Abstract
Soil management significantly affects the soil labile organic factors. Understanding carbon and nitrogen dynamics is extremely helpful in conducting research on active carbon and nitrogen components for different kinds of soil management. In this paper, we examined the changes in microbial biomass carbon (MBC), microbial biomass nitrogen (MBN), dissolved organic carbon (DOC), and dissolved organic nitrogen (DON) to assess the effect and mechanisms of land types, organic input, soil respiration, microbial species, and vegetation recovery under Deyeuxia angustifolia freshwater marshes (DAMs) and recovered freshwater marsh (RFM) in the Sanjiang Plain, Northeast China. Identifying the relationship among the dynamics of labile carbon, nitrogen, and soil qualification mechanism using different land management practices is therefore important. Cultivation and land use affect intensely the DOC, DON, MBC, and MBN in the soil. After DAM soil tillage, the DOC, DON, MBC, and MBN at the surface of the agricultural soil layer declined significantly. In contrast, their recovery was significant in the RFM surface soil. A long time was needed for the concentration of cultivated soil total organic carbon and total nitrogen to be restored to the wetland level. The labile carbon and nitrogen fractions can reach a level similar to that of the wetland within a short time. Typical wetland ecosystem signs, such as vegetation, microbes, and animals, can be recovered by soil labile carbon and nitrogen fraction restoration. In this paper, the D. angustifolia biomass attained natural wetland level after 8 years, indicating that wetland soil labile fractions can support wetland eco-function in a short period of time (4 to 8 years) for reconstructed wetland under suitable environmental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Overdevelopment and irresponsible use of soil by humans have reduced organic materials, resulting in lower soil fertility and capacity to hold water (Shi et al. 2000). Thus, effective utilization of land, which is closely related to soil nutrient cycling, is the utmost concern of land management. Land use management can influence plant litter and residue (Dalal and Mayer 1988) and can affect soil microbes and relocation of nutrients in the soil system (Kennedy and Papendick 1995). Land management can have a positive effect on the accumulation of organic carbon. Numerous plantations can lower organic carbon in soil and increase fine particles (Burdige and Gardner 1998). Generally speaking, 16–17 % of soil organic material can be lost because of agricultural activity, with an average loss of up to 29–30 %. The lighter the soil is, the larger the loss of organic matter. The same is also true for organic nitrogen (Knops and Tilman 2000). Earth biomass will decrease because of clearing of natural plantations, which increases soil ventilation, leading to mineralization of organic materials. Consequently, soil will be lost, resulting in soil degradation (Post and Kwon 2000). Lal (2002) showed that 60–70 % of lost carbon can be recovered using effective land management. Many research studies have shown that restoring agricultural land to natural plantation can increase soil organic material and total nitrogen (TN) contents (Ma and Jiao 2005), but only on the soil surface (An et al. 2003; Song et al. 2004; Zhang et al. 2005). After conducting a research on carbon and nitrogen dynamics of restored agricultural land, Landgraf (2001) showed that organic carbon within 0–10 cm of the soil increased gradually, but that in 10–30 cm of the soil decreased with the time during the restoration process. The result for TN was almost the same. Restoration speed was related to organic background, solid management, soil hydrological condition, and microbes (Saggar et al. 2001; Post and Kwon 2000). For the same type of soil, the longer the cultivation period, the longer the time for restoration of the eco-function. Restoration to 95 % of their natural use was estimated to take 180 years for nitrogen and 230 years for carbon (Knops and Tilman 2000). The active components, however, will be restored within a short period of time. Hughes et al. (2002) discovered that dissolved organic carbon (DOC) will increase after restoring the land to a forest due to the increase in organic A1 compounds and chelating organic acid. Zhou and Jiang (2004) conducted research on red-soil carbon pool and observed that total organic carbon (TOC) and different kinds of active carbon significantly increased after plantation restoration. The microbe carbon in the 0–20 cm soil after 17 years of restoration was 2.95 times that of the soil after 9 years of restoration only.
