Abstract
To assess the presence of three gastroenteritis viruses responsible for human acute gastroenteritis in surface water, a 1-year study was carried out in the city of Beijing, China. A total of 108 urban surface water samples were collected from nine collection sites which were defined with a global positioning system in rivers or lakes from September 2006 to August 2007. The water samples were subjected to virus concentration using an HA electronegative filter, followed by polymerase chain reaction (PCR) for rotavirus (RV) astrovirus (AV), and norovirus (NV). It showed that the number of viruses detected in water samples from different sites was variable, totaling 63 virus strains, with rotavirus (48.1%) verified as the most prevalent detected, followed by astrovirus (AV, 5.6%), and norovirus (NV, 4.6%). RV was also quantified by real-time PCR and the concentration of RV ranged from 0 to 18.27 genome copies·L−1. And the distributions of RV in surface water were abundant in cold weather (from September to February) while less prevailing in warm weather (from March to August). The high detection rate of RV we encountered in this study provided convincing evidence that RV circulated at a certain frequency in the Beijing population. There was no statistically significant correlation between RV levels and both fecal coliform (R 2 = 0.02) and Enterococcus faecalis (R 2 = 0.02) densities. Our study suggests prolonged virus persistence in aquatic environments and emphasizes the enteric virus group as the most reliable for environmental monitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In China, because of discharges of raw and treated sewage and manure runoff from agricultural land, pathogenic microorganisms may enter surface waters. In Beijing, the treated water, regarded as a second water source, has been used as toilet flushing water, industrial process water, environmental water, garden water, and road washing water (He et al. 2008). Sewage treatment and disinfective procedures for wastewater have only a limited effect on a number of viruses, and therefore treated sewage water still contain enteric viruses (Meleg et al. 2006; He et al. 2011). Because of discharges of treated sewage, pathogenic microorganisms may enter surface waters in Beijing, and therefore viruses are able to contaminate environmental waters thus preserving a long-term capacity for infection.
Exposure to waterborne pathogens in surface water may lead to adverse health effects and may result in symptoms such as nausea, fever, and diarrhea or more severe illness among recreants like bathers, divers, boaters, and anglers (Schets et al. 2008). Water-related diseases are associated with exposure to water environments in many other ways. These include not only waters used for drinking and recreation purposes but also those used for agricultural purposes such as crop irrigation and food processing, eventually resulting in foodborne outbreaks (Bosch et al. 2008).
Waterborne illness is believed to be most often due to viruses of human fecal origin. Because of the ability of certain viruses to persist in the surface water environment longer than bacteria, risks based on bacterial standards may seriously underestimate the risk of virus-associated waterborne illness (Gersberg et al. 2006). Rotaviruses (RV) are the leading cause of diarrhea in infants around the globe and under certain conditions they can be present in drinking water sources and systems. Ingestion of 10–100 viral particles is enough to cause disease (Gutiérrez-Aguirre et al. 2009). The reported frequency of infection by RV, astrovirus (AV), and norovirus (NV) was 59%, 8%, and 6%, respectively, during the winter season (from December 2000 to March 2001) in Beijing (Liu et al. 2006).
Several concentration methods have been developed in recent years and polymerase chain reaction (PCR)-based assays have become the most preferred techniques. The application of molecular techniques such as real-time (RT)-PCR has favored the relatively rapid, sensitive, and specific detection of viral genome sequences. Some studies imply that the naked viral RNA should be unstable in water and should disappear in a few minutes (Tsai et al. 1995; Limsawat and Ohgaki 1997). Despite the divergence observed for the data at some time points and the fact that molecular methods, such as PCR, do not provide direct information about the presence of infectious virus, a good correlation was found between the virus infectious titer and the presence of viral genomic material in surface water for both astrovirus and rotavirus, supporting the use of this technique to evaluate the presence of viral sequences in environmental samples and its use as an adequate indicator of virus contamination (Espinosa et al. 2008).
Inadequate water treatment was found to be one of the most important causes of waterborne outbreaks. The risk of illness to people who are exposed to treated water may be reduced to a negligibly small probability through the implementation of high-technology tertiary treatments and disinfection systems (Hamilton et al. 2006). However, such systems are often prohibitively expensive, particularly in developing countries.
