Abstract
Ultramafic rocks and their related soils (i.e., serpentine soils) are non-anthropogenic sources of metal contamination. Elevated concentrations of metals released from these soils into the surrounding areas and groundwater have ecological-, agricultural-, and human health-related consequences. Here we report the geochemistry of four different serpentine soil localities in Sri Lanka by coupling interpretations garnered from physicochemical properties and chemical extractions. Both Ni and Mn demonstrate appreciable release in water from the Ussangoda soils compared to the other three localities, with Ni and Mn metal release increasing with increasing ionic strengths at all sites. Sequential extraction experiments, utilized to identify “elemental pools,” indicate that Mn is mainly associated with oxides/(oxy)hydroxides, whereas Ni and Cr are bound in silicates and spinels. Nickel was the most bioavailable metal compared to Mn and Cr in all four soils, with the highest value observed in the Ussangoda soil at 168 ± 6.40 mg kg−1 via the 0.01-M CaCl2 extraction. Although Mn is dominantly bound in oxides/(oxy)hydroxides, Mn is widely dispersed with concentrations reaching as high as 391 mg kg−1 (Yudhaganawa) in the organic fraction and 49 mg kg−1 (Ussangoda) in the exchangeable fraction. Despite Cr being primarily retained in the residual fraction, the second largest pool of Cr was in the organic matter fraction (693 mg kg−1 in the Yudhaganawa soil). Overall, our results support that serpentine soils in Sri Lanka offer a highly labile source of metals to the critical zone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metamorphosed ultramafic rocks are distributed worldwide and are commonly associated with ophiolite complexes (Coleman and Jove 1992; Coleman 1977; Harrison and Rajakaruna 2011). Serpentinites, compositionally ultramafic rocks, form through the subduction and alteration of peridotite and pyroxenite along convergent plate margins (Coleman and Jove 1992; Oze et al. 2004a; O’Hanley 1996). Serpentinites and related serpentine soils comprise less than 1 % of the Earth’s total exposed surface; however, they contribute greatly to generating and maintaining biodiversity (Harrison and Rajakaruna 2011). Initially, the process of serpentinization occurs as peridotite and pyroxenite rocks [i.e., rocks dominantly composed of Fe- and Mg-rich silicate minerals such as olivine ((Mg, Fe2+)2 [Si2O4]) and pyroxene (XY(Si, Al)2O)] are altered by hydrothermal fluids and incorporated into subduction-related mélange (Gough et al. 1989; O’Hanley 1996; Oze et al. 2004a; Coleman 1977). Hydration of pyroxene and olivine forms the serpentine group of minerals (i.e., lizardite, chrysotile, and antigorite) from which these rocks and soils derive their names.
Serpentinite rocks are enriched with Cr, Co, and Ni and have the potential to adversely impact environmental and human health once metal ions are mobilized into soil, water, and dust by weathering (Alves et al. 2011; Rajapaksha et al. 2012; Cheng et al. 2011). Nickel and Cr(III) can substitute for Mg or Fe in octahedral sites in olivine and pyroxene in peridotites and in the serpentine group of minerals (Oze et al. 2004b). Since pyroxene and olivine in peridotite weather more quickly than serpentine minerals (Alexander 2004), the lability of Ni and Cr in rocks and soils derived from ultramafic sources may be a function of the degree of serpentinization. Chromium is mostly found in spinel minerals as chromian magnetite, chromite, and other mixed-composition spinels containing Al, Cr, Mg, and Fe (Oze et al. 2004b).
Weathering and other pedogenic processes form serpentine soils, a generic term used to describe any soil derived from serpentinite and other ultramafic rocks regardless of its physical or chemical properties (Oze 2003). Serpentine soils are generally characterized by: (1) low concentrations of plant nutrients such as N, P, and K; (2) high concentrations of biologically toxic elements including Ni, Co, and Cr; (3) Ca/Mg quotients ≪ 1; (4) low water holding capacity due to the rocky, shallow, and often exposed nature of the outcrops; and (5) distinct biota, particularly plants, often endemic to such soils (Brooks 1987; Harris and Rajakaruna 2009; Oze et al. 2008).
Ultramafic rocks and soils are widely but patchily distributed on Earth; they are found on every continent and in every major biome (Harrison and Kruckeberg 2008). Serpentine outcrops on some continents (Australia, Europe, North America) are relatively well-studied, but those in many other areas (Asia, Africa, Central and South America) are relatively unexplored (Boyd et al. 2009). Studies to date show that in many areas of the world serpentine outcrops harbor vegetation distinct from that of adjacent areas, often characterized by high endemism and rarity (Brooks 1987; Harrison and Rajakaruna 2011; Rajakaruna et al. 2009).
Geochemical studies on serpentinites and serpentine soils have mostly focused on Cr (Armienta et al. 1996; Becquer et al. 2003; Camachoa and Armientac 2000; Cheng et al. 2011; Gough et al. 1989; Oze et al. 2004a, b). Nickel has also received some attention (Amir and Pineau 2003; Alves et al. 2011; Cheng et al. 2011; Proctor and Baker 1994; Rajapaksha et al. 2012). However, not many studies have focused on serpentines in tropical regions where weathering rates are typically high due to high annual rainfall and temperature. In Sri Lanka, a continental island in the equatorial belt, several serpentine outcrops are present (Dissanayaka 1982). The serpentine outcrops in Sri Lanka have received some attention by botanists (Rajakaruna and Bohm 2002; Rajakaruna et al. 2002), and the studies to date have revealed several potential Ni hyperaccumulators, species accumulating over 1,000 μg Ni/g dry leaf tissue (Van der Ent et al. 2013), from the Ussangoda site (Rajakaruna and Baker 2004; Senevirathne et al. 2000; Weerasinghe and Iqbal 2011).
