Abstract
Nonylphenol is an endocrine disruptor with harmful effects including feminization and carcinogenesis on various organisms. This study aims to investigate the distribution and ecological risks of nonylphenol in the Daliao River Estuary, China. Nonylphenol, together with other phenolic endocrine disruptors (bisphenol A, 4-t-butylphenol, 4-t-octylphenol, and 2,4-dichlorophenol), was detected in surface water and sediment on three cruises in May 2009, June 2010, and August 2010, respectively. A large flooding occurred during our sampling campaign in August and its effect on nonylphenol concentrations and fluxes in the estuary was therefore evaluated. The results showed that nonylphenol with a concentration range between 83.6–777 ng l−1 and 1.5–456 ng g−1 dw in surface water and sediment was the most abundant among the phenolic compounds, accounting for 59.1–81.0 and 79.9–92.1 % of the total phenolic concentration in surface water and sediment, respectively. The concentrations recorded in May and June were comparable, whereas those in August were considerably higher, mainly due to the flush of flooding. The flooding also caused a 50 times increase in nonylphenol flux from the estuary into the adjacent Bohai Sea. Nonylphenol concentrations in the estuary have exceeded the threshold level of undesirable effects with a potential risk of harm to local species, especially benthic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alkylphenol ethoxylates (APEOs) are a group of nonionic surfactants, which have been used since 1940s (White et al. 1994) in various industries including textile processing, pulp and paper bleaching, resin and protective coating production, oil and gas recovery, and steel manufacturing (Tubau et al. 2010). About 500,000 t of APEOs is produced globally each year, among which nonylphenol ethoxylates account for about 80 % (Shao et al. 2003). APEOs are unstable in aquatic environments with a half-life of several days and are subject to degradation to alkylphenols mainly through bio- and photodegradation (Yoshimura 1986). Alkylphenols such as nonylphenol and 4-tert-octylphenol are, however, much more stable in the environment with a half-life of approximately 2 months in water and lasting years in sediment (Ying et al. 2002).
In contrast to their parent compounds, alkylphenols are more toxic to organisms with half lethal concentrations (LC50) between 20 and 1,590 μg l−1 to different species (Ferguson et al. 2001). In particular, numerous in vivo and in vitro studies demonstrate the endocrine-disrupting properties of these chemicals (Kwack et al. 2002; Soto et al. 1991; Vazquez-Duhalt et al. 2005; Verderame et al. 2011). Alkylphenols, particularly nonylphenol, have been reported in many countries and regions with a concentration range from undetected to 53 μg l−1 in water (Ahel et al. 2000; Blackburn and Waldock 1995; Fu et al. 2007) and from <0.1 to 13 μg g−1 in surface sediment (Isobe et al. 2001; Li et al. 2008; Petrovic et al. 2002). Concerning the toxicities and endocrine-disrupting effects of alkylphenols, many countries such as European Union members have passed regulations to restrict the use of APEOs in domestic applications (Jeannot et al. 2002). In many Asian countries including China, however, APEOs are still being legally used in large quantities.
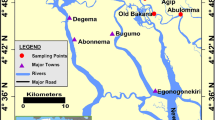
The Daliao River, located in the territory of Liaoning Province in Northeast China, stretches from the confluence of Hunhe River and Taizi River to the Bohai Sea with a length of 95 km (Fig. 1). It is among the biggest river in Northeast China with an annual freshwater discharge of 3.95 × 109 m3. Northeast China is a traditional industrial center of the country with a number of big cities including Shenyang, Benxi Anshan, and Yingkou located in the upper reaches and the estuary of Daliao River. A large amount of sewage and wastewater (∼450 × 106 t in 2008) was discharged into Daliao River, either directly or indirectly, from these cities. Consequently, the water quality in the Daliao River Estuary has deteriorated during the past two decades and was ranked the fifth grade (the worst) according to the National Surface Water Quality Guideline of China. The water quality was improved in recent years due to the implementation of various pollution control programs including the closure of 417 small paper mill companies with an annual production of <20,000 t in 2008. Previous studies on water quality in the Daliao River Estuary mainly focused on heavy metals, nutrients, and petroleum hydrocarbons (Wen et al. 2011). The distribution and risks of phenolic compounds especially nonylphenol in the estuary however remain unknown. Since phenolic compounds are usually applied in aqueous solutions and they do not tend to volatize into the atmosphere with a low vapor pressure, their main sources in surface waters are closely related to discharges of municipal sewage and industrial waste as well as to other anthropogenic activities such as storm water discharges (Soares et al. 2008). Considering the existence of numerous factories and companies including textile mills, paper pulp plants, pharmaceutical factories, and sewage treatment plants along the upper reaches and the estuary of Daliao River, these point sources are assumed to be the main origin of phenolic compounds in the river.
