Abstract
The Bay of Gokova in Southeastern Aegean Sea (Turkey) is important by the potential of agricultural, municipal, and tourism activities. In addition, there is no industrial plant within the area in the Aegean Sea. Kadın Creek (Azmak) and Akçapınar Creek (Azmak) flowing into the inner Gokova Bay are one of the important factors in determining the character of the inner Gokova Bay. In this study, nine stations were selected in the inner Gokova Bay, Kadın Creek, and Akcapınar Creek. The concentrations of Cd, Cu, Fe, Pb, and Zn were measured in suspended particulate matter (SPM) and sediments. The samples of sediments and SPM were collected in between February 2008 and September 2008. The aim of the study is searching the change of concentrations of Cd, Cu, Fe, Pb, and Zn in suspended particulate matter and sediments in the inner Gokova bay, Kadın Creek, and Akçapınar Creek. It has been identified that the metal concentrations in the creeks are higher than the metal concentrations in the marine environment. It has also been observed that the concentrations of Pb, Cd, and Cu in summer season have been higher than in the winter season, but the concentrations of Fe and Zn have not shown a significant difference between in summer and winter seasons. The obtained results show that the some heavy metals are caused by the agricultural, municipal, and tourism activities operating in the coastal areas and terrestrial environment, and they are transported from the creeks to the Gokova Bay by suspended particulate matter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The metals of natural and/or anthropogenic origin are transported by rivers and transferred to the costal marine system through estuaries (Poikane et al. 2005; Censi et al. 2006). Therefore, the estuaries constitute a natural reactor in which heterogeneous processes at the interface between dissolved phase and suspended particulate matter constitute an important part of the metals geochemical cycles (Morillo et al. 2005; Censi et al. 2006).
Many contaminants, such as heavy metals, are removed from the sea water by biological and physico-chemical processes, including scavenging by sinking particulate or organic matter, and are incorporated into sediments on the seabed (Puig et al. 1999). The metals in sediments represent a combination effect of chemical, biological, and physical processes in fluvial, estuarine, and coastal environments (Liu et al. 2007). The suspended particulate matter is one of the most important parameters for evaluating weather pollution in water systems, and pollutants such as heavy metals, polycyclic aromatic hydrocarbons, phosphorus, and organic compounds are adsorbed onto suspended solids in water column (Rossi et al. 2005). Many trace metals adsorb strongly on particles (Yiğiterhan and Murray 2008), and thus they are preferentially associated with particulate matter in rivers (Guieu and Martin 2002; Yiğiterhan and Murray 2008).
Numerous studies have demonstrated that near-shore sediments from coastal areas near large industrial and urban centers are very often highly contaminated by heavy metals (Puig et al. 1999; Turkmen and Turkmen 2004; Balkis et al. 2010). Most of the contaminants discharged by rivers, sewers, and pipelines are associated with sludge and fine river particles. Thus, the fate of contaminants introduced into the sea is closely associated with the transport and deposition of the particulate (Puig et al. 1999). The aim of the study is to investigate the change of concentrations of Cd, Cu, Fe, Pb, and Zn in suspended particulate matter and sediments of the study area, to identify possible the transport of the metals from the creeks to the Gokova Bay.
Materials and methods
Study area
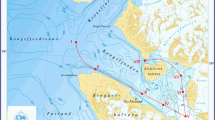
The inner Gokova Bay is located in the southwest of Turkey within the boundaries of Mugla (Fig. 1). There are two significant creeks within the study area. Kadın creek is a meandering stream with a length of approximately 1.700 m and depth of more than 6 m in some parts. While structuring is seen in the north section, the south section is largely covered by marshes. Throughout its journey from the source to the sea, the amount of water is increased by the contributions of small sources, especially from the northern side. Akcapinar creek has a length of approximately 2.250 m and an average depth of around 1.5–2 m with a more meandering and regular structure (Aydın 2010).
The amount of solid wastes in the inner Gokova bay increases considerably in summer months due to the increase in the number visitors. Wastewater treatment plant operation was transferred to the municipalities in October 2008 by the protocol between Akyaka, Gökova municipalities, and the Special Environmental Protection Organization. Moreover, the tour boats and the fishing boats are also a significant source of water pollution as sometimes they illegally dispose their waste to the water instead of using the sewage system. A large number of farmers in the inner Gökova Bay involved in agricultural activities do not get consultancy services from experts about selection and application methods of chemicals (SMAP 2009).
