Abstract
The objectives of this study are to investigate the volatile organic compound (VOC) distribution using passive samplers and to assess the resulting health risks in a high-tech science industrial park. With the advantages of passive sampling techniques, long-term and wide-area samples are collected. The results show TVOC concentrations in summer, fall, winter, and spring are 7.14 ± 5.66 ppb, 18.17 ± 5.81 ppb, 10.30 ± 3.54 ppb, and 14.56 ± 4.53 ppb, respectively; those on weekdays and weekends are 14.36 ± 6.80 ppb and 9.87 ± 4.86 ppb, respectively; and those in industrial and residential zones are 12.97 ± 0.39 ppb and 11.13 ± 0.68 ppb, respectively. Based on concentration variations, and benzene, toluene, ethylbenzene, and xylene ratios, we can resolve the source origins. Health risks are assessed based on the resulting concentrations. In the case of non-cancer chronic effects, long-term exposure to these concentrations does not support there is a risk of adverse health effects. However, potential cancer risks of exposure to these concentrations may occur, especially to carbon tetrachloride and benzene. By applying this study’s procedures, information on VOC concentration distribution, source identification, and health assessment can be obtained and they are applicable to similar studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-tech industries, such as the integrated circuit industry, optoelectronic industry, and biotech industry, are currently the main economic activities and continue to grow worldwide. Along with the growth of these industries, they bring us all kinds of chemical pollutants. Similar situations can be found in other industrial and developing countries (Cai et al. 2010; Leuchner and Rappenglück 2010; Tiwari et al. 2010). The most abundant chemicals in such industries, including silanes, silicon chemicals, halogenated chemicals, inorganic acids, caustics, and volatile organic compounds (VOCs), are released from manufacturing, cleaning, and maintenance processes (Lo and Wu 2003; Kuo 2007). Usually, high-tech industries are located in science-based industry parks. These science parks are built on the concept of the industrial community complex. Accordingly, the pollution emission patterns in these industry parks can be characterized as having many pollution chemicals, fluctuating emissions, and localized tendency (Lo and Wu 2003; Chiu et al. 2005), causing significant impact on the neighboring environment and people.
Among the pollutants, VOCs are widely used in each industry and account for a considerable volume of emissions (Wu et al. 2003; Wu et al. 2004; Kuo 2007). For example, isopropyl alcohol, acetone, toluene, methyl ethyl ketone, and butyl acetate are used in the integrated circuit and optoelectronic industries; methylene chloride and n-hexane are used in the biotechnology industry; and ethanol, n-heptane, and trichloroethane are used in the precision machinery industry (Wu et al. 2003; Wu et al. 2004; Chiu et al. 2005). According to an environmental impact report of a high-tech science park, the allowable emission amount of VOCs is 3,694 ton/year, which accounts for 35% of total emissions and the largest emission pollution species (Kuo 2007). Thus, Taiwan EPA formulates VOC emission standards of 0.6 and 0.4 kg/h for the semiconductor and optoelectronics industries, respectively (Taiwan EPA 2002, 2006) to control the VOC emissions. VOCs arouse concerns due to their photochemical activity and adverse health effects (Ho et al. 2002). For example, alkenes and alkynes have higher photochemical potential (Carter 1994); benzene is a confirmed carcinogen (IARC 2010), trichloroethene and tetrachloroethene can damage liver and kidney and cause cancers in animals (Masters 1996; IARC 2010). Accordingly, different classes of VOCs may have distinctive environmental impacts and health effects.
There are few studies on VOC concentrations in the high-tech science parks. Most of them have measured VOCs in workplaces. Wu et al. (2003; 2004) applied the multi-sorbent adsorption technique to measure VOCs in class-100 cleanrooms, and results showed that isopropyl alcohol, acetone, toluene, and 2-heptanone were frequently present. Some other techniques were implemented in VOC investigations of semiconductor foundries, such as Fourier transform infrared spectrometry and canister sampling techniques, and similar results were shown (C. P. Chang 1997; Yeh et al. 2000). For ambient VOC concentrations in the science-based industrial parks, Chiu et al. (2005) used the canister sampling technique and found that isopropyl alcohol, acetone, toluene, and benzene were dominant compounds. This indicated ambient VOC distribution reflected workplace concentrations; additionally, there were some other chemicals (benzene and methyl-tert-butyl ether), with lower concentrations found in ambient air due to other emission sources, such as traffic exhaust (Lo and Wu 2003). Another study investigated VOCs in the Central Taiwan Science Park. Results were found in which VOCs were classified into industrial- and traffic-related emissions (Kuo 2007). The studies mentioned above had relatively short sampling durations (only 1 or 2 h), and limited sampling sites. Source contribution was not able to be determined nor could representative concentration distributions be obtained. In order to comprehensively understand VOC distributions in science-based industrial parks, more data should be collected, and longer sampling times and wider areas should be implemented to include all possible emission patterns and sources. Furthermore, measuring data should be applied for source contribution and health risk assessments.
