Abstract
The human health impact of the historic and current mining and industrial activities in Tunisia is not known. This study assessed the exposure to metals in the population of Southern Tunisia, using biomonitoring. The aim of this pilot study was to evaluate metal exposure on 350 participants living near mining and active industrial areas in the South of Tunisia. Blood specimens were analyzed for metals (Cd, Cr, As, and Ni) by Atomic Absorption Spectrometer equipped with Zeeman background correction and AS-800 auto sampler by graphite furnace and graphite tubes with integrated L'vov platform. The sample population was classified according to different age groups, sex, smoking habit, sea food and water drinking consumption, occupational exposure, amalgam fillings and place of residence. The blood As, Cd, Cr and Ni values expressed as mean ± SD were 1.56 ± 2.49, 0.74 ± 1.15, 35.04 ± 26.02 and 30.56 ± 29.96 μg/l, respectively. Blood Cd and Ni levels in smokers were 2 and 1.2 times, respectively, higher than in non-smokers. Blood Cd levels increase significantly with age (p = 0.002). As, Cd and Ni were significantly correlated with gender and age (p < 0.05). Cd level in blood samples of subjects occupationally exposed was 1.3 times higher than that of non-exposed. Blood metals were not significantly affected by amalgam fillings, place of living and sea food and drinking water consumption. This first biomonitoring study of metal exposure in the South of Tunisia reveals a substantial exposure to several metals. The pathways of exposure and health significance of these findings need to be further investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the preindustrial period of human civilization, a heavy load of toxic metals being discharged into the biosphere was generally typical for endemic regions only. Environmental exposure to metals remains a worldwide public health problem and mining is a significant contributor to metal pollution in air, soil and water (EPA 2007; Greenberg et al. 2003). Currently, the majority of the world's population is exposed to toxic chemical elements from increasing anthropogenic pollution. Toxic metals present a health threat for populations living near mining and hazardous industrial objects.
In the case of Tunisia, published data about environment heavy metal contamination showed a relatively high content of these elements (Moldenhauer et al. 2008; Achiba et al. 2009; Klay et al. 2010; Gargouri et al. 2010; Ghannem et al. 2011; Houda et al. 2011; Serbaji et al. 2012). The South of Tunisia is considered as a relatively polluted area. In fact, elevated concentrations of heavy metals in the Tunisia country environment can originate from a variety of industrial processes, including metal plating, fertilizer production, mining, metallurgy, battery manufacturing and textile dyeing, among others (Zaghden et al. 2007; Barhoumi et al. 2009; Messaoudi et al. 2009; Eloussaief and Benzina 2010; Gannouni et al. 2011; Alaya-Ltifi et al. 2012; Garnit et al. 2012). Moreover, a phosphate treatment plant is now being developed along the coasts of Southeastern Tunisia, exposing coastal waters and marine organisms to increasing contamination (Zairi and Rouis 1999; Hamza-Chaffai et al. 2000, 2003). Crude phosphate mining has started in Tunisia in four regions of Gafsa City since 1899 (in Metlaoui, Redeyef, Moulares and Mdhilla), whereas phosphate treatment activity has been existing in Sfax City since 1952 (Thyna and Skhira) and in Ghannouch-Gabes City since 1972 (TPI: http://www.gct.com.tn/). During the first 20 years following the installation of these activities, 50 million tons of phosphogypsum have been released in the environment (Guillaumont et al. 1995). Heavy metals and metalloids, especially Cd, Cr, Ni and As, are waste products of industrial processes associated with smelting operations of phosphates (Sarbaji 2000; Azri et al. 2002a, b). In addition, South of Tunisia receives heavy metals from other sources including atmospheric fallout deposition (Sarbaji 2000) and outfall of untreated domestic sewage and wastewater (Tarchouna-Gharbi et al. 2010; Belaid et al. 2012). Moreover, the waters deriving from the treatment of a mix of industrial and domestic effluents have been used in Sfax City for forage crop irrigation for 17 years. The long-term use of this wastewater often results in the build-up of metals content in soils (Tarchouna-Gharbi et al. 2010; Belaid et al. 2012). As a matter of fact, the health of inhabitants living in these areas and chronically exposed to such contaminants for a long time could be affected. Therefore, the global burden of exposure to potentially heavy metals is unknown, and information concerning health risks to human exposed to metals in the Tunisia Country environment is scarce and remains an area to be studied.
In this context, several recent Tunisian studies have been conducted to document the contamination levels of heavy metals in various environmental samples such as sediment (Kharroubi et al. 2012; Serbaji et al. 2012), sedimentary phosphatic samples (Garnit et al. 2012), fish and mollusks (Barhoumi et al. 2009; Bellassoued et al. 2012) and plants (Kachout et al. 2012). However, only one study (of our research group) is available regarding the impact of arsenic on the population of Tunisia (Feki-Tounsi et al. 2013a, b). On the other hand, no data is available regarding the impact of Cd, Ni and Cr on humans in Tunisia. Therefore, this study represents the first human biomonitoring of Cd, Ni and Cr exposure in the Tunisian population. The present study aimed to determine the levels of Cd, Ni, Cr and As in whole blood of randomly selected Tunisian residents; assess the significance of the metal exposure and parameters including place of residence, gender and age; and compare the determined metal/metalloid reference values of Tunisian residents with those from other countries.
Materials and methods
Study areas and subjects
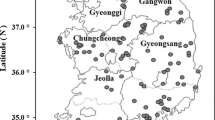
In the present study, 350 healthy unrelated individuals (257 men and 93 women) from Tunisia were enrolled after approval from the Ethics Committee of the Institute. In this essentially exploratory study, the sampling of subjects was not performed according to a predefined strategy that would provide an epidemiologically valid representation of the exposure of the population of the area. Rather, the choice of study areas was based on anticipated degrees of high or low exposure, as well as on pragmatic considerations, such as accessibility. Figure 1 shows localization of the study areas in South Tunisia and distribution map of mines and industrial types. A total of 265 subjects lived in areas situated very close to mines or industrial activities (0.5–10 km). Areas were located more than 10 km from mines or industrial activities was supposed to be less intense, even in subjects (n = 85) from these areas with supposedly low exposure. In these areas, local residents were simply approached and asked (after receiving background explanation about the study) if they were willing to provide blood samples and respond to a simple questionnaire about social habits and health problems of each individual. Subjects were interviewed to collect detailed information on their demographic (age, gender, and residential history), smoking habit, amalgam fillings, occupation and occupational exposure, as well as other lifestyle characteristics including water drinking and fish consumption. Lifetime consumption of tobacco smoking was collected. The average number of cigarettes smoked per day and the total number of years of smoking were used to calculate the cumulative smoking dose as "pack-years" (PY = [cigarettes per day/20] × years smoked). Lifetime of occupational exposure was also collected. The following occupational exposures were assessed (the number of subject ever exposed is given in parentheses): builders (23), painters (18), farmers (18), phosphogypsum (8), wood fumes (6), and others (16).
Positive responses were obtained from more than 98 % of those approached. Whole blood samples where As, Cr, Cd and Ni were measured, respectively, were also collected from all the selected subjects who lived in these areas. Because (1) the important industrial and mining activities which caused environmental pollution (Serbaji et al. 2012) and (2) the high contamination of fish species were collected from the Sfax and Gabès coasts (Barhoumi et al. 2009; Bellassoued et al. 2012), we have tried to study the association between Cd, Ni, Cr and As blood levels in the general population living in these polluted areas and fish consumption and tap water drinking. Additionally, because of the high number of subjects continuously exposed to chemical compounds (such as heavy metals) via tobacco smoking, occupational exposure and dental filling, we have also tried to determine the levels of these four metals in whole blood of the selected Tunisian subjects.