Despite the awareness of sources possibly contributing to surface water contamination in Beijing, no data were available on the occurrence of pathogenic organisms in both the rivers and recreational lakes. And little is known regarding the correlation between fecal indicators and viruses in surface waters in Beijing. These data are particularly important for local water quality monitoring authorities in China who use fecal indicators to assess the microbiological quality of surface waters. The goal of this study was to assess viral contamination by the three main viruses (RV, AV, and NV) responsible for acute gastroenteritis in the surface water environment inside the city of Beijing and investigate the levels of RV and the fecal indicators (fecal coliforms (FC) and Enterococcus). Investigation and determination of the viruses that are discharged into rivers or lakes from sewage treatment plants could better understand the possible link between the aquatic environment as a reservoir for three main viruses and their potential for disease transmission in Beijing.
Materials and methods
Sampling sites
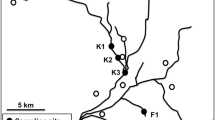
A total of 108 urban surface water samples were collected from nine collection sites which were defined with a global positioning system in rivers or lakes from September 2006 to August 2007. Ninety-six samples are from lakes within 8 urban parks and 12 from a canal in Beijing (Fig. 1). Most of them are recreational waters for boating in summer. Site no.1, 3, and 4 has begun to receive the discharge of treated sewage from sewage treatment plants nearby directly. Two-liter samples of surface water were collected in sterile bottles and transferred to the laboratory, where they were immediately stored at 4°C for viral and bacterial investigations.
Bacterial indicator
FC and Enterococcus faecalis were determined using a membrane filtration method following standard protocols (Jiang and Chu 2004). In brief, samples were serially diluted, filtered onto 0.45 μm pore size 47 mm diameter sterile filters (Millipore, Beijing, China), which were incubated on solid medium for the development of colonies. Commercially available m-FC and m-E medium (BD, USA) were used for cultivation of FC and Enterococcus, respectively. EIA agar plates (BD, USA) were used to confirm Enterococcus colonies that grew on m-E medium after 48-h incubation at 41.5°C. FC was incubated at 44.5°C for 24 h. Each assay was performed using replicate samples from each site.
Virus concentration
A modified method which was developed for concentrating viruses in freshwater, such as tap water or river water, was used for the concentration of surface water samples (Haramoto et al. 2005). Five milliliters of 250 mmol·L−1 AlCl3 was passed through an HA filter of diameter 90 mm (Millipore, Beijing, China) attached to a glass filter holder (Millipore, Beijing, China) to form a cation (Al3+)-coated filter. Subsequently, filtration of 500 mL of the river water sample was performed using the filter. Two hundred milliliter of 0.5 mmol L−1 H2SO4 (pH = 3) was passed through the filter to remove aluminum ions and other electropositive substances, followed by filtration of 10 mL of 1 mmol L−1 NaOH (pH = 11) for elution of viruses from the filter. The filtrate was recovered in a tube containing 50 μL of 100 mmol L−1 H2SO4 (pH = 1) and 100 μL of 100 × Tris-EDTA buffer (pH = 8) for neutralization.
RNA extraction and reverse transcription for viruses
RNA was extracted using the Qiagen viral RNA extraction kit (QIAgen, Germany) following the manufacturer’s instruction. The 50 μl RNA eluates were stored at −80°C until amplification of nucleic acid was performed. Primer characteristics and references for the amplification conditions of different PCR or nested PCR protocols used for nucleic acid detection of rotavirus, astrovirus, and norovirus were all described previously (Liu et al. 2006; He et al. 2008). Briefly a single-step RT-PCR reaction for viruses was performed with the following reaction condition: 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTPs (Promega, Beijing, China), 200U M-MLV RT (Promega, Beijing, China), 2.5 U Ex-Taq DNA polymerase (TaKaRa, Dalian, China), 20 U RNasin (Promega, Beijing, China), and 1 M each of primers. To avoid false-positive results, quality control measures were followed as recommended and for each set of amplifications; negative and positive control samples were included. All methodologies were standardized with reference strains of each virus. In the present study, previously characterized virus strains obtained from fecal samples were used as positive controls. The PCR products were resolved on 1.8% agarose gel followed by ethidium bromide staining (0.5 mg·mL−1), and images were obtained with the image capture system (BioRad) with the Quantity One software program. PCR products were sequenced at the Chinese National Human Genome Center using the same primers. The sequences were aligned and searched for nearly identical sequences using the Basic Local Alignment Search Tool (BLAST) program available on the National Center for Biotechnology Information (NCBI) network server.
