Abstract
Sewage sludge may be used as an agricultural fertilizer, but the practice has been criticized because sludge may contain trace elements and pathogens. The aim of this study was to compare the effectiveness of total and pseudototal extractants of Cu, Fe, Mn, and Zn, and to compare the results with the bioavailable concentrations of these elements to maize and sugarcane in a soil that was amended with sewage sludge for 13 consecutive years and in a separate soil that was amended a single time with sewage sludge and composted sewage sludge. The 13-year amendment experiment involved 3 rates of sludge (5, 10, and 20 t ha−1). The one-time amendment experiment involved treatments reflecting 50, 100, and 200 % of values stipulated by current legislation. The metal concentrations extracted by aqua regia (AR) were more similar to those obtained by Environmental Protection Agency (EPA) 3052 than to those obtained by EPA3051, and the strongest correlation was observed between pseudo(total) concentrations extracted by AR and EPA3052 and bioavailable concentrations obtained by Mehlich III. An effect of sewage sludge amendment on the concentrations of heavy metals was only observed in samples from the 13-year experiment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vehicular emissions, solid waste, household wastes, and effluents from laundries and industries are all capable of polluting air, water, and soils. Many of these pollutants end up in urban soils, which are for that reason considered an important factor in developing sustainable urban development strategies (Lu et al. 2007).
There are several options for disposing of sewage sludge, including application to soils, advanced treatments, burial in landfills, and incineration (USEPA 1999). Sewage sludge is commonly used as an agricultural fertilizer, but the practice has raised questions due to sludge’s potential for containing trace elements and pathogens (Renner 2000). The increasing volumes of sludge being produced, together with the decreasing concentrations of pollutants in that sludge, are strong incentives for the development of new disposal alternatives.
Amending soils with amended sewage sludge (biosolids) improves their physical and chemical attributes by increasing porosity, organic matter (OM) content, and water retention capacity (Metzger and Yaron 1987; O’Connor et al. 2004), among other things. Sewage sludge may contain trace elements that, when added to soils, can be adsorbed on the surfaces of oxides, clays, and organic matter. Trace elements can also be precipitated or leave residual particles in the soil (Alloway and Jackson 1991). Metallic pollutants are especially problematic, because they are not biodegradable like organic pollutants and can accumulate in living tissues throughout the food chain (Shrisvastava et al. 2003). Trace elements in sludge can also have adverse effects on crops (Sukreeyapongse et al. 2002). In wet tropical conditions, the biological availability of trace elements is not strictly linked to the retention of elements on organic surfaces, as is the case in temperate regions, since the decomposition of OM is faster in the tropics. However, large quantities of elements may be retained in highly weathered tropical soils because of their high concentrations of Fe and Al oxides.
The potential for trace elements pollution following the amendment of soils with sewage sludge has raised concerns that these elements may be absorbed by plants, build up in soils, leach into and pollute ground waters, and thereby enter the food chain, all of which represent long-term environmental risks (Wong et al. 2007).
Soil concentrations of trace elements, which are commonly determined via extraction in an acidic medium, can exceed the limits calculated by models designed to assess human health risks. It is therefore important to continue improving the analytical methods used to quantify the concentrations of these elements (Väisänen and Suontamo 2002). Digestion in a microwave oven offers clear advantages compared to more traditional techniques, including: (1) shorter times for acidic digestion, (2) greater recovery of elements and volatile compounds, (3) lower levels of pollution due to the small amounts of reagent needed, and (4) a more easily replicable process (Agazzi and Pirola 2000).
The object of this study was to compare the effectiveness of extractants of total and pseudototal concentrations of Cu, Fe, Mn, and Zn in soils amended with sewage sludge for 13 consecutive years, and in a soil amended just once with sewage sludge and composted sewage sludge.
