Abstract
Diffuse sources of surface water pathogens and nutrients can be difficult to isolate in larger river basins. This study used a geographical or nested approach to isolate diffuse sources of Escherichia coli and other water quality constituents in a 145.7-km2 river basin in south central Texas, USA. Average numbers of E. coli ranged from 49 to 64,000 colony forming units (CFU) per 100 mL depending upon season and stream flow over the 1-year sampling period. Nitrate-N concentrations ranged from 48 to 14,041 μg L−1 and orthophosphate-P from 27 to 2,721 μg L−1. High concentrations of nitrate-N, dissolved organic nitrogen, and orthophosphate-P were observed downstream of waste water treatment plants but E. coli values were higher in a watershed draining an older part of the city. Total urban land use explained between 56 and 72 % of the variance in mean annual E. coli values (p < 0.05) in nine hydrologically disconnected creeks. Of the types of urban land use, commercial land use explained most of the variance in E. coli values in the fall and winter. Surface water sodium, alkalinity, and potassium concentrations in surface water were best described by the proportion of commercial land use in the watershed. Based on our nested approach in examining surface water, city officials are able to direct funding to specific areas of the basin in order to mitigate high surface water E. coli numbers and nutrient concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Point source discharges are often a relatively constant source of contamination, as the discharge permit holder typically operates at a steady capacity (Cotman et al. 2008). One major point source in urban watersheds is wastewater treatment plant effluent which has been shown to have a large impact on stream water quality (Petersen et al. 2006; Carey and Migliaccio 2009; Aitkenhead-Peterson et al. 2009, 2011). Yet, wastewater treatment is imperative for maintaining clean cities, preserving healthy surface waters and lowering the incidence of devastating water-borne diseases. Nonpoint or diffuse sources, on the other hand, contribute Escherichia coli, nutrients, and other chemical constituents to surface waters diffusely from a large area, often without immediate or obvious signs of contamination. Common nonpoint sources in urban and rural watersheds include landscape and lawn fertilizer, topsoil erosion, municipal tap water used for irrigation, domestic animal and wildlife waste, and runoff from impervious surfaces. These sources generally have a varying influence based on frequency and intensity of precipitation events (Cotman et al. 2008). Two notable exceptions to this are the impacts of irrigation water chemistry seen more often during dry weather and the contribution of septic systems, which yield a steady-state nutrient enrichment and also have more of an impact on water quality during low flow when dilution effects are minimal (Clinton and Vose 2006).
In 2002, only 19 % of rivers and streams in the USA had been evaluated for designated uses, and 39 % of this evaluated river mileage had bacterial impairments (Arnone and Walling 2007). In response, watershed managers with surface water fecal bacteria impairments sought methods for determining the source of bacterial impairment. A large bacterial load to a stream may come from a number of source vectors, including wildlife, birds, pets, livestock, or humans. Although bird and wildlife contributions cannot be readily changed and in many cases are encouraged in natural riparian areas (Tufford and Marshall 2002), planning, research, and management of the watershed can reduce contributions from human and domesticated animal waste through the implementation of best management practices (Dickerson et al. 2007).
E. coli are easily culturable, gram-negative coliform bacteria which grow abundantly in the lower intestines of warm-blooded animals and are expelled in feces (Winfield and Groisman 2003). The presence of fecal indicator bacteria in surface waters such as E. coli also means that there is a potential for pathogens as well. Besides pathogenic strains of E. coli (e.g., 0157:H7), other water-borne pathogenic bacteria include Salmonella spp., Shigella spp., Vibrio cholerae, and Legionella pneumophila (Arnone and Walling 2007). Reducing the amount of indicator bacteria reaching surface water by eliminating human sources will also reduce pathogens, and thus reduce the potential for water-borne diseases (Clinton and Vose 2006). Weather patterns also play a major role in fluxes of fecal bacteria. Muirhead et al. (2004) postulated that during dry periods, fecal bacteria in a watershed are stored on the land surface and in the bottom sediments of the stream channel. These two pools of fecal bacteria then are likely to become sources during a rain event, causing a dramatic increase in surface water E. coli numbers at high flow. During this “first flush,” colony forming units (CFU) per 100 mL of E. coli increased by 2 orders of magnitude compared to base flow values (Muirhead et al. 2004). The objective of this study was to examine the creek chemistry and E. coli in a large subtropical basin in south central Texas with the view of identifying nonpoint source areas of E. coli and other water quality constituents.
Materials and methods
Site description
The Carters Creek basin, situated in the Coastal Plains ecoregion, is a tributary of the Navasota River situated in Brazos County, Texas, USA. The Carters Creek basin is dominated by the twin cities of Bryan and College Station with a combined metropolitan population of 190,000. Average annual temperature is 20 °C and annual precipitation averages 1,000 mm. The underlying parent material is predominantly residuum from sandstone and shale or loamy/clayey alluvium over the Yegua geologic formation from the Eocene age. Soils are mainly alfisols underlain with marine clays (Aitkenhead-Peterson et al. 2011).
