Abstract
The effects of a sewage effluent with no treatment on the mesozooplankton structure and the environmental quality were evaluated in the Bahía Blanca Estuary, during June to November 1995. The highest values of particulate organic matter, nutrients and specially phosphate, were observed in the effluent discharge zone. In addition, taxa richness, mesozooplankton abundance and Shannon diversity values were lower in the sewage discharge area compared with the less polluted area. Eurytemora americana and Acartia tonsa as well as larvae of Balanus glandula, Neohelice granulata and Spionidae were found in the discharge area with lower densities. These results highlight the importance of sewage effluent effects on mesozooplankton community providing background data to use in other monitoring programmes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increase in sewage volume is one of the negative consequences derived from urban growth, constituting the main cause of eutrophication and associated pollution in estuaries and coastal zones. Wastewaters contain large amounts of organic matter which are used by bacteria, thus reducing the dissolved oxygen levels in aquatic environments (Curds 1982). Also, these are the major source of inorganic nutrients, particularly nitrogen and phosphate, which can produce eutrophication (Curds 1982; Smith et al. 1999; Wolf 1990). Furthermore, effluents transport large volumes of polluting chemical compounds such as heavy metals, hydrocarbons, pesticides and other toxic organic compounds (Caulleaud et al. 2009; Fleeger et al. 2003; Smith et al. 1999; Thompson et al. 2007).
On the other hand, eutrophication processes produce changes in the ecosystem which affect its communities. Several studies indicate that organic contamination, which is, in general, related to the discharge of sewage effluents, exerts its influence on phytoplanktonic and benthonic communities, modifying them significantly (Kimor 1991, 1992; Mayer-Pinto and Junqueira 2003; Meyer-Reil and Köster 2000). In this respect, previous research has demonstrated that the incorporation of nutrients in the Mediterranean and the Baltic Seas produced changes in phytoplankton composition (Kimor 1991, 1992). Also, in Bilbao estuary, in particular, where high volumes of sewage and industrial remains are deposited, a decrease in dissolved oxygen levels was observed, which caused changes in the macro-benthonic community (Saiz-Salinas 1997).
Studies on the impact produced by sewage effluents on coastal and estuarine benthos communities are abundant, while those addressing plankton are relatively scarce (Danulat et al. 2002; Siokou-Frangou and Papathanassiou 1991; Uriarte and Villate 2004). However, some studies have been carried out to define zooplankton categories taking into account their tolerance to sewage pollution influence (Siokou-Frangou and Papathanassiou 1991; Uriarte and Villate 2004). In fact, changes such as the decrease of taxa richness, diversity and abundance have been observed in environments with high concentrations of dissolved nutrients and organic matter associated to urban and industrials inputs (Bianchi et al. 2003; Vecchione 1989).
In Argentina, one of the most polluted areas affected by sewage discharges is the Bahia Blanca estuary in which effluents from the cities of the Bahía Blanca, Punta Alta, General Cerri and Ingeniero White have been poured for decades without previous treatment. Bahía Blanca city’s effluent, in particular, has been physically and chemically characterized (Lara et al. 1985; Tombesi et al. 2000; Hoffmeyer et al. 2004) and its effects on the biota have been evaluated through bioindicators such as bacteriae (Baldini and Cabezalí 1988; Baldini et al. 1999) and planktonic ciliates (Barría de Cao et al. 2003) in different areas of the estuary. The composition, structure and dynamics of meso- and macrozooplankton fractions related to environmental features of Bahía Blanca estuary are well-known (Cervellini 1986, 2001; Hoffmeyer 1983, 1994, 2004; Hoffmeyer and Cervellini 2004; Sabatini 1989, among others). However, there are no previous studies done on sewage effluent effects upon bio-ecological characteristics of planktonic fractions in this area.
In this context, we think that the mesozooplankton community is being negatively affected by the Bahía Blanca city’s sewage effluent and the mesozooplankton taxa richness, abundance and diversity decrease towards that impacted area. So, the objective of this study was to evaluate the effects of sewage effluent from Bahía Blanca city on the mesozooplankton community through information from a monitoring study performed during winter–spring 1995. This study contributes with new information on the usefulness of mesozooplankton as an indicator of water quality in estuaries and also provides a comparative baseline to contrast with recent monitoring data for this estuary.
Materials and methods
Study area
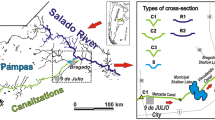
The Bahía Blanca estuary (38°44′–39°27′ S and 61°45′–62°30′ W) (Fig. 1) is a mesotidal, temperate and turbid estuary, located in the southwestern Atlantic Ocean and with a total area of ∼2,300 km2 at high tide. The inner area of this estuary is partially mixed with a strong tendency to be vertically homogeneous under low freshwater discharge, and partially stratified under high-discharge conditions. The outer area is sectionally homogeneous with mean salinities typical of shelf waters (Perillo et al. 2001). Water circulation is dominated by a semidiurnal tidal wave and winds strongly affect this estuarine circulation. In general, winds blowing from the NW sector reduce sea level and SE winds generate the opposite effect (Perillo et al. 2004).
