Abstract
Laboratory experiment was conducted to understand the persistence behavior of tetraconazole in three soils of West Bengal (alluvial, red lateritic, and coastal saline) and also in water maintained at three different pH (4.0, 7.0, and 9.2) conditions. Processed soil samples (100 g) were spiked at two treatment doses: 2.5 μg/g (T1) and 5.0 μg/g (T2). Double distilled buffered water (200 ml) was spiked at two treatment doses: 1.0 μg/ml (T1) and 2.00 μg/ml (T2). The tetraconazole dissipation followed first-order reaction kinetics and the residual half-life (T 1/2) values in soil were found to be in the range of 66.9–77.2 days for T1 and 73.4–86.0 days for T2. The persistence increased in the order red lateritic > new alluvial > coastal saline. Interestingly, the red lateritic soil exhibited the lowest pH (5.56) and organic carbon (0.52 %) content as compared to other two soils. However, the dissipation of tetraconazole in case of water was not pH dependant. The T 1/2 values in water were in the range of 94 to 125 days. The study indicated the persistent nature of tetraconazole in soil and water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tetraconazole, (RS)-2-(2,4-dichlorophenyl)-3-(1H-1,2,4-triazole-1-yl)propyl 1,1,2,2-tetrafluoroethyl ether [C13H11Cl2F4N3O] is a broad spectrum systemic fungicide of triazole group (Khalfallah et al. 1998) introduced in 1973 by Grewe and Buchel. Tetraconazole is effective in controlling a broad spectrum of diseases such as powdery mildew and scab on fruits, powdery mildew on vines and cucumbers, powdery mildew and rust on vegetables and powdery mildew, and brown rust Septoria and Rhynchosporium on cereals (Menkissoglu et al. 1998).
Like all other triazoles, tetraconazole belongs to the sterol biosynthesis inhibitors group inhibiting the metabolic pathway leading to fungal sterol production, thus blocking the lanosterol demethylation reaction. Due to the presence of the tetrafluoroethoxy group in its molecule, tetraconazole can behave like either a hydrophilic or a lipophilic compound. Its well-balanced hydrosolubility/liposolubility ratio results in excellent systemic activity. Tetraconazole quickly enters the plant and distributes evenly throughout all treated tissues; this leads to a high level of protection even in the untreated section or in the vegetation grown after the spray. Due to its particular molecular structure which has no inhibitory action upon gibberellins and phytosterols (Fukuto 1987; Ware 1986; Stevens and Sumner 1991), dwarfing and phytotoxic effects are absent.
Studies on the residual fate of the compound reported half-life values of 4–5 days in mango (Alam et al. 2011) and preharvest intervals of 12.5 and 28.5 days at recommended rates and double application rates, respectively (Banerjee et al. 2008). However, the proposed application method of tetraconazole is ground and aerial application as a foliar spray or chemigation alternated with a non-triazole fungicide (EPA 2005). Thus, soil may serve as an indirect source of pesticide residue in the final produce. Possibilities of groundwater pollution also exist as leachate from soil may contain tetraconazole residue. In addition, runoff to surface water leaves risk of contamination of aquatic bodies to a varying degree. Thus, persistence of the fungicide in soil and water needs to be evaluated for safe and judicious use of the fungicide. In this context, the present study covers dissipation of tetraconazole in three soils and water buffered at three pH levels under laboratory condition.
Materials and methods
Persistence of tetraconazole in soil
Collection and processing of soil
Three types of agricultural field soils were collected from different agroclimatic zones of West Bengal, viz., (1) alluvial soil from University Research Farm, BCKV, Gayeshpur, Nadia; (2) red lateritic soil from Regional Research Station, BCKV, Jhargram, Midnapore; and (3) coastal saline soil from Research Station of Central Soil Salinity Research Institute (ICAR) at Canning, South 24- Parganas. Soil samples were air-dried and sieved to remove extraneous material.
Analysis of physicochemical properties of soils
The physicochemical properties of the soil were described in Table 1. pH of the soil solution (soil/water 1:2) was measured following the method described by Jackson (1973), organic matter of the soils following Walkley–Black method (Black 1965), textural class of the soils by the hydrometer method (Black 1965), and water holding capacity (WHC) by Keen’s box method (Piper 1944).