The Sanjiang Plain is located in the eastern part of Heilongjiang Province, Northeast China, and is bordered by Russia. It covers an area of 10.89 Mha, mostly dominated by marshes in 1893, and is presently the second largest marsh in China (Zhao 1999; Liu and Ma 2000). Draining wetlands for agricultural purposes is significant in this area especially in the last 50 years, and this activity causes wetland loss. According to the US EPA (2001), destroying wetlands can lead to serious consequences that include increased flooding and extinction of species. In the Sanjiang Plain, this occurrence has resulted in the degradation of the ecological attributes of the natural wetlands and also significantly affected the TOC and TN of the wetlands.
Converting native marshland to agricultural soil resulted in distinct changes in soil water content and temperature (Song et al. 2004), increase in the amount of microaggregate, and a rapid decrease in labile organic C concentration and amount of macroaggregate, as well as that of soil organic C (SOC) (Zhang et al. 2006).
From the 1990s, overuse and reclamation of the Sanjiang Plain have changed in some ways. In particular, in recent years, many ecological engineering have taken place in Heilongjiang Province. In the Sanjiang Plain, restoring plants to wetland and forest helps control wetland degradation and changes carbon storage and formation. Zhang and Song (2003) conducted a related research, but extensive field studies are still needed. In the present paper, we conducted research on dissolved organic matter, microbe carbon, nitrogen, and plantation and evaluated the eco-functions after restoring the agricultural land into wetland.
Materials and methods
Study area
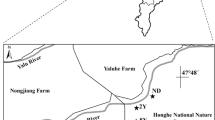
The study site was set up at the Sanjiang Mire Wetland Experimental Station, Chinese Academy of Sciences, Sanjiang Plain, China, approximately 47°35′ N, 133°31′ E (Fig. 1). Boreal climate conditions and low slope grade have led to the largest area of mire wetlands in North China. The average altitude is between 55.4 and 57.9 m, the annual mean temperature is 1.9 °C, and the non-frost period is 125 days (Song et al. 2004). The study site is in the seasonal frozen zone (Song et al. 2004) with annual precipitation between 550 and 600 mm, mainly in July and August, accounting for more than 65 % of the annual precipitation.
The study area which was a natural wetland was converted to a farmland and subsequently restored into a wetland. The study area was originally dominated by Deyeuxia angustifolia. D. angustifolia wetland is a seasonal flooded wetland and submerged only in May. This natural wetland was subjected to human interferences which resulted in significant changes in the TOC and TN of the wetland. The drainage and use of wetlands for agricultural fields occurred in the past 50 years with the population growth, resulting in the increase in cultivated land from about 0.79 × 106 ha in 1949 to 4.57 × 106 ha in 1994 (Liu and Ma 2000). Now, the Sanjiang Plain has become an intensive area of land use/cover change in China. The soils at all sites were classified as Albaquic Paleudalfs with silty clay texture (Zhang et al. 2008).
The Honghe and Qianjin farmlands have plantations existing for 20 and 40 years, respectively. The area is approximately 105 hm2, composed mainly of different natural wetlands, in addition to 6.6 hm2 of swampy area and 7.0 hm2 of dry land. In the study area, the plantation time is short, the plantation history is clear, and the structure is simple. In this area, natural wetland, artificial forest, agricultural land, and restored wetland exist, providing better conditions for research on different soil management practices.
Experimental design
The study area considered was a natural mire wetland with D. angustifolia. This natural mire wetland was converted to agricultural land. The agricultural land was subsequently restored to wetland. During the period when the area was used as an agricultural land, soy was planted for more than 10 years, which was a typical mired meadow. Data were collected over a period of 10 years at 2-year interval during the restoration period of the agricultural soil to wetland (R2, R4, R6, R8, and R10 denote data collected on the second, fourth, sixth, eighth, and tenth years, respectively). Also, a nearby soy plantation (S) and a natural D. angustifolia wetland (M) within the Sanjiang Plain were considered. In each type of soil management, a 40 × 40-m study area was chosen, and three sampling areas and ten soil volumes (20 mm in diameter) were obtained. The samples were sent to the laboratory where they were treated by removing the litter, dead roots, and small animals and preserved in refrigerator at 4 °C.
