Abstract
Recent and past studies have documented the prevalence of pyrethroid and organophosphate pesticides in urban and agricultural watersheds in California. While toxic concentrations of these pesticides have been found in freshwater systems, there has been little research into their impacts in marine receiving waters. Our study investigated pesticide impacts in the Santa Maria River estuary, which provides critical habitat to numerous aquatic, terrestrial, and avian species on the central California coast. Runoff from irrigated agriculture constitutes a significant portion of Santa Maria River flow during most of the year, and a number of studies have documented pesticide occurrence and biological impacts in this watershed. Our study extended into the Santa Maria watershed coastal zone and measured pesticide concentrations throughout the estuary, including the water column and sediments. Biological effects were measured at the organism and community levels. Results of this study suggest the Santa Maria River estuary is impacted by current-use pesticides. The majority of water samples were highly toxic to invertebrates (Ceriodaphnia dubia and Hyalella azteca), and chemistry evidence suggests toxicity was associated with the organophosphate pesticide chlorpyrifos, pyrethroid pesticides, or mixtures of both classes of pesticides. A high percentage of sediment samples were also toxic in this estuary, and sediment toxicity occurred when mixtures of chlorpyrifos and pyrethroid pesticides exceeded established toxicity thresholds. Based on a Relative Benthic Index, Santa Maria estuary stations where benthic macroinvertebrate communities were assessed were degraded. Impacts in the Santa Maria River estuary were likely due to the proximity of this system to Orcutt Creek, the tributary which accounts for most of the flow to the lower Santa Maria River. Water and sediment samples from Orcutt Creek were highly toxic to invertebrates due to mixtures of the same pesticides measured in the estuary. This study suggests that the same pyrethroid and organophosphate pesticides that have been shown to cause water and sediment toxicity in urban and agriculture water bodies throughout California, have the potential to affect estuarine habitats. The results establish baseline data in the Santa Maria River estuary to allow evaluation of ecosystem improvement as management initiatives to reduce pesticide runoff are implemented in this watershed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal estuaries are among the most ecologically important and critically threatened habitats in California. Less than 20 % of the State’s coastal wetlands remain from the time of European settlement, and many of these wetlands face threats from water quality degradation. Along California’s Central Coast, the Santa Maria River drains to a coastal estuary that provides essential habitat for early life stages of commercially and recreationally important marine fish species, threatened anadromous fish species, migratory birds, and other wildlife.
The lower Santa Maria River watershed contains year-round, intensively cultivated agricultural land that supports a \$3.5 billion/year industry producing much of the nation’s lettuce, artichokes, and crucifer crops. Runoff from irrigated agriculture constitutes a significant portion of river flow during most of the year, and a previous study documented pesticide occurrence and biological affects in the Santa Maria River (Anderson et al. 2006a).
As toxicity associated with organophosphate and pyrethroid pesticides has been shown to be prevalent in urban and agriculture watersheds throughout California (Holmes et al. 2008), the primary goal of the study was to determine whether these pesticides have the potential to affect critical estuarine habitats at the downstream end of coastal watersheds. Evidence of pesticide impacts in central California has encouraged implementation of farm management practices to reduce pesticide concentrations and toxicity in agricultural runoff. The second goal was to establish baseline conditions for the Santa Maria Estuary to facilitate detection of changes in pesticide concentrations as management practices are implemented.
Methods
Study location
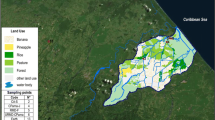
The Santa Maria River watershed drains approximately 1,880 mi2 comprising 1,203,000 acres on California’s central coast. The watershed includes the Cuyama and Sisquoc Rivers which join to form the Santa Maria River. Orcutt Creek drains approximately 50,000 acres of land southeast of the Santa Maria River estuary. Inputs to the Santa Maria River estuary are dominated by Orcutt Creek and a drainage ditch that enters the river near the entrance to the Rancho Guadalupe Dunes Preserve. Together, flows from these two sources comprise 92 % of the total input into the estuary (SAIC 2004). The Santa Maria River estuary provides critical nesting and foraging habitat to resident and migratory shorebirds, including western snowy plovers. The system functions as a lagoon during those parts of the year when beach sand blocks flow to the ocean, and it functions as an estuary when winter waves and high river flows breaches the lagoon’s western edge. The estuary is recognized as a globally important wetland along the Pacific Flyway in the western hemisphere (SAIC 2004). The estuary and lagoon also provide critical nursery and foraging habitat for numerous marine and estuarine fish and invertebrate species, including threatened tidewater gobies. In the lower Santa Maria and adjacent Oso Flaco Creek watersheds, several water bodies, including Orcutt-Solomon Creek, and the lower Santa Maria River, are currently listed as impaired by pesticides and/or nutrients under Clean Water Act §303[d]. Monitoring for pesticides and associated biological effects in the Santa Maria River estuary have been limited. Recent studies have included pesticide and toxicity monitoring in the lower Santa Maria River watershed (Anderson et al. 2006a; Phillips et al. 2006; Phillips et al. 2010a) and on-going regulatory monitoring associated with grower cooperatives in the watershed.
This study measured sediment toxicity and chemistry, benthic macroinvertebrate community structure, and water column toxicity and chemistry, combining stratified sampling designs for each component. For sediments, the Santa Maria River estuary was divided into eight sections and a station was sampled in each section for sediment toxicity and chemistry (Electronic supplementary material (ESM) Fig. 1). Samples for benthic community characterization were collected at spatially representative stations 1–5, which are the stations nearest to the ocean. Water column toxicity testing and chemical analyses were conducted at three stations. One station was located in the lower estuary (lower), another in the upper estuary (upper; square symbols in ESM Fig. 1) and a third was located in Orcutt Creek, a key input into the estuary. This station, ORC, is indicated with a circle in ESM Fig. 1 and was located at the sand plant, where the creek crosses under the road to the Guadalupe Dunes Reserve.