Collection of blood samples
For all studied subjects, 3 ml venous blood samples were drawn by nurses, stored into BD Vacutainer tubes with ethylenediaminetetraacetic acid (EDTA) anticoagulant. Samples were immediately transported in a cool box to the laboratory and then stored in a freezer at −20 °C for further analysis. Two metals were detected in these blood samples: Cr and Ni. To analyze these two metals, the blood samples were first digested with 66 % HNO3 (Sigma-Aldrich, Germany) at 80 °C with subsequent heating to a soft boil for 8 h. After mineralization, the solution was diluted with doubly distilled water to a final volume of 5 ml. It is clear that chemical digestion in appropriate conditions is required before blood analysis. Mineralization in a closed system — where the contamination problems are significantly reduced, can be recommended for such samples.
Determination of metals
Levels of metal compounds were determined by a Perkin-Elmer Analyst 800 Atomic Absorption Spectrometer (Perkin Elmer, Norwalk, USA) equipped with Zeeman background correction and an AS-800 auto sampler by graphite furnace and graphite tubes with integrated L'vov platform (Perkin Elmer). Appropriate matrix modifiers were used for the selected heavy metal studied and prepared in 0.2 % (v/v) nitric acid and 0.1 % Triton X-100. The limits of detection (LOD) were 0.03, 0.19 and 0.24 μg/l for Cd, Cr and Ni, respectively. Calibration graphs were linear until 7, 30 and 15 μg/l (for more details, see Olmedo et al. 2010). For arsenic, a direct flow-injection atomic absorption spectrometric technique (FI-HGAAS) was used to measure the levels of total arsenic. The arsenic contained in standard solutions (calibration curve 0, 0.5, 1.5 and 2.5 μg/l) or blood samples were reduced to As3+ prior to analysis with a mixture of potassium iodide and ascorbic acid. To 1 ml of the sample or reference solution, 1 ml of concentrated HCl and 1 ml of 5 % (w/v) KI-ascorbic acid were added. After 45 min at room temperature, the mixture was diluted to 10 ml with water.
The reducing agent was an aqueous solution of 0.2 % (w/v) NaBH4 in a 0.05 % (w/v) NaOH solution freshly prepared and filtered. Standard addition was required. The parameters for As were wavelength of 193.7 nm, integration time of 15 s, smoothing of 19 points or 0.5 s and temperature cell of 900 °C. An electrode less discharge lamp was used. The limit of detection (LOD) for this metalloid was 0.03 μg/l, and calibration graphs were linear until 5 μg/l (range of linearity from 0 to 5 μg/l) (Gil et al. 2006). All samples were analyzed at least twice, and in all the cases the concordance between both repetitions was not over 15 %.
Reference materials
The analytical method was controlled by using external certified reference materials (CRM). Reference samples for whole blood (three levels, refs. 201505, 201605 and 201705) were supplied by Seronorm (Billingstad, Norway). As they were supplied freeze dried, they were reconstituted by adding 5 ml of water and were run during the validation of procedures. The accuracy of CRM Seronorm As, Cd, Cr and Ni were 1.33 %, 0.45 %, 0.28 % and 5.26 %, respectively (Gil et al. 2006; Olmedo et al. 2010).
Statistical analysis
The basic statistics of data relating to 350 subjects included percentiles, mean, standard deviation (SD) and range. Differences in mean levels of metal compounds as well as the potential influence of classical confounders (tobacco, water drinking, amalgam fillings, fish consumption and gender) on these levels were assessed by using the Mann–Whitney test.
The blood metals means, SD, medians and quartiles were calculated. Statistical comparisons between age groups were carried out with one-way analysis of variance (ANOVA). Statistical calculations were performed by the statistics software SPSS for Windows, Version 13.0. A probability value (p) less than 0.05 was considered as significant.
Results
The demographic data of the subjects is presented in Table 1. Of the total, 73.4 % were males and 26.6 were females. Their age ranged from 17 to 95 years, with a mean ± SD of 53.75 ± 16.81. Regarding residence locations, 55.4 % of participants were from Sfax City, while 44.6 % were from the South of Tunisia, especially Gafsa (20.8 %) and Gabes (22.7 %) cities (Fig. 1). Three-quarters of the subjects lived in areas situated very close to mines and/or industrial activities (0.5–10 km). Overall, 71.7 % of participant consumed fish more than one time per week. The main sources of drinking water for our population are tap water and rain water. A total of 147 (42.0 %) subjects consumed tap water; 17.7 % of participants were current smokers and reported more than 20 PY of smoking. Moreover, 25.4 % of subjects were occupationally exposed and most (13.7 %) of them were exposed for more than 15 years. The subjects were classified into six categories according to occupational exposure (5.14 %, 5.14 %, 6.57 %, 1.71 %, 2.28 % and 4.57 % were exposed to dyes, pesticides, cement dust, wood fumes, phosphogypsum dust and other occupational exposure, respectively).
The distribution of As, Cd, Cr and Ni levels in whole blood in our study population are shown in Table 2. Because values did not follow a normal distribution the medians, ranges and 5–95th percentiles of the data for each element are presented in addition to mean and SD. As, Cd, Cr and Ni values in our general population were 1.56, 0.74, 35.04 and 30.56 μg/l, respectively. Mean values are given to make comparisons possible with other studies.
Table 3 presents data for the distribution of blood As, Cd, Cr and Ni levels in relation to gender. No significant differences were found between males and females for metal levels in blood sample analyzed.
The distribution of blood levels of these four elements in relation to age are shown in Table 4. Subjects were classified into seven groups by age. Blood Cd levels were found to increase significantly with age (p = 0.002). Contrary to Cd, Ni levels were found to decrease significantly with age (p < 0.001). We found a positive Spearman's correlation coefficient between blood Cd and age (p < 0.001) (Table 5), which means that blood Cd accumulates with age. However, no association was found between age and As and Cr (p > 0.05) (Table 4). Spearman's correlation for categorical variables shows that As, Cd and Ni were significantly correlated with gender and age (p < 0.05).
The magnitude of the correlation between metal concentrations in human whole blood and potential confounders were evaluated using Spearman's correlation for categorical variables (Table 5). As and Cd were significantly and positively correlated with PY number (r = 0.190 and 0.304, respectively, p < 0.001). Moreover, a significant correlation was seen with the seafood consumption rate for Cr and Ni (r = −0.115 and −0.117, respectively, p < 0.05). A positive Spearman's correlation coefficient between amalgam fillings and blood Cr and Ni (r = 0.134 and 0.137, respectively; p < 0.05) was observed. Cd levels in blood samples had a stronger correlation with occupational exposure (r = 0.106, p < 0.05). Furthermore, the correlation between metal concentrations in human whole blood showed that the metals As–Cd, Cr–As and Ni–Cr were statistically correlated (p < 0.05). There is any relationship between blood toxic element concentrations and the place of residence and water drinking in our population (Table 5).
Table 6 presents data for the bivariate analyses among As, Cd, Cr and Ni concentrations in blood as well as the major potential confounders in the study population. A positive association of Cd and Ni levels with smoking was found (comparison smoker with non-smoker) (p < 0.001 and p < 0.05, respectively). Moreover, blood Cd levels among subjects exposed occupationally (0.88 μg/l) were significantly higher than those non-exposed (0.69 μg/l). However, no association between metal blood levels and amalgam filling, water drinking and residential areas of in Tunisian population was found (Table 6).
Analysis of metal levels according to the types of some occupations is presented in Table 7. Very high blood As levels among subjects exposed occupationally to pesticides (3.57 μg/l) and wood fumes (3.07 μg/l) are shown; they were also significantly higher than those non-exposed (1.53 μg/l) (p < 0.001). Moreover, blood Cd levels among subjects exposed occupationally to dyes (1.47 μg/l) were significantly higher than those of non-exposed subjects (0.69 μg/l) (p < 0.01). Additionally, very high blood levels of Cr among builders exposed to cement dust were found (60.14 μg/l) in comparison with those of non-exposed subjects (35.37 μg/l) (p < 0.001).