Concerning seasonal distribution of nonylphenol in natural environment, there have been some reports in lakes (Li et al. 2004), bays (Fu et al. 2007; Li et al. 2005), and lagoons (Carafa et al. 2007). Cailleaud et al. (2007) also reported seasonal variation of nonylphenol in an estuary showing significant decreases in the water column during the maximum biological activity periods in spring and autumn. The seasonal distribution of nonylphenol in estuaries is however not well understood considering large variations in biological activities and geographic morphology among different estuaries around the world. This study therefore focuses on seasonal distribution and flux of nonylphenol in the Daliao River Estuary. A total of 26 stations were selected and surface water and sediment samples were collected in three seasons, i.e., the dry season (May 2009), the normal season (June 2010), and the wet season (August 2010). A flooding event with a monthly precipitation of 310 mm, the largest one in the province within the past 20 years, occurred during our sampling campaign in August 2010. The effect of flooding on nonylphenol distribution in the estuary was therefore evaluated. The flux of nonylphenol from the estuary into the Bohai Sea was calculated based on the freshwater flow rate obtained from a local hydrological station. The potential risks of nonylphenol were assessed according to its toxicity on various aquatic organisms.
Materials and methods
Chemicals
The target compounds (nonylphenol, bisphenol A, 4-t-butylphenol, 4-t-octylphenol, and 2,4-dichlorophenol) and surrogate standards (4-n-hexylphenol and bisphenol A-d14) were purchased from Sigma-Aldrich. Internal standards (naphthalene-d8, phenanthrene-d10, and pyrene-d10) were obtained from Chem Service. Bis-(trimethylsilyl)-tri-fluoroacetamide (abbreviated as BSTFA) was obtained from Supelco. HPLC-grade hexane, acetone, methanol, and dichloromethane were purchased from Tedia.
Sample collection
Samples were collected from 16 stations along the river channel in May 2009. Another 10 stations (stations 17–26) on three transects in the adjacent Bohai Sea were also sampled in June and August 2010 (Fig. 1). One liter of surface water was collected in glass bottles with a Teflon-lined cap, and 1 ml HCl solution (6 M) was added to the sample to give a pH of 1∼2 to suppress microbial activities. About 200 g surface sediment was collected with a stainless sediment grab sampler into amber glass bottles with Teflon-lined caps. The salinity was also measured with a portable electric conductivity meter (Mettler, Model SG-23B) at each station. In the laboratory, water samples were extracted and analyzed within 3 days, whereas sediment samples were stored at −20 °C before extraction and analyzed within 1 month.
Sample extraction
The method for sample extraction was based on previous reports (Li et al. 2001). Briefly, 200 ng surrogate standard (4-n-hexylphenol and bisphenol A-d14) was added to each water sample. The samples were then extracted twice with 60 ml dichloromethane (DCM). The DCM extracts were concentrated with a rotary evaporator to about 1 ml and further concentrated under a gentle flow of dry nitrogen to <0.5 ml. Then, acetone was added to change solvent, followed by derivatization with BSTFA. Internal standards were added and the final volume was adjusted to 0.5 ml by hexane before GC/MS analysis.
For sediment samples, ∼3 g of wet sample was transferred into a 50-ml Teflon tube followed by the addition of 5 ml HCl solution (0.1 M). The samples were then extracted three times, each with 5 ml DCM. Anhydrous sodium sulfate and copper were added into the extracted samples to remove water and sulfur, respectively. After concentration and derivatization, the extract was cleaned up with Florisil and eluted with hexane. Internal standard was added and the final volume was adjusted to 0.5 ml before GC/MS analysis.
