Abstract
Black pod rot is the most significant factor limiting production of cocoa (Theobroma cacao) in Malaysia with average annual losses of above 30%. This work was carried out to isolate, characterize and screen bacterial endophytes from cocoa plants for their biological control activities. Their mechanisms of action as well as abilities to reduce black pod rot disease were also investigated. In total, 103 endophytic bacterial isolates were obtained from healthy cocoa tissues (leaves, branches and fruits) from seven states of Malaysia in 2016 and screened for their antagonism against P. palmivora in vitro. The best two isolates AS1 and AS2 with more than 80% inhibition of radial growth (PIRG) were selected for subsequent experiments. Sequence analysis of the 16S rRNA region indicated that these two isolates belonged to Pseudomonas aeruginosa (AS1) and Chryseobacterium proteolyticum (AS2). Bioactive volatile compounds were identified using gas chromatography-mass spectrometry (GCMS). Major compounds present in P. aeruginosa extract were identified as Eicosane (9.11%), Hexatriacontane (6.87%), Tetratetracontane (5.17%), trans-2-Decenoic acid (17.04%) and 1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl) (3.60%). In C. proteolyticum extract, major compounds were identified as Eicosane (11.29%), Tetratetracontane (10.82%), Heneicosane (10.78%), Hexatriacontane (9.04%) and Phenol, 2,4-bis(1,1-dimethylethyl) (5.92%). Effectiveness of P. aeruginosa and C. proteolyticum in reducing black pod lesion was confirmed on detached cocoa pods with 100% inhibition for both isolates. These results indicated that these two bacterial isolates have potential to be used as bio-control agents against P. palmivora.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Black pod disease occurs in almost all cocoa-producing countries such as Malaysia, Indonesia, Papua New Guinea, Trinidad and Brazil (Padwick 1956). This disease is very destructive in nature and caused by P. palmivora in Southeast Asia. Currently, the disease is being managed using chemical control, which can be costly and cause hazardous effects on the environment. Under these circumstances, new approaches to complement current strategies in disease management for better disease control are needed. In this context, a potential biological control agent for the control of the fungal pathogen is an excellent environmentally friendly option that can minimize the negative consequences of chemicals (Sahaf et al. 2007).
Management of cocoa destructive pathogens, especially Phytophthora, has been achieved through various methods, including biological control (Arnold and Herre 2003). Galindo (1992) demonstrated that Pseudomonas fluorescence isolated from the cocoa pod was antagonistic on P. palmivora. Antagonistic bacteria under the genera Pseudomonas, Azotobacter, Azospirillum, Bradyrhizobium, Rhizobium, Burkholderia, Erwinia, Enterobacter, Lysobacter, and Bacillus have demonstrated plant growth promoting (PGP) activities in several crops (Wahyudi and Astuti 2011; Kumar and Dangar 2013). Among the genera, Bacillus and Pseudomonas had some good characteristics including the ability to produce antibiotics, siderophores, plant growth promotion compounds, hydrolytic enzymes and hormones (Compant et al. 2005; Singh et al. 2013). Jayaraj et al. (2007) reported Pseudomonas spp. as effective biocontrol agents. Pseudomonas spp. are a widespread bacterial group and prodigious colonizer, which can live in both phyllosphere and rhizosphere (Sivakamasundari and Usharani 2012). Bacteria inhabiting the phyllosphere and exposed to adverse environmental conditions can be a good source of diverse potent bacterial bioagents (Kishore et al. 2005).
Advantageous effects of the endophytes include the production of antibiotics, phytohormones, siderophores, β-1, 3-glucanase, phosphate solubilization, hydrogen cyanide (HCN), and induction of systemic resistance (Podile and Kishore 2006). Several studies have shown the cumulative effect of all mechanisms due to the production of metabolites, lytic enzymes and antibiotics (Compant et al. 2005). Biocontrol activity of microorganisms involving synthesis of allelochemicals has been studied with free-living rhizobacteria, similar mechanisms apply to endophytic bacteria (Lodewyckx et al. 2002), since they can also synthesize metabolites with antagonistic activity toward plant pathogens (Chen et al. 1995). Castillo et al. (2002) proved that antibiotics produced by the Streptomyces sp. isolated from Kennedia nigriscans, can inhibit in vitro growth of Fusarium oxysporum and Pythium ultimum. Furthermore, endophytic bacteria isolated from field-grown potato plants has been reported to reduce the in vitro growth of Xanthomonas and campestris Streptomyces scabies through production of antibiotic compounds and siderophore (Sessitsch et al. 2004). Some strains of endophytic bacteria have drawn worldwide attention for their excellent characteristics that are very significant in crop health management strategies (Liu et al. 2017). Potential biocontrol agents can produce a range of antifungal metabolites (Williams and Asher 1996). This paper reports the biological control activities of two endophytic bacteria isolated from cocoa plants through the reduction of black pod rot and induction of host plant resistance.
Materials and methods
Sample collection
Samples of healthy cocoa leaves, branches and fruits were collected from seven different states in Malaysia including Pahang, Perak, Malacca, Johor, Selangor, Sarawak and Sabah during 2016 and preserved in brown paper bags at 4 °C in a refrigerator until use.
Isolation of endophytic bacteria
Endophytic bacterial isolates were obtained from healthy cocoa pods, branches and leaves according to the method described by Arnold et al. (2000). Surface sterilized leaves were plated onto tryptic soy agar (TSA) and nutrient agar (NA) media. Segments of leaves were triturated with a mortar and pestle in 1*PBS (Phosphate buffered saline, pH 7.0) and 60 μl of the suspension were placed onto NA and TSA followed by incubation for two days at 28 ± 2 °C (room temperature). Segments of surface sterilized pods and branches were placed into 250 mL Erlenmeyer flasks containing PBS (pH 7.0) with constant shaking at 200 rpm for two days at 28 ± 2 °C (room temperature) using an orbital shaker. The resulting suspension was streaked onto NA and TSA and incubated for two days at 28 ± 2 °C (room temperature). Different colonies from isolation plates were selected based on differences in their shape, colour, and texture. Pure cultures of the obtained endophytic bacterial isolates were maintained at −80 °C in glycerol stocks for long-term and in NB slants at 4 °C for temporary storage.