Carters Creek has been identified by the Texas Commission on Environmental Quality as impaired for bacteria since 1999; in addition, Burton Creek, a tributary of Carters Creek, has been identified as impaired for bacteria since 2006. Both Carters Creek and Burton Creek were also listed as concerns for water use attainment and screening levels in June 2007 for nitrate and orthophosphate.
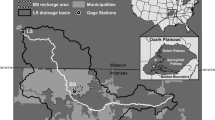
Fifteen watersheds within Carters Creek basin drain land with uses varying from agriculture and rangeland to developed urban and residential areas (Fig. 1; Table 1). A nested watershed design was used for this study to better monitor and isolate those watersheds responsible for high E. coli and nutrient concentrations in the Carters Creek basin.
Spatial distribution of our nested and independent study watersheds. All circles represent complete group of 15 nested watersheds. Black circles represent the nine hydrologically disconnected watersheds used for regression and correlation analysis and gray circles represent the watersheds sampled downstream of waste water treatment plants
Geographical analysis
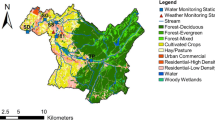
To examine the effect of land use on E. coli and nutrient concentrations, we examined land use within nine of the 15 watersheds to produce a set of independent, hydrologically disconnected watersheds (headwaters) (Fig. 1). Land use was estimated using geographical information systems in ArcView 9.3 (ESRI Inc., Redlands, CA). To determine the polygon shapes of the watersheds, a Stratmap DEM was overlaid with the National Hydrography Dataset or NHD to find the ridges of the watershed boundaries. Zoning data from 2008 were obtained from the cities of Bryan and College Station to provide the most recent land use data. For the land area outside of Bryan or College Station city limits, the USGS National Land Cover Dataset for 2001 was used. This land cover data set was clipped with the watershed polygons upstream of each sampling site, and the land use percentages for each watershed were calculated.
Sample collection and analysis
Grab samples were collected from the 15 creeks mid-channel and mid-depth using a sterile 500-mL whirl-pak bag every 2 weeks between July 2007 and June 2008. Samples were taken from bridges on the upstream side to aid ease of collection. Electrical conductivity (EC) and pH were quantified on unfiltered aliquots and the samples were transported on ice for analysis of E. coli and chemical constituents within 6 h of collection.
Determination of E. coli
Aliquots of creek samples were filtered through a sterile 0.45-μm Millipore filters and incubated on modified mTEC agar for 2 h at 35 °C and 22–24 h at 44.5 °C according to EPA Method 1603 (USEPA 2002).
Water chemistry analysis
Aliquots of creek samples were syringe filtered through ashed (400 °C for 5 h) Whatman GF/F filters (0.7 μm nominal pore size). Further aliquots were filtered through 0.2 μm Pall filters in readiness for cation and anion analysis. Samples were either analyzed on the day of collection or frozen in acid-washed high-density polyethylene bottles. Dissolved organic carbon (DOC) and total dissolved nitrogen (TDN) were measured using high-temperature Pt-catalyzed combustion with a Shimadzu TOC-VCSH and Shimadzu total measuring unit TNM-1 (Shimadzu Corp. Houston, TX, USA). Dissolved organic carbon was measured as non-purgeable carbon using USEPA method 415.1 which entails acidifying the sample and sparging for 4 min with C-free air. Ammonium was analyzed using the phenate hypochlorite method with sodium nitroprusside enhancement (USEPA method 350.1) and nitrate was analyzed using Cd-Cu reduction (USEPA method 353.3). Alkalinity was quantified using methyl orange (USEPA method 310.2) and was assumed to be in the form of bicarbonate. All colorimetric methods were performed with a Smartchem Discrete Analyzer (Westco Scientific Instruments Inc. Brookfield, CT, USA). Dissolved organic nitrogen (DON) is the difference of TDN − (NH4-N + NO3-N). Calcium, magnesium, potassium, and sodium were quantified by ion chromatography using an Ionpac CS16 analytical and Ionpac CG16 guard column for separation and 20 mM methanesulfonic acid as eluent at a flow rate of 1 mL min−1 and injection volume of 10 μL using a Dionex ICS 1000 (Dionex Corp. Sunnyvale, CA, USA). A Dionex ICS 2000 was used to quantify F−, Cl−, Br−, NO −2 , SO 2−4 , and PO 3−4 . The anions were separated using an Ionpak AS20 analytical and Ionpak AG20 guard columns, with 35 mM KOH as eluent, a flow rate of 1 mL min−1, and an injection volume of 25 μL.
Check standards and NIST traceable standards were run every 12th sample to test for instrument precision. The coefficient of variance between replicates was typically less than 2 % for colorimetric analysis and ion chromatography and less than 5 % for DOC and TDN. The sodium adsorption ratio (SAR) of each creek was calculated in units of milliequivalents per liter (Eq. 1)
SAR takes into account not only the concentration of dispersing sodium-derived positive charge but also the opposing flocculent effect of the divalent cation charge.
