Abstract
Nematodes present high potential as a biological indicator of soil quality. In this work, it was evaluated relations between soil physical properties and nematode community under sugarcane cropping and remaining of Atlantic Forest areas in Northeastern Pernambuco, Brazil. Soil samples were collected from September to November 2009 along two 200-m transects in both remaining of Atlantic Forest and sugarcane field at deeps of 0–10, 10–20, 20–30, 30–40, and 40–50 cm. For soil characterization, it was carried out analysis of soil size, water content, total porosity, bulk density, and particle density. The level of soil mechanical resistance was evaluated through a digital penetrometer. Nematodes were extracted per 300 cm3 of soil through centrifugal flotation in sucrose being quantified, classified according trophic habit, and identified in level of genus or family. Data were analyzed using Pearson correlation at 5% of probability. Geostatistical analysis showed that the penetration resistance, water content, total porosity, and bulk density on both forest and cultivated area exhibited spatial dependence at the sampled scale, and their experimental semivariograms were fitted to spherical and exponential models. In forest area, the ectoparasites and free-living nematodes exhibited spherical model. In sugarcane field, the soil nematodes exhibited pure nugget effect. Pratylenchus sp. and Helicotylenchus sp. were prevalent in sugarcane field, but in forest, there was prevalence of Dorylaimidae and Rhabditidae. Total amount of nematode did not differ between environments; however, community trophic structure in forest presented prevalence of free-living nematodes: omnivores followed by bacterial-feeding soil nematodes, while plant-feeding nematodes were prevalent in sugarcane field. The nematode diversity was higher in the remaining of Atlantic Forest. However, the soil mechanical resistance was higher under sugarcane cropping, affecting more directly the free-living nematodes; especially Dorylaimidae which was the most sensible to changes in soil physical properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cultivation of sugarcane (Saccharum spp.) has great economic importance for Brazil, which holds the title of world’s largest producer. The state of Pernambuco is currently the second largest producer of Brazil with 334,000 ha cultivated (CONAB 2010). With the expansion of the crop due to economic incentives in the 1980s, many undisturbed places have been incorporated to areas of the mills, fragmenting native vegetation areas of the Atlantic Forest (Silva and Tabarelli 2000). Besides the high waste generation, intensification of mechanization has intensified soil compaction mainly by machinery heavy traffic (Seixas and Oliveira Júnior 2001; Souza et al. 2010).
Soil compaction is a major cause of soil physical degradation. Determining the impact of management practices in the physical and biological soil attributes is imperative, because it alters the soil physical properties and reduces productivity by limiting microbial activities (Lanzanova et al. 2007). Among the soil physical attributes used to determine soil compaction, the mechanical resistance to penetration has been considered the most sensitive indicator, easy and quick application, widely used to assess management systems, because it is directly related to the growth of plants (Busscher et al. 2000; Serafim et al. 2008).
Conversely, the Atlantic Forest is considered one of the hotspots of global biodiversity, although there are only 11.73% of its original cover (Myers et al. 2000; Ribeiro et al. 2009). In the Northeast of Brazil, there are only 2% of the original area (Silva and Tabarelli 2000). As a result of intense exploitation of forest resources, it has now become fragmented and impoverished as its composition (Souza et al. 2002). Fact that makes relevant knowledge of soil physical and biological aspects of the few remaining still exists.
Nematodes constitute the group of invertebrates that are abundant and diverse, being found occupying almost all ecological niches. The structure and composition of their communities undergo change when changes occur in vegetation (Pattison et al. 2008), in soil (Gupta and Yeates 1997; Yeates and Pattison 2006), in environmental conditions, whether natural or triggered by human activities. In addition, studies related to nematode community show that the environmental disturbances will lead predominance of certain taxa (Wasilewska 1997; Yeates 1999; Goulart and Ferraz 2003; Pattison et al. 2008).
The soil properties can directly or indirectly affect the composition and structure of nematode communities (De Goede and Bongers, 1994; Yeates 1999). Specifically, changes in the soil physical properties directly influence the habitat and food chain of the nematodes (Neher 1999). Often the physical properties such as texture, water content, and soil structure are correlated with the nematodes communities (Yeates and Bongers 1999; Kandji et al. 2001; Gomes et al. 2003; Kimenju et al. 2009), presenting an important role in the abundance, distribution, and structure of nematodes communities. This is one reason that validates the potential of nematodes as bioindicators of soil quality (Kandji et al. 2001; Qi and Hu 2007; Mondino et al. 2009). Due to the abundance, morphology, rapid response to environmental changes, and management actions of the soil, short reproductive cycle, and food specificity, the nematodes have great potential bioindicator. And, consequently, the analysis of the structure of their community could complement the conventional analysis of soil (Bongers 1990; Urzelai et al. 2000; Neher 2001, Pattison et al. 2008).
Geostatistical methods quantify and characterize the spatial variability. In a certain way, the use of these methods has become popular in soil science. Many studies have been conducted on the spatial patterns of soil properties (Goovaerts 1998; Utset and Cid 2001; Amirinejad et al. 2011) and of soil nematodes (Wallace and Hawkins 1994; Shi et al. 2008; Dinardo-Miranda and Fracasso 2010). Despite, more studies for characterization of the spatial variability of soil attributes is essential to a better understanding of complex relations between soil properties and other environmental factors.
Concern about the integrity of the environment necessitates the search for sustainable strategies that maximize productivity and minimize the impact to the environment. Thus, the aim of the present study was to characterize the trophic structure of nematodes communities in soil under sugarcane continuous cropping and remaining of Atlantic Forest areas in Northeastern Pernambuco, Brazil, correlating nematode community structure with mechanical compaction of the soil.