Despite a handful of published research on the geochemistry (Dissanayaka 1982; Rajapaksha et al. 2012) and plant ecology (Brooks 1987; Rajakaruna and Baker 2004; Rajakaruna and Bohm 2002; Rajakaruna et al. 2002; Senevirathne et al. 2000; Weerasinghe and Iqbal 2011) of mostly the Ussangoda site, there is minimal research to date on the geochemistry and associated geoecology of Sri Lanka’s serpentine soils. This is rather surprising given that Sri Lanka, a biodiversity hotspot (Myers et al. 2000), is home to many endemic species and these under-explored outcrops could potentially harbor “new” species or ecotypes endemic to the substrate (Rajakaruna and Baker 2004). In this study, we examine if metal accessibility and release is comparable among the four known outcrops in Sri Lanka and assess the potential release of heavy metals, including Cr, Mn, and Ni, from the serpentines soils to the surrounding environment.
Geological setting and description of the study sites
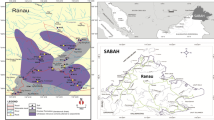
Geologically, 90 % of Sri Lanka consists of rocks of Precambrian age (Cooray 1984). The other 10 % consists mainly of Miocene Limestone, restricted to northwestern Sri Lanka (Fig. 1). Additionally, there are a few shale beds of the Jurassic period around the areas of Thabbowa and Andigama in the northwestern and western provinces of the island, respectively. Based on the rock types, their origin, and their metamorphic condition, Sri Lanka’s metamorphic terrain is divided into four main geological units (The Highland Complex, HC; the Vijayan Complex, VC; the Wanni Complex, WC) and one subordinate unit (Kadugannawa Complex, KC; Fig. 1). The central highlands, extending toward the northeast up along the east coast and to the southwest up along the southwestern coast, are included as part of HC. The rocks in HC originated under very high temperature (700–800 °C) and pressure (5–10 Kb) (Cooray 1984).
The VC occupies the eastern section of HC, and WC is found on the western portion of HC up to the Miocene Limestone (Cenozoic cover in Fig. 1). The geological boundary between HC and VC has been considered as a mini-plate boundary (Munasinghe and Dissanayake 1980) due to the mineralization that occurs along this boundary. However, there is no definite margin indicative of the plate boundary, only a widespread mineralized belt. The KC lies along the middle of HC–WC boundary.
The serpentinite outcrops of Sri Lanka lie along the boundary of the HC and VC (Fig. 1). Of these, Indikolapelessa serpentinite deposit is the only deposit studied in detail for its petrology and geochemistry (Dissanayaka 1982). The extent of this deposit is recorded to be ∼7 km2 surrounded by charnockites, calc-gneiss, migmatites, cordierite, and diopside bearing gneisses and calciphyres. Geophysical and bore logging explorations have revealed that the serpentinite bodies have a deep-seated origin. Nickel is a prominent feature of the Indikolapelessa deposit (0.05–2 %), partly as an iron oxide phase, whereas Cr is reported in the range of 300–3,100 mg kg−1 (Dissanayaka 1982).
The Ussangoda serpentine outcrop is located in the southern coastal end of the HC–VC boundary (Fig. 1). The area of the outcrop is estimated as 3 km2, and the soil has been described as hematite rich with very fine-grained clayey sand, giving a lateritic reddish hue (Rajakaruna and Bohm 2002). Previous geochemical studies at this site revealed soil Ni and Cr concentrations of 1,000 and 7,700 mg kg−1, respectively, compared to 2,100 and 10,000 mg kg−1 Ni and Cr, respectively, at the Ginigalpelessa outcrop (Ranasinghe 1987). The Ginigalpelessa outcrop is estimated to cover an area of ∼1 km2. Currently, no geochemical studies have been conducted at the Yudhaganawa serpentine deposit.
Materials and methods
Sample collection and preparation
The serpentine soils used for the study were collected from all four known serpentine outcrops in Sri Lanka: Ussangoda, Ginigalpelessa, Indikolapelessa, and Yudhaganawa (Figs. 1 and 2). Ten soil samples were obtained from each outcrop, all within comparable topographies. These soils are a direct weathering product of the rocks beneath (Dissanayake and Van Riel 1978). A composite sample for each was prepared by mixing the ten samples. Each soil sample was air-dried and mechanically sieved to obtain the <2-mm fraction for geochemical investigations. Five replicate samples from each site were used for each chemical analysis.
Physicochemical characteristics of serpentine soils
All chemicals used were of analytical grade and purchased from Fluka (Switzerland) or Sigma (USA). All laboratory glassware and plastic ware were rinsed three times with double deionized water after being soaked in a HNO3 (10 %, v/v) bath overnight. The parameters measured include pH, electrical conductivity (EC), organic carbon, and elemental composition. The pH was determined using a 1:1 mixture of soil to deionized water. EC was determined from a 2 mL of soil solution extract.
For heavy metal analysis, all the extracts were acidified with HNO3 to prevent adsorption to the polyethylene of the storage vessel and prevent growth of bacteria. The acidified supernatant was collected in polyethylene bottles and stored at 4 °C until metal analysis. Three replicates were performed for each sample and each procedure, as three independent analyses. Blanks were measured in parallel for each batch of analysis.