Statistical analysis
Data were checked for normality and transformed prior to statistical analysis if necessary. Mean values and standard deviation for high flow and low flow for each season (summer, June–August; fall, September–November; winter, December–February; and spring, March–May) were calculated for E. coli, nitrate-N, and orthophosphate-P. The mean annual concentrations and standard deviation for high flow and low flow were calculated for pH, electrical conductivity, DOC, DON, ammonium-N, cations, and anions. A two-tailed two sample t test assuming equal variance was used to determine if high or low flow produced significantly higher E. coli or creek chemical constituents during each season or over the annual sampling period. To examine relationships between surface water E. coli and watershed land use, we used linear regression analysis on an independent set of nine watersheds that were not hydrologically connected. We also used the independent set of watersheds for Pearson bivariate correlation analysis to examine correlations between surface water chemistry and surface water chemistry and land use as an aid to determine likely sources to surface waters. SPSS v.16 was used for statistical analysis.
Results
E. coli
Colony forming units of E. coli tended to be higher in creeks during high flow and during the spring and summer seasons (Table 2). Only one high flow sample was taken during the summer season so we were unable to run statistical analysis of significant differences between low and high flow. Nevertheless, summer high flow E. coli values were an order of magnitude higher during high flow relative to low flow (Table 2). During the fall, Bee, Burton 3 and 5, Carter 2 and 3, and Wolfpen had significantly higher E. coli during high flow (Table 2). In the winter, Burton 1 and 4, Carter 1, Hudson, and Wolfpen had significantly higher E. coli values during high flow (Table 2). In the spring, all creeks had significantly higher E. coli values during high flow with the exception of Briar 1, Burton 3 and 5, and Carter 4 and 5 (Table 2). No single creek had consistently highest or lowest E. coli values through seasons and flows. The Burton creeks tended to have higher E. coli values during summer and fall low flows and during the summer, fall, and winter high flows (Table 2). During low flow in the fall, winter, and spring, Burton 1 had the lowest E. coli values illustrating a dilution effect as the surface water traveled from the headwaters of Burton 3, 4, and 5 to the confluence at Burton 1 prior to joining the main stem of Carters Creek.
Because of the potential fecal source of E. coli in our creeks, we also examined nitrate-N and orthophosphate-P concentrations by season and flow. Nitrate-N concentrations were higher at the creeks sampled downstream of a wastewater treatment plant (WWTP) during all seasons and flow (Table 3). No one creek had consistently lower nitrate-N concentrations, although Carter 3, the most rural of the creeks, had lower nitrate-N concentrations in the summer and spring during low flow and during the winter and spring during high flow. There were some significant differences in nitrate-N concentration between low and high flow. During the fall, Bee and Burton 1–4 all had significantly higher nitrate concentrations during high flow (Table 3). During the winter, Carter 5 and Burton 3 had higher nitrate-N concentrations during high flow relative to low flow, and during the spring, Bee, Briar 2, Burton 1, 2, and 4, and Carter 2 and 3 had higher nitrate-N concentrations at high flow relative to low flow (Table 3).
Orthophosphate-P concentrations were also higher in those creeks sampled downstream of a WWTP during all seasons and flows except high flow in winter when Wolfpen had the highest concentration of orthophosphate-P and during high flow and in the summer when Burton 4 had the highest concentration of orthophosphate-P. The headwater creeks of the Carters Creek basins (Carter 2 and 3) tended to have lower orthophosphate-P concentrations over all seasons and flow (Table 4). There were significantly higher orthophosphate-P concentrations in some of the creeks during high flow relative to low flow and in some of the creeks significantly higher orthophosphate-P concentrations at low flow relative to high flow (Table 3). Orthophosphate-P concentrations were significantly higher during high flow in Burton 1, Burton 3, and Carter 3 during the spring, winter, and fall, respectively. Carter 5 had significantly higher orthophosphate-P concentrations during low flow in the winter and Wolfpen significantly higher concentrations during low flow in the spring (Table 3).
There was no statistically significant seasonal trend in creek orthophosphate concentrations during either low or high flow. Some creeks had lower orthophosphate concentrations in the fall and others in the winter during low flow but during high flow a similar pattern was not observed.
pH and electrical conductivity
There was no significant difference in creek pH between high and low flow (Table 5). During low flow pH ranged from 7.3 to 8.9 and during high flow it ranged from 7.4 to 8.3. Conductivity ranged from 292 to 1,198 μS cm−1 during low flow conditions and from 218 to 721 μS cm−1 during high flow conditions. There were some significant differences in conductivity between low and high flow in some of the creeks with low flow conductivity being significantly higher than high flow conductivity (Table 5).
Dissolved organic carbon and organic nitrogen
Dissolved organic carbon ranged from 26.0 to 58.9 mg L−1 during low flow and from 22.0 to 49.0 mg L−1 during high flow. There was no significant difference in DOC concentrations between high and low flow in any of the study creeks. Dissolved organic nitrogen ranged from 0.58 to 3.29 mg L−1 during low flow and from 0.76 to 1.45 mg L−1 during high flow. Similar to DOC, there was no significant difference in DON concentrations between low and high flow in any of the creeks (Table 5).
Ammonium-N
Ammonium-N ranged from 0.05 to 0.15 mg L−1 during low flow and from 0.03 to 0.17 mg L−1 during high flow. We found no significant difference in concentrations between low and high flow in any of the creeks.