This study was conducted from January 2008 until October 2009. Three irrigation season sediment samples were collected at the eight sediment stations and the additional water column stations in May and October 2008 and again in October 2009. Sediment samples were analyzed for selected herbicide, pesticides and metals, as well as grain size and total organic carbon. Sediment toxicity (H. azteca 10 days) was assessed at the eight estuary stations and the Orcutt Creek tributary station. Benthic community characterizations were conducted during the May and October 2008 sediment sampling events at five of the eight sediment stations.
For water column toxicity testing and chemical analyses, a total of 15 sampling events were conducted, divided between 11 irrigation season events, and 4 storm events. Storm events were defined as rainfall greater than or equal to 0.5 in. within 24 h preceding sampling. For all sampling events, the following parameters were monitored at the upper and lower estuary stations: water toxicity using either H. azteca (96 h), C. dubia (96 h), or both species (depending on conductivity, see below), water chemistry analyses for selected pesticides (described below), and conventional water chemistry (dissolved oxygen, pH, turbidity, and conductivity). All of these parameters were also analyzed during nine sampling events conducted concurrently at the Orcutt Creek tributary station.
Water and sediment collection
Water and sediment samples were collected using previously reported methods (Anderson et al. 2006a; Phillips et al. 2010a). Water was collected in 2.5-L amber glass bottles then stored at 4 ± 3 °C for no longer than 48 h prior to toxicity test initiation. Bed sediment was collected to a maximum depth of 5 cm using polycarbonate core tubes. Samples were homogenized in a stainless steel bowl and placed in 2-L glass jars and stored in iced coolers for transport. Testing was initiated within 2 weeks of sample collection. Additional containers of water and sediment were collected for chemical analysis and shipped or delivered to the US Geological Survey’s analytical laboratory in Sacramento, CA.
Benthic community collection
For benthic invertebrates, a 0.1-m2 area was sampled to a sediment depth of 5 cm at each site using polycarbonate cores. Samples were deposited into a 1-mm sieve and swirled gently in a few inches of water to screen out sediment. Samples were stored in plastic jars and fixed in the field with borate-buffered 10 % formalin. After a period of 3 days to 2 weeks, samples were rinsed with water and stored in 70 % ethanol. Samples were shipped to Weston Solutions in Carlsbad, CA for taxanomic analysis.
Toxicity testing
Water toxicity testing
As discussed earlier, the lower Santa Maria watershed functions as a lagoon/estuary depending on season, water flow, and the influence of wave and tidal action on beach height. As a result, the conductivity (and salinity) of the system varied, and it was necessary to use a combination of fresh to brackish water test species to assess toxicity. The upper part of the estuary nearest Orcutt Creek was dominated by this freshwater input (average conductivities, <1,000 μS/cm; salinity, <∼1 ‰) and the lower estuary nearest the ocean was usually more brackish (conductivity range = 3,180 to 13,860 μS/cm; salinity range, ∼2 to 11 ‰). Water toxicity in the estuary samples was assessed using 96 h exposures with the amphipod H. azteca (USEPA 2002), a species more tolerant of brackish water. Tests were conducted on whole, un-filtered water samples. Water toxicity in the Orcutt Creek tributary sample was assessed using 96-h exposures with the water flea C. dubia using similar procedures. Orcutt Creek samples with conductivities exceeding 3,000 μS/cm were tested with H. azteca, and estuary samples with conductivities less than 3,000 μS/cm were also tested with C. dubia side-by-side with H. azteca. Both tests were used, when appropriate, because of their differences in sensitivities to current-use pesticides such as organophosphate and pyrethroid pesticides.
Amphipod exposures were conducted in 300-mL beakers containing 100 mL of test solution and ten organisms. Daphnid tests were conducted in 50-mL glass beakers, each containing 30 mL of test solution and five organisms. Both exposures consisted of five replicate beakers. Test solutions were renewed at 48 h, at which time both organisms were fed yeast, cerophyll, and trout chow mixture (YCT). Dissolved oxygen, pH, and conductivity were measured with an Accumet meter and appropriate electrodes (Fisher Scientific, Pittsburgh, PA). Un-ionized ammonia was measured using a Hach 2010 spectrophotometer (Hach, Loveland, CO). Water temperature was recorded with a continuous recording thermometer (Onset Computer Corporation, Pocasset, MA). Additional daily temperatures were measured using a glass spirit thermometer.
Sediment toxicity testing
Sediment toxicity was assessed three times at the eight estuary stations and Orcutt Creek using the 10-day survival toxicity test with H. azteca (USEPA 2000), a resident genus in the watershed. As mentioned above, the estuary/lagoon part of the system tended to be more brackish than marine, so a species tolerant of a low to moderate salinity regime was appropriate. Each sample was thoroughly homogenized on a sediment roller and divided among eight laboratory replicates, with ten 7- to 14-day-old amphipods in each. The amphipods were exposed to 100 mL of sediment in 300 mL beakers, each containing 175 mL of overlying water. The test temperature was 23 ± 1 °C. Water quality parameters, including dissolved oxygen, pH, conductivity, and ammonia, were measured at the beginning of each test. Hardness and alkalinity were measured at the beginning of each test. Un-ionized ammonia was measured in sediment over-lying water using a Hach 2010 spectrophotometer (as above). Overlying water was renewed twice daily, and 1.5 mL YCT was added daily to each test container. Survival was compared with that of amphipods exposed to a formulated reference sediment.