The most important freshwater tributaries to this estuary are Sauce Chico River and Napostá Grande stream, both located in the northern area. The former discharges its waters at the estuarine head, whereas the Napostá Grande stream discharge is located in the mid-area of estuary. Their annual mean runoffs are 1.9 and 0.8 m3 s−1, respectively. Other less important affluents are Galván, Saladillo de García and Maldonado streams, whose altogether volumes do not reach that of the Napostá (Perillo et al. 2004). The following cities and towns are situated on the northern coast of the estuary: General Cerri, Ingeniero White, Punta Alta and Bahía Blanca. Cuatreros, Galván, Ingeniero White, Belgrano and Rosales ports are also located in this coastal area. The inner zone of this estuary is the most exposed to anthropogenic pressure. In the last 25 years, Bahía Blanca city has suffered a strong demographic expansion, increasing the maritime traffic, non-treated wastewater discharges, dredging and a significantly important growth of industrial and petrochemical activities (Perillo et al. 2001).
Sampling and data analysis
The present study was carried out in the inner zone of the Bahía Blanca estuary. This sector extends from the area affected by sewage effluent discharge to an area located near to the head of the estuary. Twenty-four mesozooplanktonic samples were collected from four sampling stations on a monthly basis, from June to November 1995. These stations were: Canal Vieja (CV), a discharging point of the sewage duct collector of Bahía Blanca city’s treatment plant, Boya 31 (B 31), Ingeniero White Port (IWP) and Cuatreros Port (CP) (Fig. 1).
Mesozooplankton samples were collected with a 200-μm mesh, 0.30-m open-mouth net containing a mechanical flowmeter. Sampling was carried out by means of horizontal tows on the surface layer during ebb tide, and samples were preserved in 4% formalin, neutralized with sodium borate (2%). Temperature (Temp) and transparency (SD, Secchi disk depth) were measured using a surface thermometer and a Secchi disk, respectively. Water samples were collected with a Van Dorn bottle at each sampling station. Nitrate (NO3), nitrite (NO2), phosphate (PO4) and silicate (SiO2) were determined using a Technicon II autoanalyser following Eberlein and Kattner (1987), Grasshoff (1983) and Treguer and Le Corre (1975). Salinity (Sal) was measured using a Beckman salinometer, and particulate organic matter (POM) was determined following Strickland and Parsons (1968). Chlorophyll-a (Chlor-a) and phaeopigments (Phaeo) concentrations were spectrophotometrically analysed according to Lorenzen (1967).
Mesozooplankton samples were qualitatively and quantitatively analysed under a Wild M5 stereomicroscope. These samples were totally analysed, and mesozooplankton abundance was expressed as individuals per cubic metre (ind. m–3). Specific diversity and dominance were calculated using the Shannon–Wiener (H′) and Simpson (λ) indexes (Pielou 1975), and it was performed using Primer 5 program (Clarke and Warwick 1994). Furthermore, two-way ANOVA without replication and least significant differences (LSD) tests were used to determine differences in abundance among the sampling stations. The Spearman’s rank correlation coefficients were calculated between environmental and biological variables (total abundance, abundance of the most important taxa Eurytemora americana and Acartia tonsa and Shannon diversity) to determine the degree of association among them.
Results
Environmental variables
Temperature varied from 5.6°C at CV (June) to 20.2°C in IWP (November). The lowest salinity values were observed at CV in August and the highest at B 31 in November (19.7–36.1, respectively). Transparency values ranged between 0.26 m at IWP in November and 1.15 m at CP in July. Chlorophyll-a concentration ranged between 1.50 μg L−1 in October (CP) and 23.89 μg L−1 in July (IWP). Phaeopigments concentration reached a maximum of 23.37 μg L−1 at B 31 (July). POM concentration was lowest at B 31 in October and highest at CV in August (725 and 7,836 μg L−1, respectively) (Fig. 2). SiO2 concentration was lowest (35.81 μZM) in IWP in August and highest (156.87 μM) in CP in June. PO4 concentration was lowest (0.64 μM) in CP and highest (50.05 μM) at CV; both values were recorded in August. NO2 concentration ranged between 0.04 and 1.88 μM; the latter was recorded at CV in August. NO3 concentrations ranging from 0.18 to 10.60 μM were recorded in CP in August and November, respectively (Fig. 3).
