Abstract
In this study, we analyzed the relationship between buried archaeological remains (masonries, pavements, and ancient ruins) and spontaneous vegetation growing above them. We carried out several vegetation surveys in the Domitian’s Stadium at the archaeological site of the Palatine (Rome). Vegetation data were collected using the Braun-Blanquet approach and elaborated using statistical analyses (cluster analysis) to assess the similarity among surveys. Structural, chorological, and ecological features of the plant communities were analyzed. Results showed that the vegetation responds significantly to the presence of sub-emerging ancient remains. The plant bioindication of this phenomenon occurs through the following floristic-vegetation variations: phenological alterations in single individuals (reduction in height, displacement of flowering/fruiting period), increase of annual species and decrease of perennial ones, decrease of total plant coverage, reduction of maturity level of the vegetation which remains blocked at a pioneer evolutive stage. The presence of sub-surfacing ruins manifests itself through the dominant occurrence of xerophilous and not-nitrophilous species (e.g., Hypochaeris achyrophorus L., Aira elegantissima Schur, Trifolium scabrum L. ssp. scabrum, Trifolium stellatum L., Plantago lagopus L., Medicago minima (L.) L., and Catapodium rigidum (L.) C.E. Hubb. ex Dony ssp. rigidum) and in a rarefaction of more mesophilous and nitrophilous species (e.g., Plantago lanceolata L., Trifolium pratense L. ssp. pratense, Trifolium repens L. ssp. repens, and Poa trivialis L.). Therefore, the vegetation can be used as bioindicator for the detection of buried ruins, contributing in the archaeological prospection for a general, fast, and inexpensive interpretation of the underground.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The first general observations on the correlation between plant coverage and buried archaeological structures date back to the seventeenth to the eighteenth centuries, when some English and French studies highlighted that there was correspondence between evident anomalies in the growth of vegetation and the presence of underground masonries and pavements (e.g., Louvet 1614; Stukeley 1776). Further investigations on this phenomenon have been carried out since the middle of last century; the majority of them have only highlighted the possibility of detecting anomalies in plant coverage/growth in relation to the presence of archaeological remains, by aerial and satellite photos (Wilson 1976; Schmidt 1982; Piccarreta 1987; Alvisi 1989; Fowler and Fowler 2005; Lasaponara and Masini 2007). Some studies have pointed out that the presence of sub-surfacing structures could limit the growth of the plants on the surface, making it more difficult and slow. In correspondence of buried remains, in fact, the soil is generally thinner, arid, and poor in nutrients and these edaphic conditions are not suitable for plant growth (Scollar 1963; Balducci 1966-1967).
Although the scientific interest, few botanical investigations have been focusing on this theme. Some studies have pointed out the potential use of plants as bioindicators of buried archaeological remains (De Marco et al. 1989; Caneva and Galotta 1994; Caneva 2002; Caneva and Ceschin 2005), but only in a few the correlations between vegetation growth and subsoil have been tested (Couderc 1983, 1985; De Marco et al. 1990; Caneva et al. 2000, 2005; Danin 2004; Ceschin et al. 2005, 2011; Jones et al. 2005; Merola et al. 2006). In these studies, researchers have emphasized that the influence of underground archaeological structures on vegetation can be highlighted analyzing both the floristic composition of the site (presence/absence of certain plant species) and the plant structure, coverage, and phenology. Several examples support this hypothesis. In the archaeological site of Moenjodaro (Pakistan), the occurrence of some calciphilous species, such as Capparis deciduas (Forsk.) Edgew., was correlated to the presence and distribution of ancient underground buildings (De Marco et al. 1990). Chromatic alterations and plant cover irregularities allowed the discover in Italy of some sectors of the Etruscan necropolis in Tarquinia, the Roman villas of Centocelle (Rome), S. Palomba (Pomezia), and the archaeological area of Paestum (Piccarreta 1987). Consistent changes in the floristic composition were recorded in the vegetation growing above buried ancient remains as in the western sector of the Domus Aurea (Caneva and Galotta 1994). A recent study in the archaeological area of the Maxentius’s villa in Rome (Ceschin et al. 2011) has highlighted that plants can be used as bioindicators of underground ruins through a decrease in plant coverage, reductions in height and vigor of plant individuals, presence of certain xerophilous species (e.g., Cota tinctoria (L.) J. Gay ssp. tinctoria, Trifolium scabrum L. ssp. scabrum), and disappearance or consistent reduction of mesophilous species (e.g., Ranunculus neapolitanus Ten., Poa trivialis L., Trifolium pratense L. ssp. pratense), which instead were frequent in close meadows.
