fields (Li et al. 2009). The wetland ecosystem in the reserve has been subject to increasing human disturbance (e.g., petroleum exploitation) since the early 1960s. Oil wells and roads have considerably fragmented the crane habitat (Bi et al. 2011). Moreover, decreased channel flow and sediment discharge of the Yellow River have resulted in frequent and prolonged channel drying up since the 1970s (Fig. 1), causing shrunk freshwater wetlands, degenerated vegetation, and decreased biodiversity (Cui 2002; Wang et al. 2006). These changes slowed down the formation of new habitat at the mouth of the Yellow River (Peng et al. 2010), while existent habitat was degenerated into other covers, such as highly saline tidal flats. Thus, the crane habitat faces grave threats and requires urgent protection.
In order to prevent the wetlands in the reserve from further deterioration and to protect the crane and other endangered birds, a wetland restoration project was initiated in July 2002 (Fig. 2). The main restoration activities included the construction of dikes to prevent seawater incursion and the artificial replenishment of freshwater to the wetlands through a newly dug canal. By 2007, the wetland ecosystem had been significantly improved as judged by the continued decline in soil salinity to over 70 % of the level in 2001 (Cui et al. 2009), creating a favorable condition for freshwater vegetation to thrive. Nevertheless, by 2008, the crane habitat has not returned to its former state at the time of reserve creation (Wang et al. 2013). The exact contribution of various factors to habitat change remains unknown. Analysis of such a habitat change and its affecting factors will yield crucial evidences for better protecting wildlife habitats and devising effective wetland restoration measures in the future (Day et al. 2000).
This study attempts to develop an appropriate remote sensing and GIS method for quantifying the exact influence of factors causing the change in the red-crowned crane habitat in the YRDNR and to explore the evolving impact that natural and anthropogenic variables exert on the change. The specific objectives are (1) to identify the spatiotemporal changes that have taken place to the habitat during 1992–2008, (2) to determine the relative importance of natural and anthropogenic drivers in causing habitat fragmentation and habitat change, (3) to critically assess the relative influence of seven variables (channel flow, rainfall, temperature, sediment discharge, number of oil wells, total length of roads, and area of settlements) on the change in potential and suitable crane habitat, and (4) to assess the effectiveness of wetland restoration efforts in rehabilitating the crane habitat.
Study area
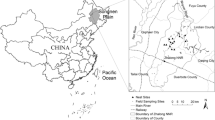
Situated in the estuary of the Yellow River in Dongying City, Shandong Province of East China (37°35′–38°12′ N, 118°33′–119°20′ E), the YRDNR comprises two parts: the floodplain of the former river mouth of the Yellow River in the north and the floodplain of the current river mouth in the east (Fig. 2), at a combined area of 153,000 ha (Zhao and Song 1995). It has a warm temperate continental monsoon climate with distinctive seasonality. Annual temperatures has an average of 11.7–12.6 °C, and mean annual precipitation varies between 530 and 630 mm, 70 % of which falls during summer. However, average annual evaporation is almost 3.5 times the average yearly precipitation. Approximately 10.5 million tons of sand and soil discharged by the river annually is deposited in the delta, forming a vast floodplain and special wetland landscape (Xu et al. 2002).
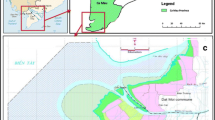
The overall pattern of wetland vegetation distribution in the study area is controlled by soil moisture and salinity. Two types of vegetation succession exist in this region (Yue et al. 2004): halophytic and hygrophyte. The former is characterized by a distribution with salinity gradient. With a landward rise in elevation, a decrease in salinity, and a rise in soil organic matter, vegetation changes from tidal flats, seablite (Suaeda salsa Pall.) community, tamarisk community, aeluropus community, Lalang grass community, to forest and dry fields (Fig. 3). Hygrophyte succession is associated with soil moisture. Radiating from the river channel, vegetation varies from floodplains, reeds, Lalang grass, willow, to dry fields. These two types of succession are intermixed spatiotemporally, both being progressive in nature (solid line in Fig. 3). In contrast, retrogressive succession refers to degeneration of reed marshes and seablite community into tidal flats due to seawater incursion (see dashed arrows in Fig. 3).
Materials and methods
Data collection
Multitemporal Landsat data have been widely used to detect coastal wetland changes (Yvonne et al. 2012). In this study, 11 Landsat Thematic Mapper (TM) images, all having a cloud cover of <10 %, were collected (Table 1). They were captured during August–October, a period corresponding to the growing and late rainy season that is ideal for extracting water and vegetation information from the images. Other satellite data collected were SPOT and ALOS images at a resolution ranging from 10 m (multispectral bands) to 2.5 m (panchromatic bands) (Table 1) for the purpose of facilitating the mapping of roads, settled areas, and oil wells inside the study area.
Given that the restoration efforts of the coastal wetlands were directed at boosting riverine inputs of freshwater and sediments to the ecosystem (Day et al. 2000), hydrological data essential in this kind of study were also collected. Average annual channel flow (F) and mean annual sediment discharge (S) were collected from the Lijin hydrographic station (1992–2008) about 100 km upstream the Yellow River mouth (Figs. 1 and 2). In addition, annual temperature (T) and precipitation (PPT) data of the same period were also collected from the Dongying meteorological station, about 60 km from the reserve.