Materials and methods
The experiment in which soils were amended with sewage sludge for 13 years is being carried out in Jaboticabal (21° 15′ 22″ S 48° 15′ 18″ W, 618 m a.s.l), São Paulo state, Brazil, on a Typic Eutrorthox. Installed during the 1997–1998 agricultural year, the experiment consists of 60 m2 plots in randomized blocks, with three treatments (rates of sewage sludge) and five replicates. The original treatments were: control (no sludge applied), 2.5, 5, and 10 t ha−1 of sewage sludge (dry weight basis). The 5 t ha−1 dose was established to provide the nitrogen (N) required by maize crops, with assumption that 1/3 of the N in the waste would become available to plants during 1 year. The treatments were modified to include mineral fertilizer in the second year of the experiment, with the goal of complementing the NPK contents of the sludge. Starting in the fourth agricultural year, the 2.5 t ha−1 rate was modified to 20 t ha−1 to cause trace elements phytotoxicity in plants. The modified rates were thus 5, 10, and 20 t ha−1 of sewage sludge (dry weight basis) and the control. During 13 years, the accumulated total doses were equivalent to 65, 130, and 207.5 t ha−1 of sewage sludge for the 5, 10, and 20 t ha−1 treatments, respectively.
The second field experiment, involving rates of sewage sludge and composted sewage sludge, is being carried out in Piracicaba (22° 43′ 31″ S, 47° 38′ 57″ W, 547 m a.s.l), São Paulo state, Brazil, in a clayey-textured Typic Hapludalfs on which the IAC91-1099 sugarcane cultivar had been cut for the second time. Following the mechanized harvest (second ratoon) at the end of July 2009, soil acidity was corrected with 3 t ha−1 of dolomitic limestone that had a 15 % water content. Sewage sludge and the organic compost were broadcasted applied one single time over the sugarcane slash left after the mechanized harvest. The fertilization experiment with rates of sewage sludge and organic compost was designed in subdivided plots with three replicates. Treatments with waste (sludge or compost) were: (1) no amendment with waste, (2) 50 % of the rate stipulated by the Brazilian Environmental National Council Resolution 375 (CONAMA 2009), (3) 100 % of the rate stipulated by the same resolution, and (4) 200 % of the rate stipulated by the same resolution. To calculate waste rates, the CONAMA resolution assumes that 20 and 10 % of the N present in organic form will be mineralized in the anaerobically digested sewage sludge and organic compost, respectively. Total N content of the sewage sludge and organic compost we used was 0.9 and 0.4 %, respectively. To supply 100 kg ha−1 of N for the sugarcane crop, 67 t ha−1 of sludge (wet basis) and 300 t ha−1 of compost (wet basis) were used; water content was 73 and 60 % for sludge and compost, respectively. The sludge and compost treatments were complemented with 120 kg ha−1 of K2O, since the sewage sludge had low levels of potassium. The experiment consisted of 24 plots each containing five lines of sugarcane measuring 7 m long and spaced 1.4 m apart. Data were collected from a 5-m section of the three central lines. Sewage sludge and organic compost were applied to the side of the sugarcane lines, on top of the slash remaining from the mechanical harvest.
Soil samples were collected from the two experiments in March 2010. The chemical characterization of sewage sludge applied over a 13-year period and the sludge and sludge compost applied once are shown in Table 1.
We combined 20 subsamples to create composite soil samples for two soil layers, at depths of 0–10 and 10–20 cm. While soils were originally collected in the 0–0.2 m layer, soils were subdivided into two depths, because the surface layer (0–10 cm) contained more OM. Samples were air-dried, sifted in a sieve with 2-mm mesh, and later sifted in a sieve with 100 mesh to extract the pseudo(total) contents. Total Cu, Fe, Mn, and Zn concentrations were extracted via microwave digestion following the Environmental Protection Agency (EPA) 3052 method (USEPA 1996), pseudototal concentrations following the EPA 3051 method (USEPA 1998) and aqua regia (McGrath and Cunliffe 1985), and biologically available concentrations were extracted using Mehlich III (Mehlich 1984). For the EPA 3052 extraction method, we used 0.5 g of soil + 2 mL of H2O2 (oxidation of the OM) and later added 9 mL of HNO3 + 3 mL of HF to the samples and placed them for digestion in the microwave. For the EPA 3051 extraction method, 10 mL of HNO3 were added to 0.5 g of soil and the samples digested in the microwave. For the aqua regia method, 9 mL of HCl + 3 mL HNO3 (3:1, v/v) were added to 0.5 g of soil and digested in the microwave. Cu, Fe, Mn, and Zn concentrations were determined via inductively coupled plasma optical emission spectrometry.