Mesozooplankton composition, abundance and diversity
Thirty-five mesozooplankton taxa were found, all of which belonged to six phyla, namely Arthropoda, Annelida, Cnidaria, Mollusca, Nematoda and Chordata. Within mesozooplankton, 24 taxa corresponded to Copepoda. In addition, the 25% of the total mesozooplankton abundance belonged to holoplankton, 35% to meroplankton and 40% to adventitious plankton. Mesozooplankton abundances were found to be much lower at CV than at the other sampling stations, except in November when the highest value was recorded (Fig. 4a). Holoplankton was lower at CV (3.77 ind. m−3) and higher at IWP (89.99 ind. m−3), while meroplanktonic abundance varied from 17.84 to 323.15 ind. m−3 (B 31 and IWP, respectively). Regarding adventitious plankton, the abundance was lowest at CV and highest in IWP (0.13 and 4.81 ind. m−3, respectively) (Fig. 4b). Within holoplankton, the most abundant taxa were E. americana and A. tonsa (29.74 and 7.50 ind. m−3, respectively). Larvae of Balanus glandula, Neohelice granulata and the family Spionidae were the most abundant taxa within meroplankton (143.30, 4.96 and 4.76 ind. m−3, respectively) (Fig. 5). Total mesozooplankton abundance varied from 23.26 at CV to 417.96 ind. m−3 at IWP. Mean diversity index values varied from 1.17 at CV to 1.73 in IWP. The dominance index showed an inverse pattern of diversity (Fig. 6).
The ANOVA revealed significant differences in the abundances between the sampling stations (F = 6.86, p < 0.05). Major differences between CV and CP stations were detected by LSD test (p < 0.05). The latter also revealed that there were not significant differences in mesozooplankton abundance among B 31, CP and IWP stations.
Association among environmental and biological variables
The Spearman’s rank correlation coefficients between environmental variables were shown in Table 1. The only Spearman’s rank significant correlations between environmental and biological variables were total mesozooplankton abundance which correlated negatively and significantly with PO4 concentration (r = −0.589, n = 24, p < 0.05). Shannon diversity index was positively correlated with NO2 and NO3 (r = 0.41, n = 24 and r = 0.51, n = 24, respectively) and negatively correlated with Phaeo (r = 0.47, n = 24). A. tonsa abundance was negatively correlated with POM concentration but positively correlated with salinity (r = −0.60, n = 18 and r = 0.44, n = 24, respectively, p < 0.05). E. americana abundance showed a negative correlation with PO4 concentration as well as with SiO2 concentration (r = −0.57 and r = −0.49, respectively, n = 24, p < 0.05).
Discussion
Environmental variables
According to previous research, the scarce spatial variation of water temperature among the stations during the sampling period seems to be due to a higher influence of air temperature on water temperature in the inner area of the Bahía Blanca estuary as a result of the low depth of this sector (Freije et al. 1981; Pucci et al. 1979). On the other hand, the lowest salinity values recorded at CV and CP stations could be due to the effect of (1) freshwater contributions from the Napostá stream at the sewage effluent discharge zone (CV) and (2) those from the Sauce Chico river in the area of CP (Pucci et al. 1979). Interestingly, in August, salinity values at CV were found to be much lower than those at the other sampling stations. This could be a consequence of the increase in both the sewage discharge and the freshwater flow from the Napostá stream, due to the strong rain prior to that sampling date (Servicio de Hidrografìa Naval, http://www.hidro.gov.ar). Both water temperature and salinity followed the typical temporal pattern known for the inner zone of the estuary with large seasonal temperature amplitude and salinity oscillations derived from precipitations in the basin. Transparency values were in general very low; product of the high contents of particulate matter in suspension composed of silt and clay, which derived from winds and tide that produce turbulence and a turbidity increase (Perillo and Piccolo 1991). On the other hand, occasional dredging operations are an anthropogenic factor which usually produces a great sediment resuspension increasing the turbidity in this estuary (Piccolo and Perillo 1990).
Chlorophyll-a values increased during winter and at the beginning of spring as a result of the typical phytoplankton bloom in this estuary which is mainly represented by diatoms. Moreover, in July, at all sampling stations, maximum concentrations of this photosynthetic pigment were observed and this seasonal behaviour agreed with that reported for the inner zone of the estuary (Gayoso 1983; 1988; Freije and Ganoso 1988; Popovich 2004; Popovich and Marcovecchio 2008). An inverse correlation was observed between Chlor-a concentrations and temperature–salinity in this study, coinciding this with those correlations reported for other estuaries, during phytoplankton blooms periods (Li et al. 2006; Uriarte and Villate 2004). Previous studies in the Bahìa Blanca estuary demonstrated that lowest water temperatures during winter produced a strong decrease in zooplankton (Hoffmeyer 1994, 2004). This would cause a lower grazing pressure on phytoplankton, thus contributing to give rise to a typical winter phytoplankton bloom (Popovich 2004). On the other hand, phaeopigments showed an inverse pattern to that of chlor-a that may be indicative of zooplankton grazing, in agreement with the observations reported by Spetter (2006) at CP station. However, the increase of phaeopigments in July in CV and B 31 stations could be due to the destruction of chlor-a for sewage’s toxic substances or/and microzooplankton grazing pressure rather than mesozooplankton grazing since mesozooplankton was recorded in low abundance.
The highest POM values found at CV station (reaching a peak of 7,835 μg L−1 in August) was possibly the result of both a higher freshwater discharge from the Napostá stream and sewage discharge, coinciding with the one mentioned by Barría de Cao et al. (2003). An inverse relationship between POM and salinity and transparency was considered as indicator of sewage pollution in Bilbao and Urdaibai estuaries on the Basque coast (Bay of Biscay) (Uriarte and Villate 2004). The inverse relationship found between POM and salinity and transparency (0.70 m) at CV station during August confirms the pattern reported by these authors and strongly supports our previous hypotheses.
