Abstract
Pectinolytic bacteria were isolated from 48 potato plants showing the symptoms of blackleg and collected in different fields of commercial potato production areas at Samsun, Amasya, Corum and Yozgat provinces in Turkey in 2015. The survey resulted in the isolation of 26 pectinolytic strains that belonged to P. atrosepticum, P. carotovorum subsp. brasiliense, P. carotovorum subsp. carotovorum and P. parmentieri species based on molecular identification with species-specific PCR and phenotypic characterization. The identified strains indicated typical biochemical characteristics of the assigned species. For 16 representative Pectobacterium isolates 10 unique rep-PCR band patterns were obtained. The 16S rRNA and recA and gapA gene fragment sequencing confirmed the species identity of the isolates. The phenotypic characterization of the strains revealed that for all assays but one (cellulase, protease activity, swimming but not swarming), the tested Pectobacterium species were significantly different from each other proving the diversity of the strains belonging to these genera. Recent outbreaks of blackleg and/or soft rot in potato production areas in Turkey may pose a threat on other crops, as tomato, pepper, cucumber, onion, cabbage, broccoli and sugar beet are cultivated in the same provinces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum L.) is one of the most important plants cultivated all over the world with the production rate of 381 million tons, which was grown on about 19 million hectares of land (FAO 2014). Potato is affected by a number of pathogenic organisms such as e. g. pectinolytic bacteria from the genus Dickeya and Pectobacterium that were listed among the top 10 most important plant pathogens (Mansfield et al. 2012). Pectinolytic bacteria can cause wilting of the plant, rotting on daughter tubers, brown discoloration of vascular tissues and blackleg on potato stem base (Pérombelon and Kelman 1980). Seed potato production is adversely affected by pectinolytic bacteria due to downgrading or rejection of seed potatoes during certification process. Moreover, Dickeya spp. and Pectobacterium spp. can spread through latently infected seed tubers, which may facilitate their introduction to other countries (Perombelon 2002; Toth et al. 2011; Czajkowski et al. 2015). Pectobacterium and Dickeya species secrete a wide range of cell wall-degrading enzymes (PCWDE) such as pectinases, cellulases and proteases that are considered to be important virulence determinants responsible for disease symptoms development (Hugouvieux-Cotte-Pattat et al. 2014).
The pectinolytic bacteria used to be classified into Enterobacteriaceae family (Lelliot and Dickey 1984). As a result of recent reclassifications, Adeolu et al. (2016) introduced a new family Pectobacteriaceae, to which the Dickeya and Pectobacterium genus were transferred. Currently, the Pectobacterium genus comprises of 10 species, and most of them were reported to cause blackleg and/or soft rot on potato, namely P. atrosepticum (Gardan et al. 2003), P. parmentieri (Khayi et al. 2016), P. carotovorum subsp. brasiliense (Duarte et al. 2004), P. carotovorum subsp. carotovorum (Hauben et al. 1998) as well as newly established P. polaris (Dees et al. 2017a, b) and P. peruviense (Waleron et al. 2017). The genus Dickeya consists of 8 species but only strains of D. dianthicola and D. solani cause blackleg and soft rot on potato in temperate climate regions (Toth et al. 2011; Potrykus et al. 2016).
Bacteria from P. atrosepticum genus are found exclusively on potato plants. They seem to be still of major concern in countries such as Scotland, where the climate conditions are cooler and wetter (G. Saddler, personal information). Additionally, in Poland and Norway, the number of potato plants infected with P. atrosepticum is substantial reaching 40% of all the samples tested in different years (Sledz et al. 2000; Waleron et al. 2002; Dees et al. 2017a, b).