Quality control of the analysis was carried out with certified soils (NIST 2002 San Joaquin Soil and NIST SRM 2711a Montana II Soil). The NIST recommends comparing methods that do not use hydrofluoric acid (3050, 3051, and their updated versions) with recovery based on leached values (NIST 2002), since the certified contents were determined based on methods of total concentration determination. The results were subjected to analysis of variance and correlation analysis (p < 0.05) using the SAS software platform (SAS 2002).
Results and discussion
Recovery of metals in the certified materials
In general, the rates of metal recovery were satisfactory for both certified materials (Table 2). For the pseudototal extraction methods (aqua regia (AR) and EPA3051), the amount recovered (quantified) was compared to that recovered by leaching, resulting in values ranging from 60 to 90 % for AR and from 58 to 88 % for EPA3051. The amounts recovered (quantified) by the EPA3052 method were compared to the certified amounts of the samples, resulting in values ranging from 81 to 110 %.
An important insight arising from this study is that when choosing extractants to monitor soils amended with organic waste, it is vital to consider the history of amendment, the depth of the soil layer sampled, and the element study. It is important to understand the preferred retention mechanisms for a given element in a given fraction (organic or mineral) of the soil and its extraction with solutions that affect organic material and the mineral fraction to different degrees, as well as the history of crop rotation throughout the study period.
13-Year sewage sludge amendment experiment
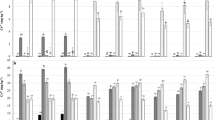
There was a linear increase in Cu concentrations extracted by AR and EPA3052 in the 0–10 and 10–20 cm layers and for Zn concentrations extracted by AR and EPA3052 in the 0–10 cm layer and by EPA3052 in the 10–20 cm layer following amendment with sewage sludge for 13 years (Table 3). Sludge rates had no effect on Fe and Mn concentrations in any of the samples, regardless of collection depth, and we attributed this finding to the fact that soils of tropical humid regions have naturally high concentrations of these elements. We did not see a quadratic effect of sewage sludge application rates for any of the elements (Table 3). Antoniadis et al. (2010) added 10, 30, and 50 t ha−1 of sewage sludge to a Typic Xerochrept for four consecutive years and showed that total concentrations of metals extracted by AR and concentrations of biologically available metals extracted by diethylene triamine pentaacetic acid were not affected by sludge rates, except for Zn and Pb, which showed increased soil concentrations with the amended rates only in the fourth year. Antoniadis et al. (2010) argued that with increasing rates of sludge there are increasing amounts of OM, and that this additional OM tends to complex metals, causing an increase in the total metal concentration without increasing its availability.
Cu–Mehlich III concentrations in the 13-year experiment varied from 3.1 to 19 mg kg−1 at the 0–10 cm depth and from 1.9 to 13.6 mg kg−1 at the 10–20 cm depth. Fe–Mehlich III concentrations varied from 112.1 to 507.6 mg kg−1 at the 0–10 cm depth and from 77.7 to 314.8 mg kg−1 at the 10–20 cm depth. Mn–Mehlich III concentrations varied from 38.2 to 66.9 mg kg−1 at the 0–10 cm depth and from 36.4 to 52.3 mg kg−1 at the 10–20 cm depth. Zn–Mehlich III concentrations varied from 6.6 to 63.2 mg kg−1 at the 0–10 cm depth and from 2.5 to 33.7 mg kg−1 at the 10–20 cm depth.
In both experiments, Cu concentrations were less than the background values (35 mg kg−1) established by Brazilian legislation (CONAMA 2009). Cu–AR concentrations were the same as those of Cu–EPA3052 and exceeded Cu–EPA3051 concentrations at the 0–10 cm depth, while Cu–AR concentrations exceeded both other methods at the 10–20 cm depth (p < 0.01; Table 3). We observed positive linear correlations with high coefficients of determination between Cu–AR and Cu–EPA3052 and between these methods and Cu–Mehlich III in the samples collected at 0–10 cm depth. At the lower soil layer (10–20 cm), there was a positive linear correlation between all the methods for extracting total and pseudototal concentrations and biologically available concentrations (Table 4).