Persistence of tetraconazole in soil
Processed soil samples (100 g) were taken in amber-colored glass bottles. Tetraconazole analytical standard solution of 100 μg/g was used to spike the soils at two treatment doses. The initial concentrations were maintained at 2.5 and 5.00 μg/g for T1 and T2, respectively. After application, the soils were thoroughly mixed and allowed to equilibrate for 1 h at room temperature. The treated soil samples were transferred into glass containers covered with perforated aluminum foils, maintained at 70 % WHC, and incubated in the dark at 25 °C. Moisture content was maintained by addition of required amount of distilled water (equivalent to 70 % WHC). Samples were withdrawn at 0, 3, 7, 15, 30, 45, 60, 90, 120, and 150 days after application and stored at −20 °C until analysis.
Persistence of tetraconazole in water
Erlenmeyer flasks (250 ml) were filled with 200 ml of double distilled water. Buffer capsules (Merck) were added (one capsule/100 ml) to bring the pH of the water at 4.0, 7.0, and 9.2. Tetraconazole analytical standard (100 μg/ml) was used to spike the water at two treatment doses: 1.0 μg/ml (T1) and 2.00 μg/ml (T2). After application, the solutions were shaken for 2 min and covered with perforated aluminum foil. The Erlenmeyer flasks were incubated in the dark at 25 °C. Samples were withdrawn at 0, 3, 7, 15, 30, 45, 60, 90, 120, and 150 days after application.
Residue analysis of tetraconazole
Soil
The soil samples were transferred to Erlenmeyer flask (500 ml) using solvent mixture of methanol/water 9:1 (200 ml, v/v) and were subjected to continuous shaking for 2 h using a mechanical shaker. The extract was filtered through a Buchner funnel followed by rinsing the flask and filter paper with methanol (50 ml).
The extracts from soil were concentrated in rotary vacuum evaporator (RVE) and partitioned thrice with dichloromethane (100, 50, and 50 ml) after addition of aqueous saturated NaCl solution (100 ml). The organic layers were collected by passing through anhydrous Na2SO4 and concentrated to a minimum volume in RVE. The concentrated dichloromethane fraction was transferred to a glass column packed with silica gel (20 g) and anhydrous sodium sulfate (5 g). Hexane/acetone 1:1 (150 ml, v/v) mixture was used as eluent after discarding 200 ml of hexane.
Water
Samples were partitioned thrice with hexane/dichloromethane 8:2 (v/v) mixture (100, 50, and 50 ml). The organic fractions were collected by passing through anhydrous Na2SO4 and concentrated in RVE. After drying, the residue was transferred to measuring tubes with distilled ethyl acetate for GC analysis and the volume was made up so as to bring the final concentration into the linear range of the detector.
Estimation of tetraconazole residues
Tetraconazole was analyzed by GC Hewlett-Packard (USA) model 5890A coupled with ECD (Ni63) attached to Chemito 5000 data processor. A DB-1701 column of length 30 m and film thickness 1.5 μm with an i.d. 0.53 mm was used for chromatography. Analysis was accomplished using an isothermal temperature condition with injector; detector and oven temperature were maintained at 275, 340, and 210 °C, respectively. Gas flow was adjusted to a constant value of 50 ml/min till the end of a complete run. The desired peak from sample chromatogram was compared with a chromatogram of analytical-grade tetraconazole having a retention time at 6.02 min.
Method validation
Preparation of standard calibration curve
The calibration curve was obtained by plotting the peak areas against the concentration of the standard solution. The limit of detection (LOD) of tetraconazole was determined by considering a signal-to-noise ratio (S/N) of 3 with reference to the background noise obtained for the blank sample, whereas the limits of quantification (LOQ) were determined by considering an S/N of 10, according to the peak-to-peak noise method as proposed by United States Environmental Protection Agency (US EPA) (Corley 2003).
Accuracy: recovery experiment
Recovery study was carried out in order to establish the authenticity of the analytical method employed. Soil samples (100 g) and water samples (200 ml) were fortified at the level of LOQ and 10 times of LOQ with the analytical standard solutions of tetraconazole and were analyzed following the above procedure. Five samples were taken at each fortification level along with two control samples.
Data analysis
The residue data of tetraconazole in soil and water were analyzed using the following mathematical expression.