Discussion
So far, the overall measure of exposure to multiple metals in the Tunisian population has not been well characterized, and data concerning metals contamination are insufficient. Our study is a pioneer investigation which provides much needed information regarding human exposure to Cd, Cr, Ni and As in Tunisia. In contrast with previous heavy metals monitoring Tunisian studies, our study was directly based on measurements of blood metal, which represents a measure of internal dose and not on environmental monitoring as metals in the sediment or in drinking water. Blood was validated as a biomarker of recent and chronic exposure to metals (Morton and Dunnette 1994; Gil and Pla 2001; Hall et al. 2006). Consequently, whole blood is the most widely used and accepted matrices for biomonitoring heavy metal exposure in environmental toxicology (Wilhelm et al. 2004; Gil and Pla 2001; Gil and Hernández 2009; Wang et al. 2011). Therefore, blood metal levels represent the most human biomonitoring of As, Cd, Ni and Cr exposure in our study population.
Blood metal levels in relation to gender and age
In the present study, blood As, Cd, Cr and Ni levels in our general population were 1.56, 0.74, 35.04 and 30.56 μg/l, respectively (Table 2). No significant differences were found between males and females for As, Cd, Cr and Ni levels in the blood samples analyzed (Table 3). Our results for Cd blood are an agreement with those observed by Gil et al. (2011), Nkolika and Benedict (2009) and Mortada et al. (2002) in Spanish, Nigerian and Egyptian populations, respectively. However, Liu et al. (2001), Huang et al. (2011) and Forte et al. (2011) reported that the Cd value was significantly higher in females than in males in Japanese, Korean and Italian populations, respectively.
We found a positive Spearman's correlation coefficient between blood Cd and age (p < 0.001) (Table 5), which means blood Cd accumulates with age (p = 0.002) (Table 4) and confirms that blood Cd traduces both recent and chronic exposures (Jarup et al. 1983, 1997). Our findings are in agreement with several other published data (Forte et al. 2011; Huang et al. 2011; Bjermo et al. 2013). This is a reasonable finding in view of this metal's well-known tendency to accumulate in the human body with increasing age (Friberg et al. 1974). Cd is not biotransformed. It is the most toxic elements, and many accumulating evidence points to negative health effects from cumulative lower level exposure (Hu 2002). These negative effects on human's health are due to its low excretion rate (half-life in tissue as long as 15–20 years and in blood is about 2.5 months) (Jin et al. 1998) and its accumulation in the organism. Hence, we can explain that the high Cd levels in the blood among older subjects can be due to their cumulative effects. Contrary to Cd, Ni blood levels were found to decrease with age (p < 0.001). While cadmium is a ubiquitous environmental pollutant whose concentrations in blood increase with the age as consistently found in the literature (Forte et al. 2011; Huang et al. 2011; Bjermo et al. 2013), Ni concentrations in blood are mainly affected by occupational exposure and decline rapidly when exposure is reduced or stopped (Von Burg 1997). Accordingly, it could be possible to observe that nickel blood levels decrease with age since a 40-year-old individual occupationally exposed to nickel could display higher nickel blood concentrations than another 60-year-old individual who is non-exposed.
Comparison between values in blood of metals selected in this study and reported in the literature
Mean values of our study are given to make comparisons possible with other studies. As, Cd, Cr and Ni are the metal compounds more widely studied in blood although large variations in their levels have been reported (Table 8). Mean concentrations are between 0.11 and 8.78 μg/l, 0.15 and 9.81 μg/l, 0.44 and 248 μg/l and 0.06 and 10.4 μg/l for As, Cd, Cr and Ni, respectively. The highest concentration of blood Cd and blood Cr is in Pakistan (Afridi et al. 2011a) and that for blood Ni is found in Russian and British populations (Ivanenko et al. 2012; Hodnett et al. 2012). However, for blood As, the highest concentration is in Tunisia (Feki-Tounsi et al. 2013a, b). When we compare our findings with those represented in previous studies (Table 8), we observed that our results for blood Cd among healthy individuals (0.74 ± 1.15 μg/l) agree with those of other published data in different localities; 0.99 (0.4–2) μg/l in Germany (Jung et al. 1993), 0.69 (0.1–1.9) μg/l in Belgium (Roels et al. 1994); and 0.1–3.4 μg/l in Italy (dell'Omo et al. 1999). Also, the results obtained in our study for blood As agree with those of Afridi et al.'s (2011b) study in Pakistan (1.7 ± 0.4 μg/l). Despite the higher blood Cr levels in our study in comparison with other populations, blood Cr levels in the Pakistani population (Afridi et al. 2011a) were 2-fold higher than our Cr values. However, our Ni values were far higher than those found by Ivanenko et al. (2012) and Hodnett et al. (2012). Cr and Ni high concentrations in blood samples of the Tunisian inhabitants might be appropriate to reflect their long-time environmental exposure to metals despite of the relatively short biological half-lives of these metals in human body. Therefore, it is reasonable to assume that the blood Cr and blood Ni levels of the study subjects were relatively consistent over a long period and could be reflected by the current metal levels in blood samples. Therefore, it might be appropriate to use the relative relationships between current blood Cr and blood Ni levels to describe the long-term relationships between blood Cr and blood Ni levels in our population.
In the literature, several epidemiologic studies indicate that a general population living near an industrial zone is a major risk factor for exposure to metals such as Cd, Cr, Ni and As (Banza et al. 2009; Chiang et al. 2010; Wang et al. 2011; Afridi et al. 2011a; Kim et al. 2011; Pino et al. 2012; Lee et al. 2012; Madeddu et al. 2012; Erraguntla et al. 2012). However, in the case of Tunisia, only one study of our research group (Feki-Tounsi et al. 2013a, b) is available regarding the impact of arsenic on the population of Tunisia. Regarding residence locations, a normal distribution of metal levels in the whole blood was found between the areas studied (Table 6). Moreover, inhabitants living in Southern Tunisian areas (Sfax, Gabes and Gafsa Cities) for years might be constantly exposed to and affected by such environmental metal pollutions. Previous studies have shown that Southern Tunisia area was highly affected by heavy metals pollution due to many industrial activities (Fig. 1), especially crude phosphate treatment industries (Azri et al. 2002a, b; Ben Amor-Magouri 2007; Elouear et al. 2008; Ghannem et al. 2011; Gargouri et al. 2010; Serbaji et al. 2012; Garnit et al. 2012; Kharroubi et al. 2012). The phosphate industry represents a potentially serious soil pollution hazard, with deposited contaminants being potentially hazardous to plants and groundwater. Phosphorus species were the principal carriers of heavy metals in soils (Tayibi et al. 2009; Garnit et al. 2012; Kassir et al. 2012; Al-Attar et al. 2012). Therefore, it is reasonable to assume that the blood-metal levels of the study subjects were relatively consistent over a long period of time and could be reflected by the current metal levels in blood samples. Additionally, with long-term exposure, blood metal can receive inputs from recent exogenous exposure such as diet, tap water drinking tobacco smoking and occupational exposure.
Associations between heavy metal concentrations in blood samples and potential confounders
In order to identify the factors influencing blood As, Cd, Cr and Ni levels, we looked for associations or correlations between these levels and occupational exposure, amalgam fillings, cultural habits such as smoking, fish consumption and the type of water consumed.
Tobacco smoking
The most outstanding finding in the current study is the positive association of Cd and Ni levels with smoking (p < 0.001 and p < 0.05, respectively) (Table 6). Moreover, As and Cd were significantly and positively correlated with PY number (p < 0.001) (Table 5). Previous studies reported that smoking constitutes an important source of chronic exposure to numerous xenobiotics, including heavy metals such as As (NRC 1999), Ni (Carter et al. 1997; Samet et al. 1997), Cr (IARC 1990; Chiba and Masironi 1992; Bernhard et al. 2005; Borgerding and Klus 2005) and in particular Cd in ionic form (Jin et al. 1998; Abshire et al. 1996; Joseph et al. 2001; Shih et al. 2003). Smoking 20 cigarettes/day has been estimated to result in an inhalation of about 2 to 4 μg Cd. Assuming a 25–50 % pulmonary absorption of this amount, smoking 20 cigarettes/day would result in a daily retention of about 1 to 2 μg Cd (Friberg et al. 1974; Elinder et al. 1983). Until now, exposure to heavy metals via smoking has been estimated based on the measurements of their concentrations in the blood, tissues or urine of smokers compared to non-smokers (Mortada et al. 2002; Stojanović et al. 2004; Batáriová et al. 2006; McKelvey et al. 2007; Son et al. 2009; Afridi et al. 2010; Gil et al. 2011; Forte et al. 2011). Therefore, our results reflect that smoking could be another major exposure source to these elements in our population and may in part explain the high variability in the blood metal levels. However, this well-known exposure source could not explain the high blood levels of metals due to exposure via tobacco smoking, suggesting that some other unknown exposure sources might be involved.