Data preprocessing
All the acquired images had already been radiometrically corrected. They were registered to the Gauss projection using ENVI v4.5 in a number of steps. First, the 1998 topographic maps (1:50,000) of the study area were used to rectify geometrically the 1999 TM images to an accuracy of <0.3 pixels. Next, this geometrically corrected image was used to rectify all the remaining TM images at an accuracy of <0.5 m (root-mean-square error or RMSE). During image rectification, all output images were resampled to 30-m resolution. Third, the SPOT and ALOS images were geometrically rectified using a total of 24 ground control points (GCPs). Deemed sufficiently adequate for the coastal plain, these GCPs were collected in the field using a GPS unit in 2006 and 2009. These GPS coordinates had a horizontal accuracy of <1 m after differential correction. Image rectification was achieved at an RMSE of <0.4 pixels. Finally, the ALOS and SPOT panchromatic bands (2.5-m spatial resolution) were fused with the 10-m ALOS and SPOT multispectral bands, respectively, to take advantage of their rich multispectral information and fine spatial resolution.
The boundary of the reserve was delimited from the Yellow River Delta spatial database obtained from the Atlas of the Yellow River Delta (Liu and Drost 1997). Although the reserve has a fixed boundary, its land component as recorded on the satellite images varies widely due to the altered river course, sedimentation, and varying tidal heights at the time of imagining. The maximal shoreline delineated from the Landsat TM images was construed as the seaside boundary of the reserve. This coastline ensures that the study area has an identical spatial extent in all the spatial data used, a prerequisite in detecting the spatiotemporal change of the crane habitat.
Assessment of the impact of human disturbance on the crane habitat requires data on roads, settlements, and oil wells. However, it is difficult to directly interpret oil wells and roads from Landsat TM images due to their relatively coarse resolution. Such information was visually interpreted from the PAN-sharpened SPOT and ALOS images acquired in 2005 and 2008, respectively (Table 1). Knowledge and experience gained from the interpretation were applied to digital image classification (unsupervised combined with supervised) of 2005 and 2008 TM images. With reference to such results, the 1998 topographic map (1:50,000), and the 1996 1:10,000 land use map, similar information was also acquired from TM images recorded in other years (see Table 1) in a similar manner. In this way, maps of roads, settlements, and oil wells at 11 different years were produced.
Habitat mapping
In the YRDNR, the habitats favored by the crane are reed marshes amid shallow water with little human disturbance and seablite tidal flats (Cao and Liu 2008; Shu et al. 2006). They offer the crane rich sources of food, such as tender shoots of reeds and other aquatic plants, seeds, roots, and stalks of seablite, in addition to fish, shellfish, and insects. Reed marshes are defined as permanent or seasonal marshes in which reeds are mixed with water of <30 % in proportion. Predominant components of seablite tidal flats are seablite and dwarf, sparse Chinese tamarisk. Given such diversity in vegetation, it is desirable to map vegetation into specific types. However, the coarse (30 m) spatial resolution of TM imagery does not allow all possible types of vegetation present inside the reserve to be mapped accurately. The final habitat mapping scheme was a compromise between the desired detail of vegetation and the image resolution. In total, nine land covers were mapped, including meadows, farmland, freshwater, aquaculture, shrub–grass, forest, reed marshes, seablite tidal flats (≥30 % vegetation cover), and highly saline tidal flats (<30 % vegetation cover). Among these covers, only reed marshes and seablite tidal flats are potential crane habitat. However, the other covers had to be mapped so as to identify the transformation between crane habitat and them and to yield information on the causes of crane habitat change.
The maximum likelihood classifier (MLC) in ENVI v4.5 was used mapping the land cover types, assisted by the 1996 land use map, and field samples collected in 1999, 2001, 2004, 2006, and 2008. The quantity of samples ranges from 56 to 204 for each of the nine ground covers. These samples were divided randomly into two parts. The first half ranging from 28 to 102 for a given type of land cover was used in supervised image classification, and the second half, for accuracy assessment. The knowledge gained from analyzing the 1999, 2001, 2004, 2006, and 2008 images was applied to the analysis of the TM images recorded in six other years (see Table 1). The mapping accuracy of each of the nine land covers (i.e., meadows, farmland, freshwater, aquaculture, shrub–grass, forest, reed marshes, seablite tidal flats, and highly saline tidal flats), averaged from the 11 TM images, was 80, 84, 97, 98, 81, 86, 88, 93, and 95 %, respectively. The accuracy for reed marshes and seablite tidal flats (i.e., defined as the potential crane habitat) was 88 and 93 %, respectively, and was suitable to analyzing its change in 1992–2008. The overall accuracy for the nine land covers (including potential crane habitat) from the 11 TM images was between 85 and 94 % with a kappa coefficient ranging from 0.84 to 0.91.
The 1992 and 2008 maps were analyzed comparatively to detect land cover change during this period because 1992 is the year when the YRDNR was founded while 2008 is the most recent date with a complete set of the most useful TM data. Eight land use and land cover types (see Table 2) were firstly identified from the TM images of 1992 and 2008 (based on MLC classification results), respectively; then, a conversion matrix was generated using spatial overlay analysis, and the types of vegetation succession (including progressive and retrogressive succession and land use change by human activities) were finally determined (Table 2). Regression analysis of the detected change in crane habitat against the seven natural and anthropogenic factors shed light on their relative importance in affecting habitat change during the study period.
In order to assess the effectiveness of wetland restoration, the potential crane habitat, water area, and human disturbance factors in 1999 and 2002 were studied in detail because in 1999, the Yellow River Conservancy Commission of the Ministry of Water Resources (YRCCMWR) took over the responsibility of managing the Yellow River solely. Since then, the river has never dried up. July 2002 was the time when the Yellow River water was pumped to the downstream wetlands to offset the low channel runoff during the dry seasons of March–May and September–November annually (Cui et al. 2009). The water area of each year was detected from the composites of Landsat TM images (R/G/B vs. TMs 7/5/1).