Material and methods
Study sites
This study was conducted in an area (19.14 ha, 7°36′48″ S; 34°58′45.9″ W) continuous cultivated with sugarcane managed under conventional tillage for over 20 years, and in a remaining area of Atlantic Forest (7°36′45.7 ″S; 35°00′47.6″ W) both located in the Engenho Bujari owned by Usina Santa Tereza—CAIG, Municipality of Goiana, Pernambuco. The areas are characterized by sandy soils. Local climate, according to Köppen Climate Classification, is humid tropical type As’ or pseudotropicalis, characterized by hot and humid, with rains from autumn to winter, and temperature annual averages ranging around 24°C.
Soil sampling
Sampling points were established from September to November 2009 along two 200-m crossed transects in both forest fragment and sugarcane cultivation. Soil cores were taken every 20 m, with Uhland sampler modified of SONDATERRA®. At each point, it was sampled soil for physical analysis and soil cores collected at depth 0–10, 10–20, 20–30, 30–40, and 40–50 cm for nematodes’ identification and density. Additionally, to verify the spatial variability, the sampling was made each 10 m at depth 0–10 cm.
Nematodes analyses
The samples for nematodes were processed from aliquots of 300 cm3 of soil by the method of centrifugal flotation in sucrose solution (Jenkins 1964). The suspensions obtained were placed in vials and stored under refrigeration until the moment of reckoning. The population estimate was obtained by counting on Peters’ slides, under an optical microscope, with two replications, and results computed per 300 cm3 of soil. Nematodes were classified according to feeding habits in five trophic groups (plant-feeders, bacterivores, fungivores, predators, and omnivores), based on the morphology of the stoma and esophagus, according to Yeates et al. (1993). Plant-feeding nematodes were identified to genus level according to the May et al. (1996). Nematode community structure was described by trophic groups and the ratios fungivores/bacterivores (F/B) and omnivores + predators/bacterivores + fungivores + plant-feeders (O + P)/(B + F + PF) (Gomes et al. 2003).
Physical properties analyses
For measure of soil compaction, it was used digital record penetrograph PNT-2000 Soil Control®. The acquisition mode was vertical with measurement of soil compaction to 400 mm in both studied sites. The cone used was the type 2 (medium) with an area of 129 mm2.
The following soil physical analyses were performed at the Laboratory of Soil Mechanics and Waste Utilization, according to EMBRAPA (1997):
-
1.
Particle size analysis: carried out by the hydrometer method using sodium hydroxide as dispersant
-
2.
Soil water content: determinate by thermogravimetric method, which is to weigh the mass of moist soil and then dry it in an oven at 105–110°C for 24 h, and after, to determine their dry weight
-
3.
Soil particle density: measured using 50-mL volumetric flask using 20 g of air-dried soil and alcohol as fluid to determine the volume occupied by the particles
-
4.
Soil bulk density: check it occurred after the assessment of water content and determined by the volumetric ring method, which relies on the use of a ring of sharp edges with known internal capacity
-
5.
Total porosity: calculated from the values of soil particle density (Dp) and soil bulk density (Ds), according to the formula: \( P = \left( {{\text{Dp}} - {\text{Ds}}} \right) \times \left( {\frac{{{100}}}{\text{Dp}}} \right) \)
Statistical and spatial variability analysis
Through analysis of data generated by physical and nematodes analysis were made calculations of Pearson’s correlation coefficients using the program Statistical Analytical System (SAS). For spatial variability analysis, the soil physical properties (penetration resistance, water content, total porosity, and bulk density), and nematodes communities (plant-feeders and free-living) were analyzed by descriptive statistic and geostatistical techniques. The normality hypothesis of data was tested by Kolmogorov–Smirnov Test using the software SAS (Schlotzhaver and Littell, 1997).
Concerning to variability of analyzed attributes was adopted the classification of Warrick and Nielsen (1980), where the variability is classified as low (CV < 12%), mean (12 ≤ CV ≤ 62%), and high (CV > 62%). For knowledge, the degree of spatial dependence was used criteria reported by Cambardella et al. (1994). In that, the parameters are computed by finding the percentage ratio of nugget to sill value of semivariogram, which if found less than 25% is considered as the indicator of strong spatial dependence and if between 26% and 75% is indicator of moderate spatial dependence; otherwise, the variable have a weak spatial dependence.
The spatial dependence was examined by geostatistical tools: GS + (Robertson 1998) and Geostatistical Environmental Assessment Software (Englund and Sparks 1992), adopting the classic estimator of semivariance (Journel and Huijbregts 1978), where γ is the estimated semivariance value since of experimental data, N(h) is the pairs number of observation Z(x i ) and Z(x i + h) separated by distances h (Landim 1998).
The parameters of theoric semivariogram were represented in the graphics defined by the range (a), sill (C 1), and nugget effect (C 0), fitted so that the mean squared errors are minimized. The semivariograms models are spherical, linear, exponential, and Gaussian (McBratney and Webster 1986). The adjustments of the models were chosen on the parameters of semivariograms, coefficients of determination (R 2), and self-validation (“Jack-Knifing”).
The trend was verified and removed using the procedure described by Davis (1986), whereby one can fit a polynomial to the property values as a function of the coordinates and cause the residual to be obtained by difference between measured and estimated values by polynomial in each point.