Occupational exposure
In the present study, 25.4 % of the participants reported exposing themselves to metal-related raw materials in the workplace. Results of the Spearman correlation and bivariate analyses indicated that Cd levels in blood samples had stronger correlation and association with occupational exposure (Tables 5 and 6) (p < 0.05). In fact, the Cd level in blood samples of subjects occupationally exposed was 1.3 times higher than that of non-exposed (Table 6). A previous follow-up study on Cd-exposed workers showed that blood Cd was a good indicator of cumulative dose many years after the cessation of exposure (Jarup et al. 1997). In addition, the classification by type of occupation shows that As, Cd and Cr levels in the blood samples of subjects among workers exposed to pesticides and wood fumes, dyes, and cement dust, respectively, were significantly higher than those of non-exposed (Table 7). In previous studies, elevated As, Cd, Cr and Ni levels in human blood have been reported, which might be attributed to the occupational exposure source (Chang et al. 2006; Stridsklev et al. 2004, 2007; Gil et al. 2011; Afridi et al. 2011a, b; Hodnett et al. 2012; Ivanenko et al. 2012; El-Shafei 2012; Feki-Tounsi et al. 2013a, b; Caciari et al. 2012). Therefore, the elevated blood metal levels among workers occupationally exposed in this study could be ascribed to occupational exposure such as workers exposed to cement dust, wood fumes, pesticides, pigments and dyes. Hence, we can explain that the high heavy metal levels in the blood of these workers can be due to the elevated concentration of As in pesticides and wood (Quandt et al. 2010; Decker et al. 2010), Cd in pigments/dyes such as cadmium pigments (IARC 1993) and Cr in cement (Sinyoung et al. 2011).
Sea food consumption and water drinking
Because seafood is a staple in the Mediterranean diet, our study population is a moderate consumer of fish. Several studies have been conducted to document the contamination levels of heavy metals in natural populations of fish collected from the Coastal areas of Sfax and Gabes governments (Hamza-Chaffai et al. 2003; Messaoudi et al. 2009; Barhoumi et al. 2009; Bellassoued et al. 2012). In our study, the correlation of blood Cr and Ni with sea food consumption (Table 5) confirms the environmental contamination by these two metals which may be concentrated throughout the dietary chain. The bioaccessibility from food to human indicates the portion of total chemical with food digested into solution and is potentially to be assimilated to reach systemic circulation by the alimentary canal (He et al. 2010; Bjermo et al. 2013). However, the bioaccessibility may provide an excellent provision of data in enhancing human health risk assessment (Man et al. 2010; Ju et al. 2012).
There is any relationship between blood toxic element concentrations and tap water drinking in the Tunisian population (Tables 5 and 6). Therefore, drinking water was not found to be incriminated as exposure source. This result involves the safety of water drinking, although there is no direct evidence to confirm this hypothesis. Indeed, standards determined by the specialized authorities are not yet updated to the last WHO (2000) guidelines, reflecting lack of control of these elements likely due to its formerly established rarity in the Tunisian environment.
Amalgam fillings
Amalgam, which was introduced more than 150 years ago, is the most frequently used material in tooth filling restoration. Dental fillings provide a major iatrogenic exposure to xenobiotic compounds such as heavy metals (Brownawell et al. 2005). Conventional dental amalgam is a mercury (Hg) and silver-based alloy and may also contain traces of cadmium, platinum and palladium. Considering the high number of subjects continuously exposed for many years to dental filling constituents, we have determined the levels of Cd, Ni, Cr and As in whole blood of these selected Tunisian subjects. Our results showed a positive Spearman's correlation coefficient between amalgam fillings and blood Cr and blood Ni (p < 0.05) (Table 5). However, no association between metal blood levels and amalgam filling was found (Table 6). Our findings were in accordance with previous studies that reported the absence of metal exposure resulting from the use of dental appliances (Bishara et al. 1993; Kerosuo et al. 1997).
There are several limitations to the current study. First, the findings were based on individuals whose age ranged from 17 to 95 years. The estimate was consistent with other studies. However, it is also important to include children because they are especially susceptible to heavy metal exposure and because of their sensitivity of the developing nervous system (Jarup 2003). Second, the designed questionnaire did not allow us to cover all the potential sources of exposure to these four metals. Therefore, future studies should focus on dietary sources. Third, the findings on metals were based only on subjects who lived in polluted areas. Therefore, a future study should focus on the evaluation of metals in people who lived in unpolluted areas, to serve as the control group.
Finally, as reported in several studies (Rautray et al. 2009; Chiang et al. 2010; Afridi et al. 2010, 2011b; Yuan et al. 2011; Chhabra et al. 2012; Pasha et al. 2010; Romanowicz-Makowska et al. 2011; Wadhwa et al. 2011; Feki-Tounsi et al. 2013a), elevated metal levels in human blood, which might be attributed to metals contaminants in some polluted areas, occupational exposure, smoking and contaminated water drinking sources, could play a significant role in the development of diseases and cancer. Therefore, we consider it necessary to study the effects of metal exposure on our general population using sensitive biomarkers of acute and chronic effect.
Conclusion
In summary, the present study is the first cadmium, chromium and nickel human biomonitoring study in Tunisia; hence, it provides values that may be useful for comparisons in future studies or when addressing public and environmental health challenges associated with heavy metal contamination. The As, Cd, Cr and Ni values in our general population were 1.56, 0.74, 35.04 and 30.56 μg/l, respectively. Levels of blood Cd and As were positively correlated with age and the levels blood Cd were accumulated with age. Despite the environmental heavy metal contamination, by mining and industrial activities, in the south of Tunisia, smoking and occupational exposure seem to be the main exposure source to heavy metals. Cd blood levels were significantly correlated with PY number (of smoking). The blood Cd levels of subjects occupationally exposed was 1.3-fold higher than that of non-exposed. Additionally, analysis of metals levels according to the type of occupational exposure showed an association between the very high blood As levels and exposure to pesticides and wood fumes, blood Cd levels and exposure to dyes and also blood Cr levels and exposure to cement dust. Finally, we consider that it is particularly important to evaluate simultaneous exposure to potentially toxic metals in areas with mining and industrial activities. This strategy may allow getting a better characterization of exposure to metals in resident subjects of these areas, which will reflect more closely the "real world" of exposed subjects. This may represent an advantage for the risk assessment analyses that should consider the results of the interaction of metals in population exposed simultaneously to mixture of metals and contribute to a better interpretation on health consequences. Overall, it is clear that more biomonitoring studies are urgently needed in regard to inhabitants' health in heavy metal-polluted areas in order to provide tools for better risk assessment, but mainly for the implementation of actions to reduce exposure.
References
Abshire, M. K., Buzard, G. S., Shiraishi, N., & Waalkes, M. P. (1996). Induction of proto-oncogene expression in rat L6 myoblasts by cadmium is inhibited by zinc preinduction of the metallothionein gene. Journal of Toxicology and Environmental Health, 48, 359–377.
Achiba, W. B., Gabteni, N., Lakhdar, A., Laing, G. D., Verloo, M., Jedidi, N., et al. (2009). Effects of 5-year application of municipal solid waste compost on the distribution and mobility of heavy metals in a Tunisian calcareous soil. Agriculture, Ecosystems and Environment, 130, 156–163.
Afridi, H. I., Kazi, T. G., Kazi, N. G., Jamali, M. K., et al. (2010). Evaluation of cadmium, lead, nickel and zinc status in biological samples of smokers and nonsmokers hypertensive patients. Journal of Human Hypertension, 24(1), 34–43.