Quantification by GC/MS
Gas chromatography–mass spectrometry was employed for identification and quantification of the target phenolic compounds. Analyte separation was achieved by gas chromatography (Agilent GC 6890) with a DB-5MS capillary column (length, 30 m; i.d., 0.25 mm; coated film thickness, 0.25 μm). An Agilent MS 5975 mass spectrometry detector was used to qualify and quantify the analytes. Selected ion monitoring mode was employed for mass scan with a sampling rate of 0.2 s. Each target compound was qualitatively and quantitatively determined based on retention time as well as relative intensities of quantification and confirmation ions (Li et al. 2001).
Quality assurance and quality control
Blank, spike, and replicate samples were processed during each batch of analysis. Surrogate standards (4-n-hexylphenol and bisphenol A-d14) were added to each sample before extraction. The method detection limit (MDL) was calculated as: MDL = SD × t + blank, where SD is the standard deviation of seven replicate samples and t = 3.71 for n = 7 at 99 % confidence level.
No target analytes were detected in the blanks in this study. The spiking recoveries in the water and sediment samples were between 87.2–99.4 and 75.3–105 %, respectively. The surrogate recoveries were between 80.2 and 105 %. Method detection limits for various phenolic compounds were around 1 ng l−1 for water samples and 0.4 ng g−1 for sediment samples.
The flux of nonylphenol entering the sea
The flux is simply estimated with the following equation: \( {Q_{\mathrm{i}}}={c_{\mathrm{i}}}\times \mathrm{q} \), where Q i represents the flux entering the sea, c i is the pollutant concentration at the upstream of the Daliao River Estuary (station 1), and q is the flow rate of the river. The flow rate was obtained from the Tangmazai Hydrological Station (41°11′ N, 122°43′ E) situated in the upper reach of the Daliao River Estuary.
Results and discussion
Compositions of phenolic compounds
Nonylphenol (NP) and bisphenol A were detected in all samples, whereas 4-t-butylphenol, 4-t-octylphenol, and 2,4-dichlorophenol were detected in most samples. The concentrations of NP and concentration ranges of other detected phenolic compounds in the Daliao River Estuary are provided in Tables 1 and 2, respectively. NP was the most abundant among the detected compounds, accounting for 59.1–81.0 and 79.9–92.1 % of the total concentration in surface water and sediment, respectively. Bisphenol A contributed 10.9–32.3 and 5.03–14.1 % of the total concentration in the two sample matrices, respectively. 4-t-Butylphenol, 4-t-octylphenol, and 2,4-dichlorophenol generally accounted for less than 5 % of the total concentration. Because of the high source attribution to the total concentration, the following detail will focus on the NP distribution.
Nonylphenol in surface water
In the river channel (stations 1–16), NP concentrations showed a clear seasonal variation with a range of between 180–277, 83.6–335, and 521–777 ng l−1 in May (the dry season), June (the normal season), and August (the wet season), respectively (Table 1). In May, the dry season, high values of NP occurred in the upstream of the river (stations 1–8), reflecting low freshwater flow (mean flow rate, 143 m3 s−1) and strong tidal dilution downstream (Table 1). However, in June, NP in the whole river channel was of similar concentration, indicating an increase of freshwater transport (mean flow rate, 211 m3 s−1) and a weak seawater dilution downstream. In August, NP in the river channel was also similar but at much higher concentrations with an average of 664 ng l−1, i.e., about three times of that observed in May and June. This could be caused by a rise in temperature and associated increase in microbial activity leading to an enhanced degradation of nonylphenol ethoxylates and an increased production of NP (Li et al. 2004). However, it is postulated that the major reason for the enhanced NP concentrations in August is due to the flooding event that occurred during our sampling campaign. The river runoff increased substantially to 2,120 m3 s−1 in August, which was approximately 10 times of that in June and 15 times of that in May. A consequence of the flood is that it reduced the deposition process of the suspended solids and enhanced the resuspension process of the sediment. A significant correlation between NP concentration and suspended solid contents in water column in August (R 2 = 0.792, p < 0.01) provides evidence of this (Fig. 2), whereas no significant correlation existed in May and June (figures omitted).