Sampling and analyses
Water samples were collected in between February 2008 and September 2008 in the inner Gokova bay (three stations) and the two creeks (six stations). The water samples were collected with Nansen Bottle, and they were immediately transported to the laboratory. Concentrations of suspended particulate matter in the water samples were measured conventionally by filtering water samples (~1,000 ml) through pre-weighted 0.45-μm paper filters, upon which the filters were dried at 105°C for 12 h and weighted. The weight difference between filter papers before and after filtration and drying was used to calculate the amount of suspended particulate matter in the water samples (Onderka and Pekárová 2008). The calculated values of suspended particulate matter for each station were noted.
The surface sediment samples were collected in April 2008, June 2008, and July 2008 with trap method. Four composite samples (three grabs per sample) were collected from the inner Gokova Bay (three stations) and the two creeks (six stations). The samples of sediments were stored in polyethylene containers and transported to the laboratory. Sediments samples were homogenized (105°C for 12 h) and were sieved through a 200-mesh sieve to normalize for particle size.
The procedure used was that described for digestion of the samples in the CEM Digestion Applications Manuel (Anon 1994). The samples of 100 mg dry sediments and SPM were placed in a Teflon vessel (100 ml capacity) with water (10 ml), HNO3 (4 ml), HF (4 ml), and HCI (2 ml). It was then subjected to treatment for 30 min, in a CEM Digestion System Model MDS 2000 (CEM Corporation Matthews NC).
Since power input, maximum 630 W, was interrupted when the pressure within the control vessel reached 0.828 Mpa, and since pressure generation within vessels was influenced largely by the organic matter content of samples, it was important on the grounds of safety to place the most labile sample in the control vessel. To facilitate this, samples were attended to in order of decreasing organic matter. Digests were treated with crystalline H3BO3 (25 ml) to neutralize excess HF, transferred to polythene containers (Dalman et al. 2006).
Analytical procedures
A GBC 9000 model flame atomic absorption spectrophotometer (FAAS) was used for the determination of the metals. Particular attention paid to the cleanliness of all wares and factory-prepared AAS standard solutions were run as samples for accuracy check after three measurements. The solutions were directly aspirated to a burner at a flow rate of 5–7 ml min−1. Operating parameters used in the FAAS are listed in Table 1. A GBC 3000 model graphite furnace atomic absorption spectrophotometer (GFAAS) was used for the determination of Cd and Pb. The program for determination of Cd and Pb by GFAAS are given in Table 2 with the instrumental setting. Argon 99.96% (v/v) was used to protective gas throughout in GFAAS (Atgin et al. 2000; Dalman et al. 2006).
The quality of data was checked bye the analysis of standard reference materials (SRM-2704, SRM-1646). The measured concentrations are in good agreement with standard reference materials. The SRMs were analyzed before starting the measurements, and results were compared with certified values. The results were within between ±5% and ±15% of the certified materials.
Statistical analysis
The Pearson’s correlation analysis was used to compare means of metal concentrations in the sediment samples and SPM samples of study area by using SPSS. Further analysis was carried out only where there a significant difference at the 5% (p < 0.05) and 1% (p < 0.01) level of significance. Gaussian Frequency Curve of SPM was lined by the following equation.
Where x − is arithmetical mean value and σ is standard deviation.
Results and discussion
Suspended particulate matter amounts by Gaussian frequency curve
The frequency curve of SPM calculated by Gauss Distribution Function is shown on the Fig. 2. As examined the curve of SPM in the Fig. 2, it has been understood that it has a regular distribution and it can be accepted that the standard error analysis is meaningful statistically.
The metals concentrations in suspended particulate matter
The metal concentrations in SPM are summarized (arithmetic mean (AM), minimum, maximum, and standard deviation (SD)) in Table 3. The mean of the metal concentrations in the SPM varied in the following ranges: Cd < Cu < Pb < Fe < Zn. The SPM dominated by Zn (>50 μg/g) and the concentrations of Fe in SPM exhibited ranged from 4 to 22 μg/g. The concentrations of Cu, Cd, and Pb are found as <0.1 μg/g.