Derivation of habitat fragmentation
As a relatively big water fowl (adult crane can have a height over 1 m with a pair of wings as wide as 4 m), the red-crowned crane requires a relatively large habitat to survive. It is very difficult for them to live in a highly fragmented landscape as it affords limited sources of food and living space. Thus, they are sensitive to external disturbance and habitat fragmentation.
There are two categories of habitat fragmentation: physical and behavioral. Physical fragmentation refers to the physical distance of a potential habitat to the nearest water over a threshold or the potential habitat area <30 m2 (Zhang et al. 2006). Given that the crane is organized as families of two to four members (two adults plus one or two juveniles) and a subcommunity of 10–20 members in structure, it was decided in this study that potential habitat must have a minimum size above 0.81 ha or 3 × 3 TM pixels. Any reed marshes and seablite tidal flats of <0.81 ha in size were deemed too physically fragmented to be of any use to the crane. Since crane habitat lies next to water, proximity to water bodies is also critical to habitat fragmentation. The exact proximity has never been defined in the literature, but was set at 500 m by Cao and Liu (2008) and <15 m by Zhang et al. (2006). The conservative threshold of <15 m was adopted. Thus, all patches of reed marshes and seablite tidal flats of <0.81 ha in size and reed marsh patches falling outside all 15-m buffers around water bodies are considered physically fragmented.
Behavioral fragmentation is induced by human disturbance. Patchy–spotted oil wells, residential areas, and linear roads are all causes of behavioral fragmentation as they impose barriers on crane behavior. According to the field survey in the 1990s (Hu and Xiao 1999; Xiao et al. 2001) in Bohai Bay in which the Yellow River Delta and Liao River Delta are located, red-crowned crane selects its habitat far from human structures, such as roads, oils wells, and residential areas, and the physical distance from these artificial structure to crane habitat should be >200 m from roads, >300 m from oil wells, and >750 m from residential areas. In our study, road is expressed as a linear object, oil well as a dot, and residential area as polygon. However, in fact, roads have certain widths and oil wells also have certain areas. Based on field observation, a road width was determined to be 30 m and the diameter of an oil well to be 100 m, and then, behavioral fragmentation was calculated by generating buffer of appropriate widths, namely 215 m (200 + 15) for roads, 330 m (300 + 30) for oil wells, and 750 m for residential areas.
Although the habitat fragmentation is also affected by a cover type that usually takes the form of sparse vegetation of 0.5–1.3 m high for the crane (Ma et al. 2000), observations over many years in the study area suggest that the red-crowned cranes are not choosy on the density and height of the cover type. For instance, it is also possible for them to choose seablite tidal flats of a sparse vegetative cover. However, the water in the reed marshes had better be shallower than 30 cm (Shu et al. 2004). Since no data on water depth in the reed marshes were available, a suitable crane habitat is assumed to be the total habitat exclusive of the total amount of fragmented habitat (sum of both physically and behaviorally fragmented habitat). All processing was carried out in ArcGIS 9.2.
Quantifying natural and anthropogenic influences
Based on the actual habitat change in the study area, causes of habitat were grouped into three types: progressive progression, retrogressive succession, and land use/cover change. They are differentiated into natural and anthropogenic. A change is mostly natural if it is caused by progressive progression, retrogressive succession, and physical fragmentation. For instance, the vegetation progressive progression is driven by soil salinity and soil moisture while vegetation retrogressive progression is accompanied by an increase in soil salinity as a result of seawater incursion. By comparison, land use/cover change exerts both positive and negative influences on a crane habitat change as it involves human activities such as wetland restoration, land reclamation for agriculture, and aquaculture over marshes. Some seablite tidal flats may be turned into acquacultural or agricultural uses, while big storm surge and land abandonment may cause woodland and farmland to become seablite tidal flats. Wetland restoration efforts may also cause Chinese tamarisk, Chinese aelurous, Lalang grass community, and even woodland and dry fields to become reed marshes (shown as thickened arrows in Fig. 3). Thus, a habitat change induced by land use change and behavioral fragmentation is the result of human activities.
Natural and human disturbance variables
The Yellow River plays a very important role in maintaining the health of the Yellow River Delta ecosystem that is governed by the conjunct influence of channel flow and sediments discharged by the river (Wang et al. 2011), with a positive correlation between channel runoff, sediment discharge, and reed marshes (Li et al. 2009). In particular, sediment discharge plays a decisive role in land accretion in the Yellow River Delta (Hu and Cao 2003) and affects the formation of seablite tidal flats indirectly. Hijmans and Graham (2006) reported that the ecological factors affecting the spatial distribution of vegetation on the regional scale are chiefly rainfall and temperature. Precipitation directly affects the area of reed marshes, while temperature plays a secondary role by affecting evaporation. A warmer climate accelerates evaporation, resulting in reduced soil moisture and shrunk marshes (Li et al. 2009). Thus, channel flow and sediment discharge of the Yellow River, rainfall, and temperature were considered the natural drivers of a habitat change while roads, settlements, and oil wells were regarded as the anthropogenic disturbance to behaviorally fragment crane habitat.
Regression analysis
In order to determine the influence of natural and human disturbance variables on the crane habitat, the climate, and hydrological and human disturbance, data were comprehensively analyzed in a number of steps. First, the dependency of potential crane habitat area (A) and suitable habitat area (A s) on mean annual temperature, precipitation, channel flow, sediment discharge, and the number of oil wells, the total length of roads and the area of settlements of the same year were estimated via correlation analysis. Next, given the colinearity between natural and human disturbance factors, principal component analysis (PCA) was undertaken for the above seven variables, with those components having an eigenvalue of >1 output. Finally, A and A s were respectively regressed linearly against the retained components. Only those components whose coefficients in a regression equation are statistically significant in a T test were retained in the overall regression equation (significant in an F test) in order to identify the variables that significantly influence the potential or suitable crane habitat.