Quantitative detection of rotavirus genomes
Real-time PCR was performed in a MX3005P real-time quantitative polymerase chain reaction system (Stratagene, La Jolla, USA) in a total volume of 25 μL, consisting of the Brilliant II SYBR Green QPCR master mix (Stratagene, La Jolla, USA), 300 nM forward primer R3 and 300 nM reverse primer Rp (Baggi and Peduzzi 2000). The thermal cycling conditions were as follows: an initial denaturation at 95°C for 10 min followed by 45 cycles of 30 s at 95°C, 40 s at 50°C, and 30 s at 72°C. Each run ended with a melting curve analysis. Fluorescence data were collected at the end of each cycle and determination of the cycle threshold line was carried out automatically by the instrument. PCR amplicons that were positive with SYBR green real-time PCR were further analyzed by gel electrophoresis through 1.8% agarose gels to confirm the amplicon size.
In order to make a standard curve, standard samples of rotavirus–DNA plasmid (1015 genome copies·μL−1) were diluted by serial tenfold dilution. The water and standard samples were subjected to real-time PCR simultaneously, followed by analysis using Mx3000P software (Stratagene, La Jolla, USA) to obtain quantitative data on the concentration of rotavirus cDNA in a well. Five wells were used for the water and standard samples, respectively, and the average was used for subsequent calculations.
Statistical analysis
The data obtained were subjected to statistical processing with “SPSS 13.0” software. A linear regression test was used to analyze the relationship between the levels of viruses and the densities of bacterial indicators collected in the present study. In all cases, the significance was determined at the 95% confidence level.
Results
Profile of rotavirus, astrovirus, and norovirus in surface water samples
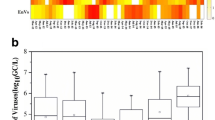
The seasonal distributions of three gastroenteritis viruses in surface water from nine collection sites were shown in Fig. 2. One hundred and eight surface water concentrates were analyzed by PCR assay during this study, and at least one virus was detected in 58 (53.7%) of these. The numbers of three viruses detected in all water samples were different. RV (52/108, 48.1%) is verified as the most predominant one, followed by AV (6/108, 5.6%), and NV (5/108, 4.6%). All positive samples were confirmed by sequence analysis after using BLAST on NCBI. The sequences of RV in positive samples were the same to that of human Wa rotavirus (K02033.1). For AV and NV, the sequences were similar to human astrovirus GGH-2004 (AY590261) and norovirus Hu/GII.4 (EF126966), respectively. It was observed that the positive signals for rotavirus occurred in most samples taken between autumn and winter (from September to February) and was less detectable during spring and summer (from March to August). The highest percentage of positive detection for rotaviruses occurred in September, October, and November 2006 (100%), followed by December 2006 (88.9%) and January 2007 (88.9%). Three positive signals for astroviruses were found in cold weather (November and December) and another three in warm weather (April and June). All five positive detections for noroviruses were found in cold weather (November, December, and February).
Quantitative survey of rotavirus genomes in surface water samples
The quantitative detections of rotavirus in surface water were carried out for each month for a 1-year period, from September 2006 to August 2007. The data of rotaviruses in surface water samples were shown in Table 1. As it was shown, the concentrations of rotavirus in nine sampling sites were quite similar, i.e., abundant in September and winter (from September to February) ranging from 0 to 18.27 genome copies L−1, and was comparably lower in spring and summer (from March to August) ranging from 0 to 3.11 genome copies·L−1 (t test, P < 0.001). The highest concentration was obtained in November 2006 (18.27 genome copies per liter) and the samples collected from April to July were all negative for rotaviruses.