During a vegetation study in the archaeological area of Palatine in Rome (Ceschin et al. 2003), we observed a marked discontinuity of vegetation in the Domitian’s Stadium. This finding gave us the opportunity to better understand how plants can indicate discontinuities of the subsoil and how vegetation anomalies can be linked to the presence of sub-surfacing archaeological remains. In this study we provide new data on this topic testing the potential use of spontaneous plants as bioindicators of buried ancient ruins.
Study area
The Domitian’s Stadium was built under the Emperor Domitian at the end of first century AD. It covers the south-east sector of the archaeological area of the Palatine, which is the oldest archaeological site in Rome, being the founding place of the city. The stadium area has an elongated rectangular shape (160 × 48 m), with smaller curved sides. At one end, there is a small semicircular construction, which was probably a fountain in the past. The Stadium was crossed by a large ring road which branched off paths and flower beds. The function of the Stadium is uncertain, although it was probably used as private garden and manège of the emperor (Tomei 1992).
From a phytoclimatic point of view, the study area, as well as most of the roman area, falls within the transitional Mediterranean region. It is characterized by a medium meso-Mediterranean thermotype and an upper subhumid ombrotype (Blasi 2001).
As regard to lithology, the soil is volcanic, specifically, composed by dark leucitic lava (Marra and Rosa 1995), with a heterogeneous accumulation of materials, primarily tufa (Funiciello et al. 1995). The stadium area is currently closed to visitors and the whole surface is subjected to periodic mechanical cutting; this management practice limits the development of the vegetation which remains blocked at a herbaceous stage.
Materials and methods
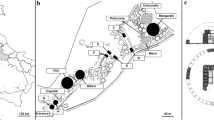
A total of 11 vegetation surveys were carried out in the whole study area during spring in 2006, using the phytosociological method (Braun-Blanquet 1928). The number of surveys is low because the stadium area is not very big and those carried out are representative of the vegetation and microenvironmental variability inside the study area. Each survey was conducted in a homogenous area of about 16 m2. Some surveys were concentrated in three sub-areas showing an evident plant discontinuity with respect to the surrounding vegetation. Among these sub-areas, one is semicircular (60 m2), located at the southern end of the Stadium, and two are in the center: one squared (36 m2), the other rectangular (180 m2) (Fig. 1).
At each survey, we listed all plant species with their coverage value according to method proposed by Braun-Blanquet (1932) (+ = sporadic species, 1 = <5 %, 2 = 5–25 %, 3 = 25–50 %, 4 = 50–75 %, and 5 = >75 %). We evaluated the species frequency in relation to the study area and considered as common those species with a frequency >50 %. Phenological features, such as size, flowering, and fruiting stage of each species, were noted in field. Differences in these features among specimens occurring in different surveys were observed.
At each sampling station, we also measured the soil depth with a graduated picket in order to verify the presence of sub-surfacing pavements or masonries. These measures were expressed into a semi-quantitative scale, as follows: very thin soil (<5 cm), with occasional emerging underground masonry (–); scarce soil (5–10 cm) with the presence of underground masonry (−+); soil depth ranging from 10 to 20 cm (+); and deep soil (more than 20 cm) with no masonry near the surface (++). The presence of human activities potentially stressful for vegetation were also noted (e.g., cutting, chemical weeding, trampling).
We followed Pignatti (1982) for the taxonomical determination of species and to obtain information on their chorotype (i.e., Mediterranean, European, Atlantic, Boreal, and Multizonal). We followed Conti et al. (2005) for the nomenclatural update of taxa. Life forms of plants were noted in field considering the Raunkiaer’s categories (1934) (therophytes-annual herbs; hemicryptophytes-perennial herbs; geophytes-perennial herbs with underground storage organs; chamaephytes-woody plants with buds at no more than 25 cm above the soil surface; phanerophytes-trees and shrubs with buds over 25 cm above the soil surface).