Longer sampling times and multiple sampling sites can be achieved by using passive sampling methods. The passive sampling technique is based on Fick’s first law of diffusion. Target compounds are drawn to a collecting medium by a concentration difference between the sampled air and the collecting medium (Górecki and Namieśnik 2002; Namieśnik et al. 2005). Several advantages over the passive sampling technique include no power requirement, easy operation, low cost, small size, and potential for large-scale field studies (Begerow et al. 1999; Zabiegała et al. 2002; McCarthy et al. 2009). To evaluate the performance of the passive sampling technique, comparisons of the passive sampling techniques and active sampling techniques (or continuous measurements) were conducted. Results of these techniques were close (Krupa and Legge 2000; Zabiegała et al. 2002; Bartkow et al. 2004). Passive sampling has been criticized as having low sampling rates and requiring longer sampling times at lower concentrations (Zabiegała et al. 2002). This prevents collection of short-term measurements and the investigation of concentration changes. This deficiency does not affect the application of this technique to ambient air VOC investigation, since ambient concentrations are relatively stable in comparison with workplaces’. On the other hand, the passive sampling technique enables measurement of the time-weighted average concentrations (Krupa and Legge 2000; Zabiegała et al. 2002). Many studies applied the passive sampling techniques to determine air quality, assess exposure concentrations, and detect pollutant sources (Ohura et al. 2006; Gonzalez-Flesca et al. 2007; Hinwood et al. 2007; Smith et al. 2007; Tovalin-Ahumada and Whitehead 2007; Kume et al. 2008). It is expected the application of passive sampling technique will continue to grow.
The objectives of this study are to investigate the VOC distribution of a high-tech industrial park by using a passive sampling method over four seasons, and to estimate the corresponding health risks for assessing the effects of VOC emissions on nearby residents. Thus, an applicable and reliable measurement and assessment method is established for future related studies.
Materials and methods
Description of the high-tech science park
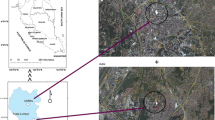
Sampling locations were in the Southern Taiwan Science Park (STSP, latitude 23.090–23.119′ N, longitude 120.270–120.290′ E), which has been established for over 10 years and has become an important industrial complex in southern Taiwan. This science park has six categories of industries that include nine integrated circuit firms, 40 optoelectronic companies, three computer and peripheral companies, nine telecommunication companies, 37 precision machinery companies, and 19 biotechnology companies as at April of 2009. This park is a complex of industrial, institutional, and residential regions with a total area of 2,565 acres.
Sampling strategy
Passive samplers (Sibata Scientific Technology, Tokyo, Japan) were used to collect long-term and wide-area ambient VOCs in four periods, which were August 2008, November 2008, February 2009, and April 2009, representing summer, fall, winter, and spring seasons, respectively. Each sampling period lasted for 12 days, during which VOCs were continuously collected for 48 h on weekdays, and 72 h on weekends, respectively, at one sampling location; thus, three sets of weekday data and two sets of weekend data were obtained in each season. There were 18 sampling locations evenly distributed in the entire sampling zone, mainly situated at major crossroads (Fig. 1). Sampling sites 1–13 were in the industrial zone, and sampling sites 14–18 were in the residential one. In addition, two field blanks and two medium blanks were included in each set, with a total of 110 samples in each season.
Map of Taiwan and sampling sites of Southern Taiwan Science Park. A Integrated circuit firm, B optoelectronic company, C integrated circuit firm, D optoelectronic company, E optoelectronic company, F optoelectronic company, G biotechnology company, H optoelectronic company, I elementary school, J junior high school, K gasoline station, L incinerator, M wastewater treatment plant, N precision machinery company, O precision machinery company, P precision machinery company. Sampling sites 1–13 are in the industrial zone, and sampling sites 14–18 are in the residential zone
Sample extraction and analysis
After sampling was complete, samplers were stored at 4°C in sealed aluminum bags until extraction. Adsorbents in each sample were taken out and extracted with 1.5-ml methylene chloride, sonicated for 60 min and centrifuged for 10 min at 3,000 rpm, 10°C. After that, the supernatant (0.99 ml) was transferred into a 2-ml vial with 0.01-ml toluene-d8 (internal standard) added (Hsiao 2009). One microliter of extract was directly injected into a gas chromatography/mass spectrometer (GC/MS, Model 6890/5973, Hewlett-Packard, Palo Alto, CA, USA). A GC temperature program increasing from 36°C to 100°C at a rate of 3°C min−1, then 100°C to 200°C at a rate of 20°C min−1, running on a HP-5MS column (30 m length, 0.25 mm ID, 0.25 μm thickness) was used to separate the compounds with different volatility. The MS detector was operated in a scan mode with a mass range of 33–250 AMU, collision energy of 70 eV, ion source temperature of 230°C, and quadruple temperature of 150°C. A VOC mix standard (catalog no.: 47537-U, Supelco Inc., Bellefonte, PA, USA) and an internal standard (toluene-d8, ASTM aromatic internal standard, Supelco Inc., Bellefonte, PA) were used for calibration and quantification. Compound identification was based on target ions and qualifier ions of mass spectrum, retention times, and comparison with library spectra. Quantification was based on regression lines, which were calculated over a range of six levels of concentrations versus the corresponding abundances.
Quality assurance
To evaluate sampling and analysis performance, field and medium blanks were taken in each set of sampling, and several analysis procedures (recovery rate, reproducibility, and method detection limit) were conducted in the laboratory before sampling. No compounds were detected in medium blanks. Several compounds, such as benzene, toluene, and octane, were found in field blanks and the amounts ranged from 0.026 to 0.108 μg, corresponding to 0.034–0.173 ppb. For analysis performance, reproducibility (in terms of coefficient of variation), linearity (in terms of the r 2 of the regression line), ranges of recovery, and method of detection limit (MDL, in terms of concentrations under sampling conditions) of target chemicals are 1.1–18.5%, 0.9413–0.9999, 44.6–99.3%, and 0.008–0.510 ppb, respectively. Detailed information is shown in Table 1.