Furthermore, a decrease in nitrate and nitrite in the area close to CV station during winter agrees with Pucci et al. (1979); however, maximum concentrations found in August could be attributed to a higher input by the sewage effluent joint to the Napostá stream. That decrease recorded in winter agreed with the winter phytoplankton bloom which was followed by a decrease in phytoplankton in late spring (Popovich and Marcovecchio 2008) when regenerative processes of these nutrients begin jointly with the increases of temperature (Freije et al. 1980; Spetter 2006). In this study, both inverse and highly significant relationships were detected between the concentration of these nutrients and Chlor-a, thus giving support to the marked decrease of nitrite and nitrate in winter. The maximum values of phosphate and silicate found at CV station in August were probably due to the same abovementioned reason for nitrate and nitrite. A high-positive significant correlation was detected between silicate and nitrate, nitrite and phosphate indicating the same pattern behaviour during the period of study. The latter agrees with the consumption behaviour of phytoplanktonic organisms (Barría de Cao et al. 2003). Furthermore, the high concentration of silicate in CP probably related to the Sauce Chico river freshwater input, is in agreement with the findings of Lara and Pucci (1982), who reported an inverse relationship between silica concentration and salinity.
Mesozooplankton response
The general decreasing pattern of mesozooplankton abundance and Shannon diversity values towards CV station seemed to correspond to the typical spatial distribution area of the taxa observed within the estuary, i.e. E. americana, A. tonsa, B. glandula, N. granulata and the family Spionidae, according to their seasonal succession. However, this pattern possibly was accentuated by the presence of high concentrations of toxic substances (such as heavy metals, polyaromatic hydrocarbons, etc.) that are discharged into the estuary with sewage, thus affecting directly and indirectly the survival of organisms (Fleeger et al. 2003; Thompson et al. 2007).
Both E. americana (exotic species) and A. tonsa (native species) were found to be the most abundant copepods within holoplankton, whose abundances were lowest at CV station. A. tonsa was recorded from June to November, coexisting with E. americana until October, after which, the latter began to decrease until it completely disappeared in November. The decrease in abundance of these copepods recorded from the inner area of Bahía Blanca estuary to CV station could be due to the coastal–estuarine characteristics of these species (Hoffmeyer 1994, 2004), which allow them to easily adapt to the fluctuating conditions of the innermost stations (CP and IWP). However, E. americana and A. tonsa were the only species within holoplankton that contributed significantly to the abundance at CV station, being this indicative of their resistance to altered environmental conditions. Furthermore, the presence of the genera Acartia and Eurytemora has been related with contaminated areas (Caulleaud et al. 2009; Uriarte and Villate 2004; Uye and Fleminger 1976). In this respect, A. tonsa has been proposed to be a bioindicator in areas with a high degree of eutrophication (Bianchi et al. 2003). The latter is indicative that these copepods tolerate high organic loads and high concentrations of phosphate, nitrate and silicate, which explains their permanence in the estuary, particularly in CV station.
The presence of nutrients (phosphate, nitrate, nitrite and silicate) at CV station induces phytoplankton growth in the estuary in winter and early spring. As we know, phytoplankton, which is mainly characterized by the presence of diatoms, was associated with a decrease in nitrate, phosphate and silicate during the period from May to August 2002 due to the consumption of these nutrients (Popovich and Marcovecchio 2008). This finding coincides with results from our study. On the other hand, E. americana was negatively correlated with the concentration of phosphate and silicate, both of which are necessary nutrients for the development of diatoms (Margalef 1977). Its mostly herbivorous habits as well as its adaptation to cold temperatures and intermediate salinities would facilitate its presence at CV station (Avent 1998; Hoffmeyer and Prado-Figueroa 1997; Sage and Herman 1972). Unlike E. americana, A. tonsa has omnivorous habits and feeds on algae, flagellates and ciliates (Petipa 1959). In a previous study conducted at CV and B 31 stations (1995–1996), it was observed that ciliate abundance is low in winter while it increases in spring and reaches a peak in summer (Barría de Cao et al. 2003). Furthermore, total abundance of aloricate ciliates correlated positively with salinity but negatively with POM in agreement with the correlations evidenced by A. tonsa, thus justifying its presence mainly at CV station.