Base cations
Sodium was the dominant base cation in all creeks. There was no significant difference between low and high flow concentrations of base cations in any of the creeks sampled (Table 6). Sodium concentrations ranged from 36 to 232 mg L−1 during low flow and from 28 to 139 mg L−1 during high flow with the highest concentrations during both flows observed at Wolfpen creek and the two sites downstream of wastewater treatment plants. Potassium concentrations ranged from 3.7 to 7.0 mg L−1 during low flow and from 3.3 to 5.9 mg L−1 during high flow with the highest concentrations observed in those creeks sampled downstream of a wastewater treatment plant. Magnesium concentrations ranged from 1.6 to 5.7 mg L−1 during low flow and from 1.6 to 3.6 mg L−1 during high flow. The highest magnesium concentrations were observed at Bee Creek during both high and low flows. Calcium concentrations ranged from 9.4 to 20.4 mg L−1 during low flow and from 10.2 to 15.6 mg L−1 during high flow with the highest concentration of calcium observed in Bee Creek during low flow and Burton 3 during high flow.
Sodium adsorption ratio was calculated for the creeks for high and low flow and tended to be higher during low flow relative to high flow though not significantly higher in any of the creeks between the two flows. SAR values ranged from 3.2 to 23.4 during low flow and from 2.5 to 14.7 during high flow. Highest SAR values were observed in Wolfpen Creek and the two creeks sampled downstream of the waste water treatment plants.
Anions
Bicarbonate was the dominant anion in all creeks and it was not significantly different between flows (Table 7). Bicarbonate concentrations ranged from 80 to 347 mg L−1 at low flow and 76 to 213 mg L−1 during high flow. Bicarbonate concentrations were highest at Wolfpen Creek during both low flow and high flow. Sulfate ranged from 7.9 to 88.6 mg L−1 during low flow and from 8.6 to 70.0 mg L−1 during high flow. The highest concentrations were observed at Bee Creek during both low and high flows. There was no significant difference in sulfate concentrations with flow at individual creeks except for Briar 1, Burton 1, and Burton 5 where concentrations were significantly higher during low flow (Table 7). Concentrations were also noticeably higher at most of the other creeks during low flow (Table 7). Chloride concentrations ranged from 17.4 to 86.6 mg L−1 during low flow and from 18.5 to 48.0 mg L−1 during high flow. There was no significant difference between high and low flow concentrations at any creek although concentrations tended to be higher during low flow (Table 7). Fluoride concentrations ranged from 0.13 to 0.92 mg L−1 during low flow and from 0.23 to 0.67 mg L−1 during high flow. Because of the high amount of variability, there was no significant difference in fluoride concentrations between high and low flow for any creek (Table 7). However, there was a pattern of higher concentrations of fluoride during high flow for many of the creeks without a WWTP and higher concentration during low flow for creeks with a WWTP (Table 7). Bromide concentrations were highest at Wolfpen Creek during both high and low flows. Bromide concentrations tended to be higher during low flow but this was only significant at Burton 5 (Table 7). Bromide concentrations ranged from 0.07 to 0.31 mg L−1 at low flow and from 0.05 to 0.57 mg L−1 at high flow.
Correlations between E. coli and other water quality constituents and land use
Between 56 and 72 % of the variance in E. coli during high flow was explained by urban land use during summer, spring, fall, and winter seasons (Fig. 2). While no one urban land use was able to significantly describe the variance in high flow E. coli during each season, urban residential land use explained the most variance in the summer and fall high flow E. coli values and urban commercial land use explained most of the variance in high flow E. coli values during the winter and spring high flow. There were no significant relationships observed between E. coli and land use during low flow.
Urban commercial land use was positively and significantly correlated with concentrations of bicarbonate and sodium and the creeks SAR value during low flow but not during high flow (Table 8). Creek water magnesium and calcium concentrations were significantly correlated with shrub and scrub land use during both low and high flow. Potassium was significantly and positively correlated with transport during low flow but with shrub and scrub land use during high flow (Table 8).
Examination of correlations among creek water quality constituents may give a better explanation of the mechanisms occurring in our watersheds. Dissolved organic carbon was significantly and positively correlated with creek SAR, bicarbonate concentrations, and pH and EC during both high and low flow (Table 9). Dissolved organic nitrogen was positively and significantly correlated with DOC and SAR but was not correlated with pH and EC during both low and high flow conditions. Most of the anions with the exception of sulfate were significantly and positively correlated with each other during high flow (Table 9). E. coli was not correlated with any water quality constituent (Table 9). However, there were positive correlations between E. coli and NH4-N (R = 0.54; p > 0.05), a positive correlation with pH (R = 0.64; p > 0.05), and a negative correlation with potassium (R = −0.65; p > 0.05) during high flow but not under low flow conditions.