Plant biomass measurements
The harvesting method involved obtaining the biomass from the ground. Three to four samplings of a 50 × 50-cm area were chosen randomly. Plants above the ground were brought to the laboratory and identified. They were dried at 80 °C for 48 h and weighed. The cutting ring method was employed to obtain the underground biomass. Two to three areas were chosen, and ten soil volumes at soil depths of 0–40 cm were obtained by using cutting ring of 4 cm in diameter and 10 cm in height.
DOC and DON measurements
Field moist soil samples (equivalent to 10 g oven dry weight) were weighed into 40-mL polypropylene centrifuge tubes. The samples were extracted using 30 mL of distilled water for 30 min on an end-over-end shaker at 30 rpm and centrifuged for 20 min at 8,000 rpm. The entire supernate was filtered through a 0.45-μm filter into separate vials for C analysis (Ghani et al. 2003). The extracts were analyzed for C using high-temperature combustion (TOC-VCPH C analyzer, Shimadzu, Kyoto, Japan). The concentrations of the total dissolved N, NH4 +, and NO3 − were determined using a continuous-flow analyzer (Skalar, Breda, Netherlands). The dissolved organic nitrogen (DON) in the extracts was calculated by subtracting NH4 + and NO3 − from the total dissolved N in the extracts [i.e., DON = total dissolved N – (NH4 +-N + NO3 −-N)].
MBC and MBN measurements
The soil microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) were determined using a fumigation–extraction method. Fumigated and non-fumigated soils were extracted with 0.5 M K2SO4 for 30 min (soil/extractant ratio = 1:5), and the extracts were analyzed for C using a TOC analyzer (TOC VCPH, Shimadzu). The total dissolved N concentrations were determined using a continuous-flow analyzer (Skalar, Breda, Netherlands). The C/N obtained from the fumigated samples minus that from the non-fumigated samples was assumed to represent the microbial − C/N flush and converted to microbial biomass C/N by using the following relationships: microbial biomass C = microbial − C flush/0.38, and microbial biomass N = microbial − N flush/0.45 (Lu 2000).
SOC and TN analyses
Air-dried samples were used for the soil organic carbon (SOC) and TN analyses. SOC was analyzed by wet digestion method using H2SO4–K2Cr2O7, and TN was determined by semi-micro Kjeldahl digestion method using Se, CuSO4, and K2SO4 as catalysts (Lu 2000).
Statistical analysis
Statistical analysis was done using the SPSS software package for Windows (SPSS 17). One-way ANOVA was used to test for significance. For all analyses where p < 0.05, the factors tested and the relationships were considered to be statistically significant.
Results
Changes in physicochemical properties of the restored wetland
The bulk density of the surface soil decreased with the restoration time (Fig. 2c). After 4 years, the bulk density at the surface reduced from 1.15 to 0.95 g/cm3. After 10 years, it decreased to 0.72 g/cm3. From the results, the change in D. angustifolia will take 25.7 years in order to be totally restored to the same state as the species in the natural wetland. After the agricultural land was restored, the porosity increased (Fig. 2a). According to the formula, the recovery process will take 29.2 years. The field capacity also increased with the restoration time (Fig. 2b), and it can be noted that it will take approximately 24.8 years to fully recover to equivalent levels of the natural wetlands.
Characteristic changes of soil TOC and TN under different restoration times
TOC and TN changed after the recovery process (Fig. 3). After 10 years of recovery, the TOC in the 0–10-cm soil was 87.91 ± 1.02 g/kg, whereas that in the 10–20-cm soil was 46.32 ± 0.51 g/kg. TN gradually increased after 10 years. The TN in the 0–10-cm soil was 5.10 ± 0.03 g/kg, whereas that in the 10–20 cm was 3.14 ± 0.38 g/kg. The total organic matter and the TN in the topsoil were higher (p < 0.0001) than those at lower soil depth (10–20 cm). Accumulation of the TOC and TN was more significant at the upper layer relative to the lower layer.