In the coastal area (stations 17–26), the concentrations of NP are lower than those in the river section, and they generally showed a decreasing trend from the river mouth to offshore (Table 1). There also existed an obvious seasonal variation in NP concentrations despite a lack of data in May. In June, NP concentrations ranged between 25.7 and 78.6 ng l−1 with an average of 51.1 ng l−1, whereas in August, they ranged between 136 and 676 ng l−1 with an average of 309 ng l−1. The average concentration in August was therefore about six times of that observed in June (Table 1). This discrepancy was again due to the dramatic hydrodynamic changes seen during the flood season. During the flood, the freshwater flow rate was much higher and the tidal dilution was therefore much weaker. Consequently, NP tends to be accumulated in the coastal area.
Nonylphenol in surface sediment
In the river section, NP concentration was recorded as between 1.5–163, 20.3–181, and 68.5–456 ng g−1 dry weight (dw) in May, June, and August, respectively. The concentration generally showed a decreasing trend from the upstream (stations 1–7) to downstream (stations 11–16). This distribution pattern in sediment is different from that in surface water, especially during normal (June) and wet (August) seasons, indicating that the suspended solids tended to deposit more quickly, whereas the water can be transported further. Seasonal variations also existed with the highest values recorded in August, again reflecting influences of flooding in summer.
In the coastal area, NP concentrations ranged between 6.4 and 41.7 ng g−1 dw with a mean of 17.2 ng g−1 dw in June and 7.89–40.0 ng g−1 dw with a mean of 20.3 ng g−1 dw in August (Table 1). The decreasing trend of NP in surface sediment from the river mouth to offshore was more obvious than that in water phase, again reflecting the fact that the water can be transported further than the suspended solids. Phenolic compounds tend to adsorb onto the suspended solids and subsequently deposit to the sediment, due to their high octanol–water partition coefficient (logKow) and low water solubility. Nonylphenol, e.g., has a logKow of 4.48 and water solubility of 5.43 mg l−1 (Isobe et al. 2001). This is also demonstrated by the high concentrations of these chemicals in the sediment relative to those in the water in this study. The vast sedimentation process near the river mouth therefore significantly contributes to the removal of phenolics in the water column.
The flux of nonylphenol into the sea
To further assess the effect of river discharge on coastal water quality, NP flux entering the Bohai Sea was estimated in this study. Based on the pollutant concentration and the freshwater flow rate in each sampling season, NP flux was estimated as 28.6, 50.9, and 1,439 mg s−1 in May, June, and August, respectively. The flux differs substantially among seasons and the flux in August (flooding season) is 28 times of that in June and 50 times of that in May. Flooding therefore significantly shapes the distribution pattern of NP in the estuary.
Based on historical data from the local hydrological station, the annual mean flow rate of the river is 3.95 × 109 m3. If the mean NP concentration recorded in our sampling seasons is assumed as the yearly average, the annual flux of NP can be estimated as 1.47 t. Similarly, the annual flux of total phenolic compounds can be estimated as 1.92 t. The flux estimation in this paper is however very preliminary since many other factors such as chemical degradation, river–sea interaction, and water–sediment exchange were not considered.
Risk assessment of nonylphenol
The concentrations of NP in this study together with those reported in other areas of the world are summarized in Table 3. A direct comparison of these data may not be suitable, since different laboratories may use different procedures with discrepant data quality. Based on this table, however, NP concentrations in the Daliao River Estuary represent a high pollution level.
As an endocrine-disrupting chemical, NP has raised widespread concern with numerous reports on its acute and chronic toxicities. NP at low concentrations (0.01–10 μg l−1) can inhibit settlement success of the barnacle, Balanus amphitrite (Billinghurst et al. 1998). It is generally agreed that the threshold level of NP for fish sex reversal is 1.0 μg l−1 (Schwaiger et al. 2002). It was also reported that the exposure of Pacific oyster larvae, Crassostrea gigas, to NP at 0.1 μg l−1 caused a significant increase in the mortality rate (Nice et al. 2000). Considering its physical–chemical properties, NP tends to be adsorbed onto suspended solids and eventually to accumulate in sediments. Research on the effects of NP on benthic species is, therefore, critical. Current research on NP effects, however, mainly focused on species living in the water column. There are only a few studies on bioaccumulation and toxicity of NP for benthos. Bettinetti et al. (2002), for example, reported an increased mortality for the larvae of the insect, Chironomus riparius, exposed to NP at 1.044 μmol g−1 (about 230 μg g−1 dw) and an absolute mortality when exposed to NP at 3.63 μmol g−1 (about 780 μg g−1 dw). A no observed effect concentration (NOEC) for the larvae of mud snail, Potamopyrgus antipodarum, was reported as 10 ng g−1 dw (Duft et al. 2003).
