Abstract
Fecal pollution may adversely impact water quality in coastal ecosystems. The goal of this study was to determine whether cattle were a source of fecal pollution in a South Carolina watershed. Surface water samples were collected in June 2002 and February through March 2003 in closed shellfish harvesting waters of Toogoodoo Creek in Charleston County, SC. Fecal coliform concentrations in 70 % of the water samples taken for this study exceeded shellfish harvesting water standards. Ribotyping was performed in order to identify animal sources contributing to elevated fecal coliform levels. Escherichia coli isolates (n = 253) from surface water samples were ribotyped and compared to a ribotype library developed from known sources of fecal material. Ribotypes from water samples that matched library ribotypes with 90 % maximum similarity or better were assigned to that source. Less than half of the unknown isolates (38 %) matched with library isolates. About half (53 %) of the matched ribotypes were assigned to cattle isolates and 43 % to raccoon. Ribotyping almost exclusively identified animal sources. While these results indicate that runoff from cattle farms was a likely source of fecal pollution in the watershed, wildlife also contributed. Given the small size of the library, ribotyping was moderately useful for determining the impact of adjacent cattle farms on Toogoodoo Creek. Increasing the number and diversity of the wildlife sources from the area would likely increase the usefulness of the method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The United States Environmental Protection Agency (USEPA) and US Food and Drug Administration (USFDA) require states to identify impaired shellfish harvesting waters to perform additional monitoring and develop plans to reduce pollution levels (USEPA 2004; USFDA 2009). Identification of pollution sources could facilitate the development of effective management strategies for reducing pollution loads. The South Carolina Department of Health and Environmental Control (SCDHEC) closed shellfish harvesting waters in the Toogoodoo Creek, SC due to fecal coliform levels which exceeded acceptable limits (geometric mean not >14 MPN/100 mL nor over 10 % of the samples >43 MPN/100 mL) (SCDHEC 2008).
Bacterial source tracking is based on the assumption that commensal enteric bacteria have evolved with their host over time, with certain strains being selected for each host due to specific host conditions such as diet and digestive system. These bacterial strains can be used to determine the type of animal from which the fecal pollution originated. Phenotypic methods such as antibiotic resistance assay (Parveen et al. 1997; Wiggins et al. 1999; Moore et al. 2005) and carbon source utilization (Hagedorn et al. 2003) have been used to identify the source of fecal pollution. Genetic fingerprinting methods used include rep-PCR (Dombek et al. 2000; Hahm et al. 2003), ribotyping (Parveen et al. 1999; Anderson et al. 2006; Kelsey et al. 2008; Klopchin et al. 2008), Enterococcus faecium esp gene detection (Scott et al. 2005), Bacteroides typing (Bernhard and Field 2000a, b), and detection of human enteric viruses (Gregory et al. 2006). Ufnar et al. (2006) and Johnston et al. (2010) typed the nifH gene of a potential indicator, Methanobrevibacter smithii.
Ribotyping is a molecular method that takes its name from the 16S ribosomal DNA (rDNA) region that is targeted for distinguishing sequences among strains of bacteria. Historically, 16S ribosomal RNA and the rDNA that codes for it have been used as a tool for bacterial phylogenetic studies (Woese and Fox 1977) because the sequences contain both highly conserved and variable regions (Chakravorty et al. 2007). The highly conserved DNA sequences provide stable markers of bacterial species and the variable regions exhibit mutations that differentiate among strains. The method results in banding patterns of fragmented DNA for each strain of bacteria analyzed. Total DNA is extracted from the bacterial cells, fragmented with a restriction enzyme, and processed in multiple steps until fragments of 16S rDNA are visualized on a nylon membrane. Each restriction enzyme, such as Hind III, recognizes only one DNA sequence at which it cleaves the strand. If a mutation occurs at the enzyme recognition sequence, the DNA strand will not be cut at that position. Mutations at restriction sites result in different sizes and numbers of DNA fragments to which the probe hybridizes and are visualized. A library is built from bacteria isolated from fecal samples of known origin that are ribotyped. Ribotypes of bacteria isolated from water samples (unknowns) can be compared with the library. Unknown isolate patterns are assigned an identification (ID) when they match a source. Specific strains can be distinguished by comparing their DNA banding patterns to a library.