Total metal concentrations in soils
Major and trace elements of the soils were analyzed via X-ray fluorescence (XRF) spectrometry using a PANalytical MagiX PRO spectrometer (Institute for Geography and Geology, University of Copenhagen). Trace elements in soils were extracted by completely dissolving the samples in a closed vessel device using temperature-controlled microwave heating system with a mixture of hot, concentrated HNO3, HCl, and HF (Sun et al. 2001) (Milestone ETHOS PLUS Labstation with HRP-1000/10S High Pressure Segmented Rotar), and analyzed by atomic absorption spectrometry (AAS) (AAS-Model GBC AAS 933A). Triplicate analyses were conducted for each sampling site for total digestion; however, only one sample was conducted for XRF analysis. X-ray diffraction (XRD) was used to identify the minerals in soils. Serpentine soils (63–105 μm) were evaluated via XRD using a Siemens D-5000 diffractometer operating at 40 kV and 40 mA (using CuKα radiation). X-ray diffraction patterns were collected between 2Ө values of 2.0–80.0 and at a scan speed of 1.0° min−1. The electron probe microanalysis (EPMA) of the serpentine soil samples was carried out to identify the Ni-, Mn-, and Cr-bound phases. Elemental mapping for metals was carried at Seoul National University.
Surface titrations
The zero point of charge (pHZPC) of the serpentine sediments was determined by conventional potentiometric titration methods (Langmuir 1997). A 2-g L−1 serpentine sediment (63–105 μm fractions) suspension was equilibrated for 24 h. Three titration experiments were performed utilizing 0.1, 0.01, and 0.001 M NaNO3. The initial pH of serpentine soil suspension was ∼5.5, and it was lowered to ∼4 with 0.10 M HNO3 prior to titrations. At each titration point, the pH value and the titrant volume (Model Orion 960 auto chemistry analyzer) were recorded. The surface charge (σ H) was calculated using the Eq. 1 below (Stumm and Morgan 1996).
where σ H is the surface charge (in coulomb per square meter), C A is the added acid concentration, C B is the added base concentration, [OH–] is the hydroxyl ion concentration, [H+] is the proton concentration, a is equilibrium OH– and H+ concentrations for a given quantity of solid used (in grams per liter), F is the Faraday constant (96,500 C mol−1), and S represents the surface area determined by N2 BET isotherm analysis.
Single extractions
Ammonium acetate extraction
The readily exchangeable and water-soluble cations (K, Ca, Mg, and Na) were determined by using ammonium acetate at pH 7.0. The procedure involved weighing 2 g of soil and adding 25 mL of ammonium acetate (20 M NH4OAc) followed by mixing on an orbital shaker for 30 min (Castilho and Rix 1993). The solution was filtered using a 0.45-μm quantitative filter paper. Potassium, Ca, Mg, and Na concentrations were analyzed using an atomic absorption spectrophotometer (GBC AAS 933A).
Diethylene triamine pentaacetic acid and CaCl2 extractions
The diethylene triamine pentaacetic acid (DTPA) and CaCl2 extraction methods provide a proxy for evaluating plant bioavailability of metals in soils and soil solutions (Kashem et al. 2007; Peijnenburg et al. 2007). Bioavailability of metals in the serpentine soil samples was quantified by the DTPA soil test (Lindsay and Norvell 1978). Approximately 20 mL of a 0.005-M DTPA, 0.01 M CaCl2, and 0.1 M triethanolamine buffered solution were added to 10 g of air-dried soil for 2 h. The filtrate was analyzed for Cr, Mn, Fe, Ni, Co, Cu, and Zn by AAS.
Additionally, the 0.01-M CaCl2 method (Houba et al. 1996) was also employed to reassess the bioavailable fraction of metals. One gram of soil was extracted with 10 mL of 0.01 M CaCl2 by stirring the solid solution for 2 h and centrifuging and filtering through a 0.45-μm pore size membrane. The supernatant was used to analyze Ni, Mn, and Cr via AAS.
Sequential extractions to determine Cr, Mn, and Ni mineral phases
Sequential extractions involve the selective extraction of trace metals from operationally defined sediment solid fractions (Tessier et al. 1979; Gleyzes et al. 2002), providing detailed information about the different availabilities of heavy metals among distinct geochemical phases. Ideally, the reagents are chosen to selectively attack a specific soil compartment with minimal dissolution of nontargeted phases. Although the separation of various chemical forms of heavy metals is difficult, sequential extraction methods provide a favorable approach (Tessier et al. 1979). The dominant mineral phase may not contribute to the highest integration with metal ions due to the low reactivity and surface area compared to the other highly reactive mineral phases. Hence, chemical extractions may provide a final “snap shot” of the dynamic processes occurring in the soil.
Sequential extraction experiments were performed on the serpentine soils following the methods of Tessier et al. (1979) and Armienta et al. (1996). A mass of 1 g of serpentine soil (dry weight) was used for the initial extraction. A total of five replicate sequential extraction analyses were completed on the sediment. Nickel, Cr, and Mn concentrations were measured in the effluent after each extraction using AAS. In the following is a list of the extraction procedures performed on the sediment:
-
(a)
Exchangeable: Sediment was reacted at room temperature for 1 h with 20 mL of magnesium chloride solution (1 M MgCl2, pH 7.0) with continuous agitation.
-
(b)
Bound to carbonates. Residue from (a) was leached at room temperature for 2 h with 20 mL of 1 M sodium acetate (NaOAc) adjusted to pH 5.0 with acetic acid (HOAc) and with continuous agitation.
-
(c)
Bound to Fe–Mn oxide: Residue from (b) was treated with 20 mL of 0.04 M hydroxylamine hydrochloride (NH2OH-HCl) in 25 % (v/v) HOAc heated at 90 °C with slow continuous agitation for 2 h.