Recently, the importance of P. parmentieri strains for potato blackleg and soft rot symptoms occurrence in field conditions has been investigated in more details. Both P. parmentieri and P. carotovorum subsp. brasiliense, happened to remain unnoticed in Europe and temperate climate countries through years due to the unavailability of the effective identification methods and finally misclassification to P. c. subsp. carotovorum (Nabhan et al. 2012; Nykyri et al. 2012; Waleron et al. 2013, 2015). P. carotovorum subsp. brasiliense strains have been shown to be highly virulent and to cause severe blackleg incidences on potato in Brazil and South Africa (Duarte et al. 2004; Van der Merwe et al. 2010). During the last ten years aggressive strains of P. c. subsp. brasiliense have been reported on potato and they have caused increasing losses in European countries and other parts of the world (Ma et al. 2007; Panda et al. 2012; Ngadze et al. 2012; Nabhan et al. 2012; De Boer et al. 2012; Leite et al. 2014; Lee et al. 2014; Onkendi and Moleleki 2014; Waleron et al. 2015; van der Wolf et al. 2017). Nevertheless, about 30% of the isolates from potato plants showing symptoms of blackleg and/or soft rot are identified as P. c. subsp. carotovorum, which is a very heterologous group (Sledz et al. 2000; Dees et al. 2017a, b).
Soft rot bacteria were reported to cause disease symptoms in a number of plants in Turkey. In the previous investigations concerning potato plantations both P. c. subsp. carotovorum (Erwinia carotovora subsp. carotovora) and P. atrosepticum (Erwinia carotovora subsp. atroseptica) were causal agents of blackleg in field and soft rot in storage in Central Anatolian region of Turkey (Benlioglu 1991; Benlioglu et al. 1991). Moreover, P. c. subsp. carotovorum (Erwinia carotovora subsp. carotovora) and Dickeya spp. (Erwinia chrysanthemi) were reported on tomato plants (Aysan et al. 2005) and Dieffenbachiae amonea (Cetinkaya-Yildiz et al. 2004), while P. carotovorum strains were reported to cause disease on tulips (Boyraz et al. 2006) and several ornamental hosts: Cactus spp., Dieffenbachia spp., Drecena massangena, Drecena marginata, Primula sp., Schefflera actinophylla, Senecio cruentus, Syngonium podphyllum and Yucca aloifolia (Aysan et al. 2009). Additionally, bacteria from P. atrosepticum were reported to cause stalk and head rot on sunflower in Turkey (Bastas et al. 2009).
The current status of the causal agents of serious losses in Turkish potato production remains unknown since the last information about pectinolytic bacteria in potato production was published 25 years ago (Benlioglu 1991; Benlioglu et al. 1991). To our knowledge, this is the first up-to-date report detailing the occurrence and characterization of the pectinolytic bacteria isolated from potato in Turkey.
Materials and methods
Bacterial strains, isolation and media conditions
Twenty six pectinolytic strains were isolated from diseased plants with blackleg and/or soft rot symptoms in commercial potato production. Pieces of infected tissue were homogenized in sterile water and plated on Nutrient Agar (Himedia, India). Uniform bacterial colonies were plated on Luria Agar (Himedia, India) or Crystal Violet Pectate (CVP) medium (Hélias et al. 2012). Bacteria were kept for long-term storage, in 40% glycerol at −80 °C. Prior to genomic DNA isolation and performance of the phenotypic and pathogenicity tests bacteria were cultured in Lysogeny Broth (Btl, Poland) with shaking (200 rpm) or LA at 28 °C for 24–48 h. Sixteen representative strains isolated in Turkey and reference strains used in this study are listed in Table 1.
Identification of pectinolytic strains with species-specific PCR
Identification of P. atrosepticum, P. carotovorum/P. parmentieri and Dickeya spp. was performed with multiplex PCR (Potrykus et al. 2014). To discriminate the strains of P. parmentieri and P. c. subsp. brasiliense species, species-specific PCR with PhF/R primers (De Boer et al. 2012) and Br1/L1r primers (Duarte et al. 2004) were performed, respectively.
Genotypic profiling of Pectobacterium strains using rep-PCR
Bacterial genomic DNA was isolated from bacterial suspensions using a Genomic Mini AX Bacteria Kit (A&A Biotechnology, Gdynia, Poland). The strains were analyzed using the primers corresponding to the conserved regions of the REP, ERIC and BOX repetitive sequences while the amplified products were separated with electrophoresis, visualized and compared according to Degefu et al. (2013).