We chose Escherichia coli because it is used as a bacterial indicator of fecal pollution, it was convenient and useful to isolate from positive cultures used for the enumeration of fecal coliforms from the same samples, and a body of data existed in the literature with which our results could be compared. For example, Parveen et al. (1999) used this method to determine sources of fecal pollution in Apalachicola Bay, FL. Anderson et al. (2006) examined the diversity and distribution of E. coli in humans, cattle, and horses by ribotyping. Kelsey et al. (2008) identified wild animal ribotypes in coastal South Carolina. We chose HindIII as the restriction enzyme because it provided a useful number of fragments (12–14 per strain) and has been used in a number previous studies including Anderson et al. (2005, 2006), Carson et al. (2001, 2003); Hartel et al. (2002, 2003), Kelsey et al. (2008), Klopchin et al. (2008), Moore et al. (2005), Parveen et al. (1999), and Scott et al. (2002, 2003).
The objective of the project presented here was to use ribotyped E. coli isolates recovered from fecal, sewage, and surface water samples to determine if cattle contributed to the elevated fecal coliform levels in Toogoodoo Creek.
Materials and methods
Study sites
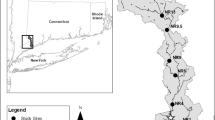
Study sites were chosen by SCDHEC in Toogoodoo Creek, Charleston County, SC due to fecal coliform levels that exceeded the shellfish harvesting limit of 14 MPN/100 mL (geometric mean) of water (SCDHEC 2008). The agency was interested in determining whether cattle in adjacent pastures were contributing to the fecal coliform load in the creek. The creek is roughly Y-shaped with two major arms and a small arm running north–south near the large east branch (Fig. 1). The towns of Hollywood and Meggett, SC border the creek. The study area comprised rural, low density development as well as agriculture and small livestock farms along the creek. The population of Hollywood consisted of 3,946 residents in 21.2 square mile in 2000 and Meggett was smaller with 1,230 residents in 14.8 square mile. For comparison, nearby Charleston, SC had a population of 96,650 residents in108 square mile (US Census Bureau 2012).
Sample collection
As part of a statewide shellfish study including Toogoodoo Creek, surface water (n = 3) and fecal samples (n = 3) were collected concurrently in June 2002 after a rain event. Additional samples (28 water and 18 source samples) were collected from February through March 2003, without regard to weather. Fresh fecal samples (n = 14) from cattle and from horse (n = 2) pastures were collected from farms bordering Toogoodoo Creek that were likely to contribute fecal coliforms due to their close proximity to the creek. One sewage sample was collected from a pump station located in the study area to represent a composite of local human fecal samples. This study focused on cattle but archived isolates from one deer and three raccoon samples collected from Kiawah Island, SC in August 2002 were also included for comparison. Deer isolates were obtained from fresh fecal material. Raccoon isolates were obtained from anal swabs of captured and sedated animals.
E. coli enumeration and isolation
Fecal coliform concentrations of the 31 surface water samples were determined at the DHEC laboratory in North Charleston, SC. Enumeration of fecal coliforms was performed by SCDHEC using the multiple tube fermentation method (MTF) with A-1 broth (APHA 1998). Positive fecal coliform broth cultures were sent to the National Ocean Service (NOS) in Charleston, SC for isolation of E. coli. Fecal coliforms were enumerated from fresh fecal samples at the NOS Environmental Microbiology laboratory in A-1 broth using the MTF method (APHA 1998). All E. coli were isolated from A-1 broth by streaking a loopful of broth to violet red bile agar and choosing colonies with the characteristic red color and purple–red halo. These presumptive E. coli colonies were confirmed with growth and fluorescence on nutrient agar with MUG. E. coli colonies isolated from fecal samples and sewage were used to build the known source library to which the isolates from the water samples were compared (Table 1). In order to provide multiple strains to build the library while keeping a manageable number, 15 isolates were randomly chosen from each source sample. Ten isolates from each water sample were chosen for ribotyping. Sources of E. coli for the ribotyping library included isolates from cattle (n = 186), deer (n = 9), horses (n = 28), raccoons (n = 38), and sewage (n = 12). Each colony (isolate) was grown in brain–heart infusion broth overnight and DNA was extracted from each culture using the DNeasy kit (Qiagen, Valencia, CA). The resulting DNA was analyzed by ribotyping.