Relationship between fecal indicators and rotavirus in surface water samples
The temporal trend indicated a higher mean concentration of the fecal indicator in the months of May, June, July, and August (Figs. 3 and 4). For nine sampling sites, all measurements of fecal coliform were below 200 CFU·(10 mL)−1 (grade IV of the Chinese standard) and the highest value was 167 CFU·(10 mL)−1 in May indicating an acceptable bacteriological water quality for recreational water without body contact in China (Fig. 3). Fecal coliform was detected in 27 of 54 samples in autumn and winter and 45 of 54 samples in spring and summer with levels ranging from 0 to 19.67 CFU·(10 mL)−1 and from 0 to 167 CFU (10 mL)−1 (Fig. 3). All measurements of E. faecalis were below 40 CFU·(10 mL)−1 and the highest value was 34 CFU·(10 mL)−1 in May (Fig. 4). E. faecalis was detected in 42 of 54 samples in autumn and winter and 49 of 54 samples in spring and summer with levels ranging from 0 to 19.60 CFU (10 mL)−1 and from 0 to 33.60 CFU·(10 mL)−1 (Fig. 4).
The statistical analysis indicated that in the absence of the rotavirus (56 samples), FC values were between 0 and 125.7 CFU·(10 mL)−1, with a mean concentration of 14.4 CFU (10 mL)−1. In contrast, in the positive samples, FC values ranged from 0 to 24.2 CFU·(10 mL)−1 and the mean concentration was 3.2 CFU·(10 mL)−1. There was no statistically significant correlation between RV levels and FC (R 2 = 0.02) densities. In the absence of the rotavirus (56 samples), the E. faecalis values were between 0 and 33.60 CFU·(10 mL)−1, with a mean concentration of 6.7 CFU (10 mL)−1. In contrast, in the positive samples, the E. faecalis values ranged from 0 to 19.6 CFU (10 mL)−1 and the mean concentration was 2.5 CFU·(10 mL)−1. And there was also no statistically significant correlation between RV levels and E. faecalis (R 2 = 0.02) densities, even if they have been suggested as more useful indicators of the presence of enteric viruses in the environment.
Discussion
For the first time, the monthly distributions of rotavirus, astrovirus, and norovirus in surface water were surveyed quantitatively in China. The detection frequencies for rotavirus, astrovirus, and norovirus were 48.1%, 5.6%, and 4.6%, respectively, which were in accordance with the reported frequencies of 32.3%, 6.3%, and 3.1% by rotavirus, astrovirus, and norovirus in sewerage systems (He et al. 2011). The present study showed a higher prevalence of rotavirus, astrovirus, and norovirus during the cold seasons, but the difference was statistically significant only for rotavirus. The longer persistence of the rotavirus genetic material, as compared to that of astrovirus and norovirus, could be, at least in part, related to the nature of their genomes, since double-stranded RNA is known to be resistant to common and ubiquitous endonucleases that cut single-stranded RNA (Espinosa et al. 2008).For both viruses, the decay of genomic material in surface water was clearly associated with components present in the water, most probably endonucleases, rather than with environmental factors.
The prevalence of positive samples for three viruses was similar compared with previous studies. For example, a study in Manaus from Brazil reported that RV was the most frequent (62.5%), followed by human RV (25%), and NV (12.5%) in river water samples of urban area (Miagostovich et al. 2008). In a study performed in Germany, RV RNA was detected and confirmed in 3–24% of the effluent and surface water samples tested (Pusch et al. 2005). In Otranto (Italy), 37.5% of the surface waters samples were positive for RV using by tangential flow ultrafiltration and RT-nested PCR (Grassi et al. 2010). In the Netherlands, NV, RV, and other enteric viruses were also detected in surface waters (Lodder and de Roda Husman. 2005).