Discussion
This study investigated the geographical patterns of bacteria and nutrients using a nested watershed approach. “Targeted sampling” is a method developed by Kuntz et al. (2003), who used repeated, geographically narrowing sample collection and visual observation to pinpoint the source of fecal contamination. The nested sampling strategy of our study had a similar aim, which was to determine the contributing area for bacteria and nutrient impairment of the Carters Creek basin. Although no nutrient in a river basin has only one source, the pattern of their concentrations and land uses in the various watersheds in the basin can help pinpoint major contributors as a first step to mitigation. Using the nested design, we were able to pinpoint the major watersheds contributing to E. coli; during the summer and fall, Burton 4 had significantly higher values than the other headwater creeks within the Burton Creek group and numbers remained elevated at Burton 2 but were diluted prior to the confluence of Burton 2 and 3 to form Burton 1.
The state water geometric mean criterion for E. coli is 126 CFU/100 mL for primary contact recreation with a single sample criterion of 399 CFU/100 mL. For secondary contact recreation 1, the geometric mean criterion is 630 CFU/100 mL, and for secondary contact recreation 2 the geometric mean criterion is 1,030 CFU/100 mL. For non-contact recreation, the geometric mean criterion is 2,060 CFU/100 mL (EPA 2010). The mean seasonal E. coli counts in all our watersheds during high flow were higher than the secondary contact recreation standard of 630 CFU/100 mL and some sites exceeded this threshold during low flow as well. In a similar study conducted in southern California, both non-urban creek and urban creek headwaters exceeded contact recreation standards for bacteria and were within an order of magnitude of each other and the watershed outlets, indicating a diffuse, widespread source for bacteria in both sub-catchments (Schiff and Kinney 2001). Tufford and Marshall (2002) found that commercial and urban open land uses contributed more heavily to bacterial loads because of increased impervious surface, compacted soil such as athletic fields, higher runoff, and a tendency to attract urban birds and rodents. We also observed that commercial land use explained more of the variance in E. coli especially during the winter and spring season during high flow whereas residential land use explained a greater portion of the variance in E. coli during the summer season during high flow. In a runoff experiment, Muirhead et al. (2006) found that tap water accumulated 26,000 MPN/100 mL E. coli as it traveled the length of a 5-m grass plot containing fresh cowpats. In an urban setting, it is highly likely that fecal matter derived from birds, typically grackles in commercial lots in Texas, and dogs in residential lots may be the greater contributors to surface water E. coli. Grackles tend to roost in ornamental trees in commercial parking lots over night and the buildup of fecal matter beneath the trees can be considerable. Homeowners are less likely to pick up after their pets during the summer because the summer temperatures tend to decompose fecal matter within a few days and regular irrigation of residential lots ensures that the decomposed fecal matter is removed. In another recent study in Texas, Harmel et al. (2010) reported mean E. coli concentrations of 1,159 CFU/100 mL for a relatively unimpacted rural stream and 1,473 CFU/100 mL for a rural stream with dairy and WWTP inputs. These high E. coli numbers from other studies and our own research put into question the feasibility of obtaining current water quality standards. For example, even if all the sections of the Carters Creek basin were to be reclassified from secondary contact recreation to no recreation, the state requirement of <2,060 CFU/100 mL would still render the majority of our creeks impaired during high flow.
Apart from the two wastewater treatment plants which increased concentrations of nitrate-N and orthophosphate in the Carters Creek basin, the nested design also helped to identify which watersheds in the basin were contributors to surface water quality for high nutrients. While none of the watersheds contributed significantly to high nitrate-N concentrations, Carter 2, Hudson, and Wolfpen watersheds all contributed significantly higher orthophosphate. Hudson and Wolfpen both have large golf courses in their headwaters and Carter 2 drains a commercial area comprised of parking lots and landscape plantings.
Stream chemistry and land use
One of the dominant features in the urban watersheds of Carters Creek basin is the ubiquitous elevated concentration of DOC (Aitkenhead-Peterson et al. 2009; Steele and Aitkenhead-Peterson 2012a). While no land use was significantly related to high DOC concentrations in our watersheds, there was a relatively high but nonsignificant correlation between DOC and commercial land use during both low and high flows (low flow R = 0.59, p = 0.09 and high flow R = 0.60, p = 0.09). Commercial land use was also significantly correlated to stream SAR during low flow (R = 0.73, p < 0.05). These results differ somewhat compared to the findings of Aitkenhead-Peterson et al. (2009) who reported that irrigation of urban open areas was likely responsible for increasing DOC concentrations in surface waters. The difference between the two studies is the range of watersheds sampled and the method of classifying watershed land use. Aitkenhead-Peterson et al. (2009) examined 12 independent watersheds along a rural to urban gradient and included some of the watersheds used in our study, namely Hudson, Wolfpen, Carter 4, and Bee, but the land use classes for each watershed were derived from the 2000 USGS LULC database. In our study, land use classes were derived from the city’s zoning maps in which commercial land use included urban open area land use such as golf courses and athletic parks. What was evident in this study was that residential land use did not contribute to excess irrigation and hence increased low flow surface water DOC and SAR as much as commercial land use. The importance of increasing sodium and SAR in surface waters cannot be ignored. A recent study found that as SAR increased from 2 to 30 the percentage of carbon leached from St Augustine grass more than doubled (Steele and Aitkenhead-Peterson 2012a). Transport of senescent vegetation to surface waters is a natural phenomenon but the higher release of DOC to surface waters with high SAR values may compromise treatment at facilities downstream which extract water for potable use. It is well documented that DOC is a precursor for trihalomethanes production when water is chlorinated (e.g., Peterson et al. 1993).