In general, the high SD values for the metals (especially Pb) in SPM are observed. It can be an effect of the storm conditions in the sampling period (Table 3). The contaminants discharged by the creeks can be associated with the particles. The heavy metal concentrations in the SPM also indicate the degree of contamination of particles discharged by the two creeks onto the inner Gokova Bay. The metal concentrations in the sediments are much higher than the metal concentrations in SPM (Table 4). This result shows that the metals in the SPM may be precipitate on the surface of sediments.
Significant differences were observed in the mean metal concentrations of SPM between the three stations. In general, the mean of the metal concentrations in SPM varied in the following ranges: Gokova Bay < Akçapınar Creek < Kadın Creek. The concentrations of the metals in SPM at Kadın Creek were higher than other stations, except Zn. The metal concentrations in the creeks are higher than the metal concentrations in the inner Gokova Bay. There is no industrial activity and mine. But Kadın Creek is mainly polluted by domestic waste, and Akcapınar Creek is affected by agricultural activities. The results show that both the creeks are extremely susceptible to pollution. The creeks may not have the environmental capacity to eliminate any anthropogenic activity around them. Table 5 shows the physico-chemical parameters of water samples in the study area. According to these results, it was seen that Akçapınar creek is mostly exposed to agricultural pollution, and Kadın creek is exposed to domestic pollution (Erdinç 2010). The results of the study are similar to the results of this study.
In other words, they have a low capacity of assimilating pollution. Fertilizers and pesticides used for agricultural purpose in the region can be carried into the creeks and indirectly to the marine environment.
Unless the necessary measures are taken and environment-friendly agricultural practices are adopted in the fields around Akcapinar Creek, eutrofication will occur in 20–30 years due to the excessive use of agricultural chemicals, and the organisms living in water will decrease altogether (SMAP 2009).
The concentrations of Zn in SPM had the highest mean values at three stations, while the concentrations of Cd had the lowest mean values. The highest mean value of Zn at Kadın Creek was 497 ± 193 μg/g.
With the start of the tourism season, the values of the Cu and Pb increase in the SPM in the three stations (Fig. 3). Daily boat trips increase remarkably in summer, especially at weekends in Gokova, increasing the physical pollution load in the harbor and the water nearby. Despite warnings, the tour boats discharge their bilge water to the sea. The solid and liquid wastes of bigger boats arranging “blue tours” in Gokova Bay also contribute to the marine pollution. The waste from the human activities in the creeks, especially agricultural waste, waste from the fishing boats and small restaurants, and waste from the street water, get mixed up with the creek water and the coastal water (SMAP 2009). The concentrations of Zn and Fe in SPM have the high mean values when compared with those other metals, and these metals do not show too much change depending on the study period. They may be geological origin. Fe, especially, is a main constituent of sediments and SPM. Thus, Fe is not subjected to anthropogenic changes (Woitke et al. 2003). The concentrations of the other metals (Cu, Pb, Cd) depend on the study period. In April, May, and June, the high concentrations of the metals (Cu, Pb, Cd) were observed. The waste from the human activities in the creeks, especially agricultural waste, waste from the fishing boats and small restaurants, and waste from the street water get mixed up with the creek water and the coastal water. These activities may be an important source of the metals (Cu, Pb, Cd).
The metals concentrations in sediments
The metal concentrations in sediments are summarized (AM, minimum, maximum, and SD) in Table 4. The mean concentrations of the metals in sediment samples varied in the following ranges: Cd < Cu < Pb < Zn < Fe. The most abundant metal in sediments was Fe at concentrations >4,000 μg/g. The low concentrations of Pb, Zn, Cu, and Cd were observed. In April, the concentrations of the all the metals in the sediment samples were determined at the three stations (Fig. 4).