Dual culture test
All of the endophytic bacterial isolates were pre-evaluated against P. palmivora. Mycelial discs of P. palmivora were taken from the 4-day old culture and inoculated in a 9 cm plate containing Vegetable Juice Agar (VJA). A full loop of endophytic bacteria isolates (48 h old) grown on NA was streaked at the centre of the plate and three cm apart from the mycelial disc of P. palmivora. The plates were incubated for seven days at 28 ± 2 °C (room temperature) and the radial growth of P. palmivora was observed daily for the development of inhibition zone. Inhibition of mycelial growth was assessed by measuring the radial growth of P. palmivora in the treated plate. The mycelial disc of P. palmivora was also inoculated on the plate without endophytic bacteria as a control. Four replicate plates were measured per isolate and the experiment was repeated twice to confirm the results. The percentage inhibition radial growth (PIRG) of treatments compared to the control was calculated as follows:
Where.
- Rc:
-
radial growth of P. palmivora in the control plate
- Rt:
-
radial growth of P. palmivora toward the antagonist in a dual culture plate
DNA extraction, PCR amplification and 16S rDNA gene sequencing
The cetyltrimethylammonium bromide (CTAB) method (Sambrook et al. 1989) was used for genomic DNA extraction of the selected bacterial isolates with high efficacy to inhibit P. palmivora. The 16S rDNA gene was amplified using the universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) (Lane 1991). The PCR products were sent to MyTACG Bioscience Enterprise for sequencing.
Phylogenetic analysis
Phylogenetic tree for endophytic bacterial isolates was constructed using sequence of the 16S rDNA region. The Neighbor-joining method was applied by Mega software (version 7) with substitution model of Kimura 2-parameter (Tamura et al. 2013). Branching of the tree obtained from phylogenetic analysis has been assessed through boot-strapping with 1000 replications to estimate the reliability of inferred monophyletic groups. All position contains gaps or missing data were eliminated. Different bacterial species sequences were chosen from the NCBI GenBank database for comparison purposes.
Culture filtrate test
Based on the dual culture test, selected endophytic bacterial isolates with high antagonistic activity (>50% PIRG) were further investigated for their ability to produce inhibitory metabolites. The test was conducted using a method described by Intana et al. (2008). The selected bacterial isolates were inoculated in 250 mL Erlenmeyer flask containing 100 mL nutrient broth (NB) and incubated at 25 ± 2 °C on a rotary shaker (150 rpm) for 72 h. The bacterial culture was centrifuged (ALC Multispeed Centrifuge, PK 121) at 10,000 rpm for 10 min; the supernatant was collected, and the pellets were discarded. The supernatant was filtrated with germ filter (d = 0.25 μm). The filtrate from each isolate was incorporated into sterilized double strength CMA in ratio 2:1, 25 mL of the amended agar was poured into each Petri plate and allowed to solidify. P. palmivora mycelial disc of 4 mm was centrally inoculated on each plate. Sterilized water mixed with the CMA was used as the control. The diameter of the mycelial growth of P. palmivora was measured over 7 days. The antagonistic activity was expressed as PIRG in relation to the mycelial growth of P. palmivora in the control plate (Montealegre et al. 2003).
Volatile metabolites production
The ability of the selected endophytic bacterial isolates to produce volatile compound(s) was also evaluated using the method described by Afsharmanesh et al. (2010). Nutrient agar (NA) medium amended with glycine (4.4 g L−1) was poured into the bottom lids of 9 cm plates. The selected bacterial cultures were inoculated by streaking on the plates and on the other halves (containing CMA), the 5-day old pure culture of P. palmivora was put at the center of the Petri plate. Both half plates were placed face to face to avoid physical contact between P. palmivora and bacteria. The plates were sealed with parafilm to prevent the leaking of volatiles produced from the plates. Control plates were maintained by streaking with sterile water, and P. palmivora culture. After seven days of incubation at room temperature, the growth of P. palmivora was recorded and a comparison was made with the control plates. The experiment was carried out with six replications and repeated twice.
Chitinase production
Chitinase enzyme activity by the selected endophytic bacterial strains was qualitatively estimated by amending agar plates with colloidal chitin suspension as described by Kamala and Devi (2012). Two-day old bacterial cultures were spot inoculated by an inoculating loop on the chitin agar (CA) medium followed by incubation for four days at 28 ± 2 °C (room temperature). The development of a halo clear zone around the colony of endophytic bacteria was used to determine chitinolytic activity. Six replicate plates were conducted and the experiment was repeated twice.
Protease production
Qualitative proteolytic activity of the selected endophytic bacterial strains was determined on Skim Milk agar medium using the method described by Maurhofer et al. (1995). After four days of incubation at room temperature (28 ± 2 °C), proteolytic activity was observed by the development of a halo clear zone around the colony of endophytic bacterial isolates. Six replicate plates were conducted and the experiment was repeated twice.
Cellulase and pectinase production
The method described by Hu et al. (2008) was used for cellulase and pectinase activities of the selected endophytic bacterial isolates. After three days of incubation at room temperature (28 ± 2 °C), cellulase and pectinase activities were noticed. Existence or absence of clear halos around the bacterial colonies was indicated after flooding the plates with 2 M HCl. Six replicate plates were used and the experiment was repeated twice.
Lipase production
Lipase production by the antagonistic endophytic bacterial isolates was evaluated on lipase agar medium according to Smibert and Krieg (1994). After 4 days of incubation at room temperature (28 ± 2 °C), the production of lipase was revealed by halos zone around the colonies. The experiment was carried out with six replications and repeated twice.
Ammonia (NH3) production
The ability to produce ammonia was tested as described by Cappuccino and Sherman (2008) with slight modifications. After incubation for 48 h in a shaker, the ammonia production development was observed by faint yellow to the dark brown color. The experiment was carried out with six replications and repeated twice.
Phosphate solubilization
National Botanical Research Institute’s phosphate (NBRIP) growth medium was employed for detecting solubilization of tricalcium phosphate [Ca3 (PO4)2] activity by antagonistic endophytic bacterial isolates as described by Nautiyal (1999). After 5 days of incubation at room temperature (28 ± 2 °C), phosphate solubilizing activity (P-solubilizing positive) was observed by the development of a clear zone around the colonies. The experiment was carried out with six replications and repeated twice.