-
(d)
Bound to organic matter: Residue from (c) was treated with 3 mL of 0.02 M HNO3 and 5 mL of 30 % H2O2 adjusted to pH 2 with HNO3, heated to 85 °C for 2 h with occasional agitation. A 3-mL aliquot of 30 % H2O2 (pH 2 with HNO3) was added, and the sample was heated again to 85 °C for 3 h with intermittent agitation. After cooling, 5 mL of 3.2 M NH4OAc in 20 % (v/v) HNO3 was added, and the sample was diluted to 20 mL and agitated continuously.
-
(e)
Residual: Residue from (d) was treated with a mixture of 10 mL concentrated HF and 2 mL concentrated HClO4 and heated to near dryness. It was then treated with 1 mL HClO4 + 10 mL HF and heated again to near dryness; 1 mL HClO4 was added, heated until the appearance of white fumes, and finally dissolved with 12 N HC1 and diluted to 25 mL with deionized water.
Between each successive extraction listed above [(b) to (e)], the sample was centrifuged at 3,500 rpm for 15 min. Additionally, the supernatant was filtered using 0.45 μm filter paper prior to AAS analysis.
Effect of ionic strength and water for metal ion release
Metal release batch experiments were carried out using distilled water to observe Ni, Cr, and Mn release from the sediment with respect to time. This experiment was performed to investigate the dependence of toxic metal release due to the variation in ionic strength. Since one of the serpentine sites is located along the coast (Ussangoda), sea spray may contain salts which can increase the ionic strength of the soil solution. Thus, the experiment was conducted to characterize any variation of metal release due to changes in ionic strength, resulting from salt spray. A mass of 5 g of solid sample per L of water (5 g L−1 sediment suspension) was initially used where subsamples of 25 mL suspension were taken from the batch experiments over 20 days. Nickel, Cr, and Mn release with different ionic strengths was analyzed using 0.1, 0.01, and 0.001 M NaNO3 solutions by changing the solution pH (4–9). A 5-g L−1 sediment suspension was prepared and adjusted to pH ∼4 in desired ionic strengths (0.1, 0.01, and 0.001) by 5 M NaNO3. The system pH was incrementally increased at ∼1.0 pH intervals up to ∼9. At each point, a 25-mL sample portion was transferred to a capped polypropylene tube. These tubes were equilibrated for 24 h at 75 rpm (EYELA B603 shaker), and the pH of the suspension was re-measured and recorded. Membrane-filtered supernatant was used for Ni, Cr, and Mn analysis via AAS.
Inorganic and organic acid extractions
Organic and inorganic acids can be found in the environment due to plant and microbial activity in the rhizosphere and via dissolved ions in rainwater. These acids can play a role in metal release due to the change of pH of the soil solution. Therefore, three inorganic (sulfuric, nitric, hydrochloric) and three organic (citric, acetic, oxalic) acids of different concentrations (0.05, 0.1, 0.5, 1.0, 5.0, and 10 mM) were used to evaluate Cr, Ni, and Mn release from the sediment. Approximately 1 mL (15–20 drops) of chloroform was added per liter of all organic acid solutions to prevent microbial breakdown of the organic acids. Half a gram of sediment was placed in polypropylene tubes, and 25 mL of each acid was added. The tubes were equilibrated for 24 h at room temperature and agitated at 75 rpm (Model EYELA B603 shaker). The supernatant was transferred by membrane filtration (0.45 μm) after centrifugation, and the solutions were analyzed for Mn and Ni using the flame method of AAS.
Results and discussion
Serpentine soil chemistry
Chemical properties (pH, electrical conductivity, organic matter, cation exchange capacity) for four soil samples from Ussangoda, Yudhaganawa, Ginigalpelessa, and Indikolapelessa are listed in Table 1. The pH values of all soils are near neutral (pH from 6.26 to 7.69). EC in the soils range from 33.50 to 129.90 μS cm−1, indicative of relatively few dissolved salts and/or major dissolved inorganic solutes. The highest EC is reported from the Ussangoda soil, potentially due to the deposition of salt spray from the sea. The organic carbon content of the soil ranges from 1.09 to 2.58 %. The highest organic carbon percentage is reported from Yudhaganawa soil which is adjacent to a forested habitat. Furthermore, a considerable color difference was identified among the soil samples collected from the four localities (Fig. 3).
Depending on soil pH, mineral surfaces can bear net negative, positive, or no charge. The pH where the net electrical charge is zero is the zero point of charge (pHZPC), and it is a parameter used to describe variable-charge surfaces (Morais et al. 1976; Parks and de Bruyn 1961). Titrations performed at three ionic strengths provide the pHZPC at pH 8.57, 8.90, 8.30, and 8.01 for Ussangoda, Yudhaganawa, Ginigalpelessa, and Indikolapelessa, respectively (Table 1). As observed, it is evident that the surface hydroxyl functional groups in the serpentine soils behave amphoterically.
XRD analysis is important for revealing the mineralogical composition of the soils as shown for the Ussangoda soil in Fig. 4. Our results also document XRD patterns similar to those reported from previous studies (Sucik et al. 2008; Camachoa and Armientac 2000). Analyses reveal that antigorite ((Mg, Fe)3 Si2O5 (OH)4) is often the dominant mineral present with minor amounts of chrysotile (Mg3(Si2O5) (OH)4), magnetite (Fe3O4), spinels, and clays.
Soil chemical properties (pH, electrical conductivity, organic matter, cation exchange capacity), surface titrations, EPMA, and SEM analysis of serpentine soils collected from different localities in Sri Lanka are not significantly different. pH of the soil is near neutral and the range of EC is indicative of relatively few dissolved salts and/or major dissolved inorganic solutes (Oze et al. 2004b). These data corroborate with serpentine soil data reported from CA, USA (Oze et al. 2004b). However, serpentine soils from the coastal Ussangoda site show high EC and CEC compared to the other inland serpentine soils, which may be due to the deposition of salts from sea spray.