The presence of the three main viruses responsible for acute gastroenteritis observed in the water of urban surface water provides evidence that these viruses circulate at certain frequencies among the population of Beijing. In sampling sites 3, which received the discharge of treated sewage directly, the average concentration of rotavirus was 4.30 genome copies L−1. The amount of average concentration in this site is the top one among that in all sampling sites. The data in Table 1 could provide an assessment of the risk of human disease associated with effluent disposal into the lakes of Beijing. Even though microbiological parameters such as fecal coliform or E. faecalis indicated acceptable microbiological water quality, the virological data of this study suggested the possibility that surface waters might be a source for enteric viral infections.
The high percentage of RV detection in the present samples indicates that this virus should be considered for use as a potential indicator of fecal environmental contamination in developing countries. The presence of viral genomes in areas where fecal contamination was not demonstrated by bacterial indicators suggests prolonged virus persistence in aquatic environments and emphasizes the enteric virus group as the most reliable for environmental monitoring (Miagostovich et al. 2008). It has been recognized that these viruses are more stable than bacteria in water and sewage, constituting not only a potential hazard for public health but also good indicators of fecal pollution, as well as the potential presence of other viruses (Miagostovich et al. 2008). Previous work has shown that there is a poor correlation between levels of viruses and the current surface water indicators enterococci and Escherichia coli (Wong et al. 2009). The poor correlation between fecal indicators and rotaviruses also has been reported in studies for surface waters in other countries (Miagostovich et al. 2008; Grassi et al. 2010). This is due to their different levels of fecal excretion from humans and animals, different rates of inactivation during conventional sewage treatment, and different survival rates upon exposure to the environment.
However, there are still problems existing in using enteric viruses like RV as an indicator. Generally, it is not easy for molecular protocols to be used as routine water quality tools especially in developing countries since expensive devices are indispensable. Recently in our group, we have developed the method to detected RV based on loop-mediated isothermal amplification (LAMP). LAMP assay is a rapid, accurate, and cost-effective diagnostic method which amplifies the target nucleic acid under isothermal conditions usually between 60°C and 65°C. Hence, only simple equipment, such as a heating block or a water bath, is required and LAMP products could be judged with HNB by different colors with the naked eyes.
It is a fact that water reuse worldwide is increasing, and one major issue comprises re-injection of treated water into surface water. It is necessary to continue studying the behavior of enteric viruses and other microorganisms in the environment, particularly in water, because the latter comprises a rapid pathogen distribution route to the population. In addition, it is necessary to increase the efficiency of the distribution systems of water intended for human consumption, as well as to improve treatment processes for the reuse of water that would be injected into groundwater systems (Espinosa et al. 2008).
References
Baggi, F., & Peduzzi, R. (2000). Genotyping of rotaviruses in environmental water and stool samples in Southern Switzerland by nucleotide sequence analysis of 189 base pairs at the 5′ end of the VP7 gene. Journal of Clinical Microbiology, 38, 3681–3685.
Bosch, A., Guix, S., Sano, D., & Pinto, R. (2008). New tools for the study and direct surveillance of viral pathogens in water. Current Opinion in Biotechnology, 19, 295–301.
Espinosa, A. C., Mazari-Hiriart, M., Espinosa, R., Maruri-Avidal, L., Mendez, E., & Arias, C. F. (2008). Infectivity and genome persistence of rotavirus and astrovirus in groundwater and surface water. Water Research, 42, 2618–2628.
Gersberg, R., Rose, M., Robles-Sikisaka, R., & Dhar, A. (2006). Quantitative detection of hepatitis A virus and enteroviruses near the United States–Mexico border and correlation with levels of fecal indicator bacteria. Applied and Environmental Microbiology, 72, 7438.
Grassi, T., Bagordo, F., Idolo, A., Lugoli, F., Gabutti, G., & De Donno, A. (2010). Rotavirus detection in environmental water samples by tangential flow ultrafiltration and RT-nested PCR. Environmental Monitoring and Assessment, 164, 199–205.
Gutiérrez-Aguirre, I., Banjac, M., Steyer, A., Polj ak-Prijatelj, M., Peterka, M., Trancar, A., et al. (2009). Concentrating rotaviruses from water samples using monolithic chromatographic supports. Journal of Chromatography A, 1216, 2700–2704.