Benthic community characterization
Samples were sorted into major taxonomic groups and identified to the lowest practical taxonomic level, most often species. Taxonomic quality was assured by re-identification and re-enumeration of 10 % of the samples by independent taxonomists. Species lists were tabulated and community indices were calculated including the Relative Benthic Index (RBI) for each sample. RBIs values were calculated using central coast lagoon habitat methodology (Barnett et al. 2008). The RBI was developed for application to California bay and estuarine habitats. A detailed description of the methods used to calculate the RBI for central coast estuaries is provided in Hunt et al. (2001). The community pattern metrics used in the RBI include number of species and individuals (total number of all taxa, total number of mollusc species and individuals, and total number of crustacean species and individuals), the abundance of species indicative of relatively disturbed benthic habitats, and the abundance of species indicative of relatively undisturbed benthic habitats. Negative indicators included Capitella sp. complex and oligochaetes. Positive indicators included the amphipods Grandifoxus grandis and Eohaustorius estuarius and the bivalve Tellina modesta. The overall RBI was calculated by summing the values of the total fauna, total molluscs, crustacean species, and indicator species and standardizing it to the total range. This resulted in a range of values from 0.00 (most impacted) to 1.00 (least impacted). The RBI is scaled from 0 to 1 based on the range of values in the development dataset. The scaling is based on the Habitat E dataset (coastal wetlands and estuaries) (Ranasinghe et al. 2010). During application to the present data, if the raw RBI value was less than the minimum in the development dataset, the result is a negative scaled value. This occurred in several of the estuary samples presented below.
RBI developer thresholds were based on the distribution of index values, following Hunt et al. (2001). The RBI values were subdivided into four categories (Weisberg et al. 2008; Ranasinghe et al. 2009): (1) Unaffected—a community that would occur at a reference site for that habitat; (2) Marginal deviation from reference—a community that exhibits some indication of stress, but might be within measurement variability of reference condition; (3) Affected—a community that exhibits clear evidence of physical, chemical, natural, or anthropogenic stress; (4) Severely affected—a community exhibiting a high magnitude of stress. Affected and severely affected communities are those believed to be showing clear evidence of disturbance, while unaffected and marginal communities do not. Disturbed communities could be due to the effects of one or more types of anthropogenic or natural stress while undisturbed communities likely indicate minimal stress of all types (Ranasinghe et al. 2010).
The RBI is the only index available for use in west coast wetlands and estuaries, designated Habitat E in Ranasinghe et al. (2010). These are low-salinity coastal wetlands and estuaries ranging from southern California to the Pacific Coast of Washington State. We note that the application of the RBI in Habitat E has not been validated, although it has been validated for use in other areas such as Habitats C (southern California marine bays) and D (polyhaline central San Francisco Bay) (Ranasinghe et al. 2009) and was used to evaluate central California sites as part of a California statewide analysis using various benthic community index methods (Ranasinghe et al. 2010).
Chemistry methods
Water
Water samples were analyzed for a suite of 59 pesticides by extracting one liter of sample water onto Oasis HLB SPE cartridges. Prior to extraction, all water samples were filtered using a 0.7-μm glass fiber filter to separate suspended material. The filter and filtered particles were eluted with ethyl acetate and this extract was analyzed separately using the same GC procedure described below. All samples were spiked with 13C-atrazine and 13C-diazinon as recovery surrogates. Following extraction, the SPE cartridges were dried with carbon dioxide, eluted with 12 mL of ethyl acetate, reduced to 200 μL and deuterated internal standards were added. All sample extracts were analyzed on a Varian Saturn 2000 (Walnut Creek, CA, USA) gas chromatograph/ion trap mass spectrometer (GC/ITMS). Reported chemicals for each sample represent the sum of compounds eluted from the glass fiber filter plus those eluted from the SPE column. Additional details are given in Hladik et al. (2008).
Sediment
Sediment samples were extracted based on methods described in Smalling and Kuivila (Smalling and Kuivila 2008). Briefly, sediment samples were extracted by pressurized liquid extraction using a Dionex 200 Accelerated Solvent Extractor with dichloromethane. Sample matrix was removed using stacked pre-packed Carbon/Alumina SPE cartridges. Finally, sulfur was removed using a gel-permeation/high-pressure liquid chromatography system. Sample extracts were analyzed for current-use pesticides by GC/ITMS. In addition, moisture content, percent organic carbon, and percent nitrogen were measured for each sediment sample. As the lower part of this watershed is dominated by agriculture irrigation water runoff, the organic chemical analysis emphasized legacy organochlorine pesticides, current use organophosphate, carbamate and pyrethroid pesticides, fungicides, and herbicides. Organic chemicals associated with urban inputs were not analyzed (e.g., polynuclear aromatic hydrocarbons and flame retardants). Sediment samples were also analyzed for trace metals (As, Ag, Cd, Cr, Cu, Hg, Mg, Ni, Pb, and Zn) by inductively coupled plasma mass spectroscopy using US EPA method 6010A.
Data interpretation
To ensure the integrity of the data collected, quality assurance/quality control (QA/QC) procedures were conducted. Pesticide concentrations in all matrices (water, sediment, and biota) were validated against a comprehensive set of quality control parameters including laboratory and field blanks, matrix spikes, replicate samples, certified reference material, and surrogate recovery. Environmental and QA/QC data met or exceeded applicable California Surface Water Ambient Monitoring Program (SWAMP 2008) guidelines. Samples were defined as toxic if the following two criteria were met: (1) there was a significant difference (p < 0.05) in mean organism response (e.g., percent survival) between a sample and the laboratory control, as determined using a separate-variance t test, and (2) the difference in organism response between the sample and control was greater than 20 % (Phillips et al. 2001).