Results and discussion
Soil nematodes
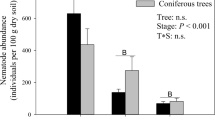
Total nematode abundance
The nematode community composition varied within studied environments (Table 1). In sugarcane field, it was identified seven genera of plant-feeders and five families: bacterivores (two families), fungivores (one family), omnivores (one family), and predators (one family). In the forest soil, it was identified, in addition to previous measurements, more five genera of plant-feeders (Table 1).
In this study, omnivores were more representatives in forest rather than sugarcane soil, with 30% of dominance followed by bacterivores Rhabditidae, with 19%. This group has been considered more sensitive to environmental changes, because they occupy higher levels in food chains. In stable environments, they are numerous and plentiful (Wasilewska 1997; Yeates 1999). Gomes et al. (2003) and Goulart and Ferraz (2003) pointed out the sensitivity of Dorylaimidae to cultural practices, resulting in low densities in cultivated soils, whereas its presence is synonymous of stability in the ecosystem.
Plant-feeding nematodes tend to be favored by monocultures (Pattison et al. 2008). Sites with many stresses or environmental disturbances, such as the intensively cultivated agroecosystems, provide the prevalence of the plant-feeder communities (Cares and Huang 1991; Niles and Freckman 1998; Gomes et al. 2003; Mondino et al. 2006, 2009). Thus, the results hereby are consistent with this assertion; plant-feeders prevailed under monoculture with dominance of 81%, highlighting the genera Pratylenchus and Helicotylenchus with dominance of 39% and 25%, respectively. These ratios were similar to those found by Mondino et al. (2009) in experiments with pineapple, beans, maize-bean intercropping and arrowroot, as well those results of Kimenju et al. (2009) in crops with beans and corn, and Li et al. (2007) in maize cultivation.
In the forest, there was a predominance of free-living nematodes, which corresponded to 64% of total abundance, confirming the results found by Kimenju et al. (2009) also in the forest. This response must be linked to the fact that forests have a higher content of organic matter in soil, which favors microbian fauna, allowing dominance of free-living nematodes (Papatheodorou et al. 2004). However, bacterivores nematodes corresponded to 21%. These usually are associated with the decomposition of organic matter, which explains its prominence in the forest. Yeates (2003) observed that soil water content and soil organic matter provide a relative increase in the number of species in Rhabditidae. Yeates and Bongers (1999) emphasized bacterivores dominance in forest under temperate conditions also and found low contribution of predators and plant-feeders in that environment. According to Niles and Freckman (1998), the Rhabditidae family predominates in initial phases of organic matter decomposition and over time will be replaced by Cephalobidae family, which is predominating in a mature community. Thus, nematode communities have not reached the climax in the areas of study, since Rhabditidae was prevalent.
The plant-feeders in forest area accounted for 36%, in which the predominant genera were Xiphinema and Tylodorus, both with 12% of dominance. Mattos (2002) also noted the prevalence of Xiphinema, although in the native area of Savannah. Mattos et al. (2008) observed dominance of this genus in cultivation of Eucalyptus spp. and Pinus caribaea, associating it with cultural systems with minimum tillage corroborating Cadet et al. (2003).
Mononchidae accounted for 5% in sugarcane area and 12% in the forest. Regulating the food web by predation on other nematodes (Grewal et al. 2005), the low trophic dominance of predators is a characteristic feature of annual crops (Mattos 2002). On the other hand, the contrast of omnivore dominance on soil under sugarcane and forest (8% and 30%, respectively) corroborates with Gomes et al. (2003) that reported omnivore prevalence higher than 25% under low human intervention.
Nematode community structure
In the sugarcane area, the plant-feeders are correlated to the other trophic groups (Table 2). Gomes et al. (2003) found a strong correlation between plant-feeders and both fungivores and predators and also between predators and fungivores. In this study, Meloidogyne correlated negatively with Pratylenchus. Umesh and Ferris (1994) observed that species feeding the roots first inhibited penetration by the latter species. The free-living nematodes stood out, and Rhabditidae correlated with Dorylaimidae, Mononchidae, Aphelenchidae, and plant-feeders. On forest, there was significant correlation between Rhabditidae and plant ectoparasites and between Helicotylenchus and Hemicycliophora. Tylodorus correlated with Meloidogyne, Pratylenchus, and Xiphinema (Table 3).
Nematode community indices
The ratio of fungivores to bacterivores nematodes (F/B) is known to be an important indicator of the relative organic matter decomposition pathway in detrital food webs (Sohlenius and Sandor 1987). In this study, the F/B ratio was lower than 1 in both sugarcane (0.30 ± 0.78) and in forest (0.04 ± 0.18) areas. Liang et al. (2001) recorded similar values in peanut cropping; Tong et al. (2010) in forest and Steinberger et al. (2001) in desert. The low F/B ratio indicates decomposition of organic matter was based more on bacterial than fungal action. Bacterial population favors the bacterivore communities directly reflecting in the decomposition of organic matter (Freckman and Caswell 1985). Thus, the organic material decays faster (Porazinka and Coleman 1995), because the decomposition rate of bacteria-based food chain is faster than that based on fungi, reflecting the structure of soil microbial communities (Liliana 2003).
The ratio O + P/B + F + PF reflected lower incidence of omnivores and predators in relation to other trophic groups, with higher value in forest (0.86) than in sugarcane area (0.16). This low value is reflection of annual crops (Neher and Campbell 1994).