Hamilton, A., Stagnitti, F., Premier, R., Boland, A., & Hale, G. (2006). Quantitative microbial risk assessment models for consumption of raw vegetables irrigated with reclaimed water. Applied and Environmental Microbiology, 72, 3284–3290.
Haramoto, E., Katayama, H., Oguma, K., & Ohgaki, S. (2005). Application of cation-coated filter method to detection of noroviruses, enteroviruses, adenoviruses, and torque teno viruses in the Tamagawa River in Japan. Applied and Environmental Microbiology, 71, 2403–2411.
He, X., Cheng, L., Zhang, D., Xie, X., Wang, D., & Wang, Z. (2011). One-year monthly survey of rotavirus, astrovirus and norovirus in three sewage treatment plants in Beijing, China and associated health risk assessment. Water Science and Technology, 63, 191–198.
He, X. Q., Cheng, L., Li, W., Xie, X. M., Ma, M., & Wang, Z. J. (2008). Detection and distribution of rotavirus in municipal sewage treatment plants (STPs) and surface water in Beijing. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 43, 424–429.
Jiang, S. C., & Chu, W. (2004). PCR detection of pathogenic viruses in southern California urban rivers. Journal of Applied Microbiology, 97, 17–28.
Limsawat, S., & Ohgaki, S. (1997). Fate of liberated viral RNA in wastewater determined by PCR. Applied and Environmental Microbiology, 63, 2932–2933.
Liu, C., Grillner, L., Jonsson, K., Linde, A., Shen, K., Lindell, A. T., et al. (2006). Identification of viral agents associated with diarrhea in young children during a winter season in Beijing. China Journal of Clinical Virology, 35, 69–72.
Lodder, W. J., & de Roda Husman, A. M. (2005). Presence of noroviruses and other enteric viruses in sewage and surface waters in The Netherlands. Applied and Environmental Microbiology, 71, 1453–1461.
Meleg, E., Jakab, F., Kocsis, B., Banyai, K., Melegh, B., & Szucs, G. (2006). Human astroviruses in raw sewage samples in Hungary. Journal of Applied Microbiology, 101, 1123–1129.
Miagostovich, M., Ferreira, F., & Guimaraes, F. R. (2008). Molecular detection and characterization of gastroenteritis viruses occurring naturally in the stream waters of Manaus, Central Amazonia, Brazil. Applied and Environmental Microbiology, 74, 375.
Pusch, D., Oh, D. Y., Wolf, S., Dumke, R., Schröter-Bobsin, U., Höhne, M., et al. (2005). Detection of enteric viruses and bacterial indicators in German environmental waters. Archives of Virology, V150, 929–947.
Schets, F., van Wijnen, J., Schijven, J., Schoon, H., & de Roda Husman, A. (2008). Monitoring of waterborne pathogens in surface waters in Amsterdam, The Netherlands, and the potential health risk associated with exposure to Cryptosporidium and Giardia in these waters? Applied and Environmental Microbiology, 74, 2069–2078.
Tsai, Y., Tran, B., & Palmer, C. (1995). Analysis of viral RNA persistence in seawater by reverse transcriptase- PCR. Applied and Environmental Microbiology, 61, 363–366.
Wong, M., Kumar, L., Jenkins, T. M., Xagoraraki, I., Phanikumar, M. S., & Rose, J. B. (2009). Evaluation of public health risks at recreational beaches in Lake Michigan via detection of enteric viruses and a human-specific bacteriological marker. Water Research, 43, 1137–1149.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51108029), the National Key Technology R&D Program (2009ZX07527-005), National High Technology Research and Development Program (2008AA062501), and the Foundation of State Key Laboratory of Environmental Aquatic Chemistry (2009–001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, X., Wei, Y., Cheng, L. et al. Molecular detection of three gastroenteritis viruses in urban surface waters in Beijing and correlation with levels of fecal indicator bacteria. Environ Monit Assess 184, 5563–5570 (2012). https://doi.org/10.1007/s10661-011-2362-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2362-6