Chemistry data in water and sediment were compared with known toxicity thresholds, where available, and to other water quality criteria. The thresholds used for assessing the potential for pesticide toxicity to C. dubia and H. azteca in water are provided in ESM Table 1a. Pesticide toxic units (TUs) for C. dubia and H. azteca were calculated by dividing the measured chemical concentration by the organism-specific LC50s. Total organic carbon concentrations in the sediment were used to normalize total sediment chemical concentrations to organic carbon-corrected concentrations. Corrected concentrations are considered to be more representative of the bioavailable fraction of contaminants in sediment. Higher concentrations of TOC can reduce the bioavailability of sediment contaminants.
Previous research has shown that chlorpyrifos and diazinon are additive when they occur in mixtures (Bailey et al. 1997), as are some mixtures of pyrethroids (Trimble et al. 2010). Based on this, and the fact that all of the pesticides that were measured at toxic concentrations are neurotoxins, the TUs for these pesticides were added to calculate a total TU value for each sample. TUs for H. azteca were calculated by adding individual TUs from chlorpyrifos, diazinon, bifenthrin, permethrin. Sum TUs for C. dubia were calculated by adding individual TUs from chlorpyrifos, diazinon, malathion, bifenthrin, and permethrin.
In addition, concentrations of unionized ammonia were also measured in water and these were compared with LC50s for toxicity to C. dubia and H. azteca. No unionized ammonia concentrations exceeded these LC50s in any of the samples during this study.
Sediment toxicity thresholds and sediment quality guideline values used to assess the sediment chemistry data are provided in ESM Table 1b. In addition, concentrations of unionized ammonia were also measured in sediment overlying water and these were compared with the LC50 for toxicity to H. azteca. No unionized ammonia concentrations exceeded the LC50 in any of the samples during this study.
Results and discussion
Water toxicity
A high incidence of water toxicity was observed in the upper and lower Santa Maria River estuary stations (Table 1). At the upper estuary station, nine of the eleven (82 %) irrigation season water samples, and two of the four storm season water samples were toxic to H. azteca. In addition, 36 % of the irrigation season water samples from the lower estuary station, and 50 % of the storm season samples were toxic to H. azteca. Toxicity to H. azteca was also observed in two of the three Orcutt Creek water samples, and 95 % of the water samples tested with C. dubia were significantly toxic (Table 1). Water quality parameters in the water samples were compared with established acceptability ranges for each parameter to verify that they were not exceeded (e.g., dissolved oxygen, pH, conductivity, and alkalinity). None of the samples exceeded the acceptability ranges.
Elevated chlorpyrifos concentrations accounted for water toxicity to C. dubia and H. azteca in the majority of samples in Orcutt Creek and in the Santa Maria River estuary (Table 2). The LC50s for chlorpyrifos toxicity to H. azteca and C. dubia are 86 and 53 ng/L, respectively (Phipps et al. 1995; Bailey et al. 1997). Many of the samples had very high concentrations of diazinon, which contributed to toxicity to C. dubia (LC50 = 320 ng/L) (Bailey et al. 1997). The diazinon concentrations in these water samples likely had a negligible effect on H. azteca (LC50 = 6,510 ng/L) (Ankley and Collyard 1995). Summed chemical TUs in Orcutt Creek ranged from 0.47 to 12.6 in samples toxic to H. azteca. Sum TUs in the upper estuary ranged from 0.56 to 6.44 in the samples toxic to H. azteca. Sum TUs in the lower estuary ranged from 0.55 to 4.51 in the samples toxic to H. azteca (Table 2).
In no cases were there sufficient concentrations of pyrethroids in water to account for H. azteca mortality. In a concurrent study in 2008 and 2009, five pyrethroid pesticides were detected in Orcutt Creek water samples and in samples from the lower Santa Maria River (Phillips et al. 2010a), that was approximately 100 meters east of the upper estuary station sampled in the current study. Concentrations of two pyrethroids, cyhalothrin, and cypermethrin, were at or above water toxicity thresholds for H. azteca in the 2008–2009 study (Phillips et al. 2010a).
While the majority of samples from Orcutt Creek and the Santa Maria River estuary had sufficient chlorpyrifos to account for the observed C. dubia mortality, many of the samples also had toxic concentrations of diazinon (Table 2). Sum TUs in Orcutt Creek ranged from 0.45 to 7.9 in the samples toxic to C. dubia. Sum TUs ranged as high as 5.40 and 4.77, respectively, in the upper and lower estuary samples toxic to C. dubia.
Sediment toxicity
As was observed with water toxicity testing, a high frequency of sediment toxicity was observed in samples from the Santa Maria estuary during this study. Although both amphipod survival and growth were measured in the sediment tests, minimal growth effects were observed (data not shown). The following discussion pertains to impacts on amphipod survival. Eleven of 24 sediment samples collected from June 2008 to October 2009 were toxic to the amphipod H. azteca (46 %, Table 3). The highest magnitude of toxicity was observed in samples from the upper estuary stations, reflecting the proximity of these stations to the Orcutt Creek confluence. Sediments from all of the Orcutt Creek samples were highly toxic to amphipods. Moderate toxicity was also observed in October 2009 in samples from stations 1 and 2, the two stations nearest the mouth of the estuary. All samples were screened to assess whether unionized ammonia exceeded the toxicity threshold for H. azteca, no samples were above this concentration (4.17 mg/L; MPSL unpublished data).