XRF analysis and total metal concentrations of the soils are reported in Table 2. The elemental composition of serpentine soils was obtained using XRF and total digestion techniques. Both techniques are complimentary; major elements in metal oxides were determined by XRF spectrophotometry and in elemental form via total digestion. The XRF results (Table 2) show that the soils consist of Fe–Cr–Ni-rich aluminosilicates. Additionally, Mn is high in the samples, especially in the Yudhaganawa (2,609 mg kg−1) and Ginigalpelessa (2,224 mg kg−1) soils. Nickel is the highest in Ussangoda soils (6,459 mg kg−1), while Cr is higher in Yudhaganawa (>10,000 mg kg−1) soil compared to soils from the other localities.
EPMA analysis maps show the distribution of Ni, Mn, and Cr with Al, Fe, and Si phases (Fig. 5). Overall, these maps reveal that Ni, Mn, and Cr are not homogeneously distributed in the soil, suggesting that the metals are bound in specific mineral phases. EPMA results of different serpentine soils corroborate with data from XRF values. Cursory observations demonstrate that the Cr distribution is related to the Fe and Al phases of the serpentine soils as shown in Fig. 5, whereas the Ni distribution is consistent with the Si phases. Even though antigorite is a dominant mineral in the soil as shown by XRD, it has a relatively low surface area compared to clays or organic matter and, therefore, would contribute/release less metal compared to a high surface area/highly reactive mineral/phase in the soil. This is a major critique of chemical extractions. In chemical extractions, surface area and reactivity of a given fraction/phase is a major factor influencing metal release; however, these extractions do provide a means to begin differentiating the abundance and release of metals in a very complex medium (i.e., soil).
Metal-bound phases
Sequential extraction results for the serpentine soils are presented in Table 3. Mean values with standard deviations are reported. Nickel, Mn, and Cr concentrations for each chemical extraction step are shown (in milligrams per kilogram) as well as the percentage (in percent) extracted from the total value (Fig. 6). Manganese is equally bound in the Fe–Mn oxide fraction (420.7 mg kg−1, 37 %) as in the residual fraction (351 mg kg−1, 31 %). The residual fraction is associated with silicates as well as with other primary oxides such as spinels. Nickel is dominantly bound in the residual fraction (4,697 mg kg−1, 72 %), and Cr predominates in the residual fraction and is organic matter bound (8,567 mg kg−1, 83 % and 508 mg kg−1, 4.6 %, respectively). The order of the individual geochemical fractions where Cr, Ni, and Mn are bound from the greatest to least are: (1) Cr: residual > organic matter bound > Fe and Mn bound > exchangeable > carbonate bound, (2) Ni: residual > Fe and Mn oxide bound > organic matter bound > exchangeable > carbonate bound, and (3) Mn: Fe and Mn oxide bound > residual > organic matter bound > exchangeable > carbonate bound.
Heavy metals are present as exchangeable or associated with organic matter, carbonates, Fe–Mn oxides, and sulfides in the soil matrix fractions. Chemical extractions are utilized to assess the geochemical partitioning of metals as well as to evaluate metal mobility and bioavailability in soils and sediments. However, changes in soil pH, ionic strength, and other environmental factors may affect metal mobilization in soil environments, especially with respect to time and land use. By coupling single and sequential extractions with chemical kinetic interpretations, it is possible to gain better insight with respect to where and how Ni, Mn, and Cr are bound. More importantly, the fate and behavior of Ni, Mn, and Cr as they are mobilized and/or affected by critical zone processes can also be interpreted for a wide variety of potential chemical changes, including those related to the addition of fertilizers and changes in rainwater chemistry (Rajapaksha et al. 2012).
Sequential extraction experiments provide information on association of species, enabling the differentiation between “elemental pools” based on how they are attached and/or what minerals they are associated with, including carbonates, (oxy)hydroxides, or silicates. Even though these metals are dominantly bound in relatively unavailable forms, changes in the critical zone such as soil acidity, microbial activity, availability of chelating materials, and redox conditions can enhance the mobility, providing a continually changing flux into the environment. It is important to note that bioavailable, exchangeable, and carbonate-bound fractions may have less overall Ni, Mn, and Cr; however, these fractions potentially offer a more labile source in soil environments.
Several differences are present among serpentine soils from different localities. The exchangeable fraction of Ni was higher in Ussangoda and Yudhaganawa soils (Table 3) compared to Indikolapelessa and Ginigalpelessa soils, which may be due to the slightly higher pH and lower organic matter content at the latter two sites. In the case of Mn, all soils except those from Ussangoda show the second highest Mn in the organic matter-bound fraction and third in the residual fraction. This difference may be due to the changes in mineralogy. In soils derived from serpentinite (Becquer et al. 2003; Gasser and Dahlgren 1994; Oze et al. 2004a), most Cr is bound in the structure of primary minerals such as Cr-rich spinels (i.e., chromite) and Cr-substituted Fe oxides. The results support that antigorite (i.e., the dominant mineral identified in these serpentine soils via XRD) could be a contributor to Ni and Mn release, whereas the Cr-spinel (chromite/Cr-muscovite) is a potential major source of Cr (Rajapaksha et al. 2012). A substantial proportion of Cr was linked with organic matter, and the high proportion substantiates the high affinity of Cr for organic matter (Kabata-Pendias and Pendias 2001).
Single extractions
The DTPA treatment extracted 323 mg kg−1 (5.0 %) of Ni and 76.3 mg kg−1 (6.8 %) of Mn for the Ussangoda soil. Nickel and Mn extractions with 0.01 M CaCl2 are lower than that of DTPA in the slightly acidic serpentine soils and are 167.6 (2.6 %) for Ni and 45.51 (4.1 %) mg kg−1 for Mn (Table 4). Similarly, NaNO3 and distilled water (at pH 6.5) extractable Ni and Mn are comparatively higher in the Ussangoda soils (Table 5).