Correlations between soil physical properties and nematode communities
The nematode communities associated with the study sites was correlated with soil physical properties (Table 4). The soils of the sites evaluated are characterized by sandy to sandy loam texture. In the forest, the positive correlations were expressed between the sand particle size fraction and the Rhabditidae and Hemiciclyophora. This can be supported by the fact that bacterivores require larger pore spaces for their activities while the plant-feeders are favored in soils with sandy texture. Olabiyi et al. (2009), studying nematode communities in environments with different soil textures, found prevalence of plant-feeders in sandy soils also. Negative correlations were expressed between sand and the Acrobeles, silt and both Aphelechoide and Tylodorus, clay and Hemicycliophora.
The positive correlation between soil water content and Mononchidae could be favored by higher water content in the soil, facilitating their movement in search of their prey. In contrast, the plant-feeder Hemicycliophora showed negative correlation. In the forest area, the correlation between soil water content and both Mononchidae and Tylodorus was negative. This behavior may be associated with greater competition among the representatives of other trophic groups.
Criconemella was the only taxon that showed positive correlation with soil depth; although Li et al. (2007) claim that the plant-feeders distribution in the soil profile is a reflection of the roots distribution. However, in forest area, there was a negative correlation between soil depth and the free-living nematodes: Rhabditidae, Dorylaimidae, and Mononchidae, showing that they are concentrated in the surface layers of soil, which agrees with the results of Tita et al. (2002) and Meng et al. (2006).
In the sugarcane area, the soil bulk density was positively correlated with the plant-feeders Hemicycliophora and Pratylenchus, and negatively with Meloidogyne, Mononchidae, and Xiphinema, although Meloidogyne and Xiphinema correlated positively with soil total porosity, demonstrating these genera are pore spaces dependent, essential for gas exchange taking place via the cuticle. Olabiyi et al. (2009) also observed the predominance these nematodes in sandy soils, whereas Hemicycliophora and Pratylenchus had negative correlations with the soil total porosity. These results agree with those on Pratylenchus observed by Li et al. (2007), highlighting the plight of this genus by pore spaces.
In the forest, just Paratrichodorus correlated negatively with soil total porosity, which shows the preference of this genus by pore spaces, unlike Rhabditidae and Tylodorus which presented negative correlation with soil bulk density. Moreover, Rhabditidae was positively correlated with soil total porosity, confirming that the free-living nematodes react negatively to the decrease of pore spaces in which concentrate their activities (Jones and Thomasson 1976; De Goede and Bongers 1994; Bouwman and Arts 2000).
The free-living nematodes were negatively correlated with soil penetration resistance, which may have occurred due to the decrease of pore spaces by increasing of the soil bulk density. Cephalobidae inversely correlated with soil total porosity, which was also revealed by Yeates (2003), suggesting a possible relationship between soil degradation and this family. The positive correlation between Criconemella and penetration resistance agree with the results found by Bouwman and Arts (2000), revealing that the plant-feeders are influenced positively in more compacted soils, as well as by the greater soil bulk density.
Soil physical properties
Soil penetration resistance
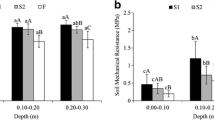
The soil mechanical resistance correlated with the soil bulk density (Table 5). Similar results were obtained by Ribon et al. (2003), Araújo et al. (2005) and Serafim et al. (2008). In contrast, penetration resistance correlated negatively with soil water content, corroborating the claims of Imhoff et al. (2001), Utset and Cid (2001), Vaz et al. (2001), Castrignano et al. (2002), Cunha et al. 2002, Genro Júnior et al. (2004), and Kılıç et al. (2004).
Both the bulk density as the penetration resistance were correlated with depth (Table 5), as reported by Franzen et al. (1994) and Bouwman and Arts (2000). Consequently, both were negatively correlated with the soil total porosity. This result resembles that of Lopes et al. (2007). Soil penetration resistance was higher in areas under sugarcane, showing higher levels of compaction when compared to the native area (Fig. 1). According to Bouwman and Arts (2000), penetration resistance is higher in areas more compact. Costa et al. (2003) showed that conventional tillage compared with native vegetation, increased penetration resistance due to degradation of properties related to the shape and stability of soil structure, also shown by the increase in soil bulk density. Results also correlated with the observations of Valpassos et al. (2001) and Araújo et al. (2004), which show that the soil bulk density was lower in the native area when compared with agricultural systems managed or not.
Soil water content
In sugarcane area, the soil water content was negatively correlated with soil bulk density and sand. And there was positive correlation with total porosity, silt, and clay. This result emphasizes that the increase in soil water content decreases the soil bulk density, and consequently also decreases total porosity, corroborating to Timm et al. (2006) that claims that the soil water content influences important process in the soil, such as: soil compaction, water movement, soil aeration, and roots development.
Soil bulk density
In both studied sites, the soil bulk density showed positive correlation with the depth, sand, clay, and penetration resistance. The bulk density typically increases with the depths because the subsurface layers have reduced organic matter compared surface layer. Thus, the organic matter has been used to improve soil conditions, consequently, preventing the soil compaction. On the other hand, the soil bulk density had negative correlation with the water content, total porosity, and silt.
Soil total porosity
In both areas, the particle density had positive correlation with the soil total porosity and bulk density negative. However, penetration resistance presented negative correlation in sugarcane area. This result means the bulk density and the penetration resistance are directly influenced by total porosity. Confirming data found by Araújo et al. (2004) that also compare forest and cultivated soil observed that bulk density and total porosity affected the penetration resistance.
Soil texture
Regarding the size fractions, the clay was positively correlated with the bulk density, similar to the result of Kılıç et al. (2004). The clay was also positively correlated with water content and silt. The sand was negatively correlated with water content and positively with total porosity and bulk density. The silt was positively correlated with the water content and total porosity and negatively with sand and soil bulk density.