Vegetation data were organized in a binary matrix (86 species × 11 surveys) and analyzed using statistical multivariate procedures, particularly cluster analysis, with chord distance and average linkage algorithm (Anderberg 1973), in order to group the surveys on a similarity ratio (Westhoff and Van der Maarel 1978). Statistical analyses were run using Syntax 2000 (Podani 2001).
For each survey, and vegetation group obtained through the cluster analysis, we calculated the floristic-vegetation indices of the bioindicators system elaborated by Taffetani and Rismondo (2009) and integrated by Rismondo et al. (2011), which are usable in transformed ecosystems (e.g., farmlands, urban areas). In particular, we used: index of maturity (IM), which provides a measure of the actual stage of maturity of a vegetation community; index of floristic biodiversity (IFB), which expresses the number of species for each community; edaphic index of xerophilia (IX), which indicates the presence of plant species adapted to xeric environments; indexes of the life forms, to calculate the percentage of therophytes (IT), hemicryptophytes (IH), and of all perennial species (IP). We also evaluated the chorological features of the single vegetation survey/group by calculating the percentages of each chorotype. All calculations, with exception for the IFB, were carried out considering the coverage value of species.
The Ellenberg’s indices (1974), adapted to the Italian Flora (Pignatti et al. 2005), were used to define the ecological characteristics of the different vegetation groups in relation to environmental factors, such as light (L), temperature (T), continentality (C), moisture (U), pH (R), and soil nitrogen content (N). For each vegetation group, we elaborated ecological weighted spectra for each environmental factor.
Results
The vegetation surveys that we have conducted in the stadium area are shown in Table 1. The cluster analysis grouped the surveys into two main groups, A and B (Fig. 2). Group A includes all surveys carried out in the identified three sub-areas, which have thin substrates (soil classes: −, −+), characterized by a sandy matrix (for the presence of emerging wall fragments) that facilitates the water drainage. Group B includes the surveys conducted in the meadows within the Stadium but outside the three sub-areas, where substrates are more deep (soil classes: +, ++) and characterized by higher moisture.
The analysis of the life forms pointed out that the vegetation structure of group A is characterized by the dominance of therophytes (56.6 %), while that of group B by hemicryptophytes (66.9 %). In both vegetation groups, woody species (i.e., chamaephytes and phanerophytes) are absent (Table 2). The maturity level of the vegetation according to the IM is higher for the communities of group B, consisting of perennial meadows, than those of group A distinguished by nano-therophytic communities.
The weighed chorological spectra (Table 3) showed that the vegetation of the group A is mainly constituted by species with euri- and steno-Mediterranean chorotype (67.3 %). These chorotypes are less represented in the vegetation of group B (43.0 %), where European, Atlantic, and Boreal species not are negligible (21.8 %). The Multizonal species are frequent in both vegetation groups.
The ecological analysis highlighted that plant communities of both groups have similar ecological features. Specifically, both groups show a certain tolerance for medium-high values of light and temperature, and they are generally linked to medium-low values of continentality and pH, and low values of soil moisture and nitrogen content. The comparison of the two ecological spectra shows, however, some differences: plant communities of group A have more heliophilous, thermophilous, but less nitrophilous and hygrophilous character than those of group B (Fig. 3).
The calculation of the mean value of IX for the two vegetation groups shows how in the surveys of the group A there are many more species adapted to xeric edaphic conditions (56.6 %), if compared to those of the group B, in which the lower value of IX (27.4 %) highlights more mesophilous edaphic conditions.
Considering the phenological features, we observed that plant individuals occurring in the surveys of group A showed more stunted growth, precocious yellowing, and reduction in size in comparison with those of group B (neighboring meadows). Particularly, the size of individuals varied almost with a 1:2 ratio, in case of plants growing on sub-emerging ruins in relation to plants growing on thicker substrates.
Other meaningful differences between the two vegetation groups were noted and particularly in respect of floristic composition and total plant coverage. Firstly, in the surveys of group A, we observed a predominance of certain species, such as Hypochaeris achyrophorus, T. scabrum ssp. scabrum, Trifolium stellatum, Aira elegantissima, Catapodium rigidum ssp. rigidum, Plantago lagopus, Cerastium glomeratum, and Medicago minima, which are instead uncommon in group B. On the other hand, species as T. pratense ssp. pratense, Trifolium repens ssp. repens, Plantago lanceolata, Silene latifolia ssp. alba, and P. trivialis are dominant in the surveys of group B, while are absent or have low coverage values in those of group A. In Table 1, we reported the differential species of each vegetation group and the species that are more common in the study area.