Afridi, H. I., Kazi, T. G., Kazi, N., Abbas Kandhro, G., et al. (2011a). Evaluation of cadmium, chromium, nickel, and zinc in biological samples of psoriasis patients living in Pakistani cement factory area. Biological Trace Element Research, 142, 284–301.
Afridi, H. I., Kazi, T. G., Kazi, A. G., Shah, F., Wadhwa, S. K., et al. (2011b). Levels of arsenic, cadmium, lead, manganese and zinc in biological samples of paralysed steel mill workers with related to controls. Biological Trace Element Research, 144(1–3), 164–182.
Al-Attar, L., Al-Oudat, M., Shamali, K., Abdul, G. B., & Kanakri, S. (2012). Case study: heavy metals and fluoride contents in the materials of Syrian phosphate industry and in the vicinity of phosphogypsum piles. Environmental Technology, 33(1–3), 143–152.
Alaya-Ltifi, L., Chokri, M. A., & Selmi, S. (2012). Breeding performance of passerines in a polluted oasis habitat in southern Tunisia. Ecotoxicology and Environmental Safety, 79, 170–175.
Azri, C., Maalej, A., Tlili, A., & Medhioub, K. (2002a). Caractérisation du niveau de pollution atmosphérique dans la ville de Sfax (Tunisie): influence des sources et des facteurs mété orologique. Technical Sciences Information, 1, 78–92. http://cat.inist.fr/?aModele=afficheN&cpsidt=13488648.
Azri, C., Tlili, A., Serbaji, M. M., & Medhioub, K. (2002b). Etude des résidus de combustion des fuels liquide et solide et de traitement chimique du phosphate brut dans la ville de Sfax (Tunisie). Pollution Atmosphere, 174, 297–308. http://cat.inist.fr/?aModele=afficheN&cpsidt=13783104.
Banza, C. L., Nawrot, T. S., Haufroid, V., Decrée, S., De Putter, T., et al. (2009). High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environmental Research, 109(6), 745–752.
Barhoumi, S., Messoudi, I., Deli, T., Said, K., & Kerkn, A. (2009). Cadmium bioaccumulation in three benthic fish species, Salaria basilisca, Zosterisessor ophiocephalus and Solea vulgaris collected from the Gulf of Gabes in Tunisia. Journal of Environmental Sciences, 21, 980–984.
Batáriová, A., Spevácková, V., Benes, B., Cejchanová, M., Smíd, J., & Cerná, M. (2006). Blood and urine levels of Pb, Cd and Hg in the general population of the Czech Republic and proposed reference values. International Journal of Hygiene and Environmental Health, 209(4), 359–366.
Belaid, N., Neel, C., Lenainc, J. F., Buzierc, R., Kallel, M., Ayoub, T., et al. (2012). Assessment of metal accumulation in calcareous soil and forage crops subjected to long-term irrigation using treated wastewater: case of El Hajeb–Sfax, Tunisia. Agriculture, Ecosystems and Environment, 158, 83–93.
Bellassoued, K., Hamza, A., van Pelt, J., Elfeki, A. (2012). Seasonal variation of Sarpa salpa fish toxicity, as related to phytoplankton consumption, accumulation of heavy metals, lipids peroxidation level in fish tissues and toxicity upon mice. Environ Monit Assess. Apr 27.
Ben Amor-Magouri, R. (2007). Géochimie des eaux et des sédiments du littoral Ghannouche-Gabès (Golfe de Gabès). Impact des rejets de phosphogypse. Thèse de Doctorat., Fac. Sc. Tunis.
Bernhard, D., Rossmann, A., & Wick, G. (2005). Metals in cigarette smoke. IUBMB Live, 57, 805–809.
Bishara, S. E., Barrett, R. D., & Selim, M. I. (1993). Biodegradation of orthodontic appliances: Part II. Changes in blood level of nickel. American Journal of Orthodontics and Dentofacial Orthopedics, 103, 115–119.
Bjermo, H., Sand, S., Nälsén, C., Lundh, T., Enghardt Barbieri, H., et al. (2013). Lead, mercury, and cadmium in blood and their relation to diet among Swedish adults. Food and Chemical Toxicology, 57C, 161–169.
Borgerding, M., & Klus, H. (2005). Analysis of complex mixtures — cigarette smoke. Experimental and Toxicologic Pathology, 57(Suppl. 1), 43–73.
Brownawell, A.M., Berent, S., Brent, R.L., et al. (2005). The potential adverse health effects of dental amalgam. Toxicological Reviews, 24(1), 1–10.
Brune, D., Aitio, A., Nordberg, G., Vesterberg, O., & Gerhardsson, L. (1993). Normal concentrations of chromium in serum and urine — a TRACY project. Scandinavian Journal of Work, Environment & Health, 19, 39–44.
Caciari, T., Capozzella, A., Tomei, F., Nieto, H. A., et al. (2012). Arsenic and peripheral blood count in workers exposed to urban stressors. Clinica Terapeutica, 163(5), 293–302.
Carter, J. D., Ghio, A. J., Samet, J. M., & Devlin, R. B. (1997). Cytokine production by human airway epithelial cells after exposure to an air pollution particle is metal-dependent. Toxicology and Applied Pharmacology, 146, 180–188.
Chang, F. H., Wang, S. L., Huang, Y. L., Tsai, M. H., Yu, S. T., & Chang, L. W. (2006). Biomonitoring of chromium for residents of areas with a high density of electroplating factories. Journal of Exposure Science & Environmental Epidemiology, 16, 138–146.
Chappuis, P., Poupon, J., Deschamps, J. F., Guillausseau, P. J., & Rousselet, F. (1992). Physiological chromium determination in serum by Zeeman graphite furnace atomic absorption spectrometry. A serious challenge. Biological Trace Element Research, 32, 85–89.
Chhabra, D., Oda, K., Jagannath, P., Utsunomiya, H., Takekoshi, S., & Nimura, Y. (2012). Chronic heavy metal exposure and gallbladder cancer risk in India, comparative study with Japan. Asian Pacific Journal of Cancer Prevention, 13(1), 187–190.
Chiang, C. T., Lian, I. B., Su, C. C., Tsai, K. Y., Lin, Y. P., & Chang, T. K. (2010). Spatiotemporal trends in oral cancer mortality and potential risks associated with heavy metal content in Taiwan soil. International Journal of Environmental Research and Public Health, 7(11), 3916–3928.
Chiang, C. T., Chang, T. K., Hwang, Y. H., Su, C. C., Tsai, K. Y., et al. (2011). A critical exploration of blood and environmental chromium concentration among oral cancer patients in an oral cancer prevalent area of Taiwan. Environmental Geochemistry and Health, 33, 469–476.
Chiba, M., & Masironi, R. (1992). Toxic and trace element in tobacco smoke. Bulletin of the World Health Organization, 70, 269–275.
Christensen, J. M., & Kirchhoff, M. (1985). Simultaneous determination of nickel and cadmium in whole blood by Zeeman atomic absorption spectrophotometry: blood nickel and cadmium levels in the general population of Denmark. In S. S. Brown & F. W. Sunderman Jr. (Eds.), Progress in nickel toxicology (pp. 125–128). London: Blackwell Scientific.
Christensen, J. M., Poulsen, O. M., & Angiov, T. (1992). Protocol for the design and interpretation of method evaluation in AAS analysis. An application for the determination of lead and manganese in blood. Journal of Analytical Atomic Spectrometry, 7, 329–334.
Christensen, J. M., Hoist, E., Peter, J., & Knudsen, L. B. (1993). Determination of chromium in blood and serum: evaluation of quality control procedures and estimation of reference values in Danish subjects. The Science of the Total Environment, 132, 11–25.
Decker, P., Cohen, B., Butala, J. H., & Gordon, T. (2010). Exposure to wood dust and heavy metals in workers using CCA pressure-treated wood. AIHA Journal, 63, 166–171.
dell'Omo, M., Muzi, G., Piccinini, R., et al. (1999). Blood cadmium concentrations in the general population of Umbria, central Italy. Science of the Total Environment, 226(1), 57–64.