Results
Spatiotemporal change of potential habitat
Shown in Table 2 is the conversion between potential crane habitat (including reed marshes and seablite tidal flats) and other land covers. During 1992–2008, potential habitats increased 1,409 ha (6,980–5,571), owing to a gain of 6,980 ha (10,117–3,137) of reed marshes chiefly from meadow and shrub–grass. On the other hand, seablite tidal flats lost 5,571 ha (12,195–6,624) because of their conversion to highly saline tidal flats, reclamation for aquaculture, and conversion to freshwater (Table 2).
The largest conversion was triggered by land use and land cover change at 16,278 ha, of which the newly gained area exceeds the lost area by more than 2,000 ha. For example, wetland reclamation for aquaculture and farming caused a loss of 3,881 ha of habitat. On the other hand, wetland restoration caused extensive meadow and shrub–grass to be turned back to reed marshes, leading to a gain of 6,338 ha in crane habitat. Retrogressive succession caused the second-most extensive change at 9,203 ha, which resulted in 6,905 ha of seablite tidal flats being converted to highly saline tidal flats. The reduction in habitat is nearly five times the increase. Progressive succession caused the smallest change at 6,592 ha.
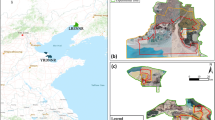
Illustrated in Fig. 4 are the spatial variations of reed marshes, seablite tidal flats, and water in 1992, 1999, 2002, and 2008. During 1992–2002, both reed marshes and seablite tidal flats experienced a large decrease, especially in the south of the reserve. During 1992–2008, extensive seablite tidal flats were turned to highly saline tidal flats via retrospective succession. However, new seablite communities emerged out of the seawater near the Yellow River mouth in 2008. Reed marshes drastically expanded along the riverbanks by 2008, owing to the efforts of wetland restoration. Such reed marshes replaced former meadows and shrub–grass. Reed marshes in the south of the northern reserve in 1992 changed to water after 1999 due to a reservoir that was constructed in the interim.
Habitat fragmentation
In 1992, the area of behaviorally fragmented habitat was larger than that of physically fragmented habitat (Table 3). Behavioral fragmentation of habitat was triggered mostly by roads. By 2008, behaviorally fragmented habitat has almost the same size as physically fragmented habitat. Furthermore, habitat fragmented by oil wells, roads, and settlements increased considerably as a result of intensified human disturbance. Although potential habitat was larger in 2008 than in 1992, suitable habitat decreased from 15,749 to 9,814 ha during the same period as a consequence of both physical and behavioral fragmentations.
The aforementioned changes in suitable habitat and its fragmentation could be better appreciated from their spatial distribution (Fig. 5). Oil wells were distributed mostly in the north of the reserve. With an increase in density, oil wells were spreading westward and seaward. This spread increasingly fragmented seablite tidal flats. In the south of the reserve, oil wells have a low density. Most of the newly developed wells are located in close proximity to reed marshes, exacerbating the fragmentation of potential habitat (Figs. 4 and 5). By comparison, roads and settlements are located more distantly from the potential crane habitat.
Natural vs. anthropogenic influences
As shown in Fig. 6, natural influence caused a change of 19,942 ha in habitat area, slightly more than 19,474 ha caused by anthropogenic factors. Thus, natural factors have played a slightly more important role in causing potential habitat change. Of the anthropogenic factors, land use change is much more important than behavioral fragmentation, accounting for 83.6 % of the total area. Of natural processes, retrogressive succession accounted for 46.1 % of the total, whereas physical fragmentation and progressive succession contributed 33.1 and 20.8 % of habitat change, respectively.
Habitat conversion caused by different influences during 1992–2008. Data related to progressive succession, retrogressive succession, and land use change are derived from Table 2. These values represent the net changes between increase and decrease
Regression results
The above observed changes in the crane habitat are caused by both natural and anthropogenic factors. Of these factors, channel flow (F) is the most closely related to the potential habitat area (A), followed by sediment discharge (S) (Table 4). However, the positive correlation between sediment discharge and potential habitat is not statistically significant. Further analysis reveals that sediment discharge and seablite tidal flats (a component of potential habitat) are correlated at a rather significant level (r = 0.74, p = 0.009). Although precipitation has a coefficient of 0.40 with A, this correlation is not as strong as that with channel flow. Temperature is negatively correlated with the potential habitat area. A higher temperature causes more evaporation, and thus, less water remains in the reserve. Of the human disturbance factors, total road length (ST) is the most closely and positively correlated with A (Table 4). Their linear configuration means that they exert a wider spread influence on the crane habitat.
Suitable habitat (A s) is positively correlated with S and negatively correlated with T at α = 0.01 significance level, but loosely correlated with human disturbance variables (Table 4). During 1992–2008, seablite tidal flats comprise the absolute majority of suitable habitat, with a percentage varying from 63.5 % in 2007 to 93.3 % in 1999. However, the overall trend is characterized by a decline consistent with the ever decrease in sediment discharge (Fig. 1).
The two components (namely PC1 and PC2) retained after the principal component analysis account for 85.4 % of the extraction sums of squared loading. PC1 captures the influence of human disturbance variables, such as total road length, settlement area, and quantity of oil wells, while PC2 captures the influence of mostly natural factors, such as channel runoff, precipitation, and sediment discharge (Table 5).