Analysis of the sediment showed that they were contaminated by mixtures of metal and organic chemicals. No metals were detected at concentrations exceeding established toxicity thresholds (for example see ESM Table 1 for copper, nickel, and arsenic). Copper concentrations in sediment were less than 17 μg/g in all samples, nickel concentrations were all less than 20 μg/g, arsenic concentrations were all less than 6 μg/g, cadmium concentrations were all less than 0.65 μg/g, and zinc concentrations were all less than 56 μg/g in all samples (data not shown). A number of organic chemicals were detected, including herbicides, fungicides, and organochlorine, organophosphate, pyrethroid and carbamate pesticides (ESM Table 2). Of the 11 estuary samples that were toxic to H. azteca, five had sum TUs greater than 0.5 and four had sum TU greater than 0.1. The sum TU values were driven by concentrations of chlorpyrifos and several pyrethroids, including bifenthrin, cyhalothrin, and cypermethrin. There were no toxic concentrations of chemicals in the two lower estuary samples that exhibited moderate toxicity. The Orcutt Creek samples had sum TU concentrations ranging from approximately 0.8 to 3.1. These values were driven by concentrations of chlorpyrifos, bifenthrin, cyhalothrin, cypermethrin, and esfenvalerate.
The relationship between sum TUs and amphipod survival in all sediment samples from the Santa Maria River estuary indicate that except for one sample, all sediments contaminated by more than 0.4 sum TUs demonstrate significant amphipod mortality (Fig. 1). Chemical analysis of Orcutt Creek and Santa Maria estuary sediments suggest that a combination of chlorpyrifos and several pyrethroid pesticides accounted for much of the toxicity observed in sediments in this system. Based on a comparison of chemicals driving the TU calculations, the majority of toxic sediments contained toxic concentrations of chlorpyrifos, pyrethroids, or mixtures of the two classes of pesticides (Table 3; ESM Table 2). As discussed above, unionized ammonia was below the toxicity threshold for H. azteca in these samples, as were measured metal concentrations. The two pyrethroids accounting for most of the toxicity were cyhalothrin and cypermethrin. These results are consistent with previous studies in the lower Santa Maria River watershed that have indicated sediment toxicity is caused by these same pesticides (Anderson et al. 2006a; Phillips et al. 2006; Phillips et al. 2010a). In cases where significant mortality was observed in samples with lower or no TUs detected, toxicity may be due to chemicals other than those detected during analysis or present in mixtures at low concentrations. Pyrethroid pesticides are particularly problematic in this regard because the method detection limits fall within the range of toxicity.
Benthic community characterization
Benthic macroinvertebrate (BMI) assemblages in the Santa Maria estuary were classified as severely affected in the May and November 2008 sampling periods (Table 4). The average number of taxa in the Santa Maria estuary was 3.4 and 6.0, in May and November, respectively. Ranasinghe et al. (2010) found that samples from uncontaminated Habitat E (wetland and marsh habitat) stations had an average of 15.9 taxa per sample. The May samples were dominated by oligochaetes and chironomids, likely due to the fact that the river lagoon was not open to the ocean during this time, and all stations had low salinity. There were fewer species and individuals in these samples, and all stations were described as category 4 (high disturbance) using the Relative Benthic Index. While there were negative indicator taxa in all of these samples, no positive indicator species were found.
A greater number of estuarine species and higher densities of animals were present in the November samples, reflecting higher salinities when the estuary was open to tidal influences. There was a greater range of species and higher densities of animals present, relative to May. All samples except Station 5 contained mixtures of the amphipods Americorophium sp. and Eogammarus confervicolus, as well as oligochaetes (except station 2) and chironomids. Total amphipod numbers in the five estuary stations in November 2008 were 360, 83, 87, 130, and 1, at stations 1–5, respectively (=total of Americorophium sp. + Corophium sp. + E. confervicolus). Amphipod abundance in the November 2008 samples corroborated amphipod mortality in the sediment toxicity tests conducted the previous month. For example, in the five lower estuary stations where sediment toxicity and benthic community structure were both characterized, the station with the lowest amphipod abundance in November 2008 (station 5), also demonstrated the highest amphipod mortality in October 2008 sediment toxicity tests.
As with all other benthic community samples in this study, only negative indicator species, such as Capitella sp., chironomids, and oligochaetes, were present. The positive indicators T. modesta, G. grandis, and E. estuarius were absent from all samples. However, none of the positive indicators used in the current were listed as abundant in Habitat E assemblages in Ranasinghe et al. (2010). The amphipod E. estuarius is considered rare in central coast estuaries (personal communication, Jim Oakden, Moss Landing Marine Laboratories), and its absence may not be indicative of pollution impacts. The amphipods Americorophium stimpsoni, Americorophium spinicorne, and E. confervicolus were all found in the Santa Maria Estuary. While these species are common in the Habitat E assemblages described in Ranasinghe et al. (2010), there is little pollution tolerance information for these species. Amphipod species from the genus Americorophium are found to occur at the least impacted stations in the San Francisco estuary, and were listed as sensitive taxa in tidal freshwater habitats in Thompson et al. (2012). It should be noted that there is much disagreement among west coast benthic ecologists about indicator taxa in mesohaline and tidal freshwater habitats (Thompson et al. 2012). As development and validation of benthic indices proceed with analysis of more coastal wetland and estuarine habitats, determination of the specific stressor tolerances of species common to these habitats will need to be determined. Based on the relative abundances of the amphipods A. stimpsoni, A. spinicorne, and E. confervicolus, it would be useful to determine which, if any, of these amphipods should be categorized as indicator species for Habitat E assemblages. Thompson et al. (2012) listed the two Americorophium species that were sometimes found in the Santa Maria River estuary as sensitive indicator species. To confirm whether the organophosphate and pyrethroid pesticides identified in the current project are affecting resident species, concentration-response data for selected chemicals should be developed for A. stimpsoni, A. spinicorne, and/or E. confervicolus. In addition, the relative effects of non-contaminant factors, particularly sediment grain size and TOC, should be evaluated. While the grain sizes at the BMI stations were all less than 10 % fined grain sediments in May 2008, they varied from 33 to 75 % fined grain sediments in the October 2008 samples. The percent TOC in these samples ranged from 0.02 to 0.5 % in the May 2008 samples, and from 0.7 to 3.6 % in the October 2008 samples (Table 3). Salinity and dissolved oxygen were consistent between the five BMI stations in the two sampling periods (data not shown). These and other noncontaminant factors could have influenced the distribution of macroinvertebrates in this system.