16S rRNA, recA and gapA genes analysis
To confirm the identification on species level, selected representative strains were subjected for 16S rRNA gene fragment and/or recA gene sequencing (Weisburg et al. 1991; Waleron et al. 2002). For one strain, namely P. c. subsp. brasiliense A4G1, we obtained a gapA gene fragment sequence (Cigna et al. 2017), as we were unable to amplify recA gene fragment for it. Amplicons were sequenced with forward and reverse primers by Genomed (Warsaw, Poland) or Medsantek (Turkey). The obtained 16S rRNA sequences of five strains: P. c. subsp. brasiliense A4G1 (KX548227), P. parmentieri VK5G3 (KX557265), P. atrosepticum CA2G8 (KX557266), P. c. subsp. carotovorum A10G7 (KY071198) and A11G4 (KY071199) and recA sequences of 11 strains: P. parmentieri YS18Y5 (KX548226), VK7G11(KY067396), VK5G3 (KY067397), VK10Y13 (KY067399), CA3G6 (KY077482), CA1G7 (KY077483); P. c. subsp. carovotorum A16G3 (KY067398), A11G4 (KY067400), A8G4 (KY067401), VK10G14 (KY067403); P. atrosepticum CA9G1 (KY067402) and a gap A sequence of one strain: P. c. subsp. brasiliense A4G1 (MF539826) were deposited in GenBank and compared with available sequences using BLASTN search (http://www.ncbi.nlm.nih.gov/). Phylogenetic analysis was performed with recA gene sequences. Maximum likelihood phylogenetic trees were generated with default parameters at www.phylogeny.fr (Dereeper et al. 2008, 2010).
Pathogenicity tests: disease symptoms development on potato plants and potato tubers
Potato plants (cv. Marabel) were grown in 20 cm diameter pots for 4 weeks with 16 h light at 26 °C and 8 h dark at 16 °C with 65% humidity during daytime. Two stems per potato plant, 5 cm above the stem base, were pierced by a sterile pipette tip and 20 μl of bacterial suspension (OD600 = 0.1) were inoculated into the stems and covered with a parafilm strip to avoid desiccation. The development of disease symptoms was observed daily for 4 days. The experiment was performed twice with three replicates for each of 13 strains used. Control plants were inoculated with sterile water.
Maceration ability of the eight strains was determined on the whole tubers (cv. Denar) as described previously (Potrykus et al. 2014). After 48 h, soft tissue was weighed to evaluate the amount of macerated tissue. The experiment was performed twice with six replicates. For negative control, tubers were inoculated with sterile water.
Phenotypic characterization with plate assays
Sixteen representative strains were subjected to biochemical characterization according to Schaad et al. (2001). The evaluation of the cell wall degrading enzymes (cellulase, protease) production was performed as described previously (Potrykus et al. 2014). Briefly, 2 μl of bacterial suspension (0.5 McFarland) were spotted onto a specific plate and were incubated for 48 h at 28 °C. LA media were used to assess the motility of the strains at different agar concentrations, 0.3% for swimming and 0.6% for swarming (Harshey 2003). The diameters of the colonies were measured after 24 h at 28 °C. The experiments were conducted with eight replicates and performed twice.
Quantitative evaluation of the total pectate lyase activity
Pectate lyase activity was examined by monitoring spectrophotometrically the formation of unsaturated products from polygalacturonate (Tardy et al. 1997). One unit of pectate lyase activity was defined as the amount of the enzyme required to produce 1 μmol of unsaturated product per minute. Total pectate lyases activity was expressed as μmoles of unsaturated products (UP) liberated per 1 min per mg of bacterial dry weight. The measurements were performed using spectrophotometer (Beckman DU-640, USA) at 230 nm, 37 °C for 5 min. The experiment was performed twice with two replicates.
Statistical analysis
We compared the phenotypic features of the strains isolated in Turkey and the reference strains used in the study with ANOVA followed by the Tuckey post-hoc test that was carried out using Statistica 12.5 StatSoft Polska. Error bars representing standard error are shown in charts, the data represented are the mean values. The significance level used was P < 0.05.
Results
Identification of pectinolytic bacteria isolated from potato plants and tubers
During 2015 season, potato plants with typical symptoms of blackleg and soft rot were collected on the territory of Turkish Samsun, Amasya, Çorum and Yozgat provinces. Surveyed regions are under temperate climate where potato is planted February–May and harvested in July–October. Samsun region (Havza and Vezirköprü towns), is nearly under the same climate as Amasya province with a transition climate between Middle Anatolian and Black Sea region. The other two provinces, Yozgat and Çorum, are in general cooler and they receive less precipitation than Amasya and Samsun. Approximately 8–10 fields from each province were visually inspected and 48 symptomatic plants were collected, from which 26 pectinolytic bacterial isolates were obtained. Additionally, 10 symptomless plant samples were collected, but no pectinolytic isolates were obtained. Disease symptoms were mostly observed on one or rarely on two stems per infected plant.