Ribotyping
The ribotyping method was adapted from Parveen et al. (1999) in the manner of Kelsey et al. (2008). With this method, bacterial DNA was isolated from the bacteria and restriction enzyme digested with Hind III, which cleaves at a specific DNA sequence (AAGCTT). The resulting DNA fragments were separated by agarose gel electrophoresis in 1× Tris–borate–EDTA. The DNA in the gel was treated to break hydrogen bonds (depurinated); the double helix was separated into single strands (denatured) and then stabilized by neutralization. Next, it was transferred from the gel to a positively charged nylon membrane (Southern blot) using a vacuum blotter (VacuGene XL, Pharmacia Biotech, Piscatawy, NJ), then hybridized with a 1,484-kb 16S rDNA DIG-labeled probe. The probe was synthesized according to Anderson et al. (2005) by amplifying the 16S rDNA gene of E. coli ATCC #9637 with universal bacterial primers. Hybridized probe and DNA complex was visualized on the membrane colorimetrically with nitro blue tetrazolium/(5-bromo-4-chloro-1 H-indol-3-yl) dihydrogen phosphate (NBT/BCIP). The restriction enzyme digest results in different sized fragments of the 16S gene that separate in a gel according to size, reported as molecular weight in length of base pairs. The probe binds to the fragments that have a similar DNA sequence (hybridizes) and are visible as blue bands after NBT/BCIP treatment. Small DNA fragments migrate through the gel matrix more quickly than the large ones during electrophoresis. The sizes and numbers of the bands in each banding pattern are used to discriminate between strains of E. coli.
Analysis
BioNumerics software version 4.0 (Applied Maths BVBA, Sint-Martens-Latem, Belgium) was used for analysis of ribotypes. The bands on the blot were marked on the image imported into the program and the information was automatically added to the BioNumerics database. Each blot was normalized to the same molecular weight standard (Roche Applied Science MWM II, Indianapolis, IN). Sizes were assigned to each band based on a molecular weight standard that was run concurrently with the sample isolate DNA. Source isolates were organized into a library to which water isolate patterns were compared. Maximum similarities between library and sample patterns were calculated by the program within the library comparison function by choosing the unknowns to be compared with the library. Maximum similarity requires an exact match of each band in an unidentified ribotype to a library ribotype, giving a stringent comparison. The percent similarity is reported for each match. An ID was assigned to each unknown isolate whose banding pattern was at least 90 % similar to a library isolate pattern. This threshold value was based on replicates of E. coli control (ATCC #9637) included on each gel.
Clusters of ribotypes were generated based on Jaccard similarity coefficient and a dendrogram was built using unweighted pair group method using arithmetic averages to visualize similarities and to identify ties. From this dendrogram, jackknife analysis was calculated with maximum similarities to provide rates of correct classification (RCC) in order to assess the fit of each source ribotype into its own library.
Results
Table 1 shows the number of library samples collected for each source. A total of 21 fecal samples were collected which resulted in 273 source isolates being ribotyped. The fecal samples collected were cattle (n = 14), deer (n = 1), horse (n = 2), raccoon (n = 3), and sewage (n = 1). Most E. coli ribotypes from a single fecal sample were the same but also demonstrated one to three unique ribotypes. The duplicate ribotypes (matches from a single source) were removed from each library so that one ribotype represented each strain of E. coli. Also, in three cases, assignment of an ID tied when ribotypes from two different sources matched each other or two or more sources matched one water pattern. For example, one cattle and one deer ribotype matched each other. In another comparison, a water ribotype matched both a raccoon ribotype and a deer ribotype (at less than 100 % similarity) but the two sources did not match each other. Ultimately, 117 unique, representative ribotypes comprised the library used for comparison with unknown ribotypes from water samples. For example, of the 186 cattle isolates that were ribotyped, 81 represented all the fragment patterns used for the cattle library. The numbers of water isolate ribotypes that matched library ribotypes and therefore were assigned an ID are presented in Table 1 (# match water ribotype). Of the 95 total water ribotypes assigned an ID, 50 were matched with cattle. Raccoon was the second most common ID, with 41 matched to the library. Deer made a small contribution matching with only three unknown ribotypes. Only one water isolate matched a sewage ribotype. None of the unknown ribotypes matched with the horse library.
The cutoff value used for identifying a match was determined from replicates of the E. coli ATCC #9637 ribotype included with every electrophoresis set. The maximum similarity for the replicates of E. coli ATCC #9637 ranged from 87 to 100 %, with 21 of the 24 replicates at least 90 % similar to each other. Anderson et al. (2006) also used the 90 % cutoff value to meet matching criteria. Based on experimental observations in our study, this level of similarity was equivalent to one band position difference between two patterns in the matching process, providing a stringent matching criterion.