The regression equations for potential habitat (A) and suitable habitat (A s) are presented in Eqs. (1) and (2). Equation (1) (R 2 = 0.42, p = 0.03, statistically significant) suggests that PC2 is crucial to the change in the potential habitat. Namely, such natural variables as channel runoff, precipitation, and sediment discharge are the main factors affecting the potential habitat. Since coefficients of PC1 in the regression equation are not statistically significant in the T test, PC1 was removed from Eq. (1). In order to compare the relative contribution of the two components, it was decided to drop the constant term by standardizing regression coefficients (Eq. (2)). In the second regression equation (R 2 = 0.60, p = 0.026) for the suitable habitat, the coefficients of the two components are significant (p = 0.047 for the coefficient of PC2, p = 0.035 for the coefficient of PC1). Equation (2) suggests that A s is negatively related to PC1 and positively related to PC2. If the regression coefficients of PC1 and PC2 sum up to 1, then PC1 mounts to 51.8 % and PC2 contributes 48.2 % of the total change in the suitable habitat. Thus, the suitable habitat is subject slightly more to human disturbance (captured by PC1) than to natural factors.
Discussion
Many researchers have reported similar results as those we derived from this study. For example, Wan et al. (2002) found that red-crowned crane’s nesting habitat had been seriously fragmentized based on field observations in the Shuangtaihekou National Natural Reserve of China. Cao and Liu (2008) analyzed the habitat suitability change of the red-crowned crane due to habitat loss and fragmentation by selecting a series of landscape pattern indices based on the habitat suitability maps in the YRDNR. Wang et al. (2013) also found that the crane suitable habitat in YRDNR was the most fragmented in 2001, but the least fragmented in 1992 based on multitemporal remote sensing data from 1992 to 2008 in a geographic information system. However, compared to previous studies, our study was more emphasized on the cause of the crane habitat change. We first determined how the change and fragmentation in the red-crowned crane habitat were driven by natural drivers and anthropogenic forces. Then, a principal component analysis with seven variables (i.e., natural factors: channel flow, rainfall, temperature, and sediment discharge; and human disturbances: number of oil wells, total length of roads, and area of settlements) and linear regression analyses of potential and suitable habitats against the retained principal components were performed in order to explore natural and anthropogenic influences on the crane suitable habitat change.
The YRDNR is the northernmost wintering habitat for the red-crowned crane (Zhao and Song 1995). It serves mainly as a resting or transit spot during the migration to their breeding ground in Northeast China in spring and to the largest wintering site in the Yancheng Biosphere Reserve in Jiangsu Province, China in autumn (Ma et al. 1999), even though some also opt for wintering here (Zhao and Song 1995). Naturally, habitat requirements by the crane vary with geography. Reed swamp is the nesting habitat favored by the crane in the Zhalong Nature Reserve of Northeast China (Wu and Zou 2011). Here, whether patches of remnant reeds with nearby water bodies appear are the main considerations in crane habitat selection. However, in the Yellow River Delta, reed marshes are the most favored habitat during all seasons (Cao and Liu 2008), due probably to the rich sources of food they supply. Apart from geography, even the same environmental setting will have a varying influence on habitat choice. For instance, the migration distance from human disturbance is the most important consideration. But in winter, food availability becomes more important as human disturbance dwindles or disappears (Shu et al. 2006).
In addition, the distribution of the red-crowned cranes in different places changes with the distribution of freshwater and human activities (Ma et al. 1999) and even with seasonality. For instance, they preferentially favored tidal grasslands and fish ponds in the Yancheng Biosphere Reserve. In a dry season, they may select lowland reed beds or the river mouths with abundant freshwater as their habitat. They even select wheat fields as a new type of habitat, which was not recorded before. So the habitats used by the red-crowned cranes are diverse as they adapt to the changed environments (Ma et al. 1999). Such changes need to be considered in future studies.
In the Zhalong Wetland, Li et al. (1999) conducted a field survey and found crane nest sites located in reed marshes less than 30 m to the water area (pond) with its size of 1–25 m2. In the Yellow River Delta Nature Reserve, Shu et al. (2006) found that the crane favorites a habitat that is far from human disturbance and lower reed marsh coverage with shallow water whenever in the migration or winter season. To the best of our knowledge, no reports on the size of the water area could be seen in the literature. The allocation of reed marshes and water area is important (Wang et al. 2013), for crane’s favorite habitat—reed marshes are defined as permanent or seasonal marshes in which reeds are mixed with water of <30 % in proportion. Therefore, the size of the water bodies is not so important in studying the red-crowned crane habitat; on the contrary, water depth in conjunction with reed density and height should be paid more attention in the future study.
Human activities are one of the most important factors affecting ecosystems (Deffontaine et al. 1995; Forman and Godron 1985). All human activities are a kind of disturbance to nature. Some activities such as constructing corridors to link disjoined habitats (Fleury and Brown 1997) and establishment of nature reserves (Wei et al. 1999) may have a positive impact, while most disturbances are negative (Suchanek 1996; Letnic and Fox 1997). In this study, both positive and negative impacts of human activities on the crane habitat in the reserve have been observed. On the one hand, potential crane habitat lost 1,606 and 2,534 ha to land reclamation and aquaculture, respectively, during 1992–2008 (Table 2). During the study period, the number of oil wells, settled areas, and road length increased from 219, 102 ha, and 344 km to 595, 223 ha, and 592 km, respectively. These activities caused a loss of 3,196 ha in suitable habitat (Table 3). On the other hand, the establishment of the YRCCMWR successfully resolved the channel drying off problem. In particular, the wetland restoration efforts in 2002 caused an increase of 1,409 ha in reed marshes (Table 3). Nevertheless, such reed marshes are not rationally colocated with water (e.g., no water in the vicinity of reed marshes). The amount of physically fragmented habitat still rose considerably (Table 3). Therefore, it is inadequate to just inject more freshwater into the wetlands to enlarge reed marshes. Consideration also needs to be given to its spatial distribution and water depth. In order to increase crane habitat effectively, it is vital to create open water in the vicinity of reed marshes, especially sparse (<30 % cover) reed marshes favored by the crane. Dense reeds may have to be removed during wetland restoration to create suitable habitat for the crane and other water birds. In restoring wetlands, water depth should be controlled within 50 cm as deeper water hampers crane’s hunt for food and movement (Shu et al. 2004).