Characteristic changes of soil DOC and DON under different restoration times
DOC and DON increased significantly after the restoration of the agricultural land into wetland (Fig. 4). Compared with the reference soy field, the DOC of the recovered wetland was significant within a short period of time (2 years; p < 0.001). After 4 years of recovery, the soil DOC and that of natural D. angustifolia wetland were the same for the 4–10 years of recovery. No significant difference (p > 0.5) existed. After 4 years of recovery, no difference in the 0–10 and 10–20-cm soils was apparent.
After restoration of the agricultural land into wetland, the DON in the 10–20 cm soil was higher than that in the 0–10 cm (p < 0.001). The difference in DON in R2 and R4 was not significant (p > 0.5), but higher than that of R8 and R10. Compared with the 0–10-cm soil of D. angustifolia and in the 10–20 cm of the agricultural land, DON was higher in natural D. angustifolia wetland. After 2 years of recovery, the DON was almost the same as that of the agricultural land (p > 0.5). Between the fourth year and the tenth year of recovery, the DON increased, but the difference was negligible.
Dynamics of MBC and MBN
The MBC and MBN increased significantly and reached the level of natural D. angustifolia wetland (p > 0.5; Fig. 5). Within 2 years of recovery, the MBC of the surface soil (0–10 cm) recovered in some manner, which was higher than the MBC of the cultivated agricultural land (481.72 ± 70.54 mg/kg). After 10 years of recovery, the MBC was almost the same as that of natural D. angustifolia wetland. The MBC in the 10–20-cm soil was similar to that of the 0–10-cm soil. However, the MBN in the 0–10-cm soil was higher than that of the 10–20-cm soil. After recovery, the MBN in the 0–10-cm soil for different years was almost the same as that of the 10–20-cm soil. In the 0–10-cm soil, a small difference in the natural D. angustifolia wetland (60.30 ± 12.00 mg/kg; p > 0.5) was observed after 4 years of recovery. In the 10–20-cm soil, the MBN of natural D. angustifolia wetland was 46.52 ± 1.85 mg/kg, with no significant difference after 2 years of recovery. The MBN of natural D. angustifolia wetland for all the years of recovery was higher than that of the soy soil.
The effects of soil depth on the TOC/TN and DOC/DON over the restoration period
The TOC/TN in the 0–10-cm soil increased with time within the initial 4-year restoration period but decreased afterwards. However, the TOC/TN in the 10–20-cm soil decreased with time over the entire restoration period except for the period between the fourth and the eighth year where there was a slight increment (Fig. 6a). The DOC/DON of the 0–10-cm soil increased with time over the entire restoration period. The DOC/DON in the 10–20-cm soil increased slightly after 2 years of recovery then remained almost the same for the rest of the restoration period (Fig. 6b). After recovery, the MBC/MBN of the 0–10- and 10–20-cm soils increased (Fig. 6c).
Vegetation changes after agricultural land restoration
After 2 years of recovery, the number of wetland D. angustifolia was 26.3 % of that of the reference natural wetland (Table 1). The higher proportion of the plant community was made up of Xerophytes. After 4 years of recovery, the number of wetland D. angustifolia was 80.3 % of that of the natural wetland. After 2 years of recovery, the number of Pedicularis L (a typical Xerophyte) was lower than that before the 2-year recovery. Aquatic plants occupied the higher proportion of the plant community. After 8 years of recovery, the number of wetland D. angustifolia became the dominant community, which was 103.9 % of the natural wetland. No significant difference was observed for the D. angustifolia between the eighth and tenth years of recovery.
D. angustifolia recovery characteristics under different recovery years
After 4 years of recovery, the D. angustifolia biomass recovered was 69.54 % of that of natural D. angustifolia (Fig. 7). After 8 and 10 years of recovery, little difference existed between the D. angustifolia biomass and that of the natural D. angustifolia (p > 0.5) biomass. The D. angustifolia biomass in 2 years of recovery was 12.79 %.