As regard to the total plant coverage, we recorded a mean value of 80 % in stations of group A, and close to 100 % in those of group B (Table 1). As regards to the floristic diversity, is not very dissimilar in the two vegetation groups since in both the mean value of species occurring is approximately 30 (Table 2).
Discussions
The plant discontinuity that we noted inside the stadium area is evident observing the variation in chromatic, phenological, structural, and floristic composition between the two vegetation groups. This discontinuity could be explained assuming that the vegetation, which grows in the three identified sub-areas (surveys of the group A), insists on sub-emerging remains of ancient pavements. In fact, even if excavations have not been conducted yet, and thus up to now it is not possible to verify correlations between subsoil and plants growing above, some reconstructions of the ancient Stadium have shown that in correspondence of the semicircular sub-area, there was a fountain (with a half moon shape) that was paired with the other positioned at the opposite end of the Stadium (whose remains are still visible). These fountains were used as metae (Tomei 1992). In addition, archaeologists assume that there were lightly raised flowerbeds or gardens, in the two central sub-areas, since the stadium was not used for horse racing, but as private garden (Rojo 1985). In correspondence of these three sub-areas, it is therefore very likely that there are remains of pavement or masonries which are more superficial, if compared to the rest of the stadium area. These remains create more xeric edaphic conditions, which affect the growth of vegetation above.
We observed that the presence of underground ruins can be revealed through structural and chorological changes in vegetation. Indeed, at these three sub-areas there are exclusively nano-therophytic communities, where the number of therophytes and Mediterranean species is much higher than herbaceous perennial communities occurring in the rest of the stadium area. We explained this result considering that therophytes, characterized by a weak and thin radical apparatus, are adapted to grow on thin and primitive soils, differently from herbaceous perennial species (i.e., hemicryptophytes, geophytes), which need thicker and more mature soils. It is also well known that therophytes have the capacity, especially the Mediterranean ones, to tolerate water stress edaphic conditions, such as those found in the three subareas. This local xericity plays an important role in affecting the vegetation growth here giving rise to micro-edaphic conditions that do not allow the development of the vegetation towards more mature stages; therefore the vegetation remains blocked at an early evolutive step here. Also, the IM mean values for the two survey groups confirm that the vegetation in the three sub-areas has a maturity level lower (due to the presence of annual pioneer communities), compared to the vegetation that grows in the rest of the Stadium, which consists primarily of herbaceous perennial communities.
It should be noted that other environmental factors, such as trampling and mechanical cutting of the vegetation (common in archaeological areas), cannot be the cause of the plant discontinuity occurring in the site; in fact, the stadium area is closed to the public and therefore it is not subject to phenomena of trampling, if not sporadically, and the cutting is carried out uniformly in the entire area.
Many of the results discussed above are supported by the ecological analysis with the Ellenberg’s indices and IX, which show how the vegetation of group A is more xerophilous, much more thermophilous and less nitrophilous, if compared to the vegetation of group B.
The remarks of Scollar (1963), and more recently of Ceschin et al. (2011), which underline how the presence of buried masonries can be also linked to phenological variations in vegetation, are confirmed by this study. We observed for example that individuals of Orchis coriophora and Serapias vomeracea ssp. vomeracea, collected in the three sub-areas, showed a modified phenology respect to those growing in the surrounding meadows. Particularly, these orchids had a minor development, a shorter flowering period (about fortnight), and an early yellowing at the end of the biological cycle. These individuals, in fact, were shorter (around the 50 % of height) and already faded at the end of May, in contrast with those growing in the rest of the stadium area, where, in the same period, they were still in full bloom. This behavior, showed also by other species (e.g., Urospermum dalechampii, Salvia verbenaca, and Trigonella esculenta), gave to the three sub-areas a characteristic yellow-brown coloring in late spring, strongly in contrast with the bright green color of the other meadows in the Stadium.