A significant proportion of the Cu that reaches soils via biosolids, sludge from water and sewage treatment stations, or other wastes remains in the most superficial layers, where it is mainly bound to OM (Brun et al. 1998; Teixeira et al. 2005). Among heavy metals, Cu is one of the least mobile in soils due to its strong adsorption on organic and inorganic soil colloids. On organic matter, Cu is mainly retained by humic and fulvic acids, forming stable complexes (Sparks 1995).
AR extracted large quantities of Cu and was comparable to the EPA3052 method, which extracts total concentrations. Aqua regia is a strong oxidizing solution capable of dissolving some compounds that nitric acid alone cannot (Harris 1999). Aqua regia is a mixture of HNO3 and HCl acids, and nitrosyl chloride and molecular chlorine are formed in the reaction. These are strongly reactive compounds with high oxidizing power and the ability to dissolve even noble metals, but these reactive compounds do not totally dissolve silicates. In general, extractions with AR recover between 70 and 90 % of total Cd, Co, Cr, Cu, Fe, Mn, Ni, and Pb content (Ure 1995). However, the pseudototal concentrations extracted by AR for the metals in this study were close to the total concentrations obtained with the EPA3052 method. This result probably occurred because the digestion was carried out in a closed system (microwave), in contrast to traditional AR (in an open system); the dissolving power of acids is increased by the change in their boiling point, giving the acid mixture a stronger extraction power. Nieuwenhuize et al. (1991) studied 30 soil samples and six reference materials and compared the effectiveness of the traditional digestion method (open) and in a microwave with AR (closed) at extracting Cd, Cr, Cu, Fe, Mn, Pb, and Zn. They concluded that microwave extraction was a viable alternative for sediments, sludge, and soils, due to the greater speed and lower pollution risk.
Fe–EPA3052 concentrations were higher than those extracted by other methods at both the 0–10 and 10–20 cm depths (p < 0.01), indicating that the element was mainly bound to the mineral fraction of the soil, which is dissolved by hydrofluoric acid (Table 3). A positive linear correlation was only seen between Fe–AR and Fe–EPA3052 at both depths (Table 4).
Concentrations of Mn extracted by AR were similar to those extracted by EPA3052, and exceeded those obtained by EPA 3051 at both depths (p < 0.01; Table 3). As with Cu, there was probably a bond with soil organic material, which accumulates in the superficial layers. The organic complexes formed with Mn are relatively instable, because the complex formed with humic acid has an entirely electrostatic character, and fulvic acids have a limited number of complexing sites that are specific to the element (McBride 1994). These facts, together with the low affinity of Mn for forming covalent bonds (Harter 1991), show the ready availability of the element. We only observed a positive linear correlation between Mn–AR and Mn–EPA3051 in the sample from the 0–10 cm layer (Table 4).
AR extracted the most Zn at both depths, followed by the EPA3052 and EPA3051 methods (p < 0.01; Table 3). In both experiments, Zn concentrations exceeded background values (60 mg kg−1), but were less than prevention values (300 mg kg−1) for Brazilian soils (CONAMA 2009). At the 0–10 cm depth, there was a positive linear correlation between Zn–AR and Zn–EPA3052 and between the total and pseudototal extraction methods and the biologically available concentrations extracted by Mehlich III (Table 4). We noted positive linear correlations at the 10–20 cm depth between Zn–AR and Zn–EPA3052, between Zn–EPA3051 and Zn–EPA3052, and between the EPA methods and the biologically available concentrations extracted by Mehlich III. In this case, Zn, like Cu and Mn, was extracted in greater quantities by AR, probably because Zn was bound to organic matter. Mandal et al. (2000) have suggested that humic and fulvic acids play an important role in adsorbing Zn and Cu, through the formation of chelates.
Gheju et al. (2011) amended Romanian soils with sewage sludge to increase OM and nutrient levels and assessed the extraction of Cr, Cu, Pb, Cd, Ni, and Zn by two inorganic acids (HCl and HNO3), two organic acids (citric and oxalic), and a strong chelating agent (EDTA). They observed that Zn and Ni were the metals extracted in the highest quantities by inorganic acids, since those elements are present in the sludge matrix weakly adsorbed in functional groups or as inorganic precipitates, and can be easily dissolved by strongly acidic conditions.