Spatial variability analyses of soil physical properties and soil nematodes
The Kolmogorov–Smirnov Test for forest and sugarcane areas indicated normality for soil physical properties and soil nematodes (Table 6). According to classification proposed by Warrick and Nielsen (1980) the coefficients of variation (CV) for soil nematodes were extremely elevated in both studies sites, presenting higher differences between maximum and minimum population (Table 6), except plant-feeders nematode in sugarcane area. This result corroborate with Shi et al. (2008) when comparing degraded with meliorated area, obtaining CV in degraded area similar to this study in sugarcane area.
Soil physical properties variation was lower than the nematodes, except for total porosity in sugarcane area and bulk density in both areas. The bulk density CV was relatively low as compared to the other measured properties, this value agrees with those values reported for Utset and Cid (2001) and Amaro Filho et al. (2007).
Based in the obtained experimental semivariances in the studied sites (Table 7), the semivariogram models fitted of soil water content, soil total porosity and penetration resistance exhibited spherical model but exponential model for the soil bulk density. Spherical model was the best fitted for ectoparasites and free-living nematodes under forest. The estimated parameters for the models (C 0, C 1, a) were validated by criteria of Jack-Knifing, since the standard errors showed mean near to zero and standard deviation near to one (Table 7).
Range values obtained for soil physical properties (penetration resistance, water content, total porosity, and bulk density) in both forest and sugarcane areas ranged of 27 to 95 and 15 to 90 m, respectively (Table 7). Nematodes (plant ectoparasites and free-living) in forest presented ranges of 23 and 30 m. These results mean that the classic statistic can be applied from collected samples above of these distances, since collected points with distance higher than the range are independents.
The semivariograms corresponding to the soil nematodes in sugarcane area exhibited pure nugget effect (Fig. 2a–d). This result is in agreement with those reported by Dinardo-Miranda and Fracasso (2010) that characterized the spatial and temporal variability of plant-feeders nematodes in sugarcane and observed this behavior in 22 of 52 calculated semivariograms. In forest, the plant endoparasites and plant-feeders nematodes also could not be fitted to any model (pure nugget effect, Fig. 3a, c), indicating that the spatial distribution occurred at random. Thus, the spatial variability of the soil physical properties was similar in both forest and sugarcane areas. However, the plant endoparasites nematodes no presented spatial dependence in forest, as well as, the soil nematodes in sugarcane, presenting at random. The plant ectoparasites and free-living nematodes in forest show up in aggregate, but in sugarcane, they did not have dependence, showing up uniform. This fact can be reflex of the applied management in the conventional sugarcane system, causing the spread nematodes stay more regularly.
Conclusion
The total amount of nematodes did not differ between the environments, but in forest the free-living nematodes: omnivorous followed by bacterivores were the most prevalent. While in area under sugarcane, plant-feeders were the most prevalent.
-
Dorylaimidae demonstrated greater sensitivity to changes in soil physical properties.
-
Nematode diversity of soil nematodes was higher in the remaining of Atlantic Forest.
-
The soil penetration resistance was higher in area cultivated with sugarcane.
-
Spatial variability of the soil physical properties was similar in both forest and sugarcane areas.
-
The plant endoparasites nematodes do not present spatial dependence in forest, as well as, in sugarcane, presenting at random.
-
The plant ectoparasites and free-living nematodes in forest showed up in aggregate but in sugarcane showed up in uniform.
References
Amaro Filho, J., Negreiros, R. F. D., Assis Júnior, R. N., & Mota, J. C. A. (2007). Amostragem e variabilidade espacial de atributos físicos de um latossolo vermelho em Mossoró, RN. Revista Brasileira de Ciência do Solo, 31(3), 415–422.
Amirinejad, A. A., Kamble, K., Aggarwal, P., Chakraborty, D., Pradhan, S., & Mittal, R. B. (2011). Assessment and mapping of spatial variation of soil physical health in a farm. Geoderma. 160(3–4), 292–303.
Araújo, M. A., Tormena, C. A., & Silva, A. P. (2005). Propriedades físicas de um Latossolo Vermelho distrófico cultivado e sob mata nativa. Revista Brasileira de Ciência do Solo, 28(2), 337–345.
Araújo, E. A., Lani, J. L., Amaral, E. F., & Guerra, A. (2004). Uso da Terra e propriedades físicas e químicas de Argissolo Amarelo distrófico na Amazônia Ocidental. Revista Brasileira de Ciência do Solo, 28(2), 307–315.
Bongers, T. (1990). The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia, 83(1), 14–19.
Bouwman, L. A., & Arts, W. B. M. (2000). Effects of soil compaction on the relationships between nematodes. grass production and soil physical properties. Applied Soil Ecology, 14(3), 213–222.
Busscher, W. J., Frederick, J. R., & Bauer, P. J. (2000). Timing effects of deep tillage on penetration resistance and wheat and soybean yield. Soil Science Society of America Journal, 64(3), 999–1003.
Cadet, P., Pate, E., & Thioulouse, J. (2003). Relationship of nematode communities to human demographics and environment in agricultural fields and fallow lands in Senegal. Journal of Tropical Ecology, 19(3), 279–290.
Cambardella, C. A., Moorman, T. B., Novak, J. M., Parkin, T. B., Karlen, D. L., Turco, R. F., et al. (1994). Field scale variability of soil properties in Central Iowa soils. Soil Science Society of America Journal, 58(5), 1501–1511.
Cares, J. H., & Huang, S. P. (1991). Nematode fauna in natural and cultivated cerrados of Central Brazil. Fitopatologia Brasileira, 16(3), 199–209.