B. glandula, is an introduced species whose population has greatly increased since it was settled in Bahía Blanca estuary and which competes with Balanus amphitrite (Hoffmeyer 2004; Wagner et al. 1993). In our study, this species showed the highest abundance values within meroplankton and was followed by larvae of N. granulata and the family Spionidae. B. glandula larvae were found mainly in IWP and CP with maximum abundances in winter and early spring (Hoffmeyer and Cervellini 2004). This is related to the typical winter–spring phytoplankton bloom previously mentioned in this work. On the other hand, the fact that maximal abundances occur in IWP and CP stations is probably due to differences between benthic and pelagic habitats (Sautour and Castel 1995). Since Bahía Blanca estuary is dominated by muddy depths, benthonic communities use the fixed and floating structures of IWP and CP, among others (Bremec et al. 2004). Larvae of B. glandula, N. granulata and those of the family Spionidae were the only taxa of the meroplankton recorded at CV station, although their abundance values were minimal compared to the other sampling stations. Larvae of Polychaeta seem to constitute the organisms within mesozooplankton that best evidence the degree of pollution in estuaries (Uriarte and Villate 2004). Spionidae larvae, which seem to be particularly adapted to brackish environments (Bochert et al. 1996; Lardicci et al. 1997) with high tolerance to toxic substances (Sarda and Martin 1993) and oxygen deficit (Fritzsche and von Oertzen 1995), were the dominant group within the Polychaeta in this study. In addition, this group is considered as opportunistic organisms and very abundant in areas with a high concentration of organic matter (Mayer-Pinto and Junqueira 2003). The latter was probably the reason of the presence of Spionidae larvae at CV and B 31 stations, favoured by the great amount of particulate suspended matter at these stations.
Common taxa and similar abundances were found in CV and B 31 stations in most of the study period. Dissimilarity between stations was mainly due to the differences in mesozooplankton abundance between the discharge zones (CV) (low abundance) and the farthest station from the latter (CP) (high abundance). Probably, the higher concentrations of nutrients, particularly nitrite values, in CV station than in CP produced a different pattern distribution of mesozooplankton abundance. In CV, there were also higher concentrations of organic matter and lower temperature and salinity conditions. The general pattern of low diversity and high abundance of opportunistic taxa which are more tolerant to nutrient enrichment from an untreated sewage is a common scenario in worldwide estuaries (Danulat et al. 2002; Siokou-Frangou and Papathanassiou 1991; Vecchione 1989). These authors concluded that in the point of discharge, both diversity and total abundance of zooplankton community decrease, thus inducing the presence of a high number of individuals of few opportunistic species. In our study, A. tonsa, E. americana and Spionidae larvae are adapted to tolerate higher levels of organic enrichment.
However, other factors than those analysed in this research, which may induce changes in the mesozooplankton structure in the study area, must be taken into account. The inner zone of Bahía Blanca estuary is an environment affected not only by sewage effluents but also by other type of effluents with toxic substances such as heavy metals, pesticides and hydrocarbons (Arias et al. 2009, 2010; Fernández-Severini et al. 2009; Marcovecchio et al. 1986). Tides also exert their influence on the physical and chemical conditions of the water body and on the spatial and temporal distribution of mesozooplankton. Winds and rains directly exert their influence on water conditions and circulation as a result of the tidal effect and they also indirectly operate on plankton communities (Menendéz in press).
Conclusions
The present study shows that sewage discharge affects the physical and chemical water composition in the discharge zone of Bahía Blanca estuary (CV station). This, in turn, affects both directly and indirectly the composition, diversity and abundance of mesozooplankton in the area of highest impact. This is why marked differences were observed in the composition, abundance and diversity of the mesozooplankton between the highest impact area and the sampling stations located in the headwaters of the estuary, particularly in CP. Furthermore, the high organic and inorganic nutrients and the alleged presence of toxic substances in the discharge area seem to induce a low overall abundance of mesozooplankton and dominance of certain taxa such as the copepods A. tonsa and E. americana which favourably adapt to these conditions. These phenomena explain the low species diversity recorded in this area with respect to those recorded at the other sampling stations.
Finally, this work not only emphasise the importance of an environmental monitoring study in the Bahìa Blanca estuary but also highlight the importance of ecological analyses as an essential tool for the evaluation of anthropogenic effects on ecosystems.
References
Arias, A. H., Spetter, C. V., Freije, R. H., Marcovecchio, J. E., et al. (2009). Polycyclic aromatic hydrocarbons in water, mussels (Brachidontes sp., Tagelus sp.) and fish (Odontesthes sp.) from Bahìa Blanca Estuary, Argentina. Estuarine, Coastal and Shelf Science. doi:10.1016/j.ecss.2009.06.008.
Arias, A. H., Vazquez-Botello, A., Tombesi, N., Ponce-Vèlez, G., Freije, R. H., Marcovecchio, J. E., et al. (2010). Presence, distribution, and origins of polycyclic aromatic hydrocarbons (PAHs) in sediments from Bahìa Blanca Estuary, Argentina. Environmental Monitoring and Assessment. doi:10.1007/s10661-008-0696-5.
Avent, S. R. (1998) Distribution of Eurytemora americana (Crustacea, Copepoda) in the Dwamish River estuary, School of Oceanography Report of project: University of Washington, USA, p. 12.
Baldini, M. D., & Cabezalí, C. B. (1988). Distribución de Escherichia coli en aguas del estuario de Bahía Blanca, Argentina. Revista Latinoamericana de Microbiología, 30, 229–234.
Baldini, M. D., Cubitto, M. A., Chiarello, M. N., Cabezalí, C. B., et al. (1999). Water quality for aquaculture development in Bahía Blanca estuary. Argentina. Bacteriological studies. Revista Argentina de Microbiología, 31, 19–24.