Statistical analysis and health risk calculation
Data were analyzed using the PASW Statistics 18 (IBM SPSS Inc., Armonk, NY, USA) and organized by Microsoft Office Excel 2007 (Microsoft Corporation). Since the sample size is small, non-parametric statistical methods were used in this study. The Kruskal–Wallis H test was used for seasonal comparisons. The Mann–Whitney U test was used for weekday/weekend and spatial comparisons. Additionally, the false discovery rate (FDR) was used for correction among multiple comparisons. The concept of FDR is the fraction of false positives among all tests declared significant. The purpose of this is to further evaluate tests being claimed significant. FDR can be either computed by p value transformation or by direct calculation. The rejecting point (δ) of FDR is set at 0.05 in this study (FDR <0.05, rejecting null hypothesis). This approach not only avoids false positive but increases accuracy and power (Storey 2002; Pawitan et al. 2005).
Health risks were calculated in terms of non-cancer chronic effect and cancer risk by using California Environmental Protection Agency chronic health benchmarks (McCarthy et al. 2009; California Air Resources Board 2010). Ambient VOCs likely enter the human body through inhalation; therefore, reference concentration and unit risk were applied for estimation of non-cancer chronic effect risk and cancer risk, respectively. Non-cancer chronic effect risk was evaluated by hazard quotient (HQ) as expressed in Eq. 1:
where C is the sample concentration (μg m−3), REFc is the non-cancer chronic reference concentration (μg m−3). Furthermore, the sum of hazard quotients (denoted as hazard index, HI) of compounds which aimed at the same organ was also assessed as the following equation.
The cancer risk evaluation is by Eq. 3 which is expressed as:
where Ci is the sample concentration of a certain chemical and URFi is the unit risk value of the corresponding chemical.
Results and discussion
VOC concentration distribution
Temporal variations
Summary statistics of VOC concentrations in terms of seasonal and weekly distribution are given in Table 2. Twenty-one compounds are detected, among which 13 compounds are frequently detected in all seasons. Besides benzene, toluene, ethylbenzene, and xylene (BTEX), these include 1,2-dichloroethane, carbon tetrachloride, isooctane, heptanes, octane, undecane, tridecane, and tetradecane. The sum of BTEX and detected compound concentrations are denoted as ΣBTEX and total volatile organic compounds (TVOC), respectively. TVOC concentrations of summer, fall, winter, and spring are 7.14 ± 5.66 ppb, 18.17 ± 5.81 ppb, 10.30 ± 3.54 ppb, and 14.56 ± 4.53 ppb, respectively; moreover, TVOC concentrations of weekday and weekend are 14.36 ± 6.80 ppb, and 9.87 ± 4.86 ppb, respectively. ΣBTEX concentration of summer, fall, winter, and spring are 2.58 ± 1.55 ppb, 12.54 ± 4.91 ppb, 6.91 ± 2.91 ppb, and 9.60 ± 2.83 ppb, respectively; besides, concentrations of weekday and weekend are 9.28 ± 5.34 ppb, and 5.89 ± 3.27 ppb, respectively. According to concentration percentage data (not shown), dominant chemicals vary among seasons. Toluene is consistently abundant among all four seasons, with mean concentration percentage range between 31.3% and 47.0%; other dominant chemicals of each season are 3-ethyltoluene, 1,2,4-trimethylbenzene, and dodecane in summer; 1,2-dichloropropane, undecane, tridecane, and tetradecane in fall; benzene, dodecane, and tetradecane in winter, and benzene in spring. More compounds are found in summer, while few compounds are found in spring. In addition to dominant chemical species variations among seasons, concentration differences are found for many chemicals and show statistical significance. These include 1,2-dichloroethane, carbon tetrachloride, benzene, isooctane, heptanes, toluene, octane, ethylbenzene, xylene, undecane, tridecane, and tetradecane. Accordingly, ΣBTEX and TVOC concentrations are significantly different as well (Table 2).
The concentration magnitude of most chemicals, ΣBTEX, and TVOC in descending order is fall, spring, winter, and summer. Seasonal variation is a function of meteorological conditions, source activity, and control technology (Lo and Wu 2003; Guo et al. 2004; Chiu et al. 2005). Usually, summer has the lowest concentration due to meteorological conditions. In summer, strong dispersion and active photochemical reaction result in smaller concentrations of aromatics and alkane compounds. Target compounds in this study are mostly aromatics and alkanes that are involved in photochemical reactions and result in concentration decreases. This seasonal phenomenon is also shown in other studies (Choi and Ehrman 2004; Guo et al. 2004; C. C. Chang et al. 2005; Chiu et al. 2005; Ohura et al. 2006). The other influential factor is a source activity. This can be assessed based on sales volume. According to the STSP statistics, total sales amounts (in NT$) of sampling periods in August 2008, November 2008, February 2009, and April 2009 were 46.9 billion, 29.7 billion, 23.4 billion, and 35.0 billion, respectively (Southern Taiwan Science Park Administration 2009). Sales dropped 50% in February 2009 in comparison with that of August 2008 which represents a typical sales volume. This decrease was due to the economic recession. This recession also resulted in change in dominant compounds as mentioned earlier. The combination of influential factors resulted in seasonal variations.