The DTPA and CaCl2 extraction methods provide a proxy for evaluating plant bioavailability of Ni and Mn in soils and soil solutions (Kashem et al. 2007; Peijnenburg et al. 2007). Since DTPA forms soluble complexes with metals, thereby reducing their activity in the soil solutions, Ni and Mn ions may be desorbed from the soil and enter into the solution. Extractions with CaCl2 are commonly used to assess plant bioavailability, especially in neutral or weakly alkaline soils. High concentrations of Ni and Mn release (>19 and >7 mg kg−1, respectively) are present from all serpentine soil localities. The concentrations recorded from CaCl2 extractions were about ∼50 % lower than the DTPA extractable concentrations (Table 4). Some studies have shown that salt solutions do not accurately reflect the plant available metals, especially in the case of non-calcareous soils, whereas DTPA or hydroxylamine methods are more predictive (Gupta and Aten 1993; Aydinalp and Katkat 2004). Similar to the other extraction results, Ussangoda and Yudhaganawa soils demonstrate higher leaching capacities of Ni, Mn, and Cr than the other two soils (Table 4). This directly relates to the total metal concentration differences among soils from different localities.
Although this study did not reveal substantial differences in soil pH among the four sites, our pH results (Table 1) parallel the trend seen in previous research, documenting the lowest rhizospheric pH for soils at the Ussangoda site (4.3–4.9; Rajakaruna and Bohm 2002) followed by the Yudhaganawa site (5.05–5.65; Rajakaruna and Bohm 2002). The lower pH at these two sites may also contribute to the greater leaching capacity documented. It is widely known that the solubility and mobility of Ni in soils increases as pH decreases, at least within the pH range of physiological significance (McGrath 1995). By using ion-exchange kinetics, Echevarria et al. (2006) documented pH as the main factor influencing Ni availability in a wide range of natural soils, and Tye et al. (2004) have shown that pH is also a major influence on Ni activity in a range of contaminated soils. The more labile Ni obviously contributes to greater opportunity for plant uptake (Kukier et al. 2004) and may have contributed to the highest tissue Ni content so far observed, including levels of hyperaccumulation, for species growing at the Ussangoda site (Rajakaruna and Bohm 2002; Weerasinghe and Iqbal 2011). Chromium was not detected (detection limit was 0.001 mg kg−1) from CaCl2 extractions from any serpentine soil, although DTPA shows bioavailable concentrations for the soils from Ussangoda, Indikolapelessa, and Yudhaganawa (Table 4). Low bioavailable Cr may be due to the higher affinity of Cr to adsorb to clay surfaces and humic matter (Fendorf 1995).
Water and ionic strength extractable fraction of metals may be more representative of soils being exposed to sea spray or what is available for water pollution and plant uptake, including to those species shown to hyperaccumulate Ni at the Ussangoda site (Rajakaruna and Bohm 2002; Weerasinghe and Iqbal 2011). Distilled water and NaNO3 extractable data show high release of Ni than Mn or Cr (Table 5). The highest distilled water extractable Ni and Mn was observed from Ussangoda soil, and it shows a reduction in the sequence of Yudhaganawa, Indikolapelessa, and Ginigalpelessa. This sequence may be related with the total amount of Ni present in the soil as observed from the XRF and total digestion data. However, the behavior of Mn release with distilled water and NaNO3 is different from the total Mn present; rather, it is related to total Fe in the soil. This may be related to the Fe- and Mn-bound fraction of soils. However, the Mn release sequence is similar to that of Ni. In the case of NaNO3 extractable metal ions, with the decrease of ionic strength, the metal ion release also shows a decline. Dissolution rates are enhanced by increasing ionic strength due to surface protonation displacing ions from surface sites (Mogollón et al. 2000). Chromium does not show any release in soils except at Yudhaganawa with NaNO3, although the total concentrations reported were higher than Ni and Mn. Since Cr (III) is highly stable, strong complex formation with humic matter may have stopped it from dissolving (Fendorf 1995).
Remarks
Our study demonstrates that the weathering of ultramafic rocks and serpentinites in Sri Lanka produces soils containing high concentrations of Cr, Ni, Co, and Mn (i.e., similar to other serpentine soils) and that these metals can potentially be released to local water bodies. Our chemical characterizations show that the soils from Yudhaganawa site record the highest Cr and Mn concentrations, whereas Ussangoda soils show the highest concentration of Ni. The greater availability of Ni may explain why this is the only site where high levels of plant tissue Ni, including levels considered to be in the range of hyperaccumulation, were observed in plants tested from the four serpentine localities by Rajakaruna and Bohm (2002). Our study points to the importance of conducting a variety of extractions, ranging from single to sequential, in order to better assess bioavailable concentrations, including how labile elements can be released over a range of geochemical conditions.
References
Alexander, E. B. (2004). Serpentine soil redness, differences among peridotite and serpentinite materials, Klamath Mountains, California. International Geology Review, 46(8), 754–764.
Alves, S., Trancoso, M. A., Gonçalves, M. d. L. S., & Correia dos Santos, M. M. (2011). A nickel availability study in serpentinised areas of Portugal. Geoderma, 164(3–4), 155–163.
Amir, H., & Pineau, R. (2003). Release of Ni and Co by microbial activity in New Caledonian ultramafic soils. Canadian Journal of Microbiology, 49(4), 288–293.