Edmé, J. L., Shirali, P., Mereau, M., et al. (1997). Assessment of biological chromium among stainless steel and mild steel welders in relation to welding processes. International Archives of Occupational and Environmental Health, 70(4), 237–242.
Elinder, C. G., Kjellström, T., et al. (1983). Cadmium exposure from smoking cigarettes: variations with time and country where purchased. Environmental Research, 32(1), 220–227.
Elouear, Z., Bouzid, J., Boujelben, N., Feki, M., Jamoussi, F., & Montiel, A. (2008). Heavy metal removal from aqueous solutions by activated phosphate rock. Journal of Hazardous Materials, 156(1–3), 412–420.
Eloussaief, M., & Benzina, M. (2010). Efficiency of natural and acid-activated clays in the removal of PbII from aqueous solutions. Journal of Hazardous Materials, 178, 753–757.
EL-Shafei, H. M. (2012). Monitoring of urine and serum cellular enzymes in the chromium electroplating workers. Journal of Bioengineering & Biomedical Science, 2, 3.
EPA. (2007). Framework for Metals Risk Assessment. Washington, DC: Office of the Science Advisor, Risk Assessment Forum.
Erraguntla, N. K., Sielken, R. L., Jr., Valdez-Flores, C., & Grant, R. L. (2012). An updated inhalation unit risk factor for arsenic and inorganic arsenic compounds based on a combined analysis of epidemiology studies. Regulatory Toxicology and Pharmacology, 64(2), 329–341.
Feki-Tounsi, M., Olmedo, P., Gil, F., Khlifi, R., Mhiri, M. N., Rebai, A., Hamza-Chaffai, A. (2013a). Low-level arsenic exposure is associated with bladder cancer risk and cigarette smoking: a case–control study among men in Tunisia. Environmental Science and Pollution Research International, 20, 3923–3931. doi:10.1007/s11356-012-1335-9.
Feki-Tounsi, M., Olmedo, P., Gil, F., Khlifi, R., Mhiri, M. N., Rebai, A., Hamza-Chaffai, A. (2013b). Cadmium in blood of Tunisian men and risk of bladder cancer: interactions with arsenic exposure and smoking. Environmental Science and Pollution Research International. doi:10.1007/s11356-013-1716-8.
Fontaine, J., Dewailly, E., Benedetti, J. L., Pereg, D., Ayotte, P., & Déry, S. (2008). Re-evaluation of blood mercury, lead and cadmium concentrations in the Inuit population of Nunavik (Québec): a cross-sectional study. Environmental Health, 7, 25.
Forte, G., Madeddu, R., Tolu, P., Asara, Y., Marchal, J. A., & Bocca, B. (2011). Reference intervals for blood Cd and Pb in the general population of Sardinia (Italy). International Journal of Hygiene and Environmental Health, 214(2), 102–109.
Foster, W.G., Cheung A.P., Davis, K., Graves, G., Jarrell, J., Leblanc, A., et al. (2012). Circulating metals and persistent organic pollutant concentrations in Canadian and non-Canadian born primiparous women from five Canadian centres: results of a pilot biomonitoring study. The Science of the Total Environment, 435–436, 326–336.
Friberg, L., Piscator, M., Nordberg, G. F., & Kjellstrom, T. (1974). Cadmium in the environment (2nd ed.). Cleveland: CRC Press.
Gannouni, S., Rebai, N., Belghith, A., & Abdeljaoued, S. (2011). Estimation des teneurs en métaux lourds dans les rejets des teuneurs en métaux lourds dans les rejets miniers de Jalta et Bougrine (Tunisie) par approche spectroscopique dans le visible et proche-infrarouge et étude statistique. Revue Télédétection, 10, 1.
Gargouri, D., Azri, C., Serbaji, M. M., Jedoui, Y., & Montacer, M. (2010). Heavy metal concentrations in the surface marine sediments of Sfax Coast Tunisia. Environmental Monitoring and Assessment. doi:10.1007/s10661-010-1548-7.
Garnit, H., Bouhlel, S., Barca, D., & Chtara, C. (2012). Application of LA-ICP-MS to sedimentary phosphatic particles from Tunisian phosphorite deposits: insights from trace elements and REE into paleo-depositional environments. Chemie der Erde, 72, 127–139.
Ghannem, N., Azri, C., Serbaji, M. M., & Yaich, C. (2011). Spatial distribution of heavy metals in the coastal zone of "Sfax–Kerkennah" plateau, Tunisia. Environmental Progress and Sustainable Energy, 30(2), 221–233.
Gil, F., & Hernández, A. F. (2009). Significance of biochemical markers in applied toxicology. In B. Ballantyne, T. C. Marrs, & T. Syversen (Eds.), General and applied toxicology (Vol. 2, pp. 847–858). Chichester: Wiley.
Gil, F., & Pla, A. (2001). Biomarkers as biological indicators of xenobiotic exposure (review article). Journal of Applied Toxicology, 21, 245–255.
Gil, F., Capitán-Vallvey, L. F., De Santiago, E., et al. (2006). Heavy metal concentrations in the general population of Andalusia, South of Spain A comparison with the population within the area of influence of Aznalcóllar mine spill SW Spain. Science of the Total Environment, 372, 49–57.
Gil, F., Hernández, A. F., Márquez, C., Femia, P., Olmedo, P., López-Guarnido, O., et al. (2011). Biomonitorization of cadmium, chromium, manganese, nickel and lead in whole blood, urine, axillary hair and saliva in an occupationally exposed population. Science of the Total Environment, 409, 1172–1180.
Goullé, J.P., Mahieu, L., Maighnat, V., Bouige, D., Saussereau, Lacroix, E. (2007). Whole blood and urine ICP-MS metal and metalloid determination: usual values found in fifty-four cadavers Annales de Toxicologie Analytique, vol. XIX, n° 1.
Granadillo, V. A., Parra de Machado, L., & Romero, R. A. (1994). Determination of total chromium in whole blood, blood components, bone, and urine by fast furnace program electrothermal atomization AAS and using neither analyte isoformation nor background correction. Analytical Chemistry, 66(21), 3624–3631.
Greenberg, I., Hamilton, J., Phillips, D., McCluskey, J. (2003). Mining industry. In: Occupational, industrial, and environmental toxicology. Mosby, Pennsylvania, USA, pp. 498–517.
Guillaumont, B., BenMustapha, S., BenMoussa, H., et al. (1995). Pollution impact study in Gabes Gulf (Tunisia) using remote sensing data. Marine Technology Society Journal, 29, 46–58.
Hall, M., Chen, Y., Ahsan, H., et al. (2006). Blood arsenic as a biomarker of arsenic exposure: results from a prospective study. Toxicology, 225, 225–233.
Hamza-Chaffai, A., Amiard, J. C., Pellerin, J., Joux, L., & Berthet, B. (2000). The potential use of metallothionein in the clam Ruditapes decussatus as a biomarker of in situ metal exposure. Comparative Biochemistry and Physiology, 127, 185–197.
Hamza-Chaffai, A., Pellerin, J., & Amiard, J. C. (2003). Health assessment of a marine bivalve Ruditapes decussatus from the Gulf of Gabe’s (Tunisia). Environment International, 28, 609–617.
He, M., Ke, C. H., & Wang, W. X. (2010). Effects of cooking and subcellular distribution on the bioaccessibility of trace elements in two marine fish species. Journal of Agricultural and Food Chemistry, 58, 3517–3523.
Hodnett, D., Wood, D. M., Raja, K., Dargan, P. I., & Shah, A. D. (2012). A healthy volunteer study to investigate trace element contamination of blood samples by stainless steel venipuncture needles. Clinical Toxicology (Philadelphia, Pa.), 50(2), 99–107.
Houda, B., Dorra, G., Chafai, A., Emna, A., & Khaled, M. (2011). Impact of a mixed "industrial and domestic" wastewater effluent on the southern coastal sediments of Sfax (Tunisia) in the Mediterranean Sea. International Journal of Environmental Research, 5(3), 691–704.