Siderophore production
Siderophore production of the endophytic bacterial strains was examined using Chrome azurol S (CAS) agar medium following the protocol of Khamna et al. (2009). After five days of incubation at room temperature (28 ± 2 °C), orange halos around the colonies indicated the production of siderophore. The experiment was carried out with six replications and repeated twice.
Hydrogen cyanide (HCN) production
To determine hydrogen cyanide production, the method described by Afsharmanesh et al. (2010) was employed. After 5 days of incubation at room temperature (28 ± 2 °C), the change in color of the filter paper was observed. Five replicate plates were conducted and the experiment was repeated twice.
Extraction of antifungal metabolites by GC/MS analysis
The selected endophytic bacterial isolates with high antifungal effects were analysed with gas chromatography/mass spectrometry (GC/MS) for the production of volatile antifungal metabolites as described by Hassi et al. (2012). The compounds were detected based on a comparison of their mass spectra data and retention time as well as with the existing analytical data of specially synthesized reference compounds database (co-injection).
Efficacy of endophytic bacteria against P. palmivora
The method described by Akrofi et al. (2017) was used to evaluate the efficacy of selected endophytic bacterial isolates. Two high virulence P. palmivora isolates (MI 5 from Sabah and TI01 from Perak state) were selected for the test. The selected endophytic bacterial isolates with high PIRG (>80%) against P. palmivora were further evaluated for P. palmivora lesion inhibition on detached P. palmivora infected cocoa pods in vitro. Non-infected cocoa pods treated with sterile distilled water were included as negative control and P. palmivora infected cocoa pods treated with Halexyl 25 WP fungicide were used as positive control. Measurements of lesions were taken after seven days of incubation at room temperature (28 ± 2 °C) in the dark. Four replicate pods per isolate were used and the experiment was repeated twice.
Results
Isolation of endophytic bacteria
In total, 103 endophytic bacterial isolates were obtained from healthy cocoa tissues (leaves, branches and fruits) from seven states of Malaysia, including Pahang, Perak, Malacca, Johor, Selangor, Sabah and Sarawak states during 2016 (Table 1).
Dual culture test
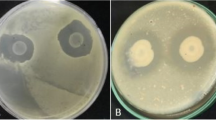
All 103 endophytic bacterial isolates were tested against P. palmivora by dual culture test on VJA, and out of the 103 bacterial isolates, only 11 isolates showed growth inhibition of P. palmivora with more than 50% PIRG. The bacterial isolates significantly reduced the growth of P. palmivora compared to the control. The minimum inhibition zones against the pathogen were ML (60.91%), AL3 (64.36%), AL2 (67.81%), AL4 (67.81%) and AF (68.96%) which showed clear inhibition zones during interaction with the pathogen of more than 60%, followed by AL1 (71.26%), CS (72.41%) and CF (72.41%) respectively. The highest inhibition zones against the pathogen over the control was obtained with isolates AS1 and AS2 (82.41% and 80.86%) (Fig. 1). Thus, AS1 and AS2 were selected for biochemical tests and further experiments. For dual culture, there was a significant difference in inhibitory effect test on VJA medium.
Inhibition of growth of P. palmivora in dual culture test on Vegetable Juice Agar (VJA) medium by Pseudomonas aeruginosa (AS1) (a), Chryseobacterium proteolyticum (AS2) (b), and control (c); Different inhibitory effects towards P. palmivora in culture filtrate test on VJA by AS1 (d), AS2 (e), and control (f); Effect of volatile metabolites on the growth of P. palmivora by AS1 (g), AS2 (h), and control (i). All the testes were measured after seven days of incubation at 28 ± 2 °C in the dark
Molecular identification of endophytic bacteria
PCR analysis of the nucleic acid extracted from the 11 endophytic bacterial isolates that showed more than 50% PIRG of P. palmivora produced an amplicon of 1500 bp on 0.8% agarose gel. Phylogenetic analysis of the 16S rDNA region revealed that the bacterial isolates AL2, ML, CS, SF, CF, AL1 and AL4 were clustered in the subclade including Bacillus altitudinis subsp. plantarum (NR_042337) with high bootstrap value of 99%. AS1 clustered in the subclade including Pseudomonas aeruginosa (NR_114471) with 100% bootstrap value, while AS2 clustered in the subclade including Chryseobacterium proteolyticum (NR_112113) with 100% bootstrap value. The identity of the 11 endophytic bacterial isolates was confirmed using molecular identification. The 16S rDNA of the bacterial isolates AL2, ML, CS, SF, CF, AL1, AL4, AS1 and AS2 were deposited into the GenBank database (Table 2; Fig. 2).
A phylogenetic tree showing the position of nine endophytic bacterial strains; phylogenetic analysis was done by the Neighbor-joining method using Mega software version 7 with substitution model of Kimura 2-parameter. The number of nodes indicates the level of bootstrap support (%) based on 1000 replicates dataset
Culture filtrate test
Eleven endophytic bacterial isolates with high antagonistic activity based on the dual culture test (>50% PIRG) were tested. P. aeruginosa isolate recorded the highest inhibition against P. palmivora (100%) followed by C. proteolyticum (62.5%) based on cultue filtrate test (Table 3; Fig. 1).
Production of volatile metabolites
In the volatile metabolites test, the growth of P. palmivora was significantly reduced by the two endophytic bacterial strains. P. aeruginosa and C. proteolyticum strains showed the pathogen growth inhibition ranging from 61.88–60.94%, respectively. The two bacterial isolates were found to produce diffusible as well as volatile metabolites, with evidence of considerable differences with the control (p < 0.05) regarding mycelial growth inhibition. Both isolates tested have a good ability to inhibit the mycelial growth of P. palmivora by producing volatile metabolites after seven days of incubation, where the isolate P. aeruginosa showed the highest inhibitory effect (61.88%) compared to the isolate C. proteolyticum, which showed a 60.94% of inhibition with respect to the control (Fig. 1).