There are several mechanisms that might explain the effect that high pH sodium bicarbonate irrigation water may have on DOC. The significant positive correlation between DOC and pH may infer solubilization of humic acids with high pH, but Kipton et al. (1992) suggested that pH needs to be at least 8.0 to solubilize a representative fraction of humic acid. The mean annual pH values for low and high flow ranged from 7.4 to 8.9 with higher pH evident during dry flow when irrigation water likely represented much of the stream flow. The mean annual surface water SAR, bicarbonate, and sodium were strongly and significantly correlated with all anions except sulfate suggesting that (a) there may be some anion competition for positively charged watershed soil minerals and (b) high sodium may have induced clay and aggregate dispersion in watershed soils which caused soil erosion including the loss of adsorped anions to surface waters.
Unlike dissolved organic carbon and the majority of anions, nitrate-N appeared to act independently of the high sodium concentrations and SAR. Nitrate-N concentrations according to Stackelberg et al. (1997) were 0.07 mg L−1 in undeveloped areas, 2.6 mg L−1 for new urban areas, 3.5 mg L−1 for old urban areas, and 13 mg L−1 for agricultural land use. Several watersheds in the Carters Creek basin had comparable values; nitrate-N concentrations between 0.07 mg L−1 NO3-N at Burton 2 and 0.23 mg L−1 NO3-N at Bee Creek is indicative of healthy concentrations of nitrate. Wolfpen Creek, our most urbanized creek, had nitrate-N concentrations ranging from 0.27 to 0.47 mg L−1, much lower than concentrations reported by Stackelberg et al. (1997). Our creeks sampled downstream of waste water treatment plants had higher concentrations of nitrate-N than reported for old urban areas and sometimes they were higher than the 13 mg L−1 reported for agricultural land use. Slow-flowing waters with high temperatures draining golf courses and neighborhood parks, such as Wolfpen, Hudson, Bee, and Briar creeks in our study, are likely to have significant denitrification potential, thus moderating the effect of urbanization on surface water nitrate-N concentrations (Schaffner et al. 2009). In addition to nitrate, Carter 4 and 5 sites also had the largest orthophosphate concentrations in the Carters Creek basin. The sites with the next highest orthophosphate concentrations were those with headwaters in golf courses. According to King et al. (2007), a golf course in Austin, Texas produced 0.51 kg PO4-P ha−1 year−1 or the equivalent of 6.2 % applied P. The phosphate contribution from point source effluent posed a threat to aquatic habitat according to the USEPA standard of 0.1 mg L−1 (King et al. 2007), and it is likely that the aquatic habitats in the Carters Creek basin are also threatened by the high orthophosphate-P inputs observed. Our study also showed a significant orthophosphate-P contribution from some watersheds with golf courses. Surface water orthophosphate-P also revealed a relationship with surface water SAR in this study. As sodium is adsorbed onto soil cation exchange sites under high pH conditions and it replaces divalent and trivalent cations, the increasing negative charge of the soil particle repels any nearby soluble orthophosphate, which will render it more transportable (Naidu and Rengasamy 1993). Curtin et al. (1995) reported that a SAR of 20 significantly decreased the binding ability of clay minerals and greatly increased the water-extractable fraction of total phosphorus. Steele and Aitkenhead-Peterson (2012b) suggested that irrigation water with a SAR of less than 5 would mitigate losses of DOC and orthophosphate based on a study of irrigation water and water-extractable soil DOC and orthophosphate under turfgrass across 26 cities in the state of Texas. This would suggest in context of our study that all but the most rural watersheds or those least likely to have in ground irrigation systems would show significantly lower concentrations of DOC and orthophosphate in surface waters.
Wastewater effluent adds significant amounts of N and P that can have a severe impact on streams (Lewis et al. 2007; Phillips et al. 2007; Fitzpatrick et al. 2007; Zampella et al. 2007). One of the significant sources for increases in both cations and anions within the urban area of Bryan/College Station was WWTP effluent discharge to the creeks. Wastewater effluent can provide the hydrologic benefit of stable flow even during periods of drought, when the creek might otherwise dry up or be reduced to a mere trickle (Cotman et al. 2008). For the creeks sampled downstream of a wastewater treatment plant in this study, the effluent-dominated creeks were enriched with calcium and magnesium but not enough to counterbalance the highly dispersive characteristics of sodium. Wastewater effluent was not found to significantly contribute to either high E. coli values or DOC concentrations in the Carters Creek basin. Several electrolytes, namely sodium, potassium, and chloride, were found by Rose (2007) to be higher in municipal wastewater effluent than in other urban streams. These electrolytes are typically added to effluent during the treatment process prior to permitted discharge; however, in the Carters Creek basin, potable water is also high in sodium and chloride and as this potable water is used for landscape irrigation which runs off to surface waters, thus observation of significantly higher sodium and chloride in those creeks downstream of a waste water treatment plant was not possible.