Although injection of a certain amount of freshwater into the wetlands from the Yellow River has been guaranteed since 2002, sediment discharge still remained at a low level (Fig. 1). The sediment load delivered from the Yellow River to the sea represents only 14 % of the widely cited estimate of 1.08 Gt year−1 (Wang et al. 2007). The combined effects of climate change and human activities in the upper, middle, and lower reaches of the river have resulted in gradual decreases in the sediment load delivered from the Yellow River to the sea. Accompanied with global warming and sea level rise (SLR), some seablite community will degenerate into tidal flats. All of these imply that seablite tidal flats will face a continued decline. Per a long-term point of view, the SLR is an important natural driver to decrease the crane habitat area. Yet, in our study, because only 17 years (from establishment of the Yellow River Delta Nature Reserve in 1992 to 2008) was considered, we assumed that the SLR influence could be ignored compared with other factors, such as channel flow and sediment discharge, and thus here, we did not consider this factor. In the future, given a long-term impact, the SLR would play an important and indispensable role in coastal ecological and environmental change, and it should be included in natural factors.
The most effective means of protecting endangered species is to protect their habitat (Ferrier 2002; Rushton et al. 2004). The prerequisite of predicting endangered species population and planning for their protection is a clear understanding of the main forces driving them to extinction (Ricklefs 2004). This study has revealed that both natural and anthropogenic drivers played an almost equally important role in causing crane habitat change during 1992–2008 (Fig. 6). Furthermore, the change in suitable crane habitat is subject more (51.8 %) to the influences of such anthropogenic factors as oil wells, roads, and settlements than those (48.2 %) of natural variables including precipitation, temperature, channel flow, and sediment discharge.
Results of regression analysis demonstrate that natural factors such as channel flow, sediment load, and climate variables are critical to the size of reed marshes and seablite tidal flats. However, human disturbance in the forms of oil well development and road construction is even more important in determining whether reed marshes will be favored by the cranes as their habitat. Therefore, anthropogenic influences should be minimized by enclosing important natural corridors from development and by banning oil exploitation in the vicinity of crane habitat for three reasons. First, oil exploitation not only consumes land and damages the crane habitat directly but also causes water and soil pollution inside the reserve. Second, oil well development and road construction exasperate fragmentation of crane habitat (Table 2). Third, construction of artillery roads between the Yellow River and the marshes (Fig. 5) disrupts water supply to the wetlands and interferes with the natural hydrological relationship between the river and its floodplain. This has caused substantial salinization in this region and substantial degradation of both wetlands and crane habitat (Cui et al. 2009).
At present, the reserve consists of two zones separated tens of kilometres apart by oil fields and towns (Fig. 2). Between them is a zone of intensive human disturbance caused by oil exploitation, road construction, and other activities. If the reserve is not well protected or managed, these two zones will likely degenerate into areas of “habitat isolation,” resulting in the “island effect.” Earlier studies have confirmed that urbanization and human economic activities give rise to habitat scattering and fragmentation that lead ultimately to a reduced bird population (Indrawan and Wirakusumah 1995). Therefore, a mechanism should be established for properly monitoring and evaluating the environmental quality in the area around the reserve and for predicting potential environmental risks on the one hand. On the other hand, given that habitat joining is the major means of overcoming habitat fragmentation, consideration should be given to construct a green corridor to link the two zones to form an inseparable entity.
Conclusions
In this study, we analyzed the area of progressive, retrogressive, and human-induced conversion between crane potential habitat (i.e., reed marshes and seablite tidal flats) and other land covers in the YRDNR mapped from multitemporal remotely sensed data in a GIS during 1992–2008. With an inclusion of physical and behavioral fragmentation influence, the results show that both natural and anthropogenic influences have played an almost equal role in causing the observed crane habitat to change from 1992 to 2008.
Natural influences provide an inherent motivation for the habitat change of the red-crowned crane. The driving forces from human beings are more intense than those from nature; furthermore, the role of natural influences can be modified by human activities. In the reserve, natural influences have played a slightly more important role (50.6 %) in causing habitat change than anthropogenic forces. However, owing to the lack of shallow water in close proximity to reed marshes, physical fragmentation, one of natural influences in our study, is mainly induced by inappropriate wetland restoration efforts. If this influence is taken into consideration, anthropogenic effects, accounting for 60 % of the total influences, would be more important than natural factors in causing the crane habitat to change. During the period of 1992–2008, channel flow and sediment discharge from the Yellow River were the main natural factors driving crane habitat change while the gradual decreases of Yellow River flow and sediment discharge were also impacted by various human activities (Wang et al. 2007; Peng et al. 2010). This study, together with the previous ones, has supplied quantitative evidences to prove once more that the main driving force behind the change in the crane habitat in the YRDNR is mostly anthropogenic in nature. More considerations should be given to diverse factors in order to restore crane habitat more effectively.