Castrignano, A., Maiorana, M., Fornaro, F., & Lopez, N. (2002). 3D spatial variability of soil strength and its change over time in a durum wheat field in southern Italy. Soil and Tillage Research, 65(1), 95–108.
CONAB- Companhia Nacional de Abastecimento (2010). Acompanhamento da safra brasileira: cana-de-açúcar. 1° levantamento: abril. <www.conab.gov.br/conabweb/download/safra/1_levantamento2010_abr2010.pdf > Accessed 15 may 2010.
Costa, F. S., Albuquerque, J. A., Bayer, C., Fontoura, S. M. V., & Wobeto, C. (2003). Propriedades físicas de um Latossolo Bruno afetadas pelos sistemas plantio direto e preparo convencional. Revista Brasileira de Ciência de Solo, 27(3), 527–535.
Cunha, J. P. A. R., Vieira, L. B., & Magalhães, A. C. (2002). Resistência mecânica do solo à penetração sob diferentes densidades e teores de água. Engenharia na Agricultura, 10(1–4), 1–7.
Davis, J. C. (1986). Statistics and data analysis in geology (2nd ed., p. 646). New York: Wiley.
De Goede, R. G. M., & Bongers, T. (1994). Nematode community structure in relation to soil and vegetation characteristics. Applied Soil Ecology, 1(1), 29–44.
Dinardo-Miranda, L. L., & Fracasso, J. L. (2010). Spatial and temporal variability of plant-parasitic nematodes population in sugarcane. Bragantia, 69, 39–52.
Empresa Brasileira de Pesquisa Agropecuária. (1997). Manual de métodos de análises de solo. Centro Nacional de Levantamento e Conservação do Solo (2nd ed., p. 212). Rio de Janeiro: Embrapa Solos.
Englund, E., & Sparks, A. (1992). Geo-EAS. Geostatistical Environmental Assessment Software. Las Vegas: International Ground Water Modeling Center. CD-Rom.
Freckman, D. W., & Caswell, E. P. (1985). The ecology of nematodes in agroecosystems. Annual Review of Phytopathology, 23, 275–296.
Franzen, H., Lal, R., & Ehlers, W. (1994). Tillage and mulching effects on physical properties of a tropical alfisol. Soil Tillage Research, 28(3–4), 329–346.
Genro Júnior, S. A., Reinert, D. J., & Reichert, J. M. (2004). Variabilidade temporal da resistência à penetração de um latossolo argiloso sob semeadura direta com rotação de culturas. Revista Brasileira de Ciência do Solo, 28(3), 477–484.
Gomes, G. S., Huang, S. P., & Cares, J. E. (2003). Nematode community. trophic structure and population fluctuation in soybean fields. Fitopatologia Brasileira, 28(3), 258–266.
Goovaerts, P. (1998). Geostatistical tools for characterizing the spatial variability of microbiological and physico-chemical soil properties. Biology and Fertility of Soils. 27(4), 315–334.
Goulart, A. M. C., & Ferraz, L. C. C. B. (2003). Comunidades de nematóides em Cerrado com vegetação original preservada ou substituída por culturas. 1. Diversidade trófica. Nematologia Brasileira, 27(2), 123–128.
Grewal, P. S., Ehlers, R. U., & Shapiro-Ilan, D. I. (2005). Nematodes As Biocontrol Agents (p. 505). London: CABI.
Gupta, V. V. S. R., & Yeates, G. W. (1997). Soil microfauna as bioindicators of soil health. In C. E. Pankhurst, B. M. Doube, & V. V. S. R. Gupta (Eds.), Biological Indicators of Soil Health (pp. 201–233). London: CABI.
Imhoff, S., Silva, A. P., Dias Júnior, M. S., & Tormena, C. A. (2001). Quantificação de pressões críticas para o crescimento das plantas. Revista Brasileira de Ciência do Solo, 25(1), 11–18.
Jenkins, W. R. (1964). A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter, 48(9), 692–695.
Kandji, S. T., Ogol, C. K. P. O., & Albrecht, A. (2001). Diversity of plant-parasitic nematodes and their relationships with some soil physic-chemical characteristics in improved fallows in western Kenya. Applied Soil Ecology, 18(2), 143–157.
Kılıç, K., Özgöz, E., & Akbas, F. (2004). Assessment of spatial variability in penetration resistance as related to some soil physical properties of two fluvents in Turkey. Soil & Tillage Research, 76(1), 1–11.
Kimenju, J. W., Karanja, N. K., Mutua, G. K., Rimberia, B. M., & Wachira, P. M. (2009). Nematode community structure as influenced by land use and intensity of cultivation. Tropical and subtropical agroecossystems, 11(2), 353–360.
Jones, F. G. W., & Thomasson, A. J. (1976). Bulk density as an indicator of pore space in soils usable by nematodes. Nematologica, 22(2), 133–137.
Journel, A. C., & Huijbregts, C. J. (1978). Mining geostatistics (p. 600). London: Academic.
Landim, P. M. B. (1998). Análise estatística de dados geológicos (p. 226). São Paulo: UNESP.
Lanzanova, M. E., Nicoloso, R. S., Lovato, T., Eltz, F. L. F., Amado, T. J. C., & Reinert, D. J. (2007). Atributos físicos do solo em sistema de integração lavoura-pecuária sob plantio direto. Revista Brasileira de Ciência do Solo, 31(5), 1131–1140.
Li, Q., Liang, W., & Ou, W. (2007). Responses of nematode communities to different land uses in an aquic brown soil. Biodiversity Science, 15(2), 172–179.