The average rate of correct classification for the library was 52 %. The cattle library had the best RCC at 84 %. The horse library RCC was 81 %. The RCC for raccoon library was 32 %, sewage was 63 %, and deer was 0 %. Deer classified more closely with the cow library. When deer and cattle were combined into a ruminant library, the ruminant RCC was 89 %. The other RCCs remained the same.
Discussion
Results of ribotyping E. coli isolates from surface water and fecal samples indicate that both cattle and raccoons contributed to the fecal coliforms found in the adjacent creeks. Isolating E. coli from the positive A-1 broth allowed the source of some of the E. coli contributing to the fecal coliforms in the water samples to be known. Our results indicate that animals may make a greater contribution to the Toogoodoo Creek watershed than humans. This is not surprising since the area is largely undeveloped. The population density surrounding Toogoodoo Creek averaged 80 residents per square mile. Also, animal waste was deposited on surface of the pasture near the creek allowing rain or irrigation to wash fecal material into the creek. Only one ribotype was identified as being sewage. Since human waste is collected underground, it is more likely that fecal coliforms would be retained by the soil matrix or would enter the Togoodoo Creek system from a nonpoint source.
Since less than half of the unknown isolates were assigned a match when compared to the ribotype library, sources that were not tested in this study likely contributed to bacterial loading. Additional source types and additional ribotypes for each source to the library may improve confidence in RCCs and the number of IDs assigned to unknown isolates. Another factor that may have contributed to the inability to identify more isolates was that all bacterial strains do not have the definitive host specificity as assumed. McLellan (2004) found that environmental genotypes of E. coli were present in higher numbers than fecal source-related genotypes.
The information gained from this study is being used by environmental managers and stakeholders. The shellfish harvesting status of the headwater and upper reaches of the Toogoodoo Creek watershed continues to be classified as Restricted. Restricted means that shellfish may be harvested only for the purposes of relaying or depuration and only under SCDHEC supervision and by special permit. A total maximum daily load has been developed for fecal coliform bacteria reduction in watershed. In late 2012, a Clean Water Act Section 319 Nonpoint Source Management Program grant was awarded by SCDHEC to Charleston Soil and Water Conservation District.
The goal of this project will be to produce significant reductions in the fecal coliform bacteria levels in order to meet water quality standards for consumption of shellfish. This will be accomplished through the watershed-wide implementation of an array of stormwater Best Management Practices (BMPs) combined with a focused education and outreach campaign. These BMPs will include agricultural BMPs, such as livestock exclusion fencing and manure management plans, as well as residential BMPs that will include a septic system evaluation and rehabilitation program.
Where creek bank riparian zones conditions indicate, another BMP that can be considered is riparian buffers. Vegetative buffers can be planted along the water’s edge to reduce runoff-related microbes as well as nutrients that they rely on. Sullivan et al. (2007) found that a buffer of natural vegetation as narrow as 1–3 m wide between pasture and stream was effective in greatly reducing fecal coliforms. Tate et al. (2006) found similar results on natural grasslands. Vandiver (2005) also found that a planted buffer decreased fecal coliform loading. It is projected that if BMPs can be implemented to reduce inputs from agricultural sources, then it is likely that water quality standards can be met for harvesting shellfish in this watershed which would allow for harvest and increase consumption and sales of shellfish to markets.
Conclusions
Although ribotyping did not identify every source of fecal pollution, the method was useful for determining whether cattle fecal pollution affected the water quality of Toogoodoo Creek. Nearly half the isolate origins identified by ribotyping were from cattle. Our results indicate that raccoons also contribute to the fecal coliforms found in Toogoodoo Creek. This source information allows environmental managers to consider options for improving water quality.
References
Anderson, K. L., Whitlock, J. E., & Harwood, V. J. (2005). Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Applied and Environmental Microbiology, 71, 3041–3048.
Anderson, M. A., Whitlock, J. E., & Harwood, V. J. (2006). Diversity and distribution of Escherichia coli genotypes and antibiotic resistance phenotypes in feces of humans, cattle, and horses. Applied and Environmental Microbiology, 72, 6914–6922.
American Public Health Association (APHA). (1998). Standard methods for the examination of water and wastewater (20th ed.). Washington: American Public Health Association, American Water Works Association, Water Environment Federation.