These results demonstrate that the Santa Maria River estuary is contaminated with toxic concentrations of organophosphate and pyrethroid pesticides and that this contamination is associated with laboratory toxicity. In addition, stations with the greatest contamination and toxicity also demonstrated severely impacted benthic macroinvertebrate communities. All stations had relatively depauperate macroinvertebrate assemblages, and species present were primarily pollution tolerant groups such and chironomids and oligochaetes. This was particularly true during the May 2008 sampling period. Recent monitoring on the central California coast has shown that of three coastal estuaries monitored, the Santa Maria estuary is the most impacted by elevated pesticide concentrations (Anderson et al. 2010b). This likely reflects the proximity of agriculture discharge streams to the estuary. The upper estuary stations are approximately one km downstream of the confluence of Orcutt Creek and the river. Approximately 90 % of the dry-weather flow observed in the lower Santa Maria River is comprised of discharge from the flows of Solomon and Orcutt Creeks and a second drainage ditch (SAIC 2004). A number of previous studies have demonstrated that Orcutt Creek below its confluence with Solomon Creek is contaminated by toxic concentrations of chlorpyrifos, diazinon, and several pyrethroid pesticides (Anderson et al. 2006a; Phillips et al. 2006; Phillips et al. 2010a). Studies have also found impacted macroinvertebrate communities in Orcutt Creek, and in the Santa Maria River downstream of its confluence with this creek (Anderson et al. 2006a). The current study demonstrates that these impacts extend into the estuary and are persistent over time.
There is growing evidence that pyrethroids in coastal urban creeks may accumulate to toxic concentrations in nearshore marine systems. Holmes et al. (2008) found toxicity to H. azteca at a number of urban creeks in southern California including Switzer Creek in San Diego County, Peters Canyon Wash in the San Diego Creek watershed in Orange County, and Ballona Creek in Los Angeles County. Additional studies in San Diego Harbor at Switzer Creek (Anderson et al. 2010a), Upper Newport Bay at San Diego Creek (Bay et al. 2005; Anderson et al. 2007; Ranasinghe et al. 2007; Phillips et al. 2010b), and the Ballona Creek Estuary (Bay et al. 2005; Ranasinghe et al. 2007; Lao et al. 2010) have shown persistent sediment toxicity, toxicity identification evaluation evidence of toxicity due to pyrethroids, and degradation of marine infaunal communities in these receiving systems.
Coastal lagoons and estuaries are important nursery grounds for nearshore fisheries, serve as refuge and habitat for migrating salmonids, and provide nesting and foraging habitat for resident and migrating shorebirds. Pesticides may directly or indirectly affect these communities through various mechanisms. For example, loss of corophiid and gamaridean amphipods shown to decline in the Santa Maria River estuary may affect foraging behavior of salmonids and littoral estuarine fish species (Shreffler et al. 1992; Grimmaldo et al. 2009). Previous bioassessments have shown declines in populations of H. azteca in the lower Santa Maria River and in Orcutt Creek (Anderson et al. 2006a). As H. azteca has been shown to be disproportionately important as a prey item for littoral fish species (Grimmaldo et al. 2009), effects on this and other amphipod species in the lower Santa Maria River and its estuary are likely relevant to the health and survival of resident and migrating fish. Pesticides may also directly affect salmon and other fish species through disruption of olfactory sensory neurons necessary for salmon homing and predator avoidance behaviors. Concentrations of diazinon, chlorpyrifos, and cypermethrin in Orcutt Creek presented in this study and by Phillips et al. (2010a) were within the range that has been demonstrated by others to affect salmonids (Scholz et al. 2000; Moore and Waring 2001; Sandahl et al. 2004) particularly if mixture effects are considered (Tierney et al. 2008).
References
Amweg, E. L., & Weston, D. P. (2007). Whole-sediment toxicity identification evaluation tools for pyrethroid insecticides: I. Piperonyl butoxide addition. Environmental Toxicology and Chemistry, 26, 2389–2396.
Amweg, E. L., Weston, D. P., & Ureda, N. M. (2005). Use and toxicity of pyrethroid pesticides in the Central Valley, CA, U.S. Environmental Toxicology and Chemistry, 24, 966–972.
Anderson, B. S., Phillips, B. M., Hunt, J. W., Worcester, K., Adams, M., Kapellas, N., & Tjeerdema, R. (2006a). Evidence of pesticide impacts in the Santa Maria River watershed, California, USA. Environmental Toxicology and Chemistry, 25, 1160–1170.
Anderson, B. S., Phillips, B. M., Hunt, J. W., Connor, V., Richard, N., & Tjeerdema, R. S. (2006b). Identifying primary stressors impacting macroinvertebrates in the Salinas River (California, USA): relative effects of pesticides and suspended particles. Environment and Pollution, 141, 402–408.
Anderson, B.S., Hunt, J.W., Phillips, B.M., Tjeerdema, R.S. (2007). Navigating the TMDL process: sediment toxicity. 02-WSM-2. Water Environment Research Foundation, p. 194
Anderson, B. S., Phillips, B. M., Hunt, J. W., Clark, S. L., Voorhees, J. P., Tjeerdema, R. S., Casteline, J., Stewart, M., Crane, D., & Mekebri, A. (2010a). Evaluation of methods to determine causes of sediment toxicity in San Diego Bay, California, USA. Ecotoxicology and Environmental Safety, 73, 534–540.