Liang, W., Lavian, I., & Steinberger, Y. (2001). Effect of agricultural management on nematode communities in a Mediterranean agroecosystem. Journal of Nematology, 33(4), 208–213.
Liliana, R. (2003). Nematode soil faunal analysis of decomposition pathways in different ecosystems. Nematology, 5(2), 179–181.
Lopes, R. A. P., Nóbrega, L. H. P., Uribe-Opazo, M. A., Prior, M., & Pereira, J. O. (2007). Propriedades físicas de Latossolo Vermelho distroférrico típico sob sistemas de manejo na sucessão soja-milho no período de três anos. Acta Scientiarum Agronomy, 29(5), 721–727.
Mattos, J. K. A. (2002). Nematóides do solo como indicadores da interferência humana nos sistemas naturais: aspectos gerais e alguns resultados obtidos no Brasil. Revisão Anual de Patologia de Plantas – RAPP, 10, 373–390.
Mattos, J. K. A., Andrade, E. P., Teixeira, M. A., Castro, A. P. G., & Huang, P. S. (2008). Gêneros-Chaves de Onze Diferentes Comunidades de Nematóides do Solo na Região dos Cerrados do Brasil Central. Nematologia Brasileira, 32(2), 142–149.
May, W. F., Mullin, P. G., Lyon, H. H., & Loeffle, K. (1996). Plant-parasitic nematodes: a pictorial key to genera (5th ed.). Ithaca: Cornell University Press. 277.
McBratney, A. B., & Webster, R. (1986). Choosing functions for semi-variograms of soil properties and fitting them to sampling estimates. Journal of Soil Science, 37(4), 617–639.
Meng, F., Ou, W., Li, Q., Jiang, Y., & Wen, D. (2006). Vertical distribution and seasonal fluctuation of nematode trophic groups as affected by land use. Pedosphere, 16(2), 169–176.
Mondino, E. A., Chaves, E. J., & Clausen, A. M. (2006). Distribution of nematodes in potato fields soil in Andean Valleys of Argentina. Revista Facultad de Agronomia UBA, 26(2), 141–148.
Mondino, E. A., Tavares, O. C. H., Ebeling, A. G., Figueira, A. F., Quintero, E. I., & Berbara, R. L. L. (2009). Avaliação das comunidades de nematóides do solo em agroecossistemas orgânicos. Acta Scientiarum Agronomy, 31(3), 509–515.
Myers, N., Mittermeier, R. A. C. G., Fonseca, G. A. B., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403(6772), 853–858.
Neher, A. D., & Campbell, C. L. (1994). Nematode communities and microbial biomass in soils with annual and perennial crops. Applied Soil Ecology, 1(1), 17–28.
Neher, D. A. (1999). Nematode communities in organically and conventionally managed agricultural soils. Journal of Nematology, 31(2), 142–154.
Neher, D. A. (2001). Nematode communities as ecological indicators of agroecosystem health. In S. R. Gliessman (Ed.), Agroecosystem sustainability: Developing practical strategies (pp. 105–120). Boca Raton: CRC.
Niles, R. K., & Freckman, D. W. (1998). From the ground up: nematode ecology in bio-assessment and ecosystem health. In J. M. Bartles (Ed.), Plant and nematode interactions (pp. 65–85). Madison: ASA/CSSA/SSSA.
Olabiyi, T. I., Olayiwola, A. O., & Oyediran, G. O. (2009). Influence of soil textures on distribution of phytonematodes in the South Western. World Journal of Agricultural Sciences, 5(5), 557–560.
Papatheodorou, E. M., Argyropoulou, M. D., & Stamou, G. P. (2004). The effects of large and small-scale differences in soil temperature and moisture on bacterial functional diversity and the community of bacterivorous nematodes. Applied Soil Ecology, 25(1), 37–49.
Pattison, A. B., Moody, P. W., Badcock, K. A., Smith, L. J., Armour, J. A., Rasiah, V., et al. (2008). Development of key soil health indicators for the Australian banana industry. Applied Soil Ecology, 40(1), 155–164.
Porazinka, D. L., & Coleman, D. C. (1995). Ecology of nematodes under influence of Cucurbita spp. and different fertilizer types. Journal of Nematology, 27(4S), 617–623.
Qi, Y., & Hu, C. (2007). Soil nematode abundance in relation to diversity in different farming management system. World Journal of Agricultural Sciences, 3(5), 587–592.
Ribeiro, M. C., Metzger, J. P., Martensen, A. C., Ponzoni, F. J., & Hirota, M. M. (2009). The Brazilian Atlantic Forest: How much is left and how is the remaining forest distributed? Implications for conservation. Biological Conservation, 142(6), 1141–1153.
Ribon, A. A., Centurion, J. F., Centurion, M. A. P. C., & Pereira, G. T. (2003). Densidade e resistência a penetração de solos cultivados com seringueira sob diferentes manejos. Acta Scientiarum: Agronomy, 25(1), 13–17.
Robertson, G. P. (1998). GS+ geostatistics for the environmental sciences: GS+ user’s guide (p. 152). Plainwell: Gamma Design Software.
Schlotzhaver, S. D., & Littell, R. C. (1997). SAS: System for elementary statistical analysis (2nd ed., p. 905). Cary: SAS.
Seixas, F., & Oliveira Júnior, E. D. (2001). Compactação do solo devido ao tráfego de máquinas de colheita de madeira. Scientia Forestalis, 60, 73–88.