Fe had the highest mean values at the three stations, while Cd had the lowest mean values or below detectable levels (Table 4). On the other hand, the concentrations of Cd in marine sediment samples are below detectable levels whereas the concentrations of Cd in the creeks samples are over detectable levels. The concentrations of Cu in the sediment samples exhibited in the all stations during this study, the highest values are observed in April (Fig. 4). Fe occurs as magnetite, hematite, siderite, or pyrite ores in combination with manganese, carbon, and sulfur as impurities in sedimentary rock deposits (Oguzie and Izevbigie 2009). Cadmium is sourced anthropogenically from the burning of oil, use of pesticides, air, and dust of urban areas where vehicular traffic is common, since cadmium is present in tires and in several vegetable species in farmlands as well as leach from municipal solid wastes (Yong et al. 1992; Atgin et al. 2000; Oguzie and Izevbigie 2009). The concentration of Cd was below Cd mean value (0.11 mg/kg) recommended for unpolluted sediment (GESAMP 1985; Oguzie and Izevbigie 2009).
The metal concentrations in Gokova Inner Bay are compared with those reported for other areas (Tables 6 and 7). The concentrations of Fe in sediments and SPM in this study are significantly higher than those reported for other environment. Fe (III) oxides constituted the major components of the surface sediments in Gokova bay. Such amorphous oxides are known to concentrate heavy metals on their surfaces in a sulfide-poor environment like Gokova Bay where oxic surface sediments predominate (Balkis et al. 2010). Even so, in order to understand the geochemical distribution of the studied metals in the surface sediments, a study of geochemical partitioning of these elements (meaning the separation of the metal geochemical fractions which could be present in sediments: (1) exchangeable adsorbed metals; (2) oxidizable metal complexes; (3) metals in carbonates; (4) reducible compounds; (5) residual metals) is necessary and will be developed in the near future (Andrade et al. 2001).
Statistical analysis
The Pearson’s correlation analysis was performed on metal concentrations data of SPM and sediment samples in this study to investigate the relationship between the metal concentrations. All correlation signals were two-tailed.
In general, significant positive correlations (p < 0.05, p < 0.01) were found between the metals in SPM (Table 8). These results may show that these metals have similar sources and thus, having similar sources, can be related to geological structure of this area. It has been reported that many metals are strongly complexed by organic ligands. These metals may interact in solution with dissolved organic matter (chelation/complexation process) that is in turn concentrated onto fine particulates such as clay minerals (Carlosena et al. 1998; Qu and Kelderman 2001).
We also applied on the metal concentrations in SPM and sediment samples to investigate the relationship between the stations. There were also highly positive relationships between the stations (p < 0.01) for each metal in SPM (Table 9). In addition, the metal concentrations in the creeks are much higher than the metal concentrations in marine environment. These results show that the metal transfer may be from the creeks to the sea water.
There were no positive relationships between all the metals in sediments samples (Table 10). The positive correlations were found between Cd, Cu, Pb, and Zn. Pb had no strong correlations with other metals (Table 10). These results suggest that Cd, Cu, and Zn are characterized by similar hydro-geochemical behavior in sediment. The correlation of Cd, Cu, and Pb was found ascribed to industrial activities (Scerbo et al. 2002; Morselli et al. 2004). SPM samples and sediment samples did not show similar characteristics for measuring metals. Sediment matrix has a very complex structure. Interaction between the metals is very difficult in sediment (Aydın 2010).
Significant positive correlations were also observed in the metals concentrations between three stations (Table 11). These results show that these are related to the behaviors of the metals in the sediment samples.
Conclusions
We observed Cd, Cu, Fe, Pb, and Zn in suspended particulate matter and sediments in the inner Gökova Bay, Kadın Creek, and Akçapınar Creek. Suspended particulate matter in study area has regular distribution.
The mean concentrations of the metals in SPM varied following ranges: Cd < Cu < Pb < Fe < Zn. But sediment samples varied in following ranges: Cd < Cu < Pb < Zn < Fe. The dominant metals are Fe and Zn for both samples. The metal concentrations in the sediment samples are much higher than the metal concentrations in the SPM. The data show that the metals in SPM may be precipitate on the surface of sediments. The metal concentrations in SPM at the Kadın creek were higher than other stations, except Zn. Kadın creek is mainly polluted by domestic wastes. In general, the significant positive correlations were found between some the metals in the both material. The results show that these metals have similar sources and, having similar sources, can be related to geological structure of the study area. On the other hand, significant positive correlations were also observed in the metal concentration between three stations in the both materials. This situation may show that the metals transfer from the creeks to the sea water.