Discussion
Usually, when the recovery time is ongoing, the carbon and nitrogen components in the surface soil increase (Juo and Lal 2003; An et al. 2003), and the increase in organic components appears only in the surface (0–10 cm) soil (Wang et al. 2002). In this research, the TOC, TN, MBC, MBN, DOC, and DON changed after the recovery. They increased with recovery time, except for the DON in the 0–10-cm soil.
The balance between the input and dissolved components determines the composition of the soil organic content. After the restoration of the agricultural land into wetland, succession and recovery of plants occurred. The biomass on the ground and underground both increased, and soil microbes developed well. Organic residue, such as the roots, increased slightly. Thus, recovery of soil physical properties occurred, which was suitable for organic accumulation. DOC and DON come from the organic residue of plants and microbes, mainly those easily decomposed by photosynthate. After restoration into wetland, the photosynthate increased with time, providing a base for DOC and DON, enabling them to increase and reach a balance between composition and degradation (Kalbitz 2001). In the current paper, after the recovery, the DON in the 10–20-cm soil was higher than that in the 0–10-cm soil, which might be due to the DON biomass usage by soil microbes and the plant roots of the surface soil (0–10 cm). After the recovery, the plant biomass increased, enabling larger nitrogen absorption in the 0–10-cm soil than in the 10–20-cm soil because nitrogen mineralization is low in natural ecosystem; thus, meeting the need of the plant was difficult. Further, the DON might have been the main nutrient absorbed by the plants (Kieland 1994; Jonasson et al. 1999).
In addition, during seasonal flooding environment, microbe activity is active in the surface soil. In the biological process in surface soil, microbe and soil animals have higher biomass usage than the ground microbes, resulting in more usage of surface soil DON, which makes the underground DON higher than that in the surface.
In the process of ecosystem recovery, more litter results in more organic matter. Microbes can use more energy, leading to more microbe biomass (Jenkinson 1976). Therefore, microbe biomass and activity indicate the ecosystem condition through interference and recovery. Zhou and Jiang (2004) showed that TOC and MBN increased significantly after red-soil plantation recovery in Fir forest. The MBN after 17 years of recovery (0–20 cm) was 2.95 times that after 9 years of recovery. In this research, the soil microbe biomass significantly increased after recovery. Such environmental factors such as soil nutrients, water condition, and plantation affected the soil microbe biomass and population. Microbe biomass has a positive relationship with soil total nutrients. During the early stages of recovery, soil nutrients and plantation did not recover much, so the soil microbe biomass was small. After a period of nutrient accumulation, nutrient condition reached the highest community density, and the soil microbe biomass attained balance, which was almost the same as that of the natural wetland. The microbe biomass reached the level of that of natural wetland.
It was observed that the recovery of the D. angustifolia biomass significantly increased during the restoration process. It must be noted that the TOC, TN, MBC, MBN, DOC, and DON also increased during the recovery process. The changes in these chemical parameters influenced the recovery of the vegetation biomass. The recovery of the vegetation biomass was probably enhanced by these parameters directly through physiological and nutritional effects (FAO 2005).
It is also worthy to note that the sharp increase in the TOC/TN in the surface layer at the initial stages can be attributed to the effects of living roots and the soil microbial community within this layer. The absorption of TN by plant and microbes at the initial stages within the surface layer was very high. This activity, however, reduces with time leading to increases in the soil TN. Therefore, the TOC/TN decreased after the eighth year. In addition, within the lower layer of the soil (10–20 cm), the transformation of the TOC occurs at a slower rate relative to the nitrogen content of the soil. As a result, the TOC/TN decreases within this depth.
Furthermore, the increases in the DOC/DON can be ascribed to the effects of the plants and the soil microbial community. Microbes and plants biomass utilize DON, whereas DOC does not change with time. Hence, DOC/DON increased over the entire restoration period. The increment was more pronounced in the surface layers owing to the active nature of the activities of plants and the soil microbial community within this region. The increases in the MBC/MBN could be attributed to the changes in the composition of the microbes.