In addition to these observations, we noted a directly proportional relationship between the total plant coverage and soil depth, but not a strong correlation with floristic diversity, confirming results emerged in a previous study (Ceschin et al. 2011). Firstly, the vegetation growing at stations with thinner soils, where there are sub-emerging archaeological remains (group A) showed a lower mean value of coverage than that occurring in stations with deeper soils (group B), where buried ruins are not close to the surface. These data can be explained considering that ruins next to the surface create stressful edaphic conditions (e.g., reduction of edaphic moisture and nutrient content), which generally limit vegetation growth. On these results, the total plant coverage seems to be a feature of the vegetation useful in the bioindication of underground ruins. Conversely, the species richness is not equally useful as a parameter to be considered, infact, the two vegetation groups show similar mean values of the species number.
Differences in floristic composition between the two vegetation groups, allowed us to identify some species that could be used to detect subsoil features. Particularly, we report T. scabrum ssp. scabrum, T. campestre, T. stellatum, H. achyrophorus, A. elegantissima, P. lagopus, M. minima, C. rigidum ssp. rigidum, and C. glomeratum, as characteristic species of the vegetation of group A, and, therefore, as potential bioindicators of sub-emerging archaeological remains. Indeed, all these species are therophytes and well-adapted to xeric, low-in-nitrate, calcareous, and shallow soils, which in natural environment characterize the annual Mediterranean meadows on calcareous substrate (Ceschin et al. 2006). Otherwise, there are species that we frequently found in the stadium area, with the exception of the three subareas, among which P. trivialis, T. pratense ssp. pratense, T. repens ssp. repens, P. lanceolata, and S. latifolia ssp. alba. These entities are herbaceous perennial species that differ from the previous species set because they are relatively less xerophilous and need thicker soil. Therefore, the presence of these species could indicate the absence of submerging archaeological remains or which are close to the surface.
It is worth noting that T. scabrum ssp. scabrum, T. pratense ssp. pratense, and P. trivialis were already reported as biological indicators for exploration of subsoil in a similar study carried out in the archaeological area of Maxentius’s villa in Rome (Ceschin et al. 2011). This underlines that these species could really play an important role as potential bioindicators for archaeological prospection.
However, it should be underlined that in the study area we have not recorded certain species, such as Ficus carica L., Rubus ulmifolius Schott, and Ulmus minor Mill. ssp. minor, that were cited in other studies as entities occurring frequently where masonry emerges from the ground (Couderc 1983; Caneva and Galotta 1994). However, these entities are woody species which grow in areas that have been abandoned for an extended period and cannot grow when the vegetation is mechanically removed, as it occurs in this study area.
Conclusions
These data confirm that the presence of sub-emerging archaeological remains affects the vegetation that grows above, giving rise to the following structural, phenological, and floristic variations:
-
Decrease of the total plant coverage that ranges from mean value near to 100 %, in case of vegetation growing on deep soils, to a mean value of 80 %, in case of vegetation on sub-merging stonework;
-
Increase of annual species and decrease of herbaceous perennial ones that are prevailing on deeper soils;
-
Reduction of the maturity level of the vegetation which is blocked at an early evolutive stage;
-
Phenological alterations that are evident in individuals growing on minimum soils as reduction in height and displacement of the flowering and fruiting period (earlier and shorter);
-
Presence of plant species that are adapted to grow on xeric soils poor in water and nutrients, such as H. achyrophorus, A. elegantissima, T. scabrum ssp. scabrum, T. stellatum, P. lagopus, M. minima, and C. rigidum ssp. rigidum;
-
Rarefaction of species that prefer thicker soils (at least more than 10 cm deep) and soils with enough moisture and nutrients, such as P. lanceolata, T. pratense ssp. pratense, T. repens ssp. repens, and P. trivialis.
We should consider that the study area is characterized by a Mediterranean climate which already selects plant species well-adapted to xeric conditions. Therefore, we can suppose that, taking into consideration our results on the ecological features of the sampled plants in this study, in areas with temperate climates (where plants are not adapted to soil aridity), the presence of underground archaeological structures should affect even more the development of the vegetation growing above.
This study has demonstrated that the spontaneous vegetation respond significantly to the presence of edaphic discontinuities caused by the presence of buried archaeological ruins. Such considerations, therefore, underline the importance that botanical studies can have in the archaeological prospection for a general, fast, and inexpensive “interpretation” of the subsoil.
References
Alvisi, G. (1989). La fotografia aerea nell’indagine archeologica. Roma: La Nuova Italia Scientifica.