Anderson, B. S., Phillips, B. M., Hunt, J. W., Siegler, K., Voorhees, J. P., Smalling, K. L., Kuivila, K. M., & Adams, M. (2010b). Watershed-scale evaluation of agricultural BMP effectiveness in protecting critical coastal habitats: final report on the status of three Central California Estuaries. Sacramento: California State Water Resources Control Board.
Ankley, G., & Collyard, S. (1995). Influence of piperonyl butoxide on the toxicity of organophosphate insecticides to three species of freshwater benthic invertebrates. Comparative Biochemistry and Physiology - Part C, 110, 149–155.
Ankley, G. T., Dierkes, J. R., Jensen, D. A., & Peterson, G. S. (1991). Piperonyl butoxide as a tool in aquatic toxicological research with organophosphate insecticides. Ecotoxicology and Environmental Safety, 21, 266–274.
Bailey, H. C., Miller, J. L., Miller, M. J., Wiborg, L. C., Deanovic, L. A., & Shed, T. (1997). Joint acute toxicity of diazinon and chlorpyrifos to Ceriodaphnia dubia. Environmental Toxicology and Chemistry, 16, 2304–2308.
Barnett, A.M., Bay, S.M., Ritter, K.J., Moore, S.L., Weisberg, S.B., (2008). Sediment quality in California bays and estuaries. Technical Report No. 522. Southern California Coastal Water Research Project.
Bay, S. M., Mikel, T. M., Schiff, K., Mathison, S., Hester, B., Young, D., & Greenstein, D. (2005). Southern California bight regional monitoring program: i. sediment toxicity. technical report 451. Westminster: Southern California Coastal Water Research Project.
Brown, R. P., Landre, A. M., Miller, J. A., Kirk, H. D., & Hugo, J. M. (1997). Toxicity of sediment-associated chlorpyrifos with the freshwater invertebrates Hyalella azteca (amphipod) and Chironomus tentans (midge). Midland: Health and Environmental Research Laboratories Dow Chemical.
Grimmaldo, L. F., Stewart, A. R., & Kimmerer, W. (2009). Dietary segregation of pelagic and littoral fish assemblages in a highly modified tidal freshwater estuary. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science, 1, 200–217.
Hladik, M. L., Smalling, K. L., & Kuivila, K. M. (2008). A multi-residue method for the analysis of pesticides and pesticide degradates in water using HLB solid-phase extraction and gas chromatography-ion trap mass spectrometry. Bulletin of Environmental Contamination and Toxicology, 80, 139–144.
Holmes, R. W., Anderson, B. S., Phillips, B. M., Hunt, J. W., Crane, D., Mekebri, A., Blondina, G., Nguyen, L., & Connor, V. (2008). Statewide investigation of the role of pyrethroid pesticides in sediment toxicity in California’s Urban waterways. Environmental Science and Technology, 42, 7003–7009.
Hunt, J. W., Anderson, B. S., Phillips, B. M., Tjeerdema, R. S., Taberski, K. M., Wilson, C. J., Puckett, H. M., Stephenson, M., Fairey, R., & Oakden, J. (2001). A large-scale categorization of sites in San Francisco Bay, USA, based on the sediment quality triad, toxicity identification evaluations, and gradient studies. Environmental Toxicology and Chemistry, 20, 1252–1265.
Lao, W., Tsukada, D., Greenstein, D. J., Bay, S. M., & Maruya, K. A. (2010). Analysis, occurrence, and toxic potential of pyrethroids, and fipronil in sediments from an urban estuary. Environmental Toxicology and Chemistry, 29, 843–851.
Liber, K., Doig, L. E., & White-sobery, S. L. (2011). Toxicity of uranium, molybdenum, nickel and arsenic to Hyalella azteca and Chironumus dilutus in water-only and spiked-sediment toxicity tests. Ecotoxicology and Environmental Safety, 74, 1171–1179.
Maund, S. J., Hamer, M. J., Lane, M. C. G., Farrelly, E., Rapley, J. H., Goggin, U. M., & Gentle, W. E. (2002). Partitioning, bioavailability, and toxicity of the pyrethroid insecticide cypermethrin in sediments. Environmental Toxicology and Chemistry, 21, 9–15.
Moore, A., & Waring, C. P. (2001). The effects of a synthetic pyrethroid pesticide on some aspects of reproductions in Atlantic Salmon (Salmo salar L.). Aquatic Toxicology, 52, 1–12.
Nebeker, A. V., Schuytema, G. S., Griffis, W. L., Barbitta, J. A., & Carey, L. A. (1989). Effect of sediment organic carbon on survival of Hyalella azteca exposed to DDT and endrin. Environmental Toxicology and Chemistry, 8, 705–718.
Phillips, B. M., Hunt, J. W., Anderson, B. S., Puckett, H. M., Fairey, R., Wilson, C. J., & Tjeerdema, R. (2001). Statistical significance of sediment toxicity test results: threshold values derived by the detectable significance approach. Environmental Toxicology and Chemistry, 20, 371–373.
Phillips, B. M., Anderson, B. S., Hunt, J. W., Huntley, S. A., Tjeerdema, R. S., Richard, N., & Worcester, K. (2006). Solid-phase sediment toxicity identification evaluation in an agricultural stream. Environmental Toxicology and Chemistry, 25, 1671–1676.
Phillips, B. M., Anderson, B. A., Hunt, J. W., Siegler, K., Voorhees, J. P., & McNeill, K. (2010a). Pyrethroid and organophosphate pesticide-associated toxicity in two coastal watersheds (California, USA). Environmental Toxicology and Chemistry, 31, 1595–1603.