References
Abdullah, M. A. H. (2008). Trace metal behavior in Mediterranean-climate coastal bay: El-mex Bay, Egypt and its coastal environment. Global Journal of Environmental Research, 2(1), 23–29.
Amín, O., Ferrer, L., & Marcovecchio, J. (1996). Heavy metal concentrations in littoral sediments from the Beagle Channel. Tierra del Fuego, Argentina, Environmental Monitoring and Assessment, 41, 219–231.
Andrade, S., Poblet, A., Scagliola, M., Vodopivez, C., Curtosi, A., Pucci, A., & Marcovecchio, J. (2001). Distribution of heavy metals in surface sediments from Antarctic Marine ecosystems. Environmental Monitoring and Assessment, 66, 147–158.
Anon. (1994). Microwave digestion applications manual. USA: CEM.
Atgin, S. R., El-Agha, O., Zararsız, A., Kocatas, A., Parlak, H., & Tuncel, T. (2000). Investigation of the sediment pollution in Izmir Bay trace elements. Spectrochimica Acta Part B, 55, 1151–1164.
Aydın, H. (2010). Investigation of heavy metal contents in the suspended solids and sediments of Gökova Gulf, Sea and Azmaks, in Turkish. M.Sc. Thesis, Institute of Science and Technology, Muğla University.
Balachandran, K. K., Lalu Raj, C. M., Nair, M., Joseph, T., Sheeba, P., & Venugopal, P. (2005). Heavy metal accumulation in a flow restricted, tropical estuary. Estuarine, Coastal and Shelf Science, 65, 361–370.
Balkis, N., Aksu, A., Okuş, E., & Apak, R. (2010). Heavy metal concentrations in water, suspended matter and sediment from Gokova Bay, Turkey. Environmental Monitoring and Assessment, 167, 359–370.
Carlosena, A., Andrade, J. M., & Prada, D. (1998). Searching for heavy metals grouping roadside soils as a function of motorized traffic influence. Talanta, 47, 753–767.
Censi, P., Spoto, S. E., Saiano, F., Sprovieri, M., Mazzola, S., Nardone, G., Di Geronimo, S. I., Punturo, R., & Ottonello, D. (2006). Heavy metals in coastal water systems, a case study from the northwestern Gulf of Thailand. Chemosphere, 64, 1167–1176.
Dalman, O., Demirak, A., & Balcı, A. (2006). Determination of heavy metals (Cd, Pb) and trace elements (Cu, Zn) in sediments and fish of the Southeastern AegeanSea (Turkey) by atomic absorption spectrometry. Food Chemistry, 95, 157–162.
Dassenakis, M., Kloukiniotou, M., & Pavlidou, A. (1996). The influence of long existing pollution on trace metal levels in a small tidal Mediterranean Bay. Marine Pollution Bulletin, 32(3), 275–282.
Erdinç, S. Ö. (2010). Water quality investigation of Kadın Creek and Akçapınar Creek that fed Gokova Bay, in Turkish. M.Sc. Thesis, Institute of Science and Technology, Muğla University.
GESAMP (IMO) FAO/UNESCO/WMO/WHO/IAEA/UN/UNEP). (1985). Joint Group of Experts on the Scientific Aspects of Marine Pollution Review of potentially harmful substances: Cadmium, lead and tin. Rep Stud, 22.
Gonzalez, A. E., Rodriguez, M. T., Sanchez, J. C. J., Espinosa, A. J. F., & De la Rosa, F. J. B. (2000). Assessment of metals in sediments in a tributary of Guadalquivir river (Spain), heavy metal partitioning and relation between the water and the sediment system. Water, Air, and Soil Pollution, 121, 11–29.
Guieu, C., & Martin, J. M. (2002). Level and fate of metals in the Danube deltaplume, Estuarine. Coastal and Shelf Science, 54(3), 501–512.