One-time sludge amendment experiment
We observed no effects of waste amendment on any elements in the soil samples collected in the experiment with one-time rates of sewage sludge and composted sewage sludge (Table 5), regardless of collection depth. This can probably be attributed to the fact that wastes were applied only once, and not incorporated, 3 months before soils were sampled. In the experiment with rates of sewage sludge and organic compost, Cu–Mehlich III concentrations varied from 2.2 to 3.5 mg kg−1 at the 0–10 cm depth and from 2 to 3.3 mg kg−1 at the 10–20 cm depth. Fe–Mehlich III concentrations varied from 97.2 to 183.3 mg kg−1 at the 0–10 cm depth and from 92.1 to 170.4 mg kg−1 at the 10–20 cm depth. Mn–Mehlich III concentrations varied from 15.2 to 45.3 mg kg−1 at the 0–10 cm depth and from 12.6 to 40.3 mg kg−1 at the 10–20 cm depth. Zn–Mehlich III concentrations varied from 0.9 to 3.4 mg kg−1 at the 0–10 cm depth and from 0.7 to 3 mg kg−1 at the 10–20 cm depth.
Cu–AR concentrations exceeded those extracted by the EPA methods in the upper soil layer (0–10 cm). On the other hand, at the 10–20 cm depth, Cu–AR concentrations were the same as those of Cu–EPA3052 and exceeded those of Cu–EPA3051 (p < 0.01; Table 5). We only observed a positive linear correlation between Cu–AR and Cu–EPA3052 at the two depths (Table 6). Cu showed the same behavior in this experiment as in the 13-year experiment; Cu was mainly bound to OM in the 0–10 cm layer and extracted in the highest quantities by the AR extractant. In the 10–20 cm layer, the mineral fraction of the soil played a greater role in retaining the element, as shown by the similar extraction effected by the AR and EPA3052 extractants. Since waste was applied only once and not incorporated, it is likely that the inorganic fraction was mostly responsible for retaining the element in that layer. Choosing the best extractant thus requires knowledge of the history of waste application, the soil layer under consideration, and the element under study.
Fe–EPA3052 concentrations exceeded those extracted by AR and EPA3051 at both depths (p < 0.01) and only the Fe–AR and Fe–EPA3052 methods showed a positive linear correlation at the 0–10 cm depth (Tables 5 and 6). As with Fe in the 13-year experiment, the element was mainly bound to the mineral fraction of the soil and had greater concentrations extracted by the method with the greatest dissolution of the mineral fraction (EPA3052). In a study of 40 samples of Entisols, Spodosols, and Ultisols from Florida, Chen and Ma (1998) showed that for most of the elements they analyzed the concentration extracted by the EPA3052 method was greater than that obtained by other methods (EPA3050, EPA3051, and EPA3051a). This result was expected, since it is a method of total extraction of soil metals and the samples used were not influenced by anthropogenically amended organic matter.
Mn–EPA3052 concentrations were similar to those of Mn–AR, but exceeded those obtained by Mn–EPA3051 at the depths we studied (p < 0.01; Table 5). The total and pseudototal extraction methods showed positive linear correlations at both depths. The biologically available concentrations only showed correlations between Mn–EPA3051 and Mn–Mehlich III at the 0–10 cm depth and between Mn–EPA3051 and Mn–Mehlich III and Mn–EPA3052 and Mn–Mehlich III at the 10–20 cm depth (Table 6).
Zn–AR concentrations were similar to those of Zn–EPA3052 and exceeded those extracted by the EPA3051 method (p < 0.01; Table 5). We only found positive linear correlations between Zn–AR and Zn–EPA3052 at both depths. Cd and Zn are efficiently extracted by pseudototal methods, while Ba, Cr, and Ni are only efficiently extracted by digestion with HF–HNO3, since they are contained in aluminosilicates (Chen and Ma 1998).
Kabala et al. (2011) assessed the extraction of metals by AR in a microwave in superficial samples (0–15 cm) of Gleyic Cambisols, Gleyic Phaeozems, and Gleyic Arenosols that had been amended with sewage sludge for a long period and observed that Zn concentrations increased with sludge amendment (p < 0.05). Kabala et al. (2011) argued that, in soils irrigated with sludge for long periods, Zn is biologically available and mobile, which represents a threat to crops and water quality. Zn, in general, mostly exists in strong associations with soil silicates in the short term, and the transformation of residual Zn in labile forms depends on soil management (Lucho-Constantino et al. 2005).