There was a stark contrast between the ion concentrations found in Georgia (Rose 2007) and South Carolina (Lewis et al. 2007) and those found in the Carter Creek basin. Burton 1 contained at least triple the concentrations of chloride, sulfate, and sodium as those observed in southeastern USA. Whether these differences were because of the ion-rich irrigation water signature or the geologic input of inorganic solutes during base flow (Aitkenhead-Peterson et al. 2011) is unknown. Most of our creeks had lower chloride concentrations than those reported in Duchess County, New York, but this is best explained by the frequent use of road salt (NaCl or CaCl2) during the winter in northern climates, which will maintain high chloride concentrations into the summer (Cunningham et al. 2009).
In commercial areas where impervious surfaces are prevalent, storm runoff from urban streets often flushes bromide-containing gasoline residues into surface waters, increasing bromide concentrations and reducing the Cl/Br ratio (Davis et al. 1998). Other sources of bromide may include private pool maintenance chemicals, rainwater, and irrigation water residues (Aitkenhead-Peterson et al. 2011). In addition to having high concentrations of sodium and chloride, Wolfpen also had the highest bromide concentrations. Surprisingly, no significant correlation was found between chloride and any urban land use, in contrast to other studies (Cunningham et al. 2009; Zampella et al. 2007; Rose 2007).
Conclusion
This study examined diffuse sources of E. coli and other water quality constituents in a river basin in south central Texas. The sources of E. coli were likely the result of urban wildlife such as birds and skunks and domestic pets such as cats and dogs. Further work has been initiated using bacterial source tracking in this basin with a focus on Burton Creeks. Some mitigation methods might include public education on pet feces in residential areas.
The major source of nutrients nitrogen and orthophosphate was wastewater treatment effluent discharge. Minor diffuse sources of orthophosphate were observed from some watersheds containing golf courses, but not all watersheds containing golf courses had high orthophosphate-P concentrations. Irrigation with municipal tap water high in pH and sodium likely has an effect on watershed soil anion adsorption resulting in diffuse release of anions during high flow conditions.
Water quality can influence human and environmental health, thus monitoring and assessing sources of diffuse pollutants to surface waters can aid in the recognition of where problems might be occurring in our watersheds so that steps to ameliorate water quality issues can be initiated.
References
Aitkenhead-Peterson, J. A., Steele, M. K., Nahar, N., & Santhy, K. (2009). Dissolved organic carbon and nitrogen in urban and rural watersheds of south central Texas: land use and land management influences. Biogeochemistry, 96, 119–129.
Aitkenhead-Peterson, J. A., Nahar, N., Harclerode, C. L., & Stanley, N. (2011). Effect of urbanization on surface water chemistry in south-central Texas. Urban Ecosystems. doi:10.1007/s11252-010-0147-2.
Arnone, R. D., & Walling, J. P. (2007). Waterborne pathogens in urban watersheds. Journal of Water and Health, 5, 149–162.
Carey, R. O., & Migliaccio, K. W. (2009). Contribution of wastewater treatment plant effluents to nutrient dynamics in aquatic systems: a review. Environmental Management, 44, 205–217.
Clinton, B. D., & Vose, J. M. (2006). Variation in stream water quality in an urban headwater stream in the southern Appalachians. Water, Air, and Soil Pollution, 169, 331–353.
Cotman, M., Droic, A., & Zgajnar-Gotvajn, A. (2008). Assessment of pollution loads from point and diffuse sources in a small river basin: case study Ljubljanica River. Environmental Forensics, 9, 246–251.
Cunningham, M. A., O’Reilly, C. M., Menking, K. M., Gillikin, D. P., Smith, K. C., Foley, C. M., Belli, S. L., Pregnall, A. M., Schlessman, M. A., & Batur, P. (2009). The suburban stream syndrome: evaluating land use and stream impairments in the suburbs. Physical Geography, 30, 269–284.
Curtin, D., Steppuhn, H., Mermut, A. R., & Selles, F. (1995). Sodicity in irrigated soils in Saskatchewan—chemistry and structural stability. Canadian Journal of Soil Science, 75, 177–185.
Davis, S. N., Whittemore, D. O., & Fabryka-Martin, J. (1998). Uses of chloride/bromide ratios in studies of potable water. Ground Water, 36, 338–350.
Dickerson, J. W., Hayedorn, C., & Hassall, A. (2007). Detection and remediation of human-origin pollution at two public beaches in Virginia using multiple source tracking methods. Water Research, 41, 3758–3770.
Fitzpatrick, M. L., Long, D. T., & Pijanowski, B. C. (2007). Exploring the effects of urban and agricultural land use on surface water chemistry, across a regional watershed, using multivariate statistics. Applied Geochemistry, 22, 1825–1840.
Harmel, R. D., Karthikeyan, R., Gentry, T., & Srinivasan, R. (2010). Effects of agricultural management, land use, and watershed scale on E. coli concentrations in runoff and streamflow. Transactions of the ASABE, 53(6), 1833–1841.