Barría de Cao, M. S., Pettigrosso, R., Parodi, E., Freije, R., et al. (2003). Abundance and species composition of planktonic Ciliophora from the wastewater discharge zone in the Bahía Blanca estuary, Argentina. Iheringia, 93(3), 229–236.
Bianchi, F., Acri, F., Bernardi Aubry, F., Berton, A., Boldrin, A., Camatti, E., et al. (2003). Can plankton communities be considered as bio-indicators of water quality in the Lagoon of Venice? Marine Pollution Bulletin. doi:10.1016/S0025-326X(03)00111-5.
Bochert, R., Fritzschie, D., & Burckhardt, R. (1996). Influence of salinity and temperature on growth and survival of planktonic larvae of Marenzelleria viridis (Polychaeta, Spionidae). Journal of Plankton Research. doi:10.1093/plankt/18.7.1239.
Bremec, C. S., Martínez, D., & Elías, R. (2004) Asociaciones bentónicas de fondos duros y comunidades incrustantes. In M. C. Piccolo & M. S. Hoffmeyer (Eds.) Ecosistema del estuario de Bahía Blanca (pp. 171–178). Bahía Blanca, Argentina: Instituto Argentino de Oceanografía (IADO-CONICET).
Caulleaud, K., Forget-Leray, J., Peluhet, L., LeMenach, K., Souissi, S., Budzinski, H., et al. (2009). Tidal influence on the distribution of hydrophobic organic contaminants in the Seine Estuary and biomarker responses on the copepod Eurytemora affinis. Environmental Pollution, 157, 64–71.
Cervellini, P. M. (1986). Larvas y postlarvas de crustacéos Decapoda del estuario de Bahía Blanca (Argentina). Aspectos cualitativos. Spheniscus, 3, 1–23.
Cervellini, P. M. (2001). Variabilidad en la abundancia y retención de larvas de crustáceos decápodos en el estuario de Bahía Blanca, Provincia de Buenos Aires, Argentina. Investigaciones Marinas, 29(2), 25–33.
Clarke, K. R., & Warwick, R. M. (1994). Change in marine communities: An approach to statistical analysis and interpretation. Cambridge: Natural Environment Research Council of United Kingdom.
Curds, C. (1982). Pelagic protists and pollution. A review of the past decade. Annales de l’Institut océanographique, 58(S), 117–136.
Danulat, E., Muniz, P., García-Alonso, J., Yannicelli, B., et al. (2002). First assessment of the highly contaminated harbour of Montevideo, Uruguay. Baseline/Marine Pollution Bulletin, 44, 551–576.
Eberlein, K., & Kattner, G. (1987). Automatic method for the determination of orthophosphate and total dissolved phosphorous in the marine environment. Fresenius’ Journal of Analytical Chemistry, 326, 354–357.
Fernández-Severini, M. D., Bottè, S. E., Hoffmeyer, M. S., Marcovecchio, J. E., et al. (2009). Spatial and temporal distribution of cadmium and copper in water and zooplankton in the Bahìa Blanca estuary, Argentina. Estuarine, Coastal and Shelf Science. doi:10.1016/j.ecss.2009.03.019.
Fleeger, J. W., Carman, K. R., & Nisbet, R. M. (2003). Indirect effects of contaminants in aquatic ecosystems. The Science of the Total Environment. doi:10.1016/S0048-9697(03)0014-4.
Freije, R. H., & Ganoso, A. M. (1988). Producción primaria del estuario de Bahía Blanca. Informes UNESCO, Ciencias del Mar, 47, 112–114.
Freije, R. H., Zavatti, J. R., Gayoso, A. M., Asteasuain, R. O., et al. (1980). Pigmentos, producción primaria y fitoplancton del estuario de Bahía Blanca. 1) Zona interior-Puerto Cuatreros, Contribución Científica 46Instituto Argentino de Oceanografía, Bahía Blanca, p.13.
Freije, R., Asteasuain, R., Sagua de Schmidt, A., Zavatti, J., et al. (1981) Relación de la salinidad y temperatura del agua con las condiciones hidrometeorológicas en la porción interna del estuario de Bahía Blanca, Contribución Científica 57: Instituto Argentino de Oceanografía, Bahía Blanca, p. 20.
Fritzsche, D., & von Oertzen, J. A. (1995). Bioenergetics of a highly adaptable brackish water polychaeta. Thermochimica Acta, 251, 1–9.
Gayoso, A. M. (1983). Estudio del fitoplancton del estuario de Bahía Blanca (Buenos Aires, Argentina). Studia Oecologica, 2, 73–88.
Gayoso, A. M. (1988). Variación estacional del fitoplancton en la zona más interna del estuario de Bahía Blanca (Argentina). Gayana, Botánica, 45, 241–248.
Grasshoff, K. (1983). Methods of seawater analysis (2nd ed.). Weinheim: Verlag Chemier.