Bernhard, A. E., & Field, K. G. (2000a). Identification of nonpoint sources of fecal pollution in coastal waters by using host-specific 16S ribosomal DNA genetic markers from fecal anaerobes. Applied and Environmental Microbiology, 66, 1587–1594.
Bernhard, A. E., & Field, K. G. (2000b). A PCR assay to discriminate human and ruminant feces on the basis of host differences in Bacteroides-Prevotella genes encoding for 16S rRNA. Applied and Environmental Microbiology, 66, 4571–4574.
Carson, C. A., Shear, B. L., Ellersieck, M. R., & Asfaw, A. (2001). Identification of fecal Escherichia coli from humans and animals by ribotyping. Applied and Environmental Microbiology, 67, 1503–1507.
Carson, C. A., Shear, B. L., Ellersieck, M. R., & Schnell, J. D. (2003). Comparison of ribotyping and repetitive extragenic palindromic-PCR for identification of fecal Escherichia coli from humans and animals. Applied and Environmental Microbiology, 69, 1836–1839.
Chakravorty, S., Helb, D., Burday, M., Connell, N., & Alland, D. (2007). A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. Journal of Microbiological Methods, 69, 330–339.
Dombek, P. E., Johnson, L. K., Zimmerley, S. T., & Sadowsky, M. (2000). Use of repetitive DNA sequences and the PCR to differentiate Escherichia coli isolates from human and animal sources. Applied and Environmental Microbiology, 66, 2572–2577.
Gregory, J. B., Litaker, R. W., & Noble, R. T. (2006). Rapid one-step quantitative reverse transcriptase PCR assay with competitive internal positive control for detection of enteroviruses in environmental samples. Applied and Environmental Microbiology, 72, 3960–3967
Hagedorn, C., Crozier, J. B., Mentz, K. A., Booth, A. M., Graves, A. K., Nelson, N. J., et al. (2003). Carbon source utilization profiles as a method to identify sources of fecal pollution in water. Journal of Applied Microbiology, 94, 792–799.
Hahm, B.-K., Maldonado, Y., Schreiber, E., Bhunia, A. K., & Nakatsu, C. H. (2003). Subtyping of foodborne and environmental isolates of Escherichia coli by multiplex-PCR, rep-PCR, PFGE, ribotyping and AFLP. Journal of Microbiological Methods, 53, 387–399.
Hartel, P. G., Summer, J. D., Hill, J. L., Collins, J. V., Entry, J. A., & Segars, W. I. (2002). Geographic variability of Escherichia coli ribotypes from animals in Idaho and Georgia. Journal of Environmental Quality, 31, 1273–1278.
Hartel, P. G., Summer, J. D., & Segars, W. I. (2003). Deer diet affects ribotype diversity of Escherichia coli for bacterial source tracking. Water Research, 37, 3263–3268.
Johnston, C., Ufnar, J. A., Griffith, J. F., Gooch, J. A., & Stewart, J. R. (2010). A real-time qPCR assay for the detection of the nifH gene of Methanobrevibacter smithii, a potential indicator of sewage pollution. Journal of Applied Microbiology, 109, 1946–1956.
Kelsey, R. H., Webster, L. F., Kenny, D. J., Stewart, J. R., & Scott, G. I. (2008). Spatial and temporal variability of ribotyping results at a small watershed in South Carolina. Water Research, 42, 2220–2228.
Klopchin, J. L., Stewart, J. R., Webster, L. F., & Sandifer, P. A. (2008). Assessment of environmental impacts of a colony of free-ranging rhesus monkeys (Macca mulatta) on Morgan Island, SC. Environmental Monitoring and Assessment, 137, 301–313.
McLellan, S. L. (2004). Genetic diversity of Escherichia coli isolated from urban rivers and beach water. Applied and Environmental Microbiology, 70, 4658–4665.
Moore, D. F., Harwood, V. J., Ferguson, D. M., Lukasik, J., Hannah, P., Getrich, M., et al. (2005). Evaluation of antibiotic resistance analysis and ribotyping for identification of faecal pollution sources in an urban watershed. Journal of Applied Microbiology, 99, 618–628.
Parveen, S., Murphree, R. L., Edmiston, L., & Tamplin, M. L. (1997). Association of multiple-antibiotic-resistance profiles with point and non-point sources of Escherichia coli in Apalachicola Bay. Applied and Environmental Microbiology, 63, 2607–2612.