In addition, there is a weekly variation with mostly higher concentrations on weekdays and lower ones on weekends. Compounds, such as 1,2-dichloroethane, carbon tetrachloride, benzene, isooctane, heptanes, toluene, m- and p-xylene, undecane, and tridecane constantly show significant difference between weekdays and weekends over four seasons. For STSP, half of employees follow regular work hours (Mon.–Fri.), and the other half work on shifts to keep the production line on all the time. Basically, weekly VOC patterns reflect working hours. Since the production is always on, compounds with higher weekday/weekend ratios are associated with traffic or human activities; and compounds with ratios close to one are industry related. Weekday/weekend ratios are robust over all four seasons indicating the constant ratio of number of regular work hour employees/number of shift workers.
Spatial variations
The STSP is built on the concept of an industry–community complex. Therefore, this park can be divided into an industrial zone and a residential one. TVOC concentrations of the industrial and residential zones are 12.97 ± 0.39 ppb and 11.13 ± 0.68 ppb, respectively; and ΣBTEX concentrations of the industrial and residential zones are 8.14 ± 0.30 ppb and 7.17 ± 0.52 ppb, respectively (Table 2). No significant differences are found in TVOC and ΣBTEX concentrations. The reason is that both benzene and toluene are main compounds in TVOC and ΣBTEX, and these two compounds are more traffic-related instead of industry related.
Several compounds are significantly different between the industrial and residential zones. These include ethylbenzene, xylenes, and 3-ethyltoluene. These compounds are higher in the industrial zone, indicating that these chemicals are related to industrial activities. Although there is no significant spatial difference for trichloroethene, higher concentrations are found at the residential zone, sites 12 and 13 where biotech companies are nearby or located. According to the chemical inventory data, trichloroethene is not used in the integrated circuit and optoelectronic factories (Kuo 2007; Hsiao 2009), and it is more likely present in biotech companies as an extraction solvent. Additionally, trichloroethene is used as a cleaning agent for dry cleaning. These are the possible reasons that trichloroethene appears in the residential zone.
VOC origins
By looking at temporal and spatial variations, the VOC origins can be identified. Table 3 shows groups of compounds based on weekday and weekend, and industrial and residential comparisons. Compounds with higher concentrations at the industrial zone with no difference between weekday and weekend can be considered as industry related, such as ethylbenzene, o-xylene, and 3-ethyltoluene. Compounds with higher concentrations on weekday and no difference in spaces are human activity related, such as 1,2-dichloroethane, carbon tetrachloride, benzene, isooctane, heptane, toluene, 4- and 2-ethyltoluenes, 1,2,4-trimethylbenzene, undecane, dodecane, and tridecane, and the most important source of these chemicals is traffic emission. Compounds with higher concentrations at the industrial zone and on weekdays are from both industry and human activities, such as m- and p-xylene. Still, several compounds cannot have their origins identified due to limited data and larger variations; therefore, they have no difference either over time or space.
BTEX distribution and comparison with other studies
Table 4 shows a comparison of BTEX concentrations of this study with other studies. These include two science parks, six industrial areas, two petrochemical ones, and five roadside or traffic ones. BTEX are common pollutants in ambient air, and these chemicals are appropriately used for comparison between studies. Concentrations of these compounds are in the range between 0.22–8.62 ppb in this study. In comparison with other science parks, BTEX concentrations are on the same order of magnitude as those of the Hsinchu’s stack air (0.16–2.13 ppb), but much lower than those of Hsinchu’s and Taichung’s ambient air (1.30–11.4 and 5.6–77.1 ppb, respectively) (Chiu et al. 2005; Kuo 2007). By comparing with other industrial areas, this study results are similar to the concentrations of Shimizu and Fuji, Japan (Ohura et al. 2006; Kume et al. 2008), Corinthias, Greece (Kalabokas et al. 2001), and Ottawa, Canada (Zhu et al. 2005), and lower than those of Hong Kong (Lee et al. 2002), Daliao (Taiwan), and Tzoying (Taiwan) (Chiang et al. 2007). BTEX concentrations vary among these studies. Concentration differences may result from different meteorological conditions, topography, sampling methods, sampling sites, sampling periods, and source activities. Concentrations of all studies are in ppb level and the concentrations in this study rank in the middle. Additionally, seasonal variations are found in Shimizu, Japan, where winter concentrations are higher than summer ones (Ohura et al. 2006). This phenomenon agrees with our findings and possible reasons are mentioned above.
BTEX ratios are also shown in Table 4. These ratios, which are not affected by meteorological conditions, are used in several studies to determine source origins (Kuo et al. 2000; Guo et al. 2004; C. C. Chang et al. 2005; Kume et al. 2008; Massolo et al. 2010; Sofuoglu et al. 2010) especially for the toluene/benzene (T/B) ratio. Usually, the T/B around 2.0 is considered as a good predictor of traffic exhaust (Kuo et al. 2000; Massolo et al. 2010; Sofuoglu et al. 2010). These two chemicals are prevailing VOCs in traffic exhausts and the ratio of these two chemicals usually remains constant in traffic emissions. In addition to traffic emissions, toluene is widely used in polyurethane synthetic leather industries, paint industries, surface coating industries, and petrochemical industries as a solvent (Chiang et al. 2007; Cheng et al. 2008). On the other hand, benzene is not used as a solvent due to its adverse health effects (IARC 2010); thus, ambient benzene is mainly from traffic exhausts and gasoline vapors. It is expected that a higher T/B ratio is found in industrial areas. The higher the T/B ratio, the stronger the industrial source strength. One exception is the petroleum refinery industry. This industry produces benzene during the refinery processes, and uses this chemical to generate other aromatic compounds; therefore, the T/B ratio will be small, usually less than 2.0 (Kalabokas et al. 2001; Lin et al. 2004). This should be of notice when the identification of source origins/strength is based on BTEX ratios. The T/B ratio relationship is also confirmed by source strengths. According to Choi and Ehrman’s study, source composition results of B/T/E/X ratios are 1/5.9/2.1/7.2 and 1/2/0.2/0.7 for surface coating and vehicle exhaust emissions, respectively (Choi and Ehrman 2004). This represents BTEX ratios of industrial and traffic emissions, respectively.