An accumulative percentage distribution of NP in the surface water in this study was illustrated in Fig. 3. NP concentration in the surface water of the Daliao River Estuary did not reach 1.0 μg l−1, the threshold level for fish sex reversal. However, the concentration did exceed 0.1 μg l−1, the critical level for oyster larvae survival, with a probability of about 85 % (Fig. 3). The concentration also totally exceeded 0.01 μg l−1, the critical level for barnacle larvae settlement. In the coastal area, NP concentrations were lower than those in the river channel. However, during the flood season in August, the concentration was also above the critical level for oyster larvae mortality.
Concerning the sediment of the Daliao River Estuary, NP concentration has generally exceeded the NOEC (10 ng g−1 dw) for the mud snail larvae. The highest concentration was about 45 times of NOEC for the species. Considering the possible interaction of NP with other phenolic compounds, the actual effects would be more serious. The toxicological effects of NP on local species therefore warrant further study.
Conclusions
Based on an analysis of phenolic compounds in the Daliao River Estuary in three seasons, NP accounted for 59.1 to 92.1 % of the total phenolic compounds. From the upper range of the river to the downward coastal area, NP concentrations in sediment showed a more obvious decrease than those in water, indicating that the suspended solids tended to deposit more quickly, whereas the water can be transported further. NP in both water and sediment showed a clear seasonal pattern with the highest values recorded in August. NP flux showed great variation among seasons and could be 50 times higher during flood season. Extreme hydrological events such as flooding should therefore be considered for the monitoring and assessment of these pollutants in rivers and estuaries. NP concentrations in both water and sediment in this study have exceeded the threshold limit for undesirable biological effects and further research is therefore warranted for their actual effects on local organisms.
References
Ahel, M., Molnar, E., Ibric, S., & Giger, W. (2000). Estrogenic metabolites of alkylphenol ethoxylates in secondary sewage effluents and rivers. Water Science and Technology, 42, 15–22.
Bettinetti, R., Cuccato, D., Galassi, S., & Provini, A. (2002). Toxicity of nonylphenol in spiked sediment to three populations of Chironomus riparius. Chemosphere, 46, 201–207.
Billinghurst, Z., Clare, A. S., Fileman, T., Mcevoy, J., & Readman, J. (1998). Inhibition of barnacle settlement by the environmental oestrogen nonylphenol and the natural oestrogen 17β-oestradiol. Marine Pollution Bulletin, 36, 833–839.
Blackburn, M. A., & Waldock, M. J. (1995). Concentrations of alkylphenols in rivers and estuaries in England and Wales. Water Research, 29, 1623–1629.
Cailleaud, K., Forget-Leray, J., Souissi, S., Lardy, S., Augagneur, S., & Budzinski, H. (2007). Seasonal variation of hydrophobic organic contaminant oncentrations in the water-column of the Seine Estuary and their transfer to a planktonic species Eurytemora affinis (Calanoïd, copepod). Part 2: alkylphenol-polyethoxylates. Chemosphere, 70, 281–287.
Carafa, R., Wollgast, J., Canuti, E., Ligthart, J., Dueri, S., Hanke, G., et al. (2007). Seasonal variations of selected herbicides and related metabolites in water, sediment, seaweed and clams in the Sacca di Goro coastal lagoon (Northern Adriatic). Chemosphere, 69, 1625–1637.
Chen, B., Duan, J. C., Mai, B. X., Luo, X. J., Yang, Q. S., Sheng, G. Y., et al. (2006). Distribution of alkylphenols in the Pearl River Delta and adjacent northern South China Sea, China. Chemosphere, 63, 652–661.
Duft, M., Schulte-Oehlmann, U., Weltje, L., Tillmann, M., & Oehlmann, J. (2003). Stimulated embryo production as a parameter of estrogenic exposure via sediments in the freshwater mudsnail Potamopyrgus antipodarum. Aquatic Toxicology, 64, 437–449.