It was found out that the labile fractions of the organic matter responded rapidly during the restoration process. This occurrence might be due to the rapid turnover rate of the labile fractions. These easily decomposed organic fractions are released directly from cells of fresh residues. As a result, they serve as early indicators of land use on soil quality (Gregorich et al. 1994) as well as important indicators of soil quality. Unlike the labile fractions of the organic matter, the TOC and TN took a long time before they were recovered during the restoration process. This observation might be due to the slower turnover rate of the TOC and TN. This may be explained by the fact that the sources of these organic matter fractions cannot be decomposed readily because they are more difficult for soil organisms to break down (FAO 2005).
It must also be pointed out that the restoration process is influenced strongly by weather conditions. In the Sanjian Plain, air temperature changes very smoothly, increasing from January to August, and then declining until December. Nearly half of the annual rainfall occurs in July and August. From May to June, the time period for plant rejuvenescence and growth, the precipitation usually is sufficient (Xu 2004). These climatic conditions provide suitable environment for the restoration of the vegetation biomass.
Conclusion
Draining wetlands for agricultural purposes is significant in the Sanjiang Plain especially in the last 50 years, and this activity causes wetland loss. This occurrence has significantly affected the TOC and TN of the wetland.
It was observed that the duration for the TOC and TN to restore to the level at which they occur naturally in the ecosystem was very long. Meanwhile, the soil active components reached the uncultivated level within a short time. The active components supplied and recycled the soil nutrients, co-working and interacting with wetland plants, microbes, and animals. Thus, the soil active components can be one of the key factors that determine eco-function recovery after restoring the agricultural land into wetland. In conclusion, this study can be used as an effective evaluation in analyzing the dynamics of plant recovery and the active carbon and nitrogen components of a wetland ecosystem undergoing significant land use changes.
References
An, S., Chang, Q., & Liu, J. (2003). Study on the characteristic properties and genesis of land desertification in the agriculture and husbandry interlace zone. Acta Ecologica Sinica, 01, 108–113.
Burdige, D. J., & Gardner, K. G. (1998). Molecular weight distribution of dissolved organic carbon in marine sediment pore waters. Marine Chemistry, 62, 45–64.
Dalal, R. C., & Mayer, R. J. (1988). Long term trends in fertility of soils under continuous cultivation and cereal cropping in southern Queensland. Soil Research, 24, 293–300.
FAO (2005). The importance of soil organic matter: key to drought-resistant soil and sustained food and production. FAO Soils Bulletin, 80, http://www.fao.org/docrep/009/a0100e/a0100e.pdf. Accessed 9 October 2012.
Ghani, A., Dexter, M., & Perrott, K. W. (2003). Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilization, grazing and cultivation. Soil Biology and Biochememistry, 35, 1231–1243.
Gregorich, E. G., Carter, M. R., Angers, D. A., Monreal, C. M., & Ellert, B. H. (1994). Towards a minimum data set to assess soil organic matter quality in agricultural soils. Canadian Journal of Soil Science, 74, 367–385.
Hughes, R. F., Kaufman, J. B., & Cummings, D. L. (2002). Dynamics of aboveground and soil carbon and nitrogen stocks and cycling of available nitrogen along a land-use gradient in Rondônia, Brazil. Ecosystems, 5, 244–259.
Jenkinson, D. S. (1976). The effects of biocidal treatments on metabolism in soil. Soil Biology and Biochememistry, 8, 203–208.
Jonasson, S., Michelsen, A., & Schmidt, I. K. (1999). Coupling of nutrient cycling and carbon dynamics in the arctic, integration of soil microbial and plant processes. Applied Soil Ecology, 11, 135–146.
Juo, A. S. R., & Lal, R. (2003). Nutrient profile in a tropical Alfisol under conventional and no-till systems. Soil Science, 127, 168–173.
Kalbitz, K. (2001). Properties of organic matter in soil solution in a German fen area as dependent on land use and depth. Geoderma, 104, 203–214.
Kennedy, A. C., & Papendick, R. I. (1995). Microbial characteristics of soil quality. Journal of Soil and Water Conservation, 5, 243–247.
Kieland, K. (1994). Amino acid absorption by arctic plants: implications for plant nutrition and nitrogen cycling. Ecology, 75, 2373–2383.