Hu, H. (2002). Human health and heavy metals exposure. In M. McCally (Ed.), Life Support: The Environment and Human Health (pp. 65–82). Cambridge: MIT Press.
Huang, M., Choi, S. J., Kim, D. W., Kim, N. Y., Bae, H. S., et al. (2011). Evaluation of factors associated with cadmium exposure and kidney function in the general population. Environmental Toxicology. doi:10.1002/tox.20750.
IARC. (1990). Working Group. Chromium and chromium compounds. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Human, 49, 249–256.
IARC. (1993). Cadmium and cadmium compounds. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 58, 119–237.
Ivanenko, N. B., Solovyev, N. D., Ivanenko, A. A., & Ganeev, A. A. (2012). Application of Zeeman graphite furnace atomic absorption spectrometry with high-frequency modulation polarization for the direct determination of aluminum, beryllium, cadmium, chromium, mercury, manganese, nickel, lead, and thallium in human blood. Archives of Environmental Contamination and Toxicology, 63(3), 299–308.
Jarup, L. (2003). Hazards of heavy metal contamination. British Medical Bulletin, 68, 167–182.
Jarup, L., Rogenfelt, A., Elinder, C. G., Nogawa, K., & Kjellstrom, T. (1983). Biological half-time of cadmium in the blood of workers after cessation of exposure. Scandinavian Journal of Work, Environment & Health, 9, 327–331.
Jarup, L., Persson, B., & Elinder, C. G. (1997). Blood cadmium as an indicator of dose in a long-term follow-up of workers previously exposed to cadmium. Scandinavian Journal of Work, Environment & Health, 23, 31–36.
Jin, T., Lu, J., & Nordberg, M. (1998). Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology, 19(4–5), 529–535.
Joseph, P., Muchnok, T., Klishis, M., Roberts, J., Antotini, J., Whong, W. Z., et al. (2001). Cadmium induced cell transformation and tumorigenesis are associated with transcription activation of c-fos, c-jun and c-myc proto-oncogenes: role of cellular calcium and reactive oxygen species. Toxicological Sciences, 61, 295–303.
Ju, Y. R., Chen, W. Y., & Liao, C. M. (2012). Assessing human exposure risk to cadmium through inhalation and seafood consumption. Journal of Hazardous Materials, 227–228, 353–361.
Jung, K., Pergarde, M., et al. (1993). Urinary proteins and enzymes as early indicators of renal dysfunction in chronic exposure to cadmium. Clinical Chemistry, 39, 757–765.
Kachout, S. S., Mansoura, A. B., Mechergui, R., Leclerc, J. C., Rejeb, M. N., & Ouerghi, Z. (2012). Accumulation of Cu, Pb, Ni and Zn in the halophyte plant Atriplex grown on polluted soil. Journal of the Science of Food and Agriculture, 92(2), 336–342.
Kassir, L. N., Lartiges, B., & Ouaini, N. (2012). Effects of fertilizer industry emissions on local soil contamination: a case study of a phosphate plant on the east Mediterranean coast. Environmental Technology, 33(7–9), 873–885.
Kerosuo, H., Moe, G., & Hensten-Pettersen, A. (1997). Salivary nickel and chromium in subjects with different types of fixed orthodontic appliances. American Journal of Orthodontics and Dentofacial Orthopedics, 111, 595–598.
Kharroubi, A., Gargouri, D., Baati, H., & Azri, C. (2012). Assessment of sediment quality in the Mediterranean Sea–Boughrara lagoon exchange areas (southeastern Tunisia): GIS approach-based chemometric methods. Environmental Monitoring and Assessment, 184, 4001–4014.
Kim, S., Cho, Y. M., Choi, S. H., Kim, H. J., & Choi, J. (2011). The effect of exposure factors on the concentration of heavy metals in residents near abandoned metal mines. Journal of Preventive Medicine and Public Health, 44(1), 41–47.
Klay, S., Charef, A., Ayed, L., Houman, B., & Rezgui, F. (2010). Effect of irrigation with treated wastewater on geochemical properties (saltiness, C, N and heavy metals) of isohumic soils (Zaouit Sousse perimeter, Oriental Tunisia). Desalination, 253, 180–187.
Lee, C. S., Lim, Y. W., Kim, H. H., Yang, J. Y., & Shin, D. C. (2012). Exposure to heavy metals in blood and risk perception of the population living in the vicinity of municipal waste incinerators in Korea. Environmental Science and Pollution Research International, 19(5), 1629–1639.
Lind, M. P., Olsen, L., & Lind, L. (2012). Elevated circulating levels of copper and nickel are found in elderly subjects with left ventricular hypertrophy. Ecotoxicology and Environmental Safety, 86, 66–72.
Liu, X. J., Arisawa, K., Nakano, A., Saito, H., Takahashi, T., & Kosaka, A. (2001). Significance of cadmium concentrations in blood and hair as an indicator of dose 15 years after the reduction of environmental exposure to cadmium. Toxicology Letters, 123(2–3), 135–141.
Madeddu, R., Tolu, P., Asara, Y., Farace, C., Forte, G., Bocca, B. (2012). Blood biomonitoring of metals in subjects living near abandoned mining and active industrial areas. Environ Monit Assess.
Man, Y. B., Sun, X. L., Zhao, Y. G., Lopez, B. N., Chung, S. S., Wu, S. C., et al. (2010). Health risk assessment of abandoned agricultural soils based on heavy metal contents in Hong Kong, the world's most populated city. Environment International, 36, 570–576.
McKelvey, W., Gwynn, R. C., et al. (2007). A biomonitoring study of lead, cadmium, and mercury in the blood of New York City adults. Environmental Health Perspectives, 115, 1435–1441.
Messaoudi, I., Barhoumi, S., Saïd, K., & Kerkeni, A. (2009). Study on the sensitivity to cadmium of marine fish Salaria basilisca Pisces, Blennidae. Journal of Environmental Sciences, 21, 1620–1624.
Moldenhauer, K. M., Zielhofer, C., & Faust, D. (2008). Heavy metals as indicators for Holocene sediment provenance in a semi-arid Mediterranean catchment in northern Tunisia. Quaternary International, 189, 129–134.
Mortada, W. I., Sobh, M. A., el-Defrawy, M. M., & Farahat, S. E. (2002). Reference intervals of cadmium, lead, and mercury in blood, urine, hair, and nails among residents in Mansoura City, Nile Delta, Egypt. Environmental Research Section A, 90, 104–110.
Morton, W. E., & Dunnette, D. A. (1994). Health effects of environmental arsenic. In J. O. Nriagu (Ed.), Arsenic in the environment: Part II. Human health and ecosystem effects (pp. 17–34). New York: Wiley.
Newton, A. W., Ranganath, L., Armstrong, C., et al. (2012). Differential distribution of cobalt, chromium, and nickel between whole blood, plasma and urine in patients after metal-on-metal (MoM) hip arthroplasty. Journal of Orthopaedic Research, 30(10), 1640–1646.
Nkolika, I. C., & Benedict, O. C. O. (2009). Elevated cadmium levels in blood of the urban population in Enugu State Nigeria. World Applied Sciences Journal, 7(10), 1255–1262.
NRC. (1999). Arsenic in the drinking water. Washington: National Research Council Report. National Academy Press.
Olmedo, P., Pla, A., et al. (2010). Validation of a method to quantify chromium, cadmium, manganese, nickel and lead in human whole blood, urine, saliva and hair samples by electrothermal atomic absorption spectrometry. Analytica Chimica Acta, 659, 60–67.
Pasha, Q., Mali, S. A., Shaheen, N., & Shah, M. H. (2010). Investigation of trace metals in the blood plasma and scalp hair of gastrointestinal cancer patients in comparison with controls. Clinica Chimica Acta, 411(7–8), 531–539.