Conclusion
-
Metal concentrations extracted by AR were more similar to those obtained with EPA3052 than to those extracted by EPA3051, indicating an increased extraction power of the acid mixture in the microwave
-
The pseudo(total) concentrations extracted by AR and EPA3052 were the most strongly correlated with the biologically available concentrations obtained by Mehlich III
-
Increased concentrations of Cu and Zn following soil amendment with sewage sludge was only observed in samples from the experiment involving long-term amendment additions
References
Agazzi, A., & Pirola, C. (2000). Fundamentals, methods and future trends of environmental microwave sample preparation. Microchemical Journal, 67, 337–341. doi:10.1016/S0026-265X(00)00085-0.
Alloway, B. J., & Jackson, A. P. (1991). The behaviour of heavy metals in sewage sludge-amended soils. The Science of the Total Environment, 100, 151–176. doi:10.1016/0048-9697(91)90377-Q.
Antoniadis, V., Tsadilas, C. D., & Samaras, V. (2010). Trace element availability in a sewage sludge-amended cotton grown Mediterranean soil. Chemosphere, 80, 1308–1313. doi:10.1016/j.chemosphere.2010.06.047.
Brun, L. A., Maillet, J., Richarte, J., Herrmann, P., & Remy, J. C. (1998). Relationships between extractable copper soil properties and copper, uptake by wild plants in vineyard soils. Environmental Pollution, 102, 151–161. doi:10.1016/S0269-7491(98)00120-1.
Chen, M., & Ma, L. Q. (1998). Comparison of four USEPA digestion methods for trace metals analysis using certified and Florida soils. Journal of Environmental Quality, 27, 1294–1300.
Environmental National Council—CONAMA (2009) Resolution no 420 of December 28, 2009. Provides criteria and guiding values of soil quality regarding presence of chemicals and establishes guidelines for environmental management of areas contaminated by these substances resulting from human activities. Accessed on 21 Oct 2011.
Gheju, M., Pode, R., & Manea, F. (2011). Comparative heavy metal chemical extraction from anaerobically digested biosolids. Hydrometallurgy, 108, 115–121. doi:10.1016/j.hydromet.2011.03.006.
Harris, D. C. (1999). Quantitative chemical analysis. 5.ed. LTC. Rio de Janeiro, 150–167. (in Portuguese)
Harter, R. D. (1991). Micronutrient adsorption–desorption reactions in soils. In R. J. Luxmoore (Ed.), Micronutrients in agriculture (pp. 59–88). Madison: SSSA Inc.
Kabala, C., Karczewska, A., Szopka, K., & Wilk, J. (2011). Copper, zinc, and lead fractions in soils long-term irrigated with municipal wastewater. Communications in Soil Science and Plant Analysis, 42, 905–919. doi:10.1080/00103624.2011.558960.
Lu, Y., Zhu, F., Chen, J., Gan, H., & Guo, Y. (2007). Chemical fractionation of heavy metals in urban soils of Guangzhou, China. Environmental Monitoring and Assessment, 134, 429–439. doi:10.1007/s10661-007-9634-1.
Lucho-Constantino, C. A., Prieto-Garcia, F., Del Razo, F. M., Rodriguez-Vazquez, R., & Poggi-Varaldo, H. M. (2005). Chemical fractionation of boron and heavy metals in soils irrigated with wastewater in central Mexico. Agriculture, Ecosystems and Environment, 108, 57–71. doi:10.1016/j.agee.2004.12.013.
Mandal, B., Hazra, G. C., & Mandal, L. N. (2000). Soil management influences of zinc desorption for rice and maize nutrition. Soil Science Society of America Journal, 64, 1699–1705.
McBride, M. B. (1994). Environmental chemistry of soils (p. 406p). Oxford: Oxford University Press Inc.
McGrath, S., & Cunliffe, C. H. (1985). A simplified method for the extraction of the metals Fe, Zn, Cu, Ni, Cd, Pb, Cr, Co and Mn from soils and sewage sludges. Journal of the Science of Food and Agriculture, 36, 794–798. doi:10.1002/jsfa.2740360906.