King, K. W., Balogh, J. C., Hughes, K. L., & Harmel, R. D. (2007). Nutrient load generated by storm event runoff from a golf course watershed. Journal of Environmental Quality, 36, 1021–1030.
Kipton, H., Powell, J., & Town, R. M. (1992). Solubility and fractionation of humic acid: effect of pH and ionic medium. Analytica Chimica Acta, 267, 47–54.
Kuntz, R. L., Hartel, P. G., Godfrey, D. G., McDonald, J. L., Gates, K. W., & Segars, W. I. (2003). Targeted sampling protocol as prelude to bacterial source tracking with Enterococcus faecalis. Journal of Environmental Quality, 32, 2311–2318.
Lewis, G. P., Mitchell, J. D., Andersen, C. B., Haney, D. C., Liao, M. K., & Sargent, K. A. (2007). Urban influences on stream chemistry and biology in the Big Brushy Creek watershed, South Carolina. Water, Air, and Soil Pollution, 182, 303–323.
Muirhead, R. W., Davies-Colley, R. J., Donnison, A. M., & Nagels, J. W. (2004). Faecal bacteria yields in artificial flood events: quantifying in-stream stores. Water Research, 38, 1215–1224.
Muirhead, R. W., Collins, R. P., & Bremer, P. J. (2006). Numbers and transported state of Escherichia coli in runoff direct from fresh cowpats under simulated rainfall. Letters in Applied Microbiology, 42, 83–87.
Naidu, R., & Rengasamy, P. (1993). Ion interactions and constraints to plant nutrition in Australian sodic soils. Australian Journal of Soil Research, 31, 801–819.
Petersen, T. M., Suarez, M. P., Rifai, H. S., Jensen, P., Su, Y. C., & Stein, R. (2006). Status and trends of fecal indicator bacteria in two urban watersheds. Water Environment Research, 78, 2340–2355.
Peterson, H. G., Milos, J. P., Spink, D. R., Hrudey, S. E., & Sketchell, J. (1993). Trihalomenthanes in finished drinking water in relation to dissolved organic carbon and treatment process for Alberta surface waters. Environmental Technology, 14, 877–884.
Phillips, P., Russell, F. A., & Turner, J. (2007). Effect of non-point source runoff and urban sewage on Yaque del Norte River in Dominican Republic. International Journal of Environment and Pollution, 31, 244–266.
Rose, S. (2007). The effects of urbanization on the hydrochemistry of base flow within the Chattahoochee River Basin (Georgia, USA). Journal of Hydrology, 341, 42–54.
Schaffner, M., Bader, H. P., & Scheidegger, R. (2009). Modeling the contribution of point sources and non-point sources to Thachin River water pollution. Science of the Total Environment, 407, 4902–4915.
Schiff, K., & Kinney, P. (2001). Tracking sources of bacterial contamination in stormwater discharges to Mission Bay, California. Water Environment Research, 73, 534–542.
Stackelberg, P.E., Hopple, J.A., & Kauffman, L.J. (1997). Occurrence of nitrate, pesticides, and volatile organic compounds in the Kirkwood-Cohansey Aquifer system, southern New Jersey. Water Resources Investigations Report 97–4241. U.S. Geological Survey.
Steele, M. K., & Aitkenhead-Peterson, J. A. (2012a). Salt impacts on organic carbon and nitrogen leaching from senesced vegetation. Biogeochemistry. doi:10.1007/s10533-012-9722-3.
Steele, M. K., & Aitkenhead-Peterson, J. A. (2012b). Urban soils of Texas: relating irrigation sodicity to water-extractable carbon and nutrients. Soil Science Society of America Journal. doi:10.2136/sssaj2011.0274.
Tufford, D. L., & Marshall, W. D. (2002). Fecal coliform source assessment in a small, mixed land use watershed. Journal of the American Water Resources Association, 38, 1625–1635.
USEPA. (2002). Method 1603: Escherichia coli (E. coli) in water by membrane filtration using modified membrane-thermotolerant Escherichia coli agar (Modified mTEC). Washington DC: Office of Water, United States Environmental Protection Agency
Winfield, M. D., & Groisman, E. A. (2003). Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Applied and Environmental Microbiology, 69, 3687–3694.
Zampella, R. A., Procopio, N. A., Lathrop, R. G., & Dow, C. L. (2007). Relationship of land-use/land-cover patterns and surface-water quality in the Mullica River basin. Journal of the American Water Resources Association, 43, 594–604.
Acknowledgments
This study is a publication of Texas AgriLife Research Hatch Project TEX09194. Cara Harclerode was supported by an IMC Fertilizer Fellowship and a Texas Water Resource Institute Mills Fellowship to complete this research. We thank Nurun Nahar for help with laboratory analysis. Sincere thanks to the anonymous reviewers whose suggestions significantly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harclerode, C.L., Gentry, T.J. & Aitkenhead-Peterson, J.A. A geographical approach to tracking Escherichia coli and other water quality constituents in a Texas coastal plains watershed. Environ Monit Assess 185, 4659–4678 (2013). https://doi.org/10.1007/s10661-012-2895-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2895-3