Pazzini, C. A., Pereira, L. J., Carlos, R. G., de Melo, G. E. B., Zampini, M. A., & Marques, L. S. (2009). Nickel: periodontal status and blood parameters in allergic orthodontic patients. American Association of Orthodontists. doi:10.1016/j.ajodo.2009.02.036.
Pino, A., Amato, A., Alimonti, A., Mattei, D., & Bocca, B. (2012). Human biomonitoring for metals in Italian urban adolescents: data from Latium Region. International Journal of Hygiene and Environmental Health, 215(2), 185–190.
Quandt, S. A., Jones, B. T., et al. (2010). Heavy metals exposures among Mexican farm workers in eastern North Carolina. Environmental Research, 110, 83–88.
Rautray, T. R., Vijayan, V., et al. (2009). Analysis of blood and tissue in gallbladder cancer. Nuclear Instruments and Methods in Physics Research B, 267, 2878–2883.
Roels, H., Van de Voorde, R., Vargas, V. M., & Lauwerys, R. (1993) Relationship between atmospheric and urinary nickel in workers manufacturing electrical resistances using nickel oxide: role of the bioavailability of nickel. Occupational Medicine (Lond), 43(2), 95–104.
Roels, H., Lauwerys, R., Konings, J., Buchet, J. P., et al. (1994). Renal function and hyperfiltration capacity in lead smelter workers with high bone lead. Occupational and Environmental Medicine, 51, 505–551.
Romanowicz-Makowska, H., Forma, E., Bryś, M., Krajewska, W. M., & Smolarz, B. (2011). Concentration of cadmium, nickel and aluminium in female breast cancer. Polish Journal of Pathology, 62(4), 257–261.
Samet, J. M., Stonehuerner, J., Reed, W., Devlin, R. B., Daily, L. A., et al. (1997). Disruption of protein tyrosine phosphate homeostasis in bronchial epithelial cells exposed to oil fly ash. American Journal of Physiology, 272, L426–L432.
Sarbaji, M.M. (2000). Utilisation d'un SIG multi-sources pour la compréhension et la gestion intégrée de l'écosystème côtier de la région de Sfax (Tunisie). Thèse de l'Université de Tunis II. 163 pp.
Schroijen, C., Baeyens, W., Schoeters, G., Den Hond, E., Koppen, G., et al. (2008). Internal exposure to pollutants measured in blood and urine of Flemish adolescents in function of area of residence. Chemosphere, 71(7), 1317–1325.
Serbaji, M. M., Azri, C., & Medhioub, K. (2012). Anthropogenic contributions to heavy metal distributions in the surface and sub-surface sediments of the Northern Coast of Sfax, Tunisia. International Journal of Environmental Research, 6(3), 613–626.
Shih, C. M., Wu, J. S., et al. (2003). Mitochondria-mediated caspase-independent apoptosis induced by cadmium in normal human lung cells. Journal of Cellular Biochemistry, 89, 335–347.
Silva, N., Senanayake, H., & Waduge, V. (2013). Elevated levels of whole blood nickel in a group of Sri Lankan women with endometriosis: a case control study. BMC Research Notes, 14, 6–13.
Sinyoung, S., Songsiriritthigul, P., Asavapisit, S., & Kajitvichyanukul, P. (2011). Chromium behavior during cement-production processes: a clinkerization, hydration, and leaching study. Journal of Hazardous Materials, 191, 296–305.
Son, J.-Y., Lee, J., Paek, D., & Lee, J.-T. (2009). Blood levels of lead, cadmium, and mercury in the Korean population: results from the Second Korean National Human Exposure and Bio-monitoring Examination. Environmental Research, 109, 738–744.
Stojanović, D., Nikić, D., & Lazarević, K. (2004). The level of nickel in smoker's blood and urine. Central European Journal of Public Health, 12(4), 187–189.
Stridsklev, I. C., Schaller, K. H., & Langård, S. (2004). Monitoring of chromium and nickel in biological fluids of stainless steel welders using the flux-cored-wire (FCW) welding method. International Archives of Occupational and Environmental Health, 77, 587–591.
Stridsklev, I. C., Schaller, K. H., & Langård, S. (2007). Monitoring of chromium and nickel in biological fluids of grinders grinding stainless steel. International Archives of Occupational and Environmental Health, 80, 450–454.
Sunderman, F. W., Crisostomo, M. C., Reid, M. C., Hopfer, S. M., & Nomoto, S. (1984). Rapid analysis of nickel in serum and whole blood by electrothermal atomic absorption spectrophotometry. Annals of Clinical Laboratory Science, 14, 232–241.
Tarchouna-Gharbi, L., Merdy, P., & Lucas, Y. (2010). Effects of long-term irrigation with treated wastewater: Part II. Role of organic carbon on Cu, Pb and Cr behavior. Applied Geochemistry, 25, 1711–1721.
Tayibi, H., Choura, M., Lopez, F. A., Alguacil, F. J., & Lopez-Delgado, A. (2009). Environmental impact and management of phosphogypsum. Journal of Environmental Management, 90, 2377–2386.
Torra, M., Rodamilans, M., Corbella, J., Ferrer, R., & Mazzara, R. (1999). Blood chromium determination in assessing reference values in unexposed Mediterranean population. Biological Trace Element Research, 70(2), 183–189.
Von Burg, R. (1997). Nickel and some nickel compounds. Journal of Applied Toxicology, 17(6), 425–431.
Wadhwa, S. K., Kazi, T. G., Kolachi, N. F., et al. (2011). Case-control study of male cancer patients exposed to arsenic-contaminated drinking water and tobacco smoke with relation to non-exposed cancer patients. Human and Experimental Toxicology, 30(12), 2013–2022.
Walter, L. R., Marel, E., Harbury, R., & Wearne, J. (2008). Distribution of chromium and cobalt ions in various blood fractions after resurfacing hip arthroplasty. Journal of Arthroplasty, 23, 6.
Wang, M., Xu, Y., Pan, S., et al. (2011). Long-term heavy metal pollution and mortality in a Chinese population: an ecologic study. Biological Trace Element Research, 142(3), 362–379.
Wennberg, M., Lundh, T., Bergdahl, I. A., Hallmans, G., Jansson, J. H., Stegmayr, B., et al. (2006). Time trends in burdens of cadmium, lead, and mercury in the population of northern Sweden. Environmental Research, 100(3), 330–338.
WHO (2000) Guidelines for drinking water quality, volume 3. http://www.who.int/water_sanitation_health/dwq/dwqtraining/en/index.html. Accessed July 2012.
Wilhelm, M., Ewers, U., & Schulz, C. (2004). Revised and new reference values for some trace elements in blood and urine for human bio-monitoring in environmental medicine. International Journal of Hygiene and Environmental Health, 207, 69–73.
Yuan, T. H., Lian, I. B., Tsai, K. Y., Chang, T. K., Chiang, C. T., Su, C. C., et al. (2011). Possible association between nickel and chromium and oral cancer. A case–control study in central Taiwan. Science of the Total Environment, 409, 1046–1052.
Zaghden, H., Kallel, M., Elleuch, B., Oudot, J., & Saliot, A. (2007). Sources and distribution of aliphatic and polyaromatic hydrocarbons in sediments of Sfax, Tunisia, Mediterranean Sea. Marine Chemistry, 105, 70–89.
Zairi, M., & Rouis, J. (1999). Impacts environnementaux du stockage du phosphogypse à Sfax-Tunisie. Bulletin de Liaison des Laboratoires des Ponts et Chaussées, 219(4145), 29–40.
Acknowledgments
We thank all members of the Department of Toxicology and Legal Medicine, Faculty of Medicine of Granada, for their considerable assistance in the analysis of metals.
Disclosure statement
The study received ethics approval. Funding was provided by the Ministry of High Education and Scientific Research in Tunisia. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
The authors have no conflicts to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khlifi, R., Olmedo, P., Gil, F. et al. Biomonitoring of cadmium, chromium, nickel and arsenic in general population living near mining and active industrial areas in Southern Tunisia. Environ Monit Assess 186, 761–779 (2014). https://doi.org/10.1007/s10661-013-3415-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3415-9