Anderberg, M. R. (1973). Cluster analysis for applications. New York: Academic.
Balducci, L. (1966–1967). La vegetazione come indizio di resti archeologici sepolti, nell’osservazione di studiosi inglesi e francesi dal XV al XIX secolo. Annali di Facoltà di Lettere e Filosofia, 4, 447–458.
Blasi, C. (2001). Carta del fitoclima dell’area romana (1:100.000). Informatore Botanico Italiano, 33(1), 240–243.
Braun-Blanquet, J. (1928). Pflanzensoziologie. Grundzüge der vegetationskunde. Wien: Springer.
Braun-Blanquet, J. (1932). Plant sociology: the study of plant communities. New York: McGraw-Hill.
Caneva, G. (2002). Bioindicatori. In A. A. Zhigljavsky & V. V. Nekrutkin (Eds.), Il Mondo dell’archeologia (pp. 171–172). Roma: Treccani ed.
Caneva, G., & Ceschin, S. (2005). L’ecologia vegetale come strumento di interpretazione ambientale attuale e storica. In G. Caneva (Ed.), La Biologia vegetale per i Beni culturali. Conoscenza e valorizzazione (Vol. 2, pp. 435–462). Firenze: Nardini ed.
Caneva, G., & Galotta, G. (1994). Floristic and structural changes of plant communities of the Domus Aurea (Rome) related to a different weed control. In V. Fascina, H. Off, F. Zezza (Eds.), The conservation of monuments in the Mediterranean Basin (pp. 317–322). Proceedings of the 3rd International Symposium Venezia, 22–25 Giugno 1994.
Caneva, G., Ceschin, S., Serra, T. (2000). Specie e comunità vegetali come bioindicatori nelle prospezioni archeologiche. Proceedings of the 95th Italian Congress of the Botany Society, 35.
Caneva, G., Salvadori, O., Ricci, S., & Ceschin, S. (2005). Ecological analysis and biodeterioration processes over time at the Hieroglyphic Stairway in the Copàn (Honduras) archaeological site. Plant Biosystems, 139(3), 295–310.
Ceschin, S., Cutini, M., & Caneva, G. (2003). La vegetazione ruderale dell’area archeologica del Palatino (Roma). Fitosociologia, 40(1), 73–96.
Ceschin, S., Caneva, G., & Kumbaric, A. (2005). Analisi ecologica della flora nell’area archeologica centrale di Roma in relazione all’uso antropico del sito. Atti Accademia dei Lincei, 218, 421–431.
Ceschin, S., Cutini, M., & Caneva, G. (2006). Contributo alla conoscenza della vegetazione ruderale delle aree archeologiche romane (Roma). Fitosociologia, 43(1), 97–139.
Ceschin, S., Kumbaric, A., Caneva, G., & Zuccarello, V. (2011). Testing flora as bioindicator of buried structures in the archaeological area of Maxentius’s villa (Rome, Italy). Journal of Archaeological Science, 39, 1288–1295.
Conti, F., Abbate, G., Alessandrini, A., & Blasi, C. (2005). An annoted checklist of Italian flora. Roma: Palombi ed.
Couderc, J. M. (1983). Les Vegetations anthropogenes et nitrophiles et la prospection archeologique. Colloques Phytosociologique, 12, 331–347.
Couderc, J. M. (1985). Vegetation anthropogenee et prospection archeologique. Review Archives Centre France, 24(4), 53–61.
Danin, A. (2004). The impact of management of the reconstructed ancient Caesarea (Israel), on the local vegetation. Proceedings of the 40th Italian Congress of the Phytosociological Society, 12.
De Marco, G., Dinelli, A., & Caneva, G. (1989). Geobotany applied to the analysis and management of archaeological sites. Braun-Blanquetia, 3(2), 293–297.
De Marco, G., Caneva, G., & Dinelli, A. (1990). Geobotanical foundation for a protection project in Moenjodaro archaeological area. Prospezioni archeologiche, 1, 115–120.
Ellenberg, H. (1974). Zeigerwerte der gefässpflanzen mitteleuropas (Vol. 9). Göttingen: Scripta Geobotanica.