Mehlich, A. (1984). Mehlich-3 soil test extractant: a modification of Mehlich-2 extractant. Communications in Soil Science and Plant Analysis, 15, 1409–1416. doi:10.1080/00103628409367568.
Metzger, L., & Yaron, B. (1987). Influence of sludge organic matter on soil physical properties. Advances in Soil Sciences, 7, 141–163.
National Institute of Standards and Technology (2002). Standard Reference Materials—SRM 2709, 2710 and 2711. Addendum issue date: 18 Jan.
Nieuwenhuize, J., Poley-Vos, C. H., van den Akker, A. H., & van Delft, W. (1991). Comparison of microwave and conventional extraction techniques for the determination of metals in soils, sediment and sludge samples by atomic spectrometry. The Analyst, 116, 347–351. doi:10.1039/AN9911600347.
O’Connor, G. A., Sarkar, D., Brinton, S. R., Elliott, H. A., & Martin, F. G. (2004). Phytoavailability of biosolids phosphorus. Journal of Environmental Quality, 33, 703–712.
Renner, R. (2000). Sewage sludge, pros & cons. Environmental Science and Technology, 34, 430–435.
SAS Institute. (2002). SAS: user’s guide statistics (6th ed.). Cary: Institute.
Shrivastava, R., Upreti, R. K., & Chaturvedi, U. C. (2003). Various cells of the immune system and intestine differ in their capacity to reduce hexavalent chromium. FEMS Immunology and Medical Microbiology, 38, 65–70. doi:10.1016/S0928-8244(03)00107-X.
Sparks, D. L. (1995). Sorption phenomena on soils. In D. L. Sparks (Ed.), Environmental soil chemistry (pp. 99–139). California: San Diego.
Sukreeyapongse, O., Holm, P. E., Strobel, B. W., Panichsakpatana, S., Magid, J., & Hansen, H. C. B. (2002). pH-dependent release of cadmium, copper, and lead from natural and sludge-amended soils. Journal of Environmental Quality, 31, 1901–1909. doi:10.2134/jeq2002.1901.
Teixeira, S. T., Melo, W. J., & Silva, E. T. (2005). Heavy metals in a degraded soil treated with sludge from water treatment plant. Scientia Agricola, 62, 498–501. doi:10.1590/S0103-90162005000500016.
USEPA (United States Environmental Protection Agency) (1996). Method 3052: microwave assisted acid digestion of siliceous and organically based matrices (compact disc). Washington.
USEPA (United States Environmental Protection Agency) (1998). In: SW-846: test methods for evaluating solid waste, physical and chemical methods. Washington.
USEPA (United States Environmental Protection Agency) (1999). Biossolids generation, use, and disposal in the United States. Washington.
Ure, A. M. (1995). Methods of analysis for heavy metals in soils. In B. J. Alloway (Ed.), Heavy metals in soil (2nd ed., pp. 58–102). Glasgow: Blackie Academic & Professional.
Väisänen, A., & Suontamo, R. (2002). Comparison of ultrasound-assisted extraction, microwave assisted acid leaching, and reflux for the determination of arsenic, cadmium, and copper in contaminated soil samples by electrothermal atomic absorption spectrometry. Journal of Analytical Atomic Spectrometry, 17, 739–742. doi:10.1039/B202534P.
Wong, J. W. C., Li, K. L., Zhou, L. X., & Selvam, A. (2007). The sorption of Cd and Zn by different soils in the presence of dissolved organic matter from sludge. Geoderma, 137, 310–317. doi:10.1016/j.geoderma.2006.08.026.
Acknowledgments
We thank the São Paulo State Research Support Foundation (FAPESP) for a Ph.D. grant awarded to the first author, and Brazil’s National Council on Scientific and Technological Development (CNPq) for a research grant provided to the second and fourth authors. The third author was funded by CNPq Project Grant no. 575025/2008-5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nogueirol, R.C., de Melo, W.J., Bertoncini, E.I. et al. Concentrations of Cu, Fe, Mn, and Zn in tropical soils amended with sewage sludge and composted sewage sludge. Environ Monit Assess 185, 2929–2938 (2013). https://doi.org/10.1007/s10661-012-2761-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2761-3