Where [A] t is the concentration (in milligram per kilogram) of A at time t (days) and [A]1 is initial concentration of A at time 0 (days) and k is the degradation rate constant. The half-life values (DT50) in soil and water were then evaluated applying the following relation:
Results and discussion
Method validation
The linearity of the calibration curve was established in the range 1–25 ng/g with a correlation coefficient R 2 > 0.99. The LOD and LOQ were 0.001 and 0.003 μg/g, respectively for soil, and 0.0004 and 0.0001 μg/g for water, respectively. Average recovery percentages from the fortified samples of three different soils of West Bengal ranged between 83 and 87 % with relative standard deviations (RSD) from 5.1 to 15.8 (Table 2). Recovery study for water was carried out in a similar way. Average recovery percentages from the fortified samples ranged between 92 and 97 % with RSD from 6.5 to 11.6 (Table 3). Control samples were free of tetraconazole residues. Thus, there was compliance with the EU DG SANCO criterion in this regard, which requires mean recoveries within the range of 70–110 % European Commission Directorate of General Health and Consumer Protection (2000). Various methods with comparable range of limit of determination of tetraconazole residues, using either gas chromatography or liquid chromatography, have also been reported (Li et al. 2012; Fernandes et al. 2012; Amer et al. 2007)
Persistence and dissipation of tetraconazole in soil
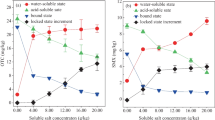
In all the three representative soils of West Bengal, dissipation of tetraconazole followed first-order kinetics. The initial concentrations were 2.43 and 4.95 μg/g for T1 and T2 doses in red lateritic soil with the calculated half-life values of 77.2 and 86.0 days, respectively (Table 4). A slight higher initial concentration of 2.50 and 5.00 μg/g was observed in case of alluvial soil with the respective half-life values of 68.4 and 75.2 days indicating a faster degradation of tetraconazole in alluvial soil than in red lateritic soil. The half-life values were the lowest in coastal saline soil, ranging from 66.9 to 73.4 days with the initial concentrations of 2.49 and 4.95 μg/g. Thus, persistence of tetraconazole was found to be in the order of red lateritic soil > new alluvial soil > coastal saline (Fig. 1). The fastest dissipation of tetraconazole in costal saline soil could be attributed to soil organic carbon content in the soils (Table 1). The higher the soil organic carbon content, the less was the persistence of the fungicide in the soil. Therefore, the red lateritic soil with the lowest organic carbon (0.52 %) content exhibited the highest persistence as compared to the other two soils. This relationship was also observed for triadimefon (Singh 2005) and propiconazole (Thorstensen and Lode 2001). The degradation behavior of other triazole fungicides, as reported earlier, shows that most of the triazole fungicides are very persistent in nature. Dissipation follows first-order kinetics with half-lives of about 200 days for propiconazole and greater than 2 years for flutriafol, epoxiconazole, and triadimenol. Degradation rates depend on the temperature, soil moisture (Bromilow et al. 1999), and organic matter content (Thorstensen and Lode 2001).
Persistence of tetraconazole in water
Tetraconazole is highly persistent in water of different pH. At pH 9.2, the initial concentrations of tetraconazole in water were 0.98 and 1.97 μg/ml for T1 and T2. The residues degraded slowly following a first-order kinetics with a half-life of 100.3 and 125.4 days for T1 and T2, respectively (Table 5). At pH 7.0, the initial concentrations of tetraconazole in water were 1.00 and 2.00 μg/ml for T1 and T2, respectively. The half-life values for T1 and T2 were found to be 115.5 and 120.4 days, respectively. Degradation kinetics has been shown graphically. A relatively low persistence of tetraconazole was obtained at pH 4.0, in comparison to pH 7.0 and pH 9.2. For T1 and T2, the corresponding initial concentration levels were 0.98 and 2.00 μg/ml with half-life values ranging from 94.1 to 115.8 days. Linear plot for first-order kinetics has been presented graphically (Fig. 2). Regression equations for the first-order kinetics along with half-life values are given (Table 5). From the results of persistence study of tetraconazole in water at acidic, neutral, and alkaline condition, it appeared that degradation of tetraconazole in water was not pH dependant. In all the cases, the residues degraded very slowly. The half-life values were in the range of 94 to 125 days. US EPA in their Pesticide Fact Sheet (2005) for tetraconazole reported that tetraconazole is stable to hydrolysis in sterile pH 5, 7, and 9 aqueous buffer solutions. It is stable to photolysis on soil (t 1/2 = 106 days) and photolysis in water (t 1/2 = 107–215 days). Therefore, it may be concluded that tetraconazole, the triazole fungicide, is very much persistent in water. The pH values of water do not affect the degradation.
Conclusions
Tetraconazole degrades slowly in soils and the dissipation was fastest in soil having maximum organic matter content. In water, only a slight increase in dissipation rate was observed as pH was decreased but not significant, indicating a reasonable persistence of the triazole pesticide in water.