Phillips, B. M., Anderson, B. S., Voorhees, J. P., Hunt, J. W., Holmes, R. W., Mekebri, A., Connor, V., & Tjeerdema, R. S. (2010b). The contribution of pyrethroid pesticides to sediment toxicity in four urban creeks in California, USA. Journal of Pesticide Science, 35, 302–309.
Phipps, G. L., Mattson, V. R., & Ankley, G. T. (1995). The relative sensitivity of three benthic test species to ten chemicals. Archives of Environmental Toxicology Chemistry, 28, 281–286.
Ranasinghe, J. A., Barnett, A. M., Schiff, K., Montagne, D. E., Brantley, C., Beegan, C., Cadien, D. B., Cash, C., Deets, G. B., Diener, D. R., Mikel, T. M., Smith, R. W., Velarde, R. G., Watts, S. D., & Weisberg, S. B. (2007). Southern California Bight 2003 Regional Monitoring Program: III. Benthic Macrofauna. Technical Report 529. Costa Mesa: Southern California Coastal Water Research Project.
Ranasinghe, J. A., Weisberg, S. B., Smith, R. W., Montagne, D. E., Thompson, B., Oakden, J. M., Huff, D. D., Cadien, D. B., Velarde, R. G., & Ritter, K. J. (2009). Calibration and evaluation of five indicators of benthic community condition in two California bay and estuary habitats. Marine Pollution Bulletin, 59, 5–13.
Ranasinghe, J. A., Welch, K. I., Slattery, P. N., Montagne, D. E., Huff, D. D., Lee, I. H., Hyland, J. L., Thompson, B., Weisberg, S. B., Oakden, J. M., Cadien, D. B., & Velarde, R. G. (2010). Habitat-related benthic macrofaunal assemblages of bays and estuaries of the western United States. Integrated Environmental Assessment and Management, 7, 1–11.
SAIC. (2004). Santa Maria Estuary Enhancement and Management Plan. Phase I Final Report. Santa Barbara: Science Applications International Corporation.
Sandahl, J. F., Baldwin, D. H., Jenkins, J. J., & Scholz, N. L. (2004). Odor-evoked field potentials as indicators of sublethal neurotoxicity in juvenile coho salmon (Oncorhynchus kisutch) exposed to copper, chlorpyrifos, or esfenvalerate. Canadian Journal of Fisheries and Aquatic Sciences, 61, 404–413.
Scholz, N. L., Truelove, N. K., French, B. L., Berejikian, B. A., Quinn, T. P., Casillas, E., & Collier, T. K. (2000). Diazinon disrupts antipredator and homing behaviors in chinook salmon (Oncorhynchus tshawytscha). Canadian Journal of Fisheries and Aquatic Sciences, 57, 1911–1918.
Shreffler, D. K., Simenstad, C. A., & Thorn, R. M. (1992). Foraging by juvenile salmon in a restored estuarine wetland. Estuaries, 15, 204–213.
Smalling, K. L., & Kuivila, K. M. (2008). Multi-residue method for the analysis of 85 current-use and legacy pesticides in bed and suspended sediments. Journal Chromatography A, 1210, 8–18.
SWAMP. (2008). Surface Water Ambient Monitoring Program—Quality Assurance Program Plan Version 1. Sacramento: California Water Boards.
Thompson, B., Weisberg, S. B., Melwani, A., Lowe, S., Ranasinghe, J. A., Cadien, D. B., Dauer, D. M., Diaz, R. J., Fields, W., Kellog, M., Montagne, D. E., Ode, P. R., Reish, D. J., & Slattery, P. N. (2012). Low levels of agreement among experts using best professional judgement to assess mesohaline and tidal freshwater benthic macrofaunal condition in the San Francisco Estuary and Delta. Ecological Indicators, 12, 167–173.
Tierney, J. B., Sampson, J. L., Ross, P. R., Sekela, M. A., & Kennedy, C. J. (2008). Salmon olfaction is impaired by an environmentally realistic pesticide mixture. Environmental Science and Technology, 42, 4996–5001.
Trimble, A. J., Weston, D. P., Belden, J. B., & Lydy, M. J. (2010). Identification and evaluation of pyrethroid insecticide mixtures in urban sediments. Environmental Toxicology and Chemistry, 28, 1687–1695.
USEPA. (2000). Methods for measuring the toxicity and bioaccumulation of sediment-associated contaminants with freshwater invertebrates. EPA/600/R-99/064. Washington, DC: Office of Research and Development.
USEPA. (2002). Methods for measuring acute toxicity of effluents and receiving water to freshwater and marine organisms. EPA-821-R-02-012. Washington, DC: Office of Research and Development.
Weisberg, S. B., Thompson, B. E., Ranasinghe, J. A., Montagne, D. E., & Cadien, D. B. (2008). The level of agreement among experts applying best professional judgment to assess the condition of benthic infaunal communities. Ecology Indicator, 8, 389–394.
Weston, D., & Jackson, C. (2009). Use of engineered enzymes to identify organophosphate and pyrethroid-related toxicity in toxicity identification evaluations. Environmental Science and Technology, 43, 5514–5520.
Wheelock, C. E., Miller, J. L., Miller, M. J., Gee, S. J., Shan, G., & Hammock, B. D. (2004). Development of toxicity identification evaluation procedure for pyrethroid detection using esterase activity. Environmental Toxicology and Chemistry, 23, 2699–2708.
Acknowledgments
This project was funded by the California State Water Resources Control Board though Proposition 50 bond funds. The authors are grateful for the help of Sheila Holt, Weston Solutions, Carlsbad, California, for managing taxonomic analyses of the benthic macroinvertebrate samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, B., Phillips, B., Hunt, J. et al. Impacts of pesticides in a Central California estuary. Environ Monit Assess 186, 1801–1814 (2014). https://doi.org/10.1007/s10661-013-3494-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3494-7