The B/T/E/X ratios of summer, fall, winter, and spring are 1/5.1/0.6/1.6, 1/5.6/0.4/1.2, 1/3.3/0.2/0.5, and 1/2.8/0.1/0.3, respectively. Ratios of four seasons are divided into two different groups by using a T/B ratio as an indicator, which are the higher-ratio group (T/B ≥5.0, summer and fall seasons) and the lower one (T/B ∼2.0, winter and spring seasons).
According to the literature data, the ratios of other science parks are 1/13.1/1.6/5.4 and 1/13.8/1.3/5.6 for Hsinchu (stack) and Taichung (ambient), respectively; however, the ratios of Hsinchu ambient air are 1/1.4/–/0.4. The ratios of industrial cities, such as Shimizu (winter season) and Fuji, Japan (Ohura et al. 2006; Kume et al. 2008) are 1/6.3/0.8/1.6 and 1/5.8/0.5/0.9, respectively, which are close to our findings in the higher-ratio group. Other industrial studies have much higher values. For example, Chiang et al. investigated two districts (Daliao and Tzoying) in the Kaohsiung metropolitan area, Taiwan. The T/B ratios of Daliao and Tzoying are 27.5–37.4 and 15.6–17.5, respectively, since there are paint industries and surface coating industries in Daliao, and petrochemical industries in Tzoying, respectively (Chiang et al. 2007). Based on the ratio information, we can conclude that differences in ratios may result from different industry type and activity strength. In comparison with the three science parks, Hsinchu’s and Taichung’s have similar B/T/E/X ratios possibly due to the same industrial activities, but they are different with those of SISP. Also, activities and solvent usages of science-based parks are different from those of paint industries, synthetic leather industries in which toluene, ethylbenzene, and xylene are common solvents, and higher T/B, E/B, or X/B ratios are expected in such industries. In conclusion, BTEX ratios may stay constant for a certain industry type, and are useful for source identification.
On the other hand, the BTEX ratios of residential areas, roadside, and traffic exhaust are 1/1.2–2.6/0.2–1.2/0.2–1.1 (Kuo et al. 2000; Barletta et al. 2002; Zhu et al. 2005; Kerbachi et al. 2006; Olson et al. 2009). For residential or roadside areas, main pollutant sources are from traffic exhausts and gasoline vapor; therefore, the T/B ratio is closer to 2.0. Hsinchu’s ambient air ratios are similar to those of traffic exhausts; this may be due to the sampling site being close to traffic sources.
In this study, the ratios of the higher T/B group are similar to those of industrial areas, whereas the ratios of the lower group are close to urban traffic or roadside ratios. The ratios of the lower group are only a little bit higher than those of residential and roadside areas, and lower than those of high ratio group. By looking at ratios, we can conclude that industrial activities are higher in the summer and fall of 2008, and lower in the winter and spring of 2009. This is correlated with sales volumes which dropped to the lowest in January 2009 and gradually are coming back. This may be due to the economic recession beginning in late 2008 and continuing in 2009.
Health risk assessment
Exposure concentrations in this study are at ppb levels and much lower than those of the reference dose for acute health effects (California Air Resources Board 2010). Therefore, only chronic health effects are investigated. According to our findings, eight commonly present compounds with significant adverse effects are included for non-cancer chronic effect risk assessment. These are carbon tetrachloride, benzene, trichloroethene, toluene, ethylbenzene, and xylene (o-, m-, and p-). Table 5 shows the hazard quotients and hazard indices of investigated compounds for different seasons. Basically, most HQs are much smaller than 0.1. It does not indicate there is a risk of adverse health effect for exposure to these concentrations. The HQ ranges of summer, fall, winter, and spring are 0.0004–0.0206, 0.0011–0.1081, 0.0006–0.0723, and 0.0007–0.1210. Generally, the HQ magnitude orders among seasons are spring > fall > winter > summer which reflect the concentration magnitude. Moreover, the HI of target organ or system is shown in Table 5. HIs of alimentary system, developmental system, nervous system, hematologic system, eye, endocrine, kidney, and respiratory system are 0.0139–0.0701, 0.0514–0.2712, 0.0541–0.2747, 0.0169–0.1210, 0.0011–0.0037, 0.0004–0.0013, 0.0004–0.0013, and 0.0237–0.1192, respectively. All HIs are smaller than one. This does not support a risk of non-cancer chronic health effects. However, developmental and nervous systems show greater HI values. This means that there might be some impact on these two systems, especially for susceptible groups, e.g., children (Sofuoglu et al. 2010).
For cancer risk, four compounds which are confirmed or suspected carcinogens (IARC 2010) are included and results are shown in Table 6. Risks of carbon tetrachloride, benzene, trichloroethene, and ethylbenzene expressed as mean (range) are 6.33 × 10−5 (2.65 × 10−6–2.42 × 10−4), 1.28 × 10−4 (5.55 × 10−7–6.86 × 10−4), 1.82 × 10−6 (3.13 × 10−7–1.84 × 10−5), and 3.73 × 10−6 (3.25 × 10−8–2.57 × 10−5), respectively. Carbon tetrachloride and benzene have the highest risks. To reduce the cancer risk, it is better to bring down the exposure concentrations, especially for carbon tetrachloride and benzene. Large variations are found for each chemical, and these are due to seasonal variations. This indicates that sampling over four seasons is more representative than sampling in one or two seasons.