Screening for lytic enzyme activities
The plate assays for the lytic enzyme activities of the two endophytic bacteria are shown in Table 4. Chitinase activities of the selected bacterial strains were assessed qualitatively. P. aeruginosa and C. proteolyticum were found to be negative to chitinase production. Protease activity by P. aeruginosa and C. proteolyticum were positive to this enzyme production (Fig. 3). P. aeruginosa and C. proteolyticum were able to produce cellulase, pectinase and lipase three days after incubation at 28 °C (Fig. 3).
Screening for plant growth promoting traits
The growth promotional activity of P. aeruginosa and C. proteolyticum and their activity against P. palmivora are shown in Table 5. Both tested strains have the ability to solubilize tricalcium phosphate used in NBRIP medium (Fig. 4) as indicated by the presence of a clear zone on the phosphate plates after incubation for five days. Responses of strains to HCN production on NA medium supplemented with glycine included change in the color of indicator paper from yellow to orange. According to grade, C. proteolyticum response was negative and P. aeruginosa response was positive in HCN production as indicated by the indicator paper’s color change (Fig. 4). Siderophore production ability of the selected bacterial isolates was observed by the changing color of Chromeazuro lS (CAS) indicator dye in agar medium. P. aeruginosa was able to produce siderophore, which was indicated by orange halos around colonies, while C. proteolyticum was unable to (Fig. 4). Moreover, a yellow-brown color was seen to develop following the addition of Nessler’s reagent, thus, showing a positive test for ammonia production.
Phosphate solubilizing efficiency of Pseudomonas aeruginosa (AS1) (a) and Chryseobacterium proteolyticum (AS2) (b) on National Botanical Research Institute’s Phosphate (NBRIP) medium; siderophore production by Pseudomonas aeruginosa (AS1) (c) and Chryseobacterium proteolyticum (AS2) (d) on CAS-agar medium; hydrogen cyanide production by Pseudomonas aeruginosa (AS1) (e) and Chryseobacterium proteolyticum (AS2) (f) on Whatman no. 1 filter paper soaked in picric acid solution. All the testes were measured after five days of incubation at 28 ± 2 °C in the dark
Extraction of antifungal metabolites
P. aeruginosa and C. proteolyticum were chosen for GC-MS analysis based on the best result shown in the antagonism assay. The presence of 102 and 63 peaks in the chromatogram indicated the presence of various compounds of P. aeruginosa and C. proteolyticum respectively. As indicated by the highest peaks, the major compounds present in P. aeruginosa extract were identified as Eicosane (9.11%); Hexatriacontane (6.87%); Tetratetracontane (5.17%); trans-2-Decenoic acid (17.04%) and 1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl) (3.60%). The major compounds present in C. proteolyticum extract as indicated by some highest peaks were identified as Eicosane (11.29%); Tetratetracontane (10.82%); Heneicosane (10.78%); Hexatriacontane (9.04%) and Phenol, 2,4-bis(1,1-dimethylethyl) (5.92%) (Table 6).
Efficacy of endophytic bacteria against P. palmivora
Treated cocoa pods with endophytic bacteria P. aeruginosa and C. proteolyticum suspension did not show any lesion development on detached cocoa pods as well as the treated cocoa pod with Halexyl 25 WP fungicide after 7 days of incubation with the pathogen. Howerve, the negetive control cocoa pods treated with sterilized distilled water and P. palmivora revealed obvious necrotic lesions with brown or black color and size around 90.8 cm2 (Fig. 5).
Discussion
Endophytic bacteria were isolated from pod, leaf, and branch of cocoa tree. The isolates were selected based on the efficiency of inhibiting P. palmivora growth. Screening of the 103 endophytic bacteria isolates yielded two promising endophytes AS1 and AS2 for black pod disease management in cocoa. When evaluated using dual culture test, the isolates AS1 and AS2 recorded over 80% suppression of P. palmivora. In the current study, AS1 and AS2 were identified by sequence comparison of conserved 16S rDNA. Based on our sequence data, the bacterial endophyte AS1 was identified as P. aeruginosa, and AS2 as C. proteolyticum, which showed nucleotide identity between 99 and 100%. P. aeruginosa produced clear inhibition zones and C. proteolyticum spread towards the P. palmivora colony rather than producing the clear zones. Akrofi et al. (2017) showed that Pseudomonas bacterial isolates 96 and 97 inhibited the P. palmivora growth by 69.7 and 65.8% respectively in the dual culture test. According to culture filtrate test, P. aeruginosa and C. proteolyticum recorded a suppression of P. palmivora over 100% and 62.5%, respectively. The performance of P. aeruginosa was superior to C. proteolyticum endophytic bacterial isolates. Several endophytes with antagonistic activities against fungal and bacterial pathogens have been reported (Sturz et al. 2000; Lodewyckx et al. 2002; Reiter et al. 2002). In addition, there are many successful reports on the diversity of fungal endophytes that are known to inhabit cocoa diseases (Bae et al. 2009; Bailey et al. 2008; Hanada et al. 2008; Herre et al. 2007). Antagonistic endophytic bacteria are not considered pathogenic to plants; however several bacterial species have been found to be pathogenic to plants (Kararah et al. 1985). Endospore-forming bacteria and actinomycetes were successfully isolated from the carposphere of cocoa pods (Macagnan et al. 2006).
Identification of endophytic bacteria through bacteriological approaches like morphological and biochemical characterization has been largely used in many studies (Silva and Nahas 2002). P. aeruginosa and C. proteolyticum strains obtained from the cocoa tissues were examined for mechanisms involved in biocontrol and plant growth promoting activities. The selected test strains were able to produce biosurfactants and a volatile metabolite that were involved in the growth inhibition of P. palmivora. These results are in agreement with results of Akrofi et al. (2015), who showed that fluorescent Pseudomonas spp. produced biosurfactants that inhibited mycelial growth of P. megakarya and P. palmivora spp. Previous studies demonstrated that antagonistic P. fluorescens and B. pumilus inhibited Botrytis cinerea through the action of volatile compounds (Swadling and Jeffries (1998). Bacillus amyloliquifaciens was reported to produce antifungal volatile compounds (Borriss et al. 2011), which are important in controlling plant pathogens. The present study had revealed that both strains P. aeruginosa and C. proteolyticum were able to produce cellulase, lipase, protease and pectinase at varying levels. These enzymes participate in the antagonistic activity in various ways. Most of them can influence the cell wall of pathogens (e.g. cellulases, chitinases and proteases produced by many bacteria) (Harman et al. 1993; Dunne et al. 1996; Ross et al. 2000). In this study, chitinase production was absent by both selected bacterial isolates. Many bacteria strains such as Erwinia, Pseudomonas, Bacillus and Xanthomonas are known as pectinase enzyme producers (Gummadi and Panda 2003). Several studies suggest that beneficial bacteria enhance plant growth, crop yield, seedling emergence, and biocontrol agents against certain pathogens (Dey et al. 2004; Minorsky 2008). Antagonistic activity of endophytic bacteria in the environment is mostly dependent on the secretion of different lytic enzymes including β-1,3-glucanase, protease, chitinase, pectinase and cellulase (Compant et al. 2005). Aspergillus niger strain UPMZ01 obtained from rhizosphere soil of rice as well as its culture filtrate inhibited growth of P. oryzae due to the activity of lytic enzymes and antimicrobial metabolites (Idan et al. 2017).