References
Alam, S., Kole, R. K., & Bhattacharyya, A. (2011). Residual fate of the fungicide tetraconazole (4 % EW) in mango. Bulletin of Environmental Contamination and Toxicology, 87(4), 444–447.
Amer, M. M., Shehata, M. A., Lotfy, H. M., & Monir, H. H. (2007). Determination of tetraconazole and diniconazole fungicide residues in tomatoes and green beans by capillary gas chromatography. Yakugaku Zasshi, 127(6), 993–999.
Banerjee, K., Oulkar, D. P., Patil, S. H., Dasgupta, S., & Adsule, P. G. (2008). Degradation kinetics and safety evaluation of tetraconazole and difenoconazole residues in grape. Pest Management Science, 64(3), 283–289.
Black, C. A. (1965). Methods of soil analysis (parts 1 & 2). Madison: American Society of Agronomy.
Bromilow, R. H., Evans, A. A., & Nicholls, P. H. (1999). Factors affecting degradation rates of five triazole fungicides in two soil types: 1. Laboratory incubations. Pesticide Science, 55(12), 1129–1134.
Corley, J. (2003). Best practices in establishing detection and quantification limits for pesticide residues in foods. In Handbook of residue analytical methods for agrochemicals (pp. 59–74). New York: Wiley.
EPA (2005). Environmental fate and effects division risk assessment for the section 3 registration of tetraconazole. http://www.epa.gov/opprd001/factsheets/tetraconazole.pdf. Accessed 27 Apr 2010.
European Commission Directorate of General Health and Consumer Protection (2000) Guidance Document on Residue Analytical Methods, SANCO/825/00 rev. 6, 20 June 2000. www.ec.europa.eu/food/plant/protection/resources/guidedoc 825–00 rev7 en.pdf. 28 Dec 2007.
Fernandes, V. C., Domingues, V. F., Mateus, N., & Delerue-Matos, C. (2012). Pesticide residues in Portuguese strawberries grown in 2009–2010 using integrated pest management and organic farming. Environmental Science and Pollution Research International, 19(9), 4184–4192.
Fukuto, R. T. (1987). Organophosphates and carbamate esters: the anticholinesterase insecticides. In J. W. Biggar & J. N. Silber (Eds.), Fate of pesticides in the environment. Davis: University of California Agricultural Experiment Station Publications. Publication number 3320.
Jackson, M. L. (1973). Soil chemical analysis. New Delhi: Prentice-Hall.
Khalfallah, S., Menkissoglu, S. U., & Constantinidou, H. A. (1998). Dissipation study of the fungicide tetraconazole in greenhouse-grown cucumbers. Journal of Agricultural and Food Chemistry, 46(4), 1614–1617.
Li, J., Wang, Y., Shi, J., Jiang, L., Yao, X., & Fang, L. (2012). Determination of 11 triazole fungicides in fruits using solid phase extraction and gas chromatography–tandem mass spectrometry. China Journal of Chromatography, 30(3), 262–266.
Menkissoglu, S. U., Xanthopoulou, N. J., & Ioannidis, P. M. (1998). Dissipation of the fungicide tetraconazole from field-sprayed sugar beets. Journal of Agricultural and Food Chemistry, 46(12), 5342–5346.
Piper, C. S. (1944). Soil and plant analysis. New York: Wiley.
Singh, N. (2005). Factors affecting triadimefon degradation in soils. Journal of Agricultural and Food Chemistry, 53(1), 70–75.
Stevens, J. T., & Sumner, D. D. (1991). Herbicides. In W. J. Hayes Jr. & E. R. Laws Jr. (Eds.), Handbook of pesticide toxicology (pp. 3–5). New York: Academic.
Thorstensen, C. W., & Lode, O. (2001). Laboratory degradation studies of bentazone, dichlorprop, MCPA and propiconazole in Norwegian soils. Journal of Environmental Quality, 30(3), 947–953.
Ware, G. W. (1986). Fundamentals of pesticides, a self-instruction guide (pp. 3–3). Fresno: Thompson.
Acknowledgments
The authors are grateful to B.C.K.V. India for providing the necessary facilities and Isagro (Asia) Agrochemical Pvt Ltd., for funding the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alam, S., Sengupta, D., Kole, R.K. et al. Dissipation kinetics of tetraconazole in three types of soil and water under laboratory condition. Environ Monit Assess 185, 9819–9824 (2013). https://doi.org/10.1007/s10661-013-3294-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3294-0