References
Bi, X. L., Wang, B., & Lu, Q. S. (2011). Fragmentation effects of oil wells and roads in the Yellow River Delta, North China. Ocean & Coastal Management, 54, 256–264.
Bird Life International (2012). Grus japonensis and G. vipio. In IUCN 2012. IUCN red list of threatened species. Version 2012.1. http://www.iucnredlist.org. Accessed 9 Jul 2012.
Cao, M. C., & Liu, G. H. (2008). Habitat suitability change of red-crowned crane in Yellow River Delta Nature Reserve. Journal of Forestry Research, 19, 141–147.
Cui, S. Q. (2002). Influence of water discharge cut-off of Huanghe on environment of its delta. Marine Sciences, 26, 42–46.
Cui, B. S., Yang, Q. C., Yang, Z. F., & Zhang, K. J. (2009). Evaluating the ecological performance of wetland restoration in the Yellow River Delta, China. Ecological Engineering, 35, 1090–1103.
Day, J. W., Shaffer, G. P., Britsch, L. D., Reed, D. J., Hawes, S. R., & Cahoon, D. (2000). Pattern and process of land loss in the Mississippi Delta: a spatial and temporal analysis of wetland habitat change. Estuaries and Coasts, 4, 425–438.
Deffontaine, J. P., Thenail, C., & Baudry, J. (1995). Agricultural systems and landscape patterns: how can we build a relationship? Landscape and Urban Planning, 31, 3–10.
Ferrier, S. (2002). Mapping spatial pattern in biodiversity for regional conservation planning: where to from here. Systems Biology, 51, 331–363.
Fleury, A. M., & Brown, R. D. (1997). A framework for the design of wildlife conservation corridors with specific application to southwestern Ontario. Landscape and Urban Planning, 37, 163–186.
Forman, R. T. T. (1997). Land mosaics. Cambridge: Cambridge University Press.
Forman, R. T. T., & Godron, M. (1985). Landscape ecology. New York: Wiley.
Gottschalk, T. K., Huettmann, F., & Ehlers, M. (2005). Thirty years of analyzing and modeling avian habitat relationships using satellite imagery data: a review. International Journal of Remote Sensing, 26, 2631–2656.
Halpern, S. (1992). Losing ground. Audubon, 7, 70–79.
Harris, J. (1997). Future for China’s cranes. ICF Bugle, 23, 1–3.
Hijmans, R. J., & Graham, C. H. (2006). The ability of climate envelope models to predict the effect of climate change on species distributions. Global Change Biology, 12, 2272–2281.
Hu, C. H., & Cao, W. H. (2003). Variation, regulation and control of flow and sediment in the Yellow River Estuary: I. Mechanism of flow-sediment transport and evolution. Journal of Sediment Research, 5, 1–8.
Hu, Y. M., & Xiao, D. N. (1999). Behavioral fragmentation of waterfowl habitat and its landscape ecological design in Shuangtai-hekou Reserve, Liaoning, China. Journal of Environmental Sciences, 11, 231–235.
Indrawan, M., & Wirakusumah, S. (1995). Jakarta urban forest as bird habitat: a conservation view. Tiger Paper, 22, 29–32.
Jiang, H. X., Qian, F. W., Liu, C. Y., Li, X. M., Hou, Y. Q., Zhang, G. G., et al. (2012). Impact of marsh changes on breeding cranes in Sanjiang Plain, northeastern China. Chinese Birds, 3, 165–179.
Lee, S. D., Jablonski, P. D., & Higuchi, H. (2007). Winter foraging of threatened cranes in the Demilitarized Zone of Korea: behavioral evidence for the conservation importance of unplowed rice fields. Biological Conservation, 139, 286–289.
Letnic, M. I., & Fox, B. J. (1997). The impact of industrial fluoride fall out on faunal succession following sand mining of dry sclerophyll forest at Tomago, NSW.—I. Lizard recolonisation. Biological Conservation, 80, 63–81.
Li, F., Yang, H. J., Zhang, H. H., & Gao, Z. X. (1999). The nest-site selection by red-crowned crane in the Zhalong wetland. Journal of Northeast Forestry University, 27(6), 57–60.
Li, S. N., Wang, G. X., Deng, W., Hu, Y. M., & Hu, W. W. (2009). Influence of hydrology process on wetland landscape pattern: a case study in the Yellow River Delta. Ecological Engineering, 35, 1719–1726.
Li, M. Y., Zhang, C. Y., Wu, J., & Xu, T. (2012). Vegetation dynamics analysis in northeastern breeding habitat of Grus japonensis under scenarios of climate warming. Journal of Central South University of Forestry & Technology, 32, 58–63.
Liu, G. H., & Drost, H. J. (1997). Atlas of the Yellow River delta. The Publishing Housing of Surveying and Mapping, Beijing.
Liu, C. Y., Jiang, H. X., Hou, Y. Q., Zhang, S. Q., Su, L. Y., Li, X. F., et al. (2010). Habitat changes for breeding waterbirds in Yancheng National Nature Reserve, China: a remote sensing study. Wetlands, 30, 879–888.
Ma, Z. J., Wang, Z. J., & Tang, H. G. (1999). Habitat use and selection by red-crowned crane Grus japonensis in winter in Yancheng Biosphere Reserve, China. Ibis, 141, 135–139.
Ma, Z. J., Li, W. J., & Wang, Z. J. (2000). The natural conservation of red-crowned crane. Beijing: Tsinghua University Press.