Several assumptions are applied for the cancer risk estimation. These are representative concentration measurements, a lifetime exposure duration, and 24-h exposure time. For concentration measurements, ambient concentrations are measured over four seasons, and there is a 12-day sampling period for each season. Therefore, they can represent annual exposure concentrations. For exposure duration and time, people work 40 years (25–65 years old) on an average, and spend 40–44 h weekly at work and the rest of time for commuting and at home. It may not be appropriate to apply these estimations for people working at the SISP but living far away from the SISP; however, these estimations are suitable for people working and living at the SISP, since there are industrial and residential zones within the SISP. In terms of exposure duration and time, these estimations may be different from the real situation. Still, these provide magnitude of cancer risks at the STSP.
Several studies also estimate health risks. For non-cancer chronic effects, Kuo (2007) calculate HIs of several compounds in the Central Taiwan Science Park. Results show the HIs of benzene, toluene, ethylbenzene, xylene, and styrene are 0.05–0.18, 0.14–0.49, <0.01, 0.02–0.07, and <0.01. Sofuoglu estimated HIs in primary schools for similar chemicals in Turkey. The HIs of benzene, toluene, ethylbenzene, and styrene are 0.044–0.47, 0.27–0.30, 0.001–0.003, and 0.001, respectively. Our findings are lower than data found in the literature.
For cancer risk, Kuo (2007) estimates the cancer risk based on benzene concentrations in the Central Taiwan Science Park. The resulting risks are in a range of 2.0 × 10−5–8.3 × 10−4. Massolo et al. also estimate benzene cancer risks in industrial and urban areas. The risks of industrial and urban areas are 2.99 × 10−5 and 6.83 × 10−6, respectively (Massolo et al. 2010). These risks have the same magnitude as those of our study. The World Health Organization and the USEPA considered the acceptable lifetime cancer risk as between 1.0 × 10−6–1.0 × 10−5 and 1.0 × 10−6, respectively (Massolo et al. 2010). Accordingly, average risks of chemicals are greater than the 10−6 risk goal. For chemicals such as carbon tetrachloride and benzene, they exceed this goal by one to two orders. The carcinogenic risk levels are not as low as those of the non-cancer chronic effects. Potential cancer risks of exposure to these concentrations may occur. Therefore, both non-cancer chronic and cancer impacts should be estimated for better health risk assessment. Our findings show greater seasonal variation (there are higher concentrations in winter and lower concentrations in summer). To obtain the representative health risk assessment, it is better to consider and deal with variations.
Limitations and application
A few limitations are present in this study. Firstly, solvents such as acetone, isopropyl alcohol, and 2-butanone, frequently used in integrated circuit and optoelectronic industries are not included due to analysis method constraint. Methylene chloride (CH2Cl2) is used as an extraction solvent, and retention times of these frequently present compounds are before that of CH2Cl2 that prevents these compounds being identified. Secondly, lack of source apportionment data for industry type restricts the confirmation of our estimations, especially for integrated circuit and optoelectronic industries. These issues should be investigated further for better understanding of source origins. Despite these limitations, this will not affect the application of this study's methods and the implication of the study's findings.
This study provides an applicable procedure of VOC measurements by using a passive sampling technique. The passive sampling technique has advantages of no power requirement, easy operation, and small size. The permeation-type passive sampler, which is independent of wind speed (Górecki and Namieśnik 2002; Ohura et al. 2006; Kume et al. 2008), is used in this study. These characteristics enable the collection of long-term and wide-area samples. Therefore, comprehensive results on temporal and spatial distributions are obtained. Additionally, this study distinguishes industrial and traffic emissions by intercomparison between weekly and spatial concentration distributions, and by calculation of BTEX ratios. The resulting information is used for assessment of chronic health effects and cancer risks. These measurement and assessment approaches are useful tools for identification of source origins and related health effects without source strength information. This expands the usage of the concentration distribution information and is applicable to other studies.
Conclusions
This study conducts comprehensive VOC measurements in terms of temporal and spatial distribution in a science-based industry park by using a passive sampling technique. Results show that several compounds have temporal and spatial variations. Seasonal concentration magnitudes are in the order of fall > spring > winter > summer. This is due to source strength, control technology, and meteorological conditions. Weekly pattern indicates higher concentrations on weekdays, which reflect working conditions. Industrial/residential area comparison reveals higher concentrations in the industrial zone. Based on weekly and spatial VOC distribution patterns, three source origins are resolved, and they are industry-related, human activity-related, and both industry- and human activity-related emissions. The resulting concentrations are used for health risk assessment in terms of non-cancer chronic effect and cancer risk. For non-cancer chronic effect, this study’s findings do not indicate there is a risk of adverse health effects. However, potential cancer risks of exposure to these concentrations may occur. This study not only provides useful and comprehensive information on VOC concentration distribution, but also expands this distribution information for source identification and health risk assessment in a high-tech science industrial park.
References
Barletta, B., Meinardi, S., Simpson, I. J., Khwaja, H. A., Blake, D. R., & Rowland, F. S. (2002). Mixing ratios of volatile organic compounds (VOCs) in the atmosphere of Karachi, Pakistan. Atmospheric Environment, 36, 3429–3443.