Knops, J. M. H., & Tilman, D. (2000). Dynamics of soil nitrogen and carbon accumulation for 61 years after agricultural abandonment. Ecology, 81, 88–98.
Lal, R. (2002). Soil carbon dynamics in cropland and rangeland. Environmental Pollution, 116, 353–362.
Landgraf, D. (2001). Dynamics of microbial biomass in Cambisols under a 3 year succession fallow in North Eastern Saxony. Journal of Plant Nutrition and Soil Science, 164, 665–671.
Liu, X. T., & Ma, X. H. (2000). Effect of large-scale reclamation on natural environment and regional environmental protection in the Sanjiang Plain. Scienta Geographic Sinica, 20, 14–19. in Chinese.
Lu, R. K. (2000). Soil agrochemistry and analytical methods (pp. 231–233). Beijing: Chinese Agricultural Science and Technology Press. in Chinese.
Ma, X. H., & Jiao J. Y. (2005). Contribution of soil nutrient in abandoned lands to vegetation restoration in hilly and gully regions on the loess plateau. Acta Botanica Boreali-occidentalia Sinica, 2, 123–130.
Post, W. M., & Kwon, K. C. (2000). Soil carbon sequestration and land-use change. Global Change Biology, 6, 317–327.
Saggar, S., Yeates, G. W., & Shepherd, T. G. (2001). Cultivation effects on soil biological properties, micro fauna and organic matter dynamics in Eutric Gleysol and Gleyic Luvisol soils in New Zealand. Soil & Tillage Research, 58, 55–68.
Shi, P., Gong, P., Li, X., et al. (2000). Methods and practice of land use/cover change. Beijing: Science Press. in Chinese.
Song, C. C., Wang, Y. Y., Bai, B. X., Lou, Y. J., & Zhao, Z. C. (2004). The changes of the soil hydrothermal condition and the dynamics of C N after the mire tillage. Environmental Science, 25(3), 168–172 (in Chinese).
US EPA (2001). Threats to wetlands. EPA 843-F-01-002d. http://www.epa.gov/owow/wetlands/pdf/threats.pdf. Accessed 9 October 2012.
Wang, G., Liu, G., & Xu, M. (2002). Effect of vegetation restoration on soil nutrient changes in Zhifanggou watershed of loess hilly region. Bulletin of Soil and Water Conservation, 22(1), 1–5.
Xu, X. (2004). Simulating study on carbon dynamics in Calamagrostis angustifolia rhizosphere micro-ecosystem. Thesis, Chinese Academy of Sciences, Changchun
Zhang, J., & Song, C. (2003). Effects of different land-use on soil physical–chemical properties in the Sanjiang Plain. Chinese Journal of Soil Science, 35(3), 371–373.
Zhang, J., Song, C., & Yang, W. (2005). Influence of land-use type on soil dissolved organic carbon in the Sanjiang Plain. China Environmental Science, 25(3), 343–347.
Zhang, J. B., Song, C. C., & Yang, W. Y. (2006). Land use effects on a distribution of labile organic carbon through soil profiles. Soil Science Society of America Journal, 70, 660–667.
Zhang, J. B., Song, C., & Wang, S. M. (2008). Short-term dynamics of carbon and nitrogen after tillage in a freshwater marsh of northeast China. Soil Tillage and Research, 99, 149–157.
Zhao, K. Y. (1999). Mires in China. Science Press, Beijing
Zhou, G., & Jiang, P. (2004). Changes in active organic carbon of erosion red soil by vegetation recovery. Journal of Soil Water Conservation, 6, 69–71.
Acknowledgments
The authors would like to thank the reviewers for their time and advice. This work was supported by the National Basic Research Program (973) of China (no. 2009CB421103) and the Natural Science Foundation (51208177). We also thank Jennifer Abena Kwofie for her excellent English editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, J., Song, C. & Nkrumah, P.N. Effects of wetland recovery on soil labile carbon and nitrogen in the Sanjiang Plain. Environ Monit Assess 185, 5861–5871 (2013). https://doi.org/10.1007/s10661-012-2990-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2990-5