Phosphorus is the second most important nutrient element for plant growth. Our study showed that P. aeruginosa and C. proteolyticum are phosphate solubilizers. Yasmin et al. (2010) demonstrated that when isolates produce clearing zones, they are phosphate solubilizers. These phosphate solubilizers could promote plant growth as they are able to solubilize phosphate into a soluble form that can be easily taken by plants. The bacterial strains solubilizing tricalcium phosphate in vitro suggest that they can be effectively used in crop fields (Fatima et al. 2009).
In this study, production of Hydrogen Cyanide (HCN) was seen only by P. aeruginosa. HCN production may contribute to the effective inhibition of mycelial growth of P. palmivora as HCN is known to suppress pathogen development and indirectly stimulates plant growth and development (Siddiqui et al. 2006). Endophytic bacteria such as Pseudomonas spp. were reported to produce HCN (Gopalakrishnan et al. 2011). HCN production was also reported by Pseudomonas spp. (Paramageetham and Prasada Babu 2012). Ammonia, which is another volatile compound, was produced by P. aeruginosa and C. proteolyticum. This result supports findings by Joseph et al. (2007) and Samuel and Muthukkaruppan (2011) that ammonia production is commonly demonstrated by rhizobacteria such as Bacillus. Bacillus and Pseudomonas were effective producers of ammonia and increased to a significant level the biomass of medicinal and aromatic plants (Mishra et al. 2010). Synthesis of ammonia by bacteria has also been observed by Nimnoi et al. (2010). Thus, from the above discussion, P. aeruginosa and C. proteolyticum strains used in this study can be regarded as effective bio-inoculants. P. aeruginosa isolate has the ability to produce siderophores as evidenced by the formation of an orange halo around the colony. However, C. proteolyticum showed a negative result. Siderophores are normally the products of different soil microbes inclusive of bacteria for binding Fe3þ from the environment and making it usable for its own growth. Bacterial isolates have been shown to be able to produce siderophores and prevent the growth of phytopathogens (Tokala et al. 2002). Plants also use these as a source of iron. Many Pseudomonas strains reported by Rassouli et al. (2005) were siderophore producers in CAS-agar medium. Bacterial isolates such as Bacillus, Pseudomonas and Azotobacter siderophores were found to significantly provide competitive inhibition to the growth of soil-borne pathogens (Husen 2016). Moreover, three Pseudomonas strains AY197009, AY197006 and AY197010 were found to be able to produce siderophores (Belimov et al. 2005). Therefore competition for iron is also a potential mechanism to inhibit the activity of P. palmivora and the above studies support the findings of the endophytic bacteria strains in this study. The results revealed that different mechanisms of the endophytic bacterial isolates had been observed for their positive performance such as phosphate solubilization, siderophore, HCN and ammonia production.
As per the GC-MS, the result demonstrated the presence of 102 and 63 different compounds by P. aeruginosa and C. proteolyticum, respectively. The chromatogram library lines and hits of both P. aeruginosa and C. proteolyticum showed that three of the bioactive compounds revealed high peaks with antifungal activity. The peak area in the chromatogram was directly proportional to the quantity of the compounds present in the extract. Chemical investigations of the extracts from both isolates used in this study indicated the presence of Eicosane, Hexatriacontane and Tetratetracontane. Eicosane is a member of the class of compounds known as alkanes, which was identified as antifungal, cytotoxic and antitumor by Hsouna et al. (2011) and Belakhdar et al. (2015). It was demonstrated to have antifungal properties against Sphaerotheca fuliginea and Botrytis cinerea on cucumber seedlings (Zhang et al. 2013). Also, Eicosane compound was reported as an antifungal compound by Karanja et al. (2012). Tetratetracontane compound has broad spectrum antimicrobial activities against a wide range of plant pathogens (Sheoran et al. 2015). It is an effective antifungal agent that can be applied practically to inhibit plant fungi such as Penicillium expansum, Pythium ultiumu and Fusarium solani (Hashem et al. 2016). Tetratetracontane was also recorded based on GC-MS studies of endophytic fungal extracts to have antimicrobial activity (Prabukumar et al. 2015). The microorganisms and plants which create hexatriacontane have been known as an antifungal and antitumor compound where it can suppress the growth of Fusarium wilt of banana (Li et al. 2012).
Detached cocoa pod test confirmed the effectiveness of P. aeruginosa and C. proteolyticum isolates in reducing black pod lesion. In our study, complete inhibition of pod rot lesion was observed on the P. aeruginosa and C. proteolyticum pretreated detached cocoa pods. This result was in agreement with findings observed by Kamil (2004) who reported complete inhibition of pod rot lesion development on detached cocoa pods treated with Pseudomonas putida. Complete inhibition of P. palmivora could be due to the production of biosurfactants by the P. aeruginosa and C. proteolyticum. Efficacy of P. aeruginosa and C. proteolyticum was on par with Halexyl 25 WP fungicide, suggesting that the two isolates could be used to manage P. palmivora on cocoa.