Nagendra, H. (2001). Using remote sensing to assess biodiversity. International Journal of Remote Sensing, 22, 2377–2400.
Peng, J., Chen, S. L., & Dong, P. (2010). Temporal variation of sediment load in the Yellow River basin, China, and its impacts on the lower reaches and the river delta. Catena, 83, 135–147.
Pimm, S. L., & Raven, P. (2000). Biodiversity–extinction by numbers. Nature, 403, 843–845.
Prugh, L. R., Hodges, K. E., Sinclair, A. R. E., & Brashares, J. S. (2008). Effect of habitat area and isolation on fragmented animal populations. PNAS, 105, 20770–20775.
Ricklefs, R. A. (2004). Comprehensive framework for global patterns in biodiversity. Ecology Letters, 7, 1–15.
Rushton, S. P., Ormerod, S. J., & Kerby, G. (2004). New paradigms for modeling species distributions. Journal of Applied Ecology, 41, 193–200.
Sala, O. E., Chapin, F. S., Armesto, J. J., Berlow, E., Bloomfield, J., Dirzo, R., et al. (2000). Global biodiversity scenarios for the year 2100. Science, 287, 1770–1774.
Shu, Y., Hu, Y. M., Guo, D. F., Shan, K., Zhu, S. Y., & Wang, L. D. (2004). The change of habitat suitable for the red-crowned crane in Yellow River Delta. Chinese Journal of Zoology, 39, 33–41.
Shu, Y., Hu, Y. M., Leng, W. F., Zhu, Y. S., & Shan, K. (2006). Habitat selection of red-crowned crane in Yellow River Delta. Chinese Journal of Ecology, 8, 954–958.
Smirenski, S. M. (1988). Chick relationships and brood sizes in red-crowned (Grus japonensis) and white-napped (Grus vipio) cranes. In N. M. Litvinenko & I. A. Neufeldt (Eds.), The Palearctic cranes. Vladivostok: Amur–Ussuri Branch of the USSE Ornithological Society.
Suchanek, T. H. (1996). Temperate coastal marine communities: biodiversity and threats. Biological Conservation, 76, 210–211.
Wan, D. M., Gao, W., Wang, Q. Y., Wang, H. T., & Liu, M. Y. (2002). Effects of habitat fragmentation on nesting site selection of red-crowned crane. Chinese Journal of Applied Ecology, 13(5), 581–584.
Wang, Q. S. (2008). Threats for red-crowned crane. China Crane News, 12, 7–12.
Wang, S., Hassan, M., & Xie, X. (2006). Relationship between suspended sediment load, channel geometry and land area increment in the Yellow River Delta. Catena, 65, 302–314.
Wang, H. J., Yang, Z. S., Saito, Y., Liu, J. P., Sun, X. X., & Wang, Y. (2007). Stepwise decreases of the Huanghe (Yellow River) sediment load (1950–2005): Impacts of climate change and human activities. Global and Planetary Change, 57, 331–354.
Wang, X., Zhang, J., & He, R. (2011). A strategy to deal with water crisis under climate change for mainstream in the middle reaches of Yellow River. Mitigation and Adaptation Strategies for Global Change, 16, 555–566.
Wang, H., Gao, J., Ren, L. L., Kong, Y., Li, H., & Li, L. (2013). Assessment of the red-crowned crane habitat in the Yellow River Delta Nature Reserve, East China. Regional Environmental Change, 13(1), 115–123.
Wei, F., Feng, Z., & Wang, Z. (1999). Current distribution, status and conservation of wild red pandas Ailurus fulgens in China. Biological Conservation, 89, 285–291.
Wilcox, B. A., & Murphy, D. D. (1985). Conservation strategy: the effects of fragmentation on extinction. American Naturalist, 125, 879–887.
Wu, Q. M., & Zou, H. F. (2011). Nest–site selection pattern of Grus japonensis in Zhalong Nature Reserve of northeast China. Journal of Forestry Research, 22, 281–288.
Xiao, D. N., Hu, Y. M., & Li, X. Z. (2001). Landscape ecology research at wetland surrounding Bohai sea delta. Beijing: China Science.
Xu, X. G., Guo, H. H., Chen, X. L., Lin, H. P., & Du, Q. L. (2002). A multi-scale study on land use and land cover quality change: the case of the Yellow River Delta in China. GeoJournal, 3, 177–183.
Yue, T. X., Xu, B., & Liu, J. Y. (2004). A patch connectivity index and its change in relation to new wetland at the Yellow River Delta. International Journal of Remote Sensing, 25, 4617–4628.
Yvonne, C. A., Brady, R. C., & John, A. B. (2012). Using multitemporal remote sensing imagery and inundation measures to improve land change estimates in coastal wetlands. Estuaries and Coasts, 35, 190–200.
Zhang, Y. H., Deng, W., & Zhang, S. W. (2006). The spatial structure analysis of the red-crown crane’s habitat in Xianghai National Nature Reserve based on RS and GIS techniques. Acta Ecologica Sinica, 26, 3725–3731.
Zhao, Y. M., & Song, C. S. (1995). Scientific survey of the Yellow River Delta Nature Reserve. Beijing: China Forestry Press.
Acknowledgments
The research was supported by the National Science Foundation of China (40871230), State Key Laboratory of Resources and Environmental Information System, and 111 Project Ministry of Education and State Administration of Foreign Experts Affairs of China (B08048).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Gao, J., Pu, R. et al. Natural and anthropogenic influences on a red-crowned crane habitat in the Yellow River Delta Natural Reserve, 1992–2008. Environ Monit Assess 186, 4013–4028 (2014). https://doi.org/10.1007/s10661-014-3676-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3676-y