Ferguson, P. L., Iden, C. R., & Brownwell, B. J. (2001). Distribution and fate of neutral alkylphenol ethoxylate metabolites in a sewage-impacted urban estuary. Environmental Science and Technology, 35, 2428–2435.
Fu, M., Li, Z., & Gao, H. (2007). Distribution characteristics of nonylphenol in Jiaozhou Bay of Qingdao and its adjacent rivers. Chemosphere, 69, 1009–1016.
Hong, S., Won, E. J., Ju, H. J., Kim, M. S., & Shin, K. H. (2010). Current nonylphenol pollution and the past 30 years record in an artificial Lake Shihwa, Korea. Marine Pollution Bulletin, 60, 303–313.
Isobe, T., Nishiyama, H., Nakashima, A., & Takada, H. (2001). Distribution and behavior of nonylphenol, octylphenol, and nonylphenol monoethoxylate in Tokyo metropolitan area: their association with aquatic particles and sedimentary distributions. Environmental Science and Technology, 35, 1041–1049.
Jeannot, R., Sabik, H., Sauvard, E., Dagnac, T., & Dohrendorf, K. (2002). Determination of endocrine-disrupting compounds in environmental samples using gas and liquid chromatography with mass spectrometry. Journal of Chromatography. A, 974, 143–159.
Jonkers, N., Laane, R., & de Voogt, P. (2003). Fate of nonylphenol ethoxylates and their metabolites in two Dutch estuaries: evidence of biodegradation in the fields. Environmental Science and Technology, 37, 321–327.
Jonkers, N., Laane, R., & de Voogt, P. (2005). Sources and fate of nonylphenol ethoxylates and their metabolites in the Dutch coastal zone of the North Sea. Marine Chemistry, 96, 115–135.
Kwack, S. J., Kwon, O. K., Hyung, S., Kim, S. S., Kim, S. H., Sohn, K. H., et al. (2002). Comparative evaluation of alkylphenolic compounds on estrogenic activity in vitro and in vivo. Journal of Toxicology and Environmental Health. Part A, 65, 419–431.
Li, D., Park, J. M., & Oh, J. R. (2001). Silyl derivatization of alkylphenols, chlorophenols and bisphenol A for simultaneous GC/MS determination. Analytical Chemistry, 73, 3089–3095.
Li, Z., Li, D., Oh, J. R., & Je, J. G. (2004). Seasonal and spatial distribution of nonylphenol in Shihwa Lake, Korea. Chemosphere, 56, 611–618.
Li, D., Dong, M., Shim, W. J., Hong, S. H., Oh, J.-R., Yim, U. H., et al. (2005). Seasonal and spatial distribution of nonylphenol and IBP in Saemangeum Bay, Korea. Marine Pollution Bulletin, 51, 966–974.
Li, X. L., Luan, T. G., Liang, Y., Wong, M. H., & Lan, C. Y. (2007). Distribution patterns of octylphenol and nonylphenol in the aquatic system at Mai Po Marshes Nature Reserve, a subtropical estuarine wetland in Hong Kong. Journal of Environmental Sciences, 19, 657–662.
Li, D., Dong, M. H., Shim, W. J., Yim, H. U., Hong, H. S., & Kannan, N. (2008). Distribution characteristics of nonylphenolic chemicals in Masan Bay environments, Korea. Chemosphere, 71, 1162–1172.
Micić, V., & Hofmann, T. (2009). Occurrence and behaviour of selected hydrophobic alkylphenolic compounds in the Danube River. Environmental Pollution, 157, 2759–2768.
Navarro, A., Tauler, R., Lacorte, S., & Barceló, D. (2010). Occurrence and transport of pesticides and alkylphenols in water samples along the Ebro River Basin. Journal of Hydrology, 383, 18–29.
Nice, H. E., Thorndyke, M. C., Morritt, D., Steele, S., & Crane, M. (2000). Development of Crassostrea gigas larvae is affected by nonylphenol. Marine Pollution Bulletin, 40, 491–496.
Peng, X. Z., Yu, Y. Y., Tan, C. M., Tan, J. H., Huang, Q. X., & Wang, Z. D. (2008). Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Science of the Total Environment, 397, 158–166.