Twenty six bacterial isolates were Gram-negative rods able to form cavities on CVP, oxidase, arginine and indole negative, positive for catalase, unable to show phosphatase activity, facultative anaerobes with an ability to macerate potato slices and to grow at 27 °C. On the basis of phenotypic characteristics, 20 of the isolates were assigned as Pectobacterium carotovorum and 6 isolates were assigned as P. atrosepticum species (Supplementary Table 1).
From 26 isolates, 16 were chosen for further, thorough characterization (Table 1). These isolates were tested with Multiplex PCR and with species-specific PCR. Out of 16 strains, four were identified as P. atrosepticum, one as P. c. subsp. brasiliense, five strains as P. c. subsp. carotovorum and six as P. parmentieri (Table 1).
rep-PCR genomic fingerprinting
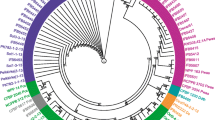
To know more about the diversity of the strains belonging to the same species, rep-PCR was performed (Fig. 1, Table 2). In general, 16 different profiles were generated with REP primers. Six P. parmentieri strains produced the same profile with BOX and ERIC primers (data not shown) while they indicated three different REP profiles (Fig. 1, Table 2). P. parmentieri CA1G7 represented profile 1, the same as P. parmentieri IFB5400 isolated in Poland. Four other P. parmentieri isolates (YS18Y5, VK10Y13, VK5G3, VK7G11) had identical profiles, and represented profile 2, the same as P. parmentieri IFB5407 strain also isolated in Poland. One strain, P. parmentieri CA3G6 represented a distinct profile from all other P. parmentieri strains, however it was more similar to profile 1 than to profile 2. Three out of 4 P. atrosepticum isolates exhibited the same REP profile 3 together with P. atrosepticum IFB5444 isolated in Poland, while one P. atrosepticum strain (YS4G4) had a distinct profile. On the contrary, high variability in the profiles of the P. c. subsp. carotovorum strains was observed. Out of 6 P. c. subsp. carotovorum strains used in this study, only A10G7 and A16G3 isolated in Turkey represented the same profile 7. Other P. c. subsp. carotovorum strains (VK10G14, A8G4, A11G4) exhibited diverse REP profiles (profile 4, 5, 6) and they were different from the profile obtained for P. c. subsp. carotovorum strain IFB5128 isolated in Tasmania (profile 10). The only P. c. subsp. brasiliense A4G1 strain represented different pattern than the reference P. c. subsp. brasiliense strains isolated in Poland and Brazil (IFB5369, IFB5390 and IFB5506) (Table 2).
Profiling of Pectobacterium sp. isolated in Turkey with regard to the reference strains with rep-PCR using REP 1 R-I and REP 2-I primers. Rep-PCR profiles were resolved and visualised with agarose gel electrophoresis. Pba – P. atrosepticum, Pcbr – P. c. subsp. brasiliense, Pcc – P. c. subsp. carotovorum, Ppa – P. parmentieri, Ddi – D. dianthicola, Dsol – D. solani, nc – negative control, 1 kb - 1 kb Gene Ruler Fermentas
recA, 16S rRNA and gapA based phylogenetic analysis
The 16S rRNA sequences obtained for P. atrosepticum CA2G8, P. c. subsp. carotovorum A11G4 and A10G7, P. parmentieri VK5G3 and P. c. subsp. brasiliense A4G1 were identical to the sequences obtained for the strains from the corresponding species, which confirmed correct identification of the isolates with Multiplex PCR and species-specific PCR. Moreover, we have obtained a gapA gene fragment sequence for P. c. subsp. brasiliense A4G1, which was highly similar to gapA sequences found in the GenBank sequenced for two P. c. subsp. brasiliense SX309 and BC1 strains (99% sequence coverage, 97% sequence identity).