Armienta, M. A., Rodríguez, R., Ceniceros, N., Juárez, F., & Cruz, O. (1996). Distribution, origin and fate of chromium in soils in Guanajuato, Mexico. Environmental Pollution, 91(3), 391–397.
Aydinalp, C., & Katkat, A. V. (2004). The comparison of extraction methods for evaluating some heavy metals in polluted soils. Plant Soil Environment, 50(5), 212–217.
Becquer, T., Quantin, C., Sicot, M., & Boudot, J. P. (2003). Chromium availability in ultramafic soils from New Caledonia. Science of the Total Environment, 301(1–3), 251–261.
Boyd, R. S., Kruckeberg, A. R., & Rajakaruna, N. (2009). Biology of ultramafic rocks and soils: research goals for the future. Northeastern Naturalist, 16(5), 422–440.
Brooks, R. R. (1987). Serpentine and its vegetation: a multidisciplinary approach. Portland: Dioscorides.
Camachoa, J. R., & Armientac, M. A. (2000). Natural chromium contamination of groundwater at Leo’n Valley, Mexico. Journal of Geochemical Exploration, 68, 167–181.
Castilho, P. D., & Rix, I. (1993). Ammonium acetate extraction for soil heavy metal speciation; model aided soil test interpretation. International Journal of Environmental Analytical Chemistry, 51(1–4), 59–64.
Cheng, C.–. H., Jien, S.–. H., Iizuka, Y., Tsai, H., Chang, Y.–. H., & Hseu, Z.-Y. (2011). Pedogenic chromium and nickel partitioning in serpentine soils along a toposequence. Soil Science Society of America Journal, 75(2), 659–668.
Coleman, R. G. (1977). Ophiolites: ancient oceanic lithosphere? Berlin: Springer.
Coleman, R. G., & Jove, C. (1992). Geological origin of serpentinites. In: A. J. M. Baker, J. Proctor, & R. D. Reeves (Eds.), The vegetation of ultramafic (serpentine) soils. Proceedings of the First International Conference on Serpentine Ecology (pp. 1–17). Hampshire: Intercept.
Cooray, P. G. (1984). An introduction to the geology of Sri Lanka (Ceylon). Colombo: National Museums of Sri Lanka.
Dissanayaka, C. B. (1982). The geology and geochemistry of the Uda Walawe serpentinite. Sri Lanka. Journal National Science Council Sri Lanka, 10, 13–34.
Dissanayake, C. B., & Van Riel, B. J. (1978). The petrology and geochemistry of a recently discovered nickeliferous serpentinite from Sri Lanka. Journal of the Geological Society of India, 19, 464–471.
Echevarria, G., Massoura, S. T., Sterckeman, T., Becquer, T., Schwartz, C., & Morel, J. L. (2006). Assessment and control of the bioavailability of nickel in soils. Environmental Toxicology and Chemistry, 25(3), 643–651.
Fendorf, S. E. (1995). Surface reactions of chromium in soils and waters. Geoderma, 67(1–2), 55–71.
Gasser, U. G., & Dahlgren, R. A. (1994). Solid-phase speciation and surface association of metals in serpentinitic soils. Soil Science and Plant Nutrition, 158, 409–420.
Gleyzes, C., Tellier, S., & Astruc, M. (2002). Fractionation studies of trace elements in contaminated soils and sediments: a review of sequential extraction procedures. TrAC Trends in Analytical Chemistry, 21(6–7), 451–467.
Gough, L. P., Meadows, G. R., Jackson, L. L., & Dudka, S. (1989). Biogeochemistry of highly serpentinized chromite rich ultramafic area, Tehma County, California. USGS Bulletin, 1901.
Gupta, S. K., & Aten, C. (1993). Comparison and evaluation of extraction media and their suitability in a simple model to predict the biological relevance of heavy metal concentrations in contaminated soils. International Journal of Environmental Analytical Chemistry, 51(1–4), 25–46.
Harris, T., & Rajakaruna, N. (2009). Adiantum viridimontanum, Aspidotis densa, Minuartia marcescens, and Symphyotrichum rhiannon: additional serpentine endemics from Eastern North America. Northeastern Naturalist, 16(sp5), 111–120.
Harrison, S., & Kruckeberg, A. R. (2008). Garden on the rocks. Natural History, 117, 40–44.
Harrison, S., & Rajakaruna, N. (2011). Serpentine: the evolution and ecology of a model system. Berkeley: University of California Press.
Houba, V. J. G., Lexmond, T. M., Novozamsky, I., & Lee, J. J. (1996). State of the art and future developments in soil analysis for bioavailability assessment. Science of the Total Environment, 178, 21–28.
Kabata-Pendias, A., & Pendias, H. (2001). Trace elements in soils and plants (3rd ed.). Boca Raton: CRC.
Kashem, M. A., Singh, B. R., Kondo, T., Imamul Huq, S. M., & Kawai, S. (2007). Comparison of extractability of Cd, Cu, Pb and Zn with sequential extraction in contaminated and non-contaminated soils. International Journal Environmental Science Technology, 4(2), 169–176.
Kukier, U., Peters, C. A., Chaney, R. L., Angle, J. S., & Roseberg, R. J. (2004). The effect of pH on metal accumulation in two alyssum species. Journal of Environmental Quality, 33(6), 2090–2102.
Langmuir, D. (1997). Aqueous environmental geochemistry. Englewood Cliffs: Prentice Hall.
Lindsay, W. L., & Norvell, W. A. (1978). Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Science Society of America Journal, 42(3), 421–428.
McGrath, S. P. (1995). Chromium and nickel. In B. J. Alloway (Ed.), Heavy metals in soils (pp. 152–174). London: Blackie Academic and Professional.