Serafim, M. E., Vitorino, A. C. T., Peixoto, P. P. P., Souza, C. M. A., & Carvalho, D. F. (2008). Intervalo hídrico ótimo em um latossolo vermelho distroférrico sob diferentes sistemas de produção. Engenharia Agrícola, 28(4), 654–665.
Shi, C., Zhang, X., Jiang, Y., Jiang, D., & Steinberger, Y. (2008). Geostatistical Analysis of Soil Nematode Communities under Degraded and Meliorated Grasslands in the Horqin Sand Land. American-Eurasian Journal of Agricultural & Environmental Sciences, 4(1), 55–61.
Silva, J. M. C., & Tabarelli, M. (2000). Tree species impoverishment and the future flora of the Atlantic forest of northeast Brazil. Nature, 404(6773), 72–74.
Sohlenius, B., & Sandor, A. (1987). Vertical distribution of nematodes in arable soil under grass (Festuca pratensis) and barley (Hordeum distichum). Biology and Fertility of Soils, 3(1–2), 19–25.
Souza, A. L., Schettino, S., Jesus, R. M., & Vale, A. B. (2002). Dinâmica da regeneração natural em uma floresta ombrófila densa secundária após corte de cipós reserva natural da Companhia Vale do Rio Doce S.A.. Estado do Espírito Santo. Brasil. Revista Árvore, 26(4), 411–419.
Souza, Z. M., Marques Júnior, J., & Pereira, G. T. (2010). Geoestatística e atributos do solo em áreas cultivadas com cana-de-açúcar. Ciência Rural, 40(1), 48–56.
Steinberger, Y., Liang, W., Savkina, E., Meshi, T., & Barness, G. (2001). Nematode community composition and diversity associated with a topoclimatic transect in a rain shadow desert. Europe Journal Soil Biology, 37(4), 315–320.
Tita, G., Desrosiers, G., Vincx, M., & Clément, M. (2002). Intertidal meiofauna of the St Lawrence estuary (Québec. Canada): diversity. biomass and feeding structure of nematode assemblages. Journal of the Marine Biology Association of the U.K., 82(5), 779–791.
Timm, L. C., Pires, L. F., Roveratti, R., Arthur, R. C. J., Reichardt, K., Oliveira, J. C. M., et al. (2006). Field spatial and temporal patterns of soil water content and bulk density changes. Scientia Agricola, 63(1), 55–64.
Tong, F., Xiao, Y., & Wang, Q. (2010). Soil nematode community structure on the northern slope of Changbai Montain. Northeast China. Journal of Forestry Research, 21(1), 93–98.
Umesh, K. C., & Ferris, H. (1994). Influence of temperature and host plant on the interaction between Pratylenchus neglectus and Meloidogyne chitwoodi. Journal of Nematology, 26(1), 65–71.
Urzelai, A., Hernandez, A. J., & Pastor, J. (2000). Biotic indices based on soil nematode communities for assessing soil quality in terrestrial ecosystems. Science of the Total Environment, 247(2–3), 253–261.
Utset, A., & Cid, G. (2001). Soil penetrometer resistance spatial variability in a Ferralsol at several soil moisture conditions. Soil and Tillage Research, 61(3–4), 193–202.
Valpassos, M. A. R., Cavalcante, E. G. S., Cassiolato, A. M. R., & Alves, M. C. (2001). Effects of soil management systems on soil microbial activity. bulk density and chemical properties. Pesquisa Agropecuária Brasileira, 36(12), 1539–1545.
Vaz, C. M. P., Bassoi, L. H., & Hopmans, J. W. (2001). Contribution of water content and bulk density to field soil penetration resistance as measured by a combined cone penetrometer-TDR probe. Soil and Tillage Research, 60(1–2), 35–42.
Wallace, M. K., & Hawkins, D. M. (1994). Applications of geostatistics in plant nematology. Journal of Nematology. 26(4s), 626–634.
Warrick, A. W., & Nielsen, D. R. (1980). Spatial variability of soil physical properties in the field. In D. Hillel (Ed.), Applications of soil physics (pp. 319–344). New York: Academic.
Wasilewska, L. (1997). Soil invertebrates as bioindicators. with special reference to soil-inhabiting nematodes. Russian Journal of Nematology, 5(2), 113–126.
Yeates, G. W. (1999). Effects of plants on nematode community structure. Annual Review of Phytopathology, 37, 127–149.
Yeates, G. W. (2003). Nematodes as soil indicators: functional and biodiversity aspects. Biology and Fertility of Soils, 37(4), 199–210.
Yeates, G. W., & Bongers, T. (1999). Nematode diversity in agroecosystems. Agriculture Ecosystem Environment, 74(1), 113–135.
Yeates, G. W., & Pattison, A. B. (2006). Moving up the Food Chain: Protozoa and nematodes. In N. Uphoff, A. S. Ball, E. Fernandes, H. Herren, O. Husson, & M. Laing (Eds.), Biological approaches to sustainable soil systems (pp. 149–162). New York: CRC/Marcel Dekker.
Yeates, G. W., Bongers, T., De Goede, R. G. M., Freckman, D. W., & Georgieva, S. S. (1993). Feeding habits in soil nematode families and genera - an outline for soil ecologists. Journal of Nematology, 25(3), 315–331.
Acknowledgements
The authors are highly thankful to Conselho Nacional de Desenvolvimento Científico e Tecnológico for providing financial support to research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveira Cardoso, M., Pedrosa, E.M.R., Rolim, M.M. et al. Effects of soil mechanical resistance on nematode community structure under conventional sugarcane and remaining of Atlantic Forest. Environ Monit Assess 184, 3529–3544 (2012). https://doi.org/10.1007/s10661-011-2206-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2206-4