Bartkow, M. E., Huckins, J. N., & Müller, J. F. (2004). Field-based evaluation of semipermeable membrane devices (SPMDs) as passive air samplers of polyaromatic hydrocarbons (PAHs). Atmospheric Environment, 38, 5983–5990.
Begerow, J., Jermann, E., Keles, T., & Dunemann, L. (1999). Performance of two different types of passive samplers for the GC/ECD-FID determination of environmental VOC levels in air. Fresenius' Journal of Analytical Chemistry, 363, 399–403.
Cai, C., Geng, F., Tie, X., Yu, Q., & An, J. (2010). Characteristics and source apportionment of VOCs measured in Shanghai, China. Atmospheric Environment, 44, 5005–5014.
California Air Resources Board (2010). Consolidated Table of OEHHA/ARB Approved Risk Assessment Health Values. http://www.arb.ca.gov/toxics/healthval/healthval.htm. Accessed 10 December 2010.
Carter, W. P. L. (1994). Development of ozone reactivity scales for volatile organic compounds. JAWMA, 44, 881–899.
Chang, C. C., Sree, U., Lin, Y. S., & Lo, J. G. (2005). An examination of 7:00–9:00 PM ambient air volatile organics in different seasons of Kaohsiung city, southern Taiwan. Atmospheric Environment, 39(5), 867–884.
Chang, C. P. (1997). Establish the open path-Fourier transform infrared (OP-FTIR) technique to detect containment in semiconductor industry (Vol. IOSH86-A310). Taipei, Taiwan: Institute of Occupational Safety and Health, Department of Labor.
Cheng, W. H., Hsu, S. K., & Chou, M. S. (2008). Volatile organic compound emissions from wastewater treatment plants in Taiwan: legal regulations and costs of control. Journal of Environmental Management, 88, 1485–1494.
Chiang, H. L., Tsai, J. H., Chen, S. Y., Lin, K. H., & Ma, S. Y. (2007). VOC concentration profiles in an ozone non-attainment area: a case study in an urban and industrial complex metroplex in southern Taiwan. Atmospheric Environment, 41, 1848–1860.
Chiu, K. H., Wu, B. Z., Chang, C. C., Sree, U., & Lo, J. G. (2005). Distribution of volatile organic compounds over a semiconductor industrial park in Taiwan. Environmental Science and Technology, 39, 973–983.
Choi, Y. J., & Ehrman, S. H. (2004). Investigation of sources of volatile organic carbon in the Batimore area using highly time-resolved measurements. Atmospheric Environment, 38, 775–791.
Górecki, T., & Namieśnik, J. (2002). Passive sampling. Trends in Analytical Chemistry, 21, 276–291.
Gonzalez-Flesca, N., Nerriere, E., Leclerc, N., Meur, S. L., Marfaing, H., Hautemanière, A., et al. (2007). Personal exposure of children and adults to airborne benzene in four French cities. Atmospheric Environment, 41, 2549–2558.
Guo, H., Lee, S. C., Louie, P. K. K., & Ho, K. F. (2004). Characterization of hydrocarbons, halocarbons and carbonyls in the atmosphere of Hong Kong. Chemosphere, 57, 1363–1372.
Hinwood, A. L., Rodriguez, C., Runnion, T., Farrar, D., Murray, F., Horton, A., et al. (2007). Risk factors for increased BTEX exposure in four Australian cities. Chemosphere, 66(3), 533–541.
Ho, K. F., Lee, S. C., & Chiu, G. M. Y. (2002). Characterization of selected volatile organic compounds, polycyclic aromatic hydrocarbons and carbonyl compounds at a roadside monitoring station. Atmospheric Environment, 36, 57–65.
Hsiao, S. (2009). The distribution of volatile organic compounds in southern Taiwan Science Park. Kaohsiung: Kaohsiung Medical University.
IARC. (2010). Agents reviewed by the IARC monographs. Lyon CEDEX: IARC.
Kalabokas, P. D., Hatzianestis, J., Bartzis, J. G., & Papagiannakopoulos, P. (2001). Atmospheric concentrations of saturated and aromatic hydrocarbons around a Greek oil refinery. Atmospheric Environment, 35, 2545–2555.
Kerbachi, R., Boughedaoui, M., Bounoua, L., & Keddam, M. (2006). Ambient air pollution by aromatic hydrocarbons in Algiers. Atmospheric Environment, 40(21), 3995–4003.
Krupa, S. V., & Legge, A. H. (2000). Passive sampling of ambient, gaseous air pollutants: an assessment from an ecological perspective. Environmental Pollution, 107, 31–45.
Kume, K., Ohura, T., Amagai, T., & Fusaya, M. (2008). Field monitoring of volatile organic compounds using passive air samplers in an industrial city in Japan. Environmental Pollution, 153(3), 649–657.
Kuo, H. W. (2007). Investigation of the air pollutants and related health risk assessment of residents in the vicinity of Central Science Park. Taipei: National Science Council.
Kuo, H. W., Wei, H. C., Liu, C. S., Lo, Y. Y., Wang, W. C., Lai, J. S., et al. (2000). Exposure to volatile organic compounds while commuting in Taichung. Taiwan Atmos Environ, 34, 3331–3336.
Lee, S. C., Chiu, M. Y., Ho, K. F., Zou, S. C., & Wang, X. (2002). Volatile organic compounds (VOCs) in urban atmosphere of Hong Kong. Chemosphere, 48(3), 375–382.