Pectobacterium strains representing different REP profiles and additionally all 6 P. parmentieri isolates were chosen for recA sequencing (Fig. 2). The phylogenetic tree based on recA sequences formed four clades. All identified Turkish strains clustered with appropriate species, which again confirmed earlier identification. Interestingly, when recA sequences of six P. parmentieri strains were compared to each other, T/A transition in the 470 position of the recA alignment, could be observed. For CA3G6 and CA1G7 strains, adenine was found in the place, while for the latter four P. parmentieri strains (YS18Y5, VK10Y13, VK5G3, VK7G11) thymine. Moreover, recA sequences of P. carotovorum strains formed one large clade, which was divided into four subclades. One subclade consisted of only P. c. subsp. carotovorum strains (A16G3 and VK10G14 and IFB5128). The other subclade was further divided into two groups, one consisting of only P. c. subsp. brasiliense strains, while the other of P. c. subsp. carotovorum ATCC15713 type strain and two more strains isolated in Turkey (A11G4 and A8G4).
Maximum Likelihood phylogeny showing evolutionary relationships of Pectobacterium sp. strains isolated in Turkey, based on recA gene seqeunces. Bootstrap values are indicated at branch points. Accession numbers of reference strains in Genbank are at the end of sequence. Dickeya solani IPO2222T was included as an outgroup. The tree was generated using www.phylogeny.fr
Pathogenicity on potato plants and ability to macerate potato tuber tissue
Six P. parmentieri strains (VK5G3, VK7G11, VK10Y13, CA1G7, CA3G6, IFB5400), 5 P. c. subsp. carotovorum strains (VK10G14, A8G4. A10G7, A11G4, A16G3), three P. atrosepticum strains (CA9G1, C10G2, IFB5444) and two P. c. subsp. brasiliense strain (A4G1, IFB5506) were used for inoculation of potato plants. Typical blackleg or rotting on the stem appeared 2–3 days post inoculation (data not shown). For all P. atrosepticum and P. parmentieri strains, severe symptoms of the disease developed as soon as 2 days post infection. Four days after inoculation plants inoculated with all tested strains collapsed completely which led to the death of the plants. The control plants treated with sterile water did not develop disease symptoms.
The ability to macerate potato tubers was tested for eight strains isolated in Turkey which were chosen on the basis of rep-PCR analysis, and which represent eight different profiles (Table 2, Fig. 3a). The P. c. subsp. carotovorum strains isolated in Turkey (A16G3, A8G4, VK10G14, A11G4) exhibited 10 times greater ability to macerate potato tubers than P. c. subsp. carotovorum IFB5128 (Fig. 3a). On the contrary, the P. c. subsp. brasiliense A4G1 was less virulent than the reference strains (2.6 fold). For the P. parmentieri and P. atrosepticum strains, their maceration ability was comparable to that of their reference strains.
Comparison of the tuber maceration ability and total pectinolytic activity of the selected Pectobacterium spp. strains. a. Maceration ability on potato tubers (cv. Denar), measured by fresh mass of macerated area (g) (n = 6); b. Total pectinolytic activity measured for bacteria grown in the presence of polygalacturonic acid (μmol min−1 mg−1) (n = 2). The experiments were performed twice. Within each figure, columns respesent the mean value and error bars represent the standard error, columns with different letters are significantly different based on ANOVA followed by Tuckey post hoc test at P < 0.05. Pba – P. atrosepticum, Pcbr – P. c. subsp. brasiliense, Pcc – P. c. subsp. carotovorum, Ppa – P. parmentieri, Dsol – D. solani, Ddia – D. dianthicola, R – the mean value obtained for reference strain of the species, S – the mean value obtained for the strains isolated in Turkey (two P. atrosepticum, two P. c. subsp. brasiliense, four P. c. subsp. carotovorum, three P. parmentieri strains)
Phenotypic characterization
First, the biochemical characterization of the strains was performed. Four P. atrosepticum strains (CA2G8, CA9G1, C10G2, YS4G4) were exclusively able to utilize maltose, could reduce sucrose and produced acid from ɑ-methyl glucoside (Supplementary Table 1). Five isolated P. c. subsp. carotovorum strains (VK10G14, A8G4, A11G4, A10G7, A16G3) exclusively utilized D + arabinose as a single carbon source, while three P. c. subsp. carotovorum strains (VK10G14, A8G4 and A16G3) could utilise also sorbitol (Supplementary Table 1). Moreover, all tested isolates could utilize L-rhamnose, D-mannitol, raffinose, D + cellobiose, D + xylose, D + galactose, D + trehalose, D-mannose, L + arabinose (data not shown). Ten isolates, namely six P. parmentieri (VK5G3, VK7G11, VK10Y13, YS18Y5, CA1G7 and CA3G6) and four P. atrosepticum (CA2G8, CA9G1, C10G2, YS4G4) were not able to grow at 37 °C, while all tested P. c. subsp. carotovorum strains could. The same six P. parmentieri strains were not able to grow in the presence of 5% NaCl (Supplementary Table 1). None of the tested Pectobacterium spp. isolates grew at 39 °C.