Petrovic, M., Lacorte, S., Viana, P., & Barcelo, D. (2002). Pressurized liquid extraction followed by liquid chromatography-mass spectrometry for the determination of alkylphenolic compounds in river sediment. Journal of Chromatography. A, 959, 15–23.
Pojana, G., Gomiero, A., Jonkers, N., & Marcomini, A. (2007). Natural and synthetic endocrine disrupting compounds (EDCs) in water, sediment and biota of a coastal lagoon. Environment International, 33, 929–936.
Schwaiger, J., Mallow, U., Ferling, H., Knoerr, S., Braunbeck, T., Kalbfus, W., et al. (2002). How estrogenic is nonylphenol? A transgenerational study using rainbow trout (Oncorhynchus mykiss) as a test organism. Aquatic Toxicology, 59, 177–189.
Shao, B., Hu, J., & Yang, M. (2003). Nonylphenol ethoxylates and their biodegradation intermediates in water and sludge of a sewage treatment plant. Bulletin of Environmental Contamination and Toxicology, 70, 527–532.
Shen, G., Zhang, Z., Yu, G., Li, X., Hu, H., & Li, F. (2005). Dissolved neutral nonylphenol ethoxylates metabolites in the Haihe River and Bohai Bay, People's Republic of China. Bulletin of Environmental Contamination and Toxicology, 75, 827–834.
Soares, A., Guieysse, B., Jefferson, B., Cartmell, E., & Lester, J. N. (2008). Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environment International, 34, 1033–1049.
Soto, A. M., Justicia, H., Wray, J. W., & Sonnenschein, C. (1991). P-Nonylphenol: an estrogenic xenobiotic released from “modified” polystyrene. Environmental Health Perspectives, 92, 167–173.
Tubau, I., Vázquez-Suñé, E., Carrera, J., González, S., Petrovic, M., López de Alda, M. J., et al. (2010). Occurrence and fate of alkylphenol polyethoxylate degradation products and linear alkylbenzene sulfonate surfactants in urban ground water: Barcelona case study. Journal of Hydrology, 383, 102–110.
Vazquez-Duhalt, R., Marquez-Rocha, F., Ponce, E., Licea, A. F., & Viana, M. T. (2005). Nonylphenol, an integrated vision of a pollutant. Applied Ecology and Environmental Research, 4, 1–25.
Verderame, M., Prisco, M., Andreuccetti, P., Aniello, F., & Limatola, E. (2011). Experimentally nonylphenol-polluted diet induces the expression of silent genes VTG and ERα in the liver of male lizard Podarcis sicula. Environmental Pollution, 159, 1101–1107.
Wang, L., Ying, G. G., Zhao, J. L., Liu, S., Yang, B., Zhou, L. J., et al. (2011). Assessing estrogenic activity in surface water and sediment of the Liao River system in northeast China using combined chemical and biological tools. Environmental Pollution, 159, 148–156.
Wen, Q., Chen, X., Shi, Y., Ma, J., & Zhao, Q. (2011). Analysis on composition and pattern of agricultural nonpoint source pollution in Liaohe River Basin, China. Procedia Environmental Sciences, 8, 26–33.
White, R., Jobling, S., Hoare, S. A., Sumpter, J. P., & Parker, M. G. (1994). Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology, 135, 175–182.
Ying, G. G., Williams, B., & Kookana, R. (2002). Environmental fate of alkylphenols and alkylphenol ethoxylates—a review. Environmental International, 28, 1–12.
Yoshimura, K. (1986). Biodegradation and fish toxicity of nonionic surfactants. Journal of American Oil Chemical Society, 63, 1590–1599.
Zhang, X., Li, Q. Z., Li, G. X., Wang, Z. S., & Yan, C. Z. (2009). Levels of estrogenic compounds in Xiamen Bay sediment, China. Marine Pollution Bulletin, 58, 1210–1216.
Acknowledgments
This work was funded by the National Science and Technology Foundation (no. 2009ZX07528-006-03) and Ocean Public Service Foundation of China (no. 201005012-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Gibson, M., Liu, C. et al. Seasonal variation of nonylphenol concentrations and fluxes with influence of flooding in the Daliao River Estuary, China. Environ Monit Assess 185, 5221–5230 (2013). https://doi.org/10.1007/s10661-012-2938-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2938-9