Leuchner, M., & Rappenglück, B. (2010). VOC source–receptor relationships in Houston during TexAQS-II. Atmospheric Environment, 44, 4056–4067.
Lin, T.-Y., Sree, U., Tseng, S.-H., Chiu, K. H., Wu, C.-H., & Lo, J.-G. (2004). Volatile organic compound concentrations in ambient air of Kaohsiung petroleum refinery in Taiwan. Atmospheric Environment, 38, 4111–4122.
Lo, J. G., & Wu, C. H. (2003). Final report—the analytic technology study on source attribution of VOCs in the atmospheric air of the high-tech industrial park. Taiwan EPA.
Massolo, L., Rehwagen, M., Porta, A., Ronco, A., Herbarth, O., & Mueller, A. (2010). Indoor-Outdoor distribution and risk assessment of volatile organic compounds in the atmosphere of industrial and urban Areas. Environmental Toxicology, 25, 339–349.
Masters, G. M. (1996). Introduction to environmental engineering and science (2nd ed.). Upper Saddle River: Prentice-Hall, Inc.
McCarthy, M. C., O'Brien, T. E., Charrier, J. G., & Hafner, H. R. (2009). Characterization of the chronic risk and hazard of hazardous air pollutants in the United States using ambient monitoring data. Environmental Health Perspectives, 117, 790–796.
Namieśnik, J., Zabiegała, B., K-W, A., Partyka, M., & Wasik, A. (2005). Passive sampling and/or extraction techniques in environmental analysis: a review. Analytical and Bioanalytical Chemistry, 381, 279–301.
Ohura, T., Amagai, T., Senga, Y., & Fusaya, M. (2006). Organic air pollutants inside and outside residences in Shimizu, Japan: levels, sources and risks. Science of the Total Environment, 366(2–3), 485–499.
Olson, D. A., Hammond, D. M., Seila, R. L., Burke, J. M., & Norris, G. A. (2009). Spatial gradients and source apportionment of volatile organic compounds near roadways. Atmospheric Environment, 43, 5647–5653.
Pawitan, Y., Michiels, S., Koscielny, S., Gusnanto, A., & Ploner, A. (2005). False discovery rate, sensitivity and sample size for microarray studies. Bioinformatics, 21(13), 3017–3024.
Smith, L. A., Stock, T. H., Chung, K. C., Mukerjee, S., Liao, X. L., Stallings, C., et al. (2007). Spatial analysis of volatile organic compounds from a community-based air toxics monitoring network in Deer Park, Texas, USA. Environmental Monitoring and Assessment, 128, 369–379.
Sofuoglu, S. C., Aslan, G., Inal, F., & Sofuoglu, A. (2010). An assessment of indoor air concentrations and health risks of volatile organic compounds in three primary schools. International Journal of Hygiene and Environmental Health. doi:10.1016/j.ijheh.2010.1008.1008.
Southern Taiwan Science Park Administration (2009). Statistics of sales, http://www.stsipa.gov.tw/web/WEB/Jsp/Page/cindex.jsp?frontTarget=ENGLISH&thisRootID=22
Storey, J. D. (2002). A direct approach to false discovery rates. Journal of the Royal Statistical Society B, 64, 479–498.
Taiwan, E. P. A. (2002). Air pollution control and emission standards of semiconductor industry. Taiwan: Taipei.
Taiwan, E. P. A. (2006). Air pollution control and emission standards of optoelectronic industry. Taiwan: Taipei.
Tiwari, V., Hanai, Y., & Masunaga, S. (2010). Ambient levels of volatile organic compounds in the vicinity of petrochemical industrial area of Yokohama, Japan. Air Quality, Atmosphere and Health, 3, 65–75.
Tovalin-Ahumada, H., & Whitehead, L. (2007). Personal exposures to volatile organic compounds among outdoor and indoor workers in two Mexican cities. Science of the Total Environment, 376(1–3), 60–71.
Wu, C. H., Feng, C. T., Lo, Y. S., Lin, T. Y., & Lo, J. G. (2004). Determination of volatile organic compounds in workplace air by multisorben adsorption/thermal desorption-GC/MS. Chemosphere, 56, 71–80.
Wu, C. H., Lin, M. N., Feng, C. T., Yang, K. L., & Lo, Y. S. (2003). Measurement of toxic volatile organic compounds in indoor air of semiconductor foundries using multisorbent adsorption/thermal desorption coupled with gas chromatography–mass spectrometry. Journal of Chromatography. A, 996, 225–231.
Yeh, M.-P., Wu, R.-T., & Yu, J.-P. (2000). Probing airborne chemicals of semiconductor work place using gas chromatography mass spectrometry. Institute of Occupational Safety and Health Journal, 8(2), 1–16.
Zabiegała, B., Górecki, T., Przyk, E., & Namieśnik, J. (2002). Permeation passive sampling as a tool for the evaluation of indoor air quality. Atmospheric Environment, 36, 2907–2916.
Zhu, J., Newhook, R., Marro, L., & Chan, C. C. (2005). Selected volatile organic compounds in residential air in the city of Ottawa, Canada. Environmental Science and Technology, 39, 3964–3971.
Acknowledgments
This project was partially supported by the National Science Council in Taiwan (NSC NSC 95-2314-B-037-082).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, CY., Hsiao, SL., Lan, CH. et al. Application of passive sampling on assessment of concentration distribution and health risk of volatile organic compounds at a high-tech science park. Environ Monit Assess 185, 181–196 (2013). https://doi.org/10.1007/s10661-012-2542-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2542-z