Conclusion
A total of 103 endophytic bacterial isolates were successfully isolated from the cocoa plants. Our study appears to be the first to report the antagonistic activity of two selected endophytic bacterial isolates P. aeruginosa (AS1) and C. proteolyticum (AS2) against the black pod pathogen. The growth inhibition obtained by these isolates in the dual culture tests indicated that they have the potential to produce essential metabolites to control the pathogen. This study showed that P. aeruginosa and C. proteolyticum had significant antifungal activity against P. palmivora.
References
Afsharmanesh, H., Ahmadzadeh, M., Javan-Nikkhah, M., & Behboudi, K. (2010). Characterization of the antagonistic activity of a new indigenous strain of Pseudomonas fluorescens isolated from onion rhizosphere. Journal of Plant Pathology, 187–194.
Ahmad Kamil, M. J. (2004). Antagonistic activities of epiphytic bacteria on black pod disease of cocoa. Master’s Thesis: The Senate of Universiti Putra, Malaysia.
Akrofi, A. Y., Amoako-Atta, I., Assuah, M., & Asare, E. K. (2015). Black pod disease on cacao (Theobroma cacao, L) in Ghana: Spread of Phytophthora megakarya and role of economic plants in the disease epidemiology. Crop Protection, 72, 66–75.
Akrofi, A. Y., Terlabie, J. L., Amoako-Attah, I., & Asare, E. K. (2017). Isolation and characterization of bacteria from different cacao progenies and their antagonistic activity against the black pod disease pathogen, Phytophthora palmivora. Journal of Plant Diseases and Protection, 124, 143–152.
Arnold, A. E., & Herre, E. A. (2003). Canopy cover and leaf age affect colonization by tropical fungal endophytes: Ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia, 95, 388–398.
Arnold, A. E., Maynard, Z., Gilbert, G. S., Coley, P. D., & Kursar, T. A. (2000). Are tropical fungal endophytes hyperdiverse? Ecology Letters, 3, 267–274.
Bae, H., Sicher, R. C., Kim, M. S., Kim, S. H., Strem, M. D., Melnick, R. L., & Bailey, B. A. (2009). The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. Journal of Experimental Botany, 60, 3279–3295.
Bailey, B. A., Bae, H., Strem, M. D., Crozier, J., Thomas, S. E., Samuels, G. J., Vinyard, B. T., & Holmes, K. A. (2008). Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biocontrol potential in Theobroma cacao. Biological Control, 46, 24–35.
Belakhdar, G., Benjouad, A., & Abdennebi, E. H. (2015). Determination of some bioactive chemical constituents from Thesium humile Vahl. Journal of Materials and Environmental Science, 6, 2778–2783.
Belimov, A., Hontzeas, A., Safronova, N., Demchinskaya, V. I., Piluzza, S. V., Bulitta, G., & Glick, B. R. (2005). Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. czern.). Soil Biology and Biochemistry, 37, 241–250.
Borriss, R., Chen, X. H., Rueckert, C., Blom, J., Becker, A., Baumgarth, B., & Junge, H. (2011). Relationship of Bacillus amyloliquefaciens clades associated with strains DSM 7T and FZB42T: A proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on complete genome sequence comparisons. International Journal of Systematic and Evolutionary Microbiology, 61, 1786–1801.
Cappuccino, J. G., & Sherman, N. (2008). Microbiology: A laboratory manual (Vol. 9). Pearson/Benjamin cummings.
Castillo, U. F., Strobel, G. A., Ford, E. J., Hess, W. M., Porter, H., Jensen, J. B., & Stevens, D. (2002). Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscansa. Microbiology, 148, 2675–2685.
Chen, C., Bauske, E. M., Musson, G., Rodriguezkabana, R., & Kloepper, J. W. (1995). Biological control of Fusarium wilt on cotton by use of endophytic bacteria. Biological Control, 5, 83–91.
Compant, S., Duffy, B., Nowak, J., Clément, C., & Barka, E. A. (2005). Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Applied and Environmental Microbiology, 71, 4951–4959.
Dey, R. K. K. P., Pal, K. K., Bhatt, D. M., & Chauhan, S. M. (2004). Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiological Research, 159, 371–394.
Dunne, C., Delany, I., Fenton, A., & O'Gara, F. (1996). Mechanisms involved in biocontrol by microbial inoculants. Agronomie, 16, 721–729.
Fatima, Z., Saleemi, M., Zia, M., Sultan, T., Aslam, M., Rehman, R., & Chaudhary, M. F. (2009). Antifungal activity of plant growth-promoting rhizobacteria isolates against Rhizoctonia solani in wheat. African Journal of Biotechnology, 8, 219–225.
Galindo, J.J. (1992). Prospects for biological control of cacao. In: Keane, P.J., & putter, C. a. J. (Eds.). Cocoa pest and disease management in Southeast Asia and Australasia. Rome, FAO plant production and protection paper 112.
Gopalakrishnan, S., Pande, S., Sharma, M., Humayun, P., Kiran, B. K., Sandeep, D., & Rupela, O. (2011). Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Protection, 30, 1070–1078.
Gummadi, S. N., & Panda, T. (2003). Purification and biochemical properties of microbial pectinases—A review. Process Biochemistry, 38, 987–996.
Hanada, R. E., de Jorge Souza, T., Pomella, A. W., Hebbar, K. P., Pereira, J. O., Ismaiel, A., & Samuels, G. J. (2008). Trichoderma martiale sp. nov., a new endophyte from sapwood of Theobroma cacao with a potential for biological control. mycological research, 112: 1335-1343.
Harman, G. E., Hayes, C. K., Lorito, M., Broadway, R. M., Di Pietro, A., Peterbauer, C., & Tronsmo, A. (1993). Chitinolytic enzymes of Trichoderma harzianum: Purification of chitobiosidase and endochitinase. Phytopathology, 83, 313–318.
Hashem, M., Alamri, S. A., Alrumman, S. A., & Moustafa, M. F. (2016). Suppression of phytopathogenic fungi by plant extract of some weeds and the possible mode of action. British Microbiology Research Journal, 15, 1–13.
Hassi, M., El Guendouzi, S., Haggoud, A., David, S., Ibnsouda, S., Houari, A., & Iraqui, M. (2012). Antimycobacterial activity of a Brevibacillus laterosporus strain isolated from a Moroccan soil. Brazilian Journal of Microbiology, 43, 1516–1522.