Hoffmeyer, M. S. (1983). Zooplancton del área interna de la Bahía Blanca (Buenos Aires, Argentina). I-Composición faunística. Historia Natural, 3(8), 73–94.
Hoffmeyer, M. S. (1994). Seasonal succession of Copepoda in the Bahía Blanca Estuary. Hydrobiologia, 292(293), 303–308.
Hoffmeyer, M. S. (2004). Decadal change in zooplankton seasonal succession in the Bahía Blanca estuary, Argentina following introduction of two zooplankton species. Journal of Plankton Research. doi:10.1093/plankt/fbh023.
Hoffmeyer, M. S., & Cervellini, P. M. (2004). Macro-zooplancton del estuario y aguas costeras adyacentes. In M. C. Piccolo & M. S. Hoffmeyer (Eds.) Ecosistema del estuario de Bahía Blanca (pp. 143–151). Bahía Blanca, Argentina: Instituto Argentino de Oceanografía (IADO-CONICET).
Hoffmeyer, M. S., & Prado-Figueroa, M. (1997). Integumental structures in the oral field of Eurytemora affinis and Acartia tonsa (Copepoda, Calanoida) in relation to their trophic habits. Crustaceana, 70, 257–271.
Hoffmeyer, M. S., Tumini, L., Pettigrosso, R., Barrìa, M. S., Contradi, E., et al. (2004). In: Estudio de la calidad de agua en la Rìa de Bahìa Blanca, Convenio MBB-UNSR Reporte final, p. 98.
Kimor, B. (1991). The impact of eutrophication on phytoplankton composition in coastal marine ecosystems. Science of the Total Environment, 131, 871–878.
Kimor, B. (1992). Changes and stress signs in planktonb communities as a result of man-induced perturbations in enclosed coastal seas (Mediterranean, Baltic). Marine Pollution Bulletin, 23, 171–174.
Lara, R. J., & Pucci, A. E. (1982). Distribución espacio temporal de nutrientes en la Bahía Blanca. Acta Oceanográfica, 3(2), 113–134.
Lara, R. J., Gomez, E. A., & Pucci, A. E. (1985). Organic matter, sediment particle size and nutrient distributions in a sewage affected shallow channel. Marine Pollution Bulletin. doi:10.1016/0025-326X(85)90087-6.
Lardicci, C., Rossi, F., & Castelli, A. (1997). Analysis of macrozoobenthic community structure after severe dystrophic crises in a mediterranean coastal lagoon. Marine Pollution Bulletin. doi:10.1016/S0025-326X(96)00164-6.
Li, K. Z., Yan, J. Q., Huang, L. M., Tan, Y. H., et al. (2006). Spatial and temporal variations of mesozooplankton in the Pearl River estuary, China. Estuaries Coastal and Shelf Research. doi:10.106/j.e.css.2005.12.008.
Lorenzen, C. L. (1967). Determination of chlorophyll-a and phaeopigments. Spectrophotometric equations. Limnology and Oceanography, 12, 343–346.
Marcovecchio, J. E., Moreno, V. J., & Pérez, A. (1986). Bio-magnification of total mercury in Bahía Blanca Estuary shark. Marine Pollution Bulletin, 16, 360–364.
Margalef, R. (1977). Ecología. Barcelona: Edición Omega, S. A.
Mayer-Pinto, M., & Junqueira, A. O. R. (2003). Effects of organic pollution on the initial development of fouling communities in a tropical Bay, Brazil. Marine Pollution Bulletin. doi:10.1016/S0025-326X(03)00249-2.
Menèndez, M. C., Piccolo, M. C., Hoffmeyer, M. S., Sassi, M., et al. (in press). Estuarine mesozooplankton dynamics on a short-term time scale: Role of semidiurnal tidal cycle. Brazilian Journal of Oceanography.
Meyer-Reil, L. A., & Köster, M. (2000). Eutrophication of marine waters: Effects of benthic microbial communities. Marine Pollution Bulletin. doi:10.1016/S0025-326X(00)00114-4.
Perillo, G. M. E., & Piccolo, M. C. (1991). Tindal response in the Bahìa Blanca estuary, Argentina. Journal of Coastal Research, 7, 437–449.
Perillo, G. M. E., Piccolo, M. C., Parodi, E., Freije, R. H., et al. (2001). The Bahía Blanca Estuary, Argentina. In U. Seeliger & B. Kjerfve (Eds.), Coastal marine ecosystem of Latin America (pp. 205–217). New York: Springer Verlag.
Perillo, G. M. E., Piccolo, M. C., Palma, E., Pérez, D., Pierini, J. et al. (2004). Oceanografía Física. In M. C. Piccolo & M. S. Hoffmeyer (Eds.), Ecosistema del estuario de Bahía Blanca (pp. 61–67). Bahía Blanca, Argentina: Instituto Argentino de Oceanografía (IADO-CONICET).
Petipa, T. S. (1959). Feeding of the copepod Acartia clausi Giesbrecht. Proceedings of the USSR AS Sevastopol Biological Station, 11, 72–99.