Fowler, M. J. F., & Fowler, Y. M. (2005). Detection of archaeological crop marks on declassified Corona KH-4B intelligence satellite photography of southern England. Archaeological Prospection, 12(4), 257–264.
Funiciello, R., Marra, F., & Rosa, C. (1995). I caratteri geologici-stratigrafici. In B. Cignini, G. Massari, & S. Pignatti (Eds.), L’Ecosistema Roma, ambiente e territorio (pp. 29–39). Roma: Fratelli Palombi.
Jones, G., Charles, M., Bogaard, A., Hodgson, J. G., & Palmer, C. (2005). The functional ecology of present-day arable weed floras and its applicability for the identification of past crop husbandry. Vegetation History and Archaeobotany, 14, 493–504.
Lasaponara, R., & Masini, N. (2007). Detection of archaeological crop marks by using satellite QuickBird multispectral imagery. Journal of Archaeological Science, 34, 214–221.
Louvet, P. (1614). Histoire de la ville de Beauvais et des antiquités du pays de Beauvoisis. Paris: Préauly ed.
Marra, F., & Rosa, C. (1995). Stratigrafia e assetto geologico dell’area di Roma. In R. Funiciello (Ed.), Memorie descrittive della Carta geologica d’Italia, Il Centro Storico (pp. 49–118). Roma: Istituto Poligrafico e Zecca di Stato.
Merola, P., Allegrini A., Guglietta D., Sampieri, S. (2006). Study of buried archaeological sites using vegetation indices. Proceedings of Conference “Remote Sensing for Environmental Monitoring, GIS Applications, and Geology VI”. Stockholm, 13 September 2006, doi:10.1117/12.689727.
Piccarreta, F. (1987). Manuale di fotografia aerea: Uso archeologico. Roma: L’Erma di Bretschneider.
Pignatti, S. (1982). Flora d’Italia. Bologna: Edagricole.
Pignatti, S., Menegoni, P., & Pietrosanti, S. (2005). Indicazione attraverso le piante vascolari. Valori di indrsicazione secondo Ellenberg (Zeigerwerte) per le specie della Flora d’Italia. Braun-Blanquetia, 3, 91–97.
Podani, J. (2001). Syntax 2000. Computer program for date analysis in ecology and systematics. User’s manual. Budapest: Scientia.
Raunkiaer, C. (1934). The life forms of plants and statistical plant geography. Oxford: Oxford University Press.
Rismondo, M., Lancioni, A., & Taffetani, F. (2011). Integrated tools and methods for the analysis of agro-ecosystem’s functionality through vegetational investigations. Fitosociologia, 48(1), 41–52.
Rojo, M. (1985) Il Palatino. In AA.VV. (Eds), Roma Antiqua. L’area archeologica centrale: “envois” degli architetti francesi (1788–1924). (pp. 326–255) Rome: Academie de France à Rome, Ecole Fraincaise de Rome & École nationale supérieure des Beaux-Arts.
Schmidt, G. (1982). Fotointerpretazione archeologica. Contributi sul restauro archeologico. Firenze: Alinea ed.
Scollar, I. (1963). Physical conditions tending to produce crop sites in the Rhineland. (pp. 39–47). Paris, Collection International d’Archéologie Aerienne.
Stukeley, W. (1776). Itinerarium curiosum (2nd ed.). London: Baker and Leigh.
Taffetani, F., & Rismondo, M. (2009). Bioindicator system for the evaluation of the environmental quality of agro-ecosystems. Fitosociologia, 46(2), 3–22.
Tomei, M. A. (1992). Il Palatino. Soprintendenza archeologica di Roma. Milano: Electa.
Westhoff, V., & Van Der Maarel, E. (1978). The Braun blanquet approach. In R. H. Whittaker & W. Den Haag (Eds.), Classification of plant Communities (pp. 287–399). The Hague: Junk.
Wilson, D. R. (1976). Air photo interpretation for archaeologists. London: BT Basford LTD.
Acknowledgments
Authors are grateful to the Archaeological Superintendence of Rome for providing historical materials on the study area and for having allowed the access to areas closed to the public.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ceschin, S., Caneva, G. Plants as bioindicators for archaeological prospection: a case of study from Domitian’s Stadium in the Palatine (Rome, Italy). Environ Monit Assess 185, 5317–5326 (2013). https://doi.org/10.1007/s10661-012-2947-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2947-8