Sixteen Pectobacterium spp. isolates and 10 reference strains belonging to Dickeya or Pectobacterium genera were screened for their characteristics regarding pectate lyase, cellulase and protease activity, as well as swimming and swarming ability (Table 2, Fig. 3, Supplementary Fig. 1).
The pectate lyase production was verified with quantitative spectrophotometric method for eight Pectobacterium spp. strains representing different profiles in REP-PCR analysis and six reference strains. The lowest pectate lyase activity in the presence of polygalacturonic acid was exhibited by P. c. subsp. carotovorum IFB5128 reference strain, which was almost 3 times smaller than that observed for P. c. subsp. carotovorum strains isolated in Turkey (Fig. 3b). The largest pectate lyase activity was exhibited by P. c. subsp. brasiliense reference strain IFB5369, but it was not significantly different from the one observed for the P. c. subsp. brasiliense A4G1 (Fig. 3b). On the contrary, the P. atrosepticum isolates produced significantly lower amounts of pectate lyases than the P. atrosepticum reference strain IFB5444 (Fig. 3b).
The lowest cellulase activity was observed for P. atrosepticum strains, while for D. solani IFB0099 it was 3 times larger (Table 2, Supplementary Fig. 1). The P. parmentieri strains expressed intermediate cellulase activity, similar to D. dianthicola. P. parmentieri CA3G6 exhibited about 30% smaller cellulase activity than the rest of P. parmentieri strains tested. The reference strains and strains isolated in Turkey that belong to P. atrosepticum and P. parmentieri exhibited a similar level of cellulase activity, contrary to strains from P. c. subsp. brasiliense and P. c. subsp. carotovorum isolated in Turkey which exhibited a significantly higher cellulase activity than the reference strains used (Table 2, Supplementary Fig. 1A). The protease activity observed for the tested strains is variable. The lowest production among strains isolated in Turkey was represented by P. parmentieri YS18Y5, while the highest two P. c. subsp. carotovorum strains A11G4 and A8G4 (Table 2). In general, P. c. subsp. brasiliense and P. c. subsp. carotovorum and D. solani exhibited the largest protease actitivy, while P. parmentieri and D. dianthicola reference strains produced a significantly lower amount of proteases among the strains tested (Supplementary Fig. 1B).
The lowest swimming motility was presented by the P. atrosepticum YS4G4 and IFB5444, while the highest by P. c. subsp. brasiliense A4G1, IFB5369 and IFB5390; and one P. c. subsp. carotovorum A11G4. Generally, the P. atrosepticum strains were the least motile in LA medium containing 0.3% agar, while the strains belonging to P. c. subsp. brasiliense were the most motile (Table 2, Supplementary Fig. 1C). Regarding swarming ability, no difference between reference strains of Pectobacterium and the strains isolated in Turkey was visible (Supplementary Fig. 1D). However, variation between P. c. subsp. carotovorum strains was observed. P. c. subsp. carotovorum VK10G14 was able to swarm even 30% more rapidly than D. solani IFB0099, while P. c. subsp. carotovorum A8G4, A11G4 and A10G7 showed much lower swarming motility. They were approximately 2 times less motile than D. solani (Table 2). Among P. parmentieri strains, the highest swarming ability was presented by P. parmentieri VK5G3.
Discussion
Soft rot Pectobacteriaceae are reported as very important plant pathogens in a wide range of hosts (Ma et al. 2007). Turkey has large areas suitable for seed and ware potato production