Parveen, S., Portier, K. M., Robinson, K. E., & Tamplin, M. L. (1999). Discriminant analysis of ribotype profiles of E. coli for differentiating human and nonhuman sources of fecal pollution. Applied and Environmental Microbiology, 65, 3142–3147.
SCDHEC (2008). R.61-47, Shellfish, http://www.scdhec.gov/environment/water/reg.htm#61-47. Accessed 20 July 2012.
Scott, T. M., Rose, J. B., Jenkins, T. M., Farrah, S. R., & Lukasik, J. (2002). Microbial source tracking: current methodology and future directions. Applied and Environmental Microbiology, 68, 5796–5803.
Scott, T. M., Parveen, S., Portier, K. M., Rose, J. B., Tamplin, M. L., Farrah, S. R., et al. (2003). Geographical variation in ribotype profiles of Escherichia coli isolates from humans, swine, poultry, beef and dairy cattle in Florida. Applied and Environmental Microbiology, 69, 1089–1092.
Scott, T. M., Jenkins, T. M., Lukasik, J., & Rose, J. B. (2005). Potential use of a host associated molecular marker in Enterococcus faecium as an index of human fecal pollution. Environmental Science and Technology, 39, 283–287.
Sullivan, T. J., Moore, J. A., Thomas, D. R., Mallery, E., Snyder, K. U., Wusterberg, M., et al. (2007). Efficacy of vegetative buffers in preventing transport of fecal coliform bacteria from pasturelands. Environmental Monitoring, 40, 958–965.
Tate, K. W., Atwill, E. R., Bartolome, J. W., & Nader, G. (2006). Significant Escherichia coli attenuation by vegetative buffers on annual grasslands. Journal of Environmental Quality, 35, 795–805.
Ufnar, J., Wang, S. Y., Christiansen, J. M., Yampara-Iquise, H., Carson, C. A., & Ellender, R. D. (2006). Detection of the nifH gene of Methanobrevibacter smithii: a potential tool to identify sewage pollution in recreational waters. Journal of Applied Microbiology, 101, 44–52.
US Census Bureau. Quickfacts.census.gov/qfd/states/45/4513330.html. Accessed 20 July 2012
US Environmental Protection Agency (2004). http://water.epa.gov/scitech/swguidance/standards/criteria/health/microbial/index.cfm. Accessed 23 July 2012
US Food and Drug Administration (2009). National Shellfish Sanitation Program guide for the control of molluscan shellfish. http://www.fda.gov/Food/FoodSafety/Product-SpecificInformation/Seafood/FederalStatePrograms/NationalShellfishSanitationProgram/ucm046353.htm. Accessed 23 July 2012
Vandiver, L. (2005). Characterizing pollutant transport through a coastal lawn and vegetative buffer. College of Charleston Master’s Thesis.
Wiggins, B. A., Andrews, R. W., Conway, R. A., Corr, C. L., Dobratz, E. J., Dougherty, D. P., et al. (1999). Use of antibiotic resistance analysis to identify nonpoint sources of fecal pollution. Applied and Environmental Microbiology, 65, 3483–3486.
Woese, C. R., & Fox, G. E. (1977). Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proceedings of the National Academy of Sciences, 74, 5088–5090.
Acknowledgments
The authors are grateful for Joan Hagan and Sharon Gilbert at SCDHEC who enumerated fecal coliforms on the water samples and coordinated transfer of cultures to NOS staff for further analysis. Thanks go to Steve Allen for his technical assistance with collecting samples and molecular work. The authors would also like to acknowledge the help of Laura Kracker for producing the maps. Brian Thompson applied labels on the maps and performed fecal coliform enumeration on fecal samples.
Conflict of interest
This publication does not constitute an endorsement of any commercial product or intend to be an opinion beyond scientific or other results obtained by the National Oceanic and Atmospheric Administration (NOAA). No reference shall be made to NOAA, or this publication furnished by NOAA, to any advertising or sales promotion which would indicate or imply that NOAA recommends or endorses any proprietary product mentioned herein, or which has as its purpose an interest to cause the advertised product to be used or purchased because of this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Webster, L.F., Graves, D.A., Eargle, D.A. et al. Assessment of animal impacts on bacterial water quality in a South Carolina, USA tidal creek system. Environ Monit Assess 185, 7749–7756 (2013). https://doi.org/10.1007/s10661-013-3132-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3132-4