Herre, E. A., Mejía, L. C., Kyllo, D. A., Rojas, E., Maynard, Z., Butler, A., & Van Bael, S. A. (2007). Ecological implications of anti-pathogen effects of tropical fungal endophytes and mycorrhizae. Ecology, 88, 550–558.
Hsouna, A. B., Trigui, M., Mansour, R. B., Jarraya, R. M., Damak, M., & Jaoua, S. (2011). Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. International Journal of Food Microbiology, 148, 66–72.
Hu, X. F., Ying, F. X., He, Y. B., Gao, Y. Y., Chen, H. M., & Chen, J. S. (2008). Characterization of Pectobacterium carotovorum subsp. carotovorum causing soft-rot disease on Pinellia ternata in China. European Journal of Plant Pathology, 120, 305–310.
Husen, E. (2016). Screening of soil bacteria for plant growth promotion activities in vitro. Indonesian Journal of Agricultural Science, 4, 27–31.
Idan, A. A., Sijam, K., Kadir, J., Rashid, T. S., Awla, H. K., & Alsultan, W. (2017). Biological control of Pyricularia oryzae using antifungal compounds produced by Aspergillus niger. American Journal of Plant Sciences, 8, 2445–2460.
Intana, W., Chamswarng, C., Chantrapromma, K., Yenjit, P., Suwanno, C., & Sattasakulchai, S. (2008). Use of pentyl pyrone extracted from ultraviolet-induced mutant strain of Trichoderma harzianum for control leaf spot of Chinese-kale. Thai Journal of Agricultural Science, 41, 75–80.
Jayaraj, J., Parthasarathi, T., & Radhakrishnan, N. V. (2007). Characterization of a Pseudomonas fluorescens strain from tomato rhizosphere and its use for integrated management of tomato damping-off. Biological Control, 52, 683–702.
Joseph, B., Patra, R. R., & Lawrence, R. (2007). Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). International Journal of Plant Production, 1, 141–151.
Kamala, T., & Devi, S. I. (2012). Biocontrol properties of indigenous Trichoderma isolates from north-East India against Fusarium oxysporum and Rhizoctonia solani. African Journal of Biotechnology, 11, 8491–8499.
Karanja, E. N., Boga, H. I., Muigai, A. W., Wamunyokoli, F., Kinyua, J., & Nonoh, J. O. (2012, June). Growth characteristics and production of secondary metabolites from selected novel Streptomyces species isolated from selected Kenyan national parks. In Scientific Conference Proceedings.
Kararah, M. A., Barakat, F. M., Mikhail, M. S., & Fouly, H. M. (1985). Pathophysiology in garlic cloves inoculated with Bacillus subtilis, Bacillus pumilus and Erwinia carotovora. Egyptian Journal of Phytopathology, 17, 131–140.
Khamna, S., Yokata, A., & Lumyong, S. (2009). Actinomycetes isolated from medicinal plant rhizosphere soils: Diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World Journal of Biotechnology, 25, 649–655.
Kishore, G. K., Pande, S., & Podile, A. R. (2005). Phylloplane bacteria increase seedling emergence, growth and yield of field-grown groundnut (Arachis hypogaea L.). Letters in Applied Microbiology, 40, 260–268.
Kumar, U., & Dangar, T. K. (2013). Functional role of plant growth promoting Endo-and Rhizobacteria in major cereal crops. Kheti, 1, 37–40.
Lane, D.J. (1991). 16S/23S rRNA sequencing in: Stackebrandt, E. and good fellow, M., Ed. nucleic acid techniques in bacterial systematics. Pp. 115-175: NewYork, Wiley.
Li, P., Ma, L., Feng, Y. L., Mo, M. H., Yang, F. X., Dai, H. F., & Zhao, Y. X. (2012). Diversity and chemotaxis of soil bacteria with antifungal activity against Fusarium wilt of banana. Journal of Industrial Microbiology & Biotechnology, 39, 1495–1505.
Liu, Y., Guo, J., Li, L., Asem, M. D., Zhang, Y., Mohamad, O. A., & Li, W. (2017). Endophytic bacteria associated with endangered plant Ferula sinkiangensis KM Shen in an arid land: Diversity and plant growth-promoting traits. Journal of Arid Land, 9, 432–445.
Lodewyckx, C., Vangronsveld, J., Porteous, F., Moore, E. R., Taghavi, S., Mezgeay, M., & der Lelie, D. V. (2002). Endophytic bacteria and their potential applications. Critical Reviews in Plant Sciences, 21, 583–606.
Macagnan, D., Romeiro, R. D. S., de Souza, J. T., & Pomella, A. W. (2006). Isolation of actinomycetes and endospore-forming bacteria from the cacao pod surface and their antagonistic activity against the witches’ broom and black pod pathogens. Phytoparasitica, 34, 122–132.
Maurhofer, M., Keel, C., Haas, D., & Défago, G. (1995). Influence of plant species on disease suppression by Pseudomonas fluorescens strain CHAO with enhanced antibiotic production. Plant Pathology, 44, 40–50.
Minorsky, P. V. (2008). On the inside. Plant Physiology 146: 323–324 Neilands J. B., Nakamura K. (1991). In G. Winkelmann (Ed.), CRC handbook of microbial iron chelates (pp. 1–14). Florida: CRC Press.
Mishra, M., Kumar, U., Mishra, P. K., & Prakash, V. (2010). Efficiency of plant growth promoting rhizobacteria for the enhancement of Cicer arietinum L. growth and germination under salinity. Advances in Biological Research, 4, 92–96.
Montealegre, J. R., Reyes, R., Pérez, L. M., Herrera, R., Silva, P., & Besoain, X. (2003). Selection of bioantagonistic bacteria to be used in biological control of Rhizoctonia solani in tomato. Electronic Journal of Biotechnology, 6, 115–127.
Nautiyal, C. S. (1999). An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters, 170, 265–270.
Nimnoi, P., Pongsilp, N., & Lumyong, S. (2010). Endophytic actinomycetes isolated from Aquilaria crassna Pierre ex Lec and screening of plant growth promoters production. World Journal of Microbiology and Biotechnology, 26, 193–203.