Mogollón, J. L., Pérez-Diaz, A., & Lo Monaco, S. (2000). The effects of ion identity and ionic strength on the dissolution rate of a gibbsitic bauxite. Geochimica et Cosmochimica Acta, 64(5), 781–795.
Morais, F. I., Page, A. L., & Lund, L. J. (1976). The effect of pH, salt concentration, and nature of electrolytes on the charge characteristics of Brazilian tropical soils. Soil Science Society of America Journal, 40, 521–527.
Munasinghe, T., & Dissanayake, C. B. (1980). Is the Highland-eastern Vijayan boundary in Sri Lanka a possible mineralized belt? Economic Geology, 75(5), 775–777.
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403(6772), 853–858.
O’Hanley, D. S. (1996). Serpentinites: records of tectonic and petrological history. Oxford monographs on geology and geophysics (34th ed.). New York: Oxford University Press.
Oze, C. (2003). Chromium geochemistry of serpentinites and serpentine soils. Stanford: Stanford University.
Oze, C., Fendorf, S., Bird, D. K., & Coleman, R. G. (2004a). Chromium geochemistry in serpentinized ultramafic rocks and serpentine soils from the Franciscan complex of California. American Journal Science, 304, 67–101.
Oze, C., Fendorf, S., Bird, D. K., & Coleman, R. G. (2004b). Chromium geochemistry of serpentine soils. International Geology Review, 46, 97–126.
Oze, C., Skinner, C., Schroth, A., & Coleman, R. G. (2008). Growing up green on serpentine soils: biogeochemistry of serpentine vegetation in the Central Coast Range of California. Applied Geochemistry, 23, 3391–3403.
Parks, G. A., & de Bruyn, P. L. (1961). The zero point of charge of oxides. The Journal of Physical Chemistry, 66, 967–973.
Peijnenburg, W. J. G., Zablotskaja, M., & Vijver, M. G. (2007). Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicology and Environmental Safety, 67, 163–179.
Proctor, J., & Baker, A. J. M. (1994). The importance of nickel for plant growth in ultramafic (serpentine) soils. In S. M. Ross (Ed.), Toxic metals in soil–plant systems (pp. 417–432). Chichester: Wiley.
Rajakaruna, N., & Baker, J. M. (2004). Serpentine: a model habitat for botanical research in Sri Lanka. Ceylon Journal of Science (Biological Sciences), 32, 1–19.
Rajakaruna, N., & Bohm, B. A. (2002). Serpentine and its vegetation: a preliminary study from Sri Lanka. Journal of Applied Botany, 76, 20–28.
Rajakaruna, N., Harris, C. S., & Towers, G. H. N. (2002). Antimicrobial activity of plants collected from serpentine outcrops in Sri Lanka. Pharmaceutical Biology, 40(3), 235–244.
Rajakaruna, N., Harris, T. B., & Alexander, E. B. (2009). Serpentine geoecology of Eastern North America: a review. Rhodora, 111(945), 21–108.
Rajapaksha, A. U., Vithanage, M., Oze, C., Bandara, W. M. A. T., & Weerasooriya, R. (2012). Nickel and manganese release in serpentine soil from the Ussangoda ultramafic complex, Sri Lanka. Geoderma, 189–190, 1–9.
Ranasinghe, N. S. (1987). Serpentinites associated with the precambrian of Sri Lanka. Geological Society of Sri Lanka special publication no. 3. Colombo: Geological Survey Department.
Senevirathne, A. S., Nandadasa, H. G., Fernando, W. S., Sanjeevani, H. H. V. M., & Rajapakshe, R. L. H. R. (2000). The serpentine vegetation of Ussangoda (Hambantota District) and nickel accumulating plant species. Paper presented at the Proceedings of the Sixth Annual Forestry and Environmental Symposium, Kandy, Sri Lanka, 29–30 December.
Stumm, W., & Morgan, J. J. (1996). Aquatic chemistry. New York: Wiley Inter-Science.
Sucik, G., Hrsak, D., Fedorockova, A., & Lazic, L. (2008). The preliminary characterization of serpentinite from Ljeskovac locality in Croatia. Acta Metallurgica Slovaca, 14, 275–280.
Sun, Y.-c., Chi, P.-h., & Shiue, M.-y. (2001). Comparison of different digestion methods for total decomposition of siliceous and organic environmental samples. Analytical Sciences, 17(12), 1395–1399.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851.
Tye, A. M., Young, S., Crout, N. M. J., Zhang, H., Preston, S., Zhao, F. J., et al. (2004). Speciation and solubility of Cu, Ni and Pb in contaminated soils. European Journal of Soil Science, 55(3), 579–590.
Van der Ent, A., Baker, A. M., Reeves, R., Pollard, A. J., & Schat, H. (2013). Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant and Soil, 362(1–2), 319–334.
Weerasinghe, H. A. S., & Iqbal, M. C. M. (2011). Plant diversity and soil characteristics of the Ussangoda serpentine site. Journal National Science Foundation Sri Lanka, 39(4), 355–363.
Acknowledgements
International Foundation for Science (Sweden) and Organization for the Prohibition of Chemical Weapons, The Hague, are kindly acknowledged for their funding (grant number W/5068-1). Authors thank Dr. J.C. Bailey at the Institute for Geography and Geology, University of Copenhagen and Dr. Steen Christensen and colleagues at the Department of Earth Sciences, University of Aarhus, Denmark for providing XRF results and Prof. Y. S. Ok at the Department of Biological Environment at Kangwon National University, South Korea for EPMA analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vithanage, M., Rajapaksha, A.U., Oze, C. et al. Metal release from serpentine soils in Sri Lanka. Environ Monit Assess 186, 3415–3429 (2014). https://doi.org/10.1007/s10661-014-3626-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3626-8