Piccolo, M. C., & Perillo, G. M. (1990). Physical characteristics of the Bahía Blanca estuary (Argentina). Estuarine, Coastal and Shelf Science. doi:10.1016/0272-7714(90)90106-2.
Pielou, E. C. (1975). Ecological diversity. New York, USA: J. Wiley.
Popovich, C. A. (2004). Fitoplancton. In M. C. Piccolo & M. S. Hoffmeyer (Eds.) Ecosistema del Estuario de Bahía Blanca (pp. 69–78). Bahía Blanca, Argentina: Instituto Argentino de Oceanografía (IADO-CONICET).
Popovich, A. C., & Marcovecchio, J. E. (2008). Spatial and temporal variability of phytoplankton and environmental factors in a temperate estuary of South America (Atlantic coast, Argentina). Continental Shelf Research. doi:10.1016/j.csr2007.08.001.
Pucci, A. E., Freije, R. H., Asteasuain, R., Zavatti, J. R., Sericano, J. L., et al. (1979). Evaluación de la contaminación de las aguas y sedimentos de la Bahía Blanca., Contribución científica 52: Instituto Argentino de Oceanografía, Bahía Blanca, p. 90.
Sabatini, M. E. (1989). Ciclo anual del copépodo Acartia tonsa Dana, 1849 en la zona interna de la Bahía Blanca (Provincia de Buenos Aires, Argentina). Scientia Marina, 53, 847–856.
Sage, L. E., & Herman, S. S. (1972). Zooplankton of the Sandy Hook Bay Area, N.J. Chesapeake Science, 13, 29–39.
Saiz-Salinas, J. I. (1997). Evaluation of adverse biological effects induced by pollution in the Bilbao Estuary (Spain). Environmental Pollution, 96(3), 351–359.
Sarda, R., & Martin, D. (1993). Population of Streblospio (Polychaeta:Spionidae) in temperate zone: demography and production. Journal of the Marine Biological Association of the United Kingdom, 73, 769–584.
Sautour, B., & Castel, J. (1995). Comparative spring distribution of zooplankton in three macrotidal European estuaries. Hydrobiologia, 311, 139–151.
Siokou-Frangou, I., & Papathanassiou, E. (1991). Differentiation of zooplankton populations in a polluted area. Marine Ecology Progress Series, 76, 41–51.
Smith, V. H., Tilman, G. D., & Nekola, J. C. (1999). Eutrophication, impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution. doi:10.1016/S0269-7491(99)00091-3.
Spetter, C. V. (2006). Ciclo biogeoquímico de nutrientes inorgánicos de nitrógeno en los humedales del estuario de Bahía Blanca. Ph. D Thesis, Universidad Nacional del Sur (UNS), Bahía Blanca, Argentina, 147 p.
Strickland, J. D., & Parsons, T. R. (1968). Determination of particulate organic carbon. In J. D. Strickland & T. R. Parsons (Eds.) A practical Handbook of seawater analysis (pp. 207–211). Bulletin 167, Fisheries Research Board of Canada: Ottawa.
Thompson, B., Adelsbach, T., Brown, C., Hunt, J., Kuwabara, J., Neale, J., et al. (2007). Biological effects of anthropogenic contaminants in the San Francisco Estuary. Environmental Pollution, 105, 156–174.
Tombesi, N. B., Pistonesi, M. F., & Freije, R. H. (2000). Physico-chemical characterization and quality improvement evaluation of primary treated municipal waste water in the city of Bahía Blanca (Argentina). Ecology Environment and Conservation, 6(2), 147–151.
Treguer, P., & Le Corre, P. (1975). Manuel d’ analisis des sels nutritifs. Brest: Université de Bretagne occidentale.
Uriarte, I., & Villate, F. (2004). Effects of pollution on zooplankton abundance and distribution in two estuaries of the Basque coast (Bay of Biscay). Marine Pollution Bulletin, 49, 220–228.
Uye, S. I., & Fleminger, A. (1976). Effects of various environmental factors on egg development of several species of Acartia in Southern California. Marine Biology, 38, 252–262.
Vecchione, M. (1989). Zooplankton distribution in three estuarine bayous with different types of anthropogenic influence. Estuaries, 12(3), 169–179.
Wagner, J. M., Hoffmeyer, M. S., Tejera, L. A., Nizovoy, A., et al. (1993). Variación estacional de larvas y adultos de Balanus en el Puerto de Ingeniero White (Estuario de Bahía Blanca, Argentina) (pp. 79–86). Puerto Madryn, Argentina: Actas II Jornadas Nacionales de Ciencias del Mar.
Wolf, W. J. (1990). Anthropogenic influences and management of estuaries. Limnologica, 20(1), 153–156.
Acknowledgements
We are grateful to the staff of the Marine Chemistry Laboratory (IADO, Argentina), for facilitating data on the physical and chemical variables analysed in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biancalana, F., Menéndez, M.C., Berasategui, A.A. et al. Sewage pollution effects on mesozooplankton structure in a shallow temperate estuary. Environ Monit Assess 184, 3901–3913 (2012). https://doi.org/10.1007/s10661-011-2232-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2232-2