Padwick, G.W. (1956). Losses caused by plant diseases in the colonies. Volume 1, Phyto pathological papers. Kew, England, commonwealth mycological institute. (no. 632 P33).
Paramageetham, C., & Prasada Babu, G. (2012). Antagonistic Activity of Fluorescent Pseudomonads against a Polyphagous Soil Born Plant Pathogen-Sclerotium rolfsii., 1, 436.
Podile, A. R., & Kishore, G. K. (2006). Plant growth-promoting rhizobacteria. In plant-associated bacteria (pp. 195-230). Springer Netherlands.
Prabukumar, S., Rajkuberan, C., Ravindran, K., & Sivaramakrishnan, S. (2015). Isolation and characterization of endophytic fungi from medicinal plant Crescentia cujete L. and their antibacterial, antioxidant and anticancer properties. International Journal of Pharmacy and Pharmaceutical Sciences, 7, 316–321.
Rassouli, M. H., Khavazi, K., Rahimian, H., Malakouti, M. J., & Asadi-Rahmani, H. (2005). An evaluation of the potential of indigenous Fluorescent Pseudomonds of wheat rhizosphere for producing siderophore. Iran Journal of Soil and Water Science, 20, 133–143.
Reiter, B., Pfeifer, U., Schwab, H., & Sessitsch, A. (2002). Response of endophytic bacterial communities in potato plants to infection with Erwinia carotovora subsp. atroseptica. Applied and Environmental Microbiology, 68, 2261–2268.
Ross, I. L., Alami, Y., Harvey, P. R., Achouak, W., & Ryder, M. H. (2000). Genetic diversity and biological control activity of novel species of closely related pseudomonads isolated from wheat field soils in South Australia. Applied and Environmental Microbiology, 66, 1609–1616.
Sahaf, B. Z., Moharramipour, S., & Meshkatalsadat, M. H. (2007). Chemical constituents and fumigant toxicity of essential oil from Carum copticum against two stored product beetles. Insect Sci., 14, 213–218.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual. 2rd edition, cold Spring Harbor laboratory press, cold spring harbour, New York. USA. pp., 9, 31–9.57.
Samuel, S., & Muthukkaruppan, S. M. (2011). Characterization of plant growth promoting rhizobacteria and fungi associated with rice, mangrove and effluent contaminated soil. Current Botany, 2, 22–25.
Sessitsch, A., Reiter, B., & Berg, G. (2004). Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities. Canadian Journal of Microbiology, 50, 239–249.
Sheoran, N., Nadakkakath, A. V., Munjal, V., Kundu, A., Subaharan, K., Venugopal, V., & Kumar, A. (2015). Genetic analysis of plant endophytic Pseudomonas putida BP25 and chemo-profiling of its antimicrobial volatile organic compounds. Microbiological Research, 173, 66–78.
Siddiqui, I. A., Shaukat, S. S., Sheikh, I. H., & Khan, A. (2006). Role of cyanide production by Pseudomonas fluorescens CHA0 in the suppression of root-knot nematode, Meloidogyne javanica in tomato. World Journal of Microbiology and Biotechnology, 22, 641–650.
Silva, P. D., & Nahas, E. (2002). Bacterial diversity in soil in response to different plans, phosphate fertilizers and liming. Brazilian Journal of Microbiology, 33, 304–310.
Singh, Y., Singh, J., & Pandey, A. K. (2013). Molecular markers in diagnosis and management of fungal pathogens: A review. International Journal of Advanced Biotechnology and Research, 4, 180–188.
Sivakamasundari, R., & Usharani, G. (2012). Studies on the influence of Pseudomonas fluorescens and chemicals on the biocontrol sheath blight incidence in rice. International Journal of Pharmaceutical & Biological Archives, 3, 973–977.
Smibert, R. M., & Krieg, N. R. (1994). Phenotypic characterization. In methods for general and molecular bacteriology. American Society for Microbiology, 611-651.
Sturz, A. V., Christie, B. R., & Nowak, J. (2000). Bacterial endophytes: Potential role in developing sustainable systems of crop production. Critical Reviews in Plant Sciences, 19, 1–30.
Swadling, I. R., & Jeffries, P. (1998). Antagonistic properties of two bacterial biocontrol agents of grey mould disease. Biocontrol Science and Technology, 8, 439–448.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Tokala, R. K., Strap, J. L., Jung, C. M., Crawford, D. L., Salove, M. H., Deobald, L. A., & Morra, M. J. (2002). Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Applied and Environmental Microbiology, 68, 2161–2171.
Wahyudi, A. T., & Astuti, R. I. (2011). Screening of Pseudomonas sp. isolated from rhizosphere of soybean plant as plant growth promoter and biocontrol agent. American Journal of Agricultural and Biological Science.
Williams, G. E., & Asher, M. J. C. (1996). Selection of rhizobacteria for the control of Pythium ultimum and Aphanomyces cochlioides on sugar-beet seedlings. Crop Protection, 15, 479–486.
Yasmin, F., Othman, R., Sijam, K., & Saad, M. S. (2010). Characterization of beneficial properties of plant growth-promoting rhizobacteria isolated from sweet potato rhizosphere. African Journal of Microbiology Research, 3, 815–821.
Zhang, X., Li, B., Wang, Y., Guo, Q., Lu, X., Li, S., & Ma, P. (2013). Lipopeptides, a novel protein, and volatile compounds contribute to the antifungal activity of the biocontrol agent Bacillus atrophaeus CAB-1. Applied Microbiology and Biotechnology, 97, 9525–9534.
Acknowledgments
I would like to express my heartfelt and deep appreciation to my late supervisor Associate. Prof. Dr. Jugah Kadir for his valuable assistance in this study which have remarkable contributed to fulfill this research with less difficulty.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals rights
No human and/or animal participants were involved in this research.
Informed consent
All authors consent to this submission.
Electronic supplementary material
ESM 1
(DOCX 3297 kb)
Rights and permissions
About this article
Cite this article
Alsultan, W., Vadamalai, G., Khairulmazmi, A. et al. Isolation, identification and characterization of endophytic bacteria antagonistic to Phytophthora palmivora causing black pod of cocoa in Malaysia. Eur J Plant Pathol 155, 1077–1091 (2019). https://doi.org/10.1007/s10658-019-01834-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01834-8