Li, Y., Yu, Z., Song, X., & Mu, Q. (2006). Trace metal concentrations in suspended particles sediments and clams (Ruditapes Philippinarum) from Jiaozhou Bay of Chine. Environmental Monitoring and Assessment 121, 491–501
Liu, W. C., Chang, S. W., Jiann, K. T., Wen, L. S., & Liu, K. K. (2007). Modeling diagnosis of heavy metal (copper) transport in an estuary. Science of the Total Environment, 388, 234–249.
Morillo, J., Usero, J., & Gracia, I. (2005). Biomonitoring of trace metals in a mine-polluted estuarine system, Spain. Chemosphere, 58, 1421–1430.
Morselli, L., Brusori, B., Passarini, F., Bernardi, E., Francaviglia, R., Gataleta, L., Marchionni, M., Aromolo, R., Benedetti, A., & Oliveri, P. (2004). Heavy metals monitoring at a Mediterranean natural ecosystem of Central, Italy: Trends in different environmental matrixes. Environment International, 30, 173–181.
Oguzie, F. A., & Izevbigie, E. E. (2009). A study of heavy metals concentrations in the sediments upstream of Ikpoba River and Reservoir in Benin City, Nigeria. Biosience Research Communications, 21(3), 119–127.
Onderka, M., & Pekárová, P. (2008). Retrieval of suspended particulate matter concentrations in the Danube River from Landsat ETM data. Science of the Total Environment, 397(1–3), 238–243.
Pekey, H. (2006). The distribution and sources of Heavy metals in Izmit Bay surface sediments affected by a polluted stream. Marine Pollution Bulletin, 52, 1197–1208.
Poikane, R., Carstensen, J., Dahllof, I., & Aigars, J. (2005). Distribution patterns of particulate trace metals in the water column and nepheloid layer of the Gulf of Riga. Chemosphere, 60, 216–225.
Puig, P., Palanques, A., Joan, A. S. C., & Masque, P. (1999). Heavy metals in particulate matter and sediments in the southern Barcelona sedimentation system north-western Mediterranean. Marine Chemistry, 63, 311–329.
Qu, W., & Kelderman, P. (2001). Heavy metal contents in the Delf Canal sediments and suspended solids of the River Rhine: Multivariate analysis for source tracing. Chemosphere, 45, 919–925.
Rossi, L., Krejci, V., Rauch, W., Kriekenbaum, S., Fankhauser, R., & Gujer, W. (2005). Stochastic modeling of total suspended solids (TSS) in urban areas during rain events. Water Research, 39, 4188–4196.
Scerbo, R., Ristori, T., Possenti, L., Lampugnani, L., Barale, R., & Barghigiani, C. (2002). Lichen (Xanthoria parietina) biomonitoring of trace element contamination and air quality assessment in Pisa Province, Tuscany—Italy. Science of the Total Environment, 286, 27–40.
SMAP III. (2009). Gökova Project: Preparation and implementation of the integrated management action plan in for the inner Gökova Bay and the Sedir Island within Gökova Special Protected Area collaboration with stakeholders. Muğla University Press
Turkmen, A., & Turkmen, M. (2004). The seasonal variation of heavy metals in the suspended particulate material in the Iskenderun Bay (North-eastern Mediterranean Sea, Turkey). E.U. Journal of Fisheries & Aquatic Sciences, 21(3–4), 307–311.
Woitke, P., Wellmitz, J., Helm, D., Kube, P., Lepom, P., & Litheraty, P. (2003). Analysis and assessment of heavy metal pollution in suspended solids and sediments of the river Danube. Chemosphere, 51, 633–642.
Yiğiterhan, O., & Murray, J. W. (2008). Trace metal composition of particulate matter of the Danube River and Turkish rivers draining into the Black Sea. Marine Chemistry, 111, 63–76.
Yong, R. N., Mohamed, A. M. O., & Warkentin, B. P. (1992). Principles of contaminant transport in soils. Developments in Geotechnical Engineering, 73, 327.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demirak, A., Yılmaz, H.A., Keskin, F. et al. Investigation of heavy metal content in the suspended particulate matter and sediments of inner Gokova Bay and creeks. Environ Monit Assess 184, 7113–7124 (2012). https://doi.org/10.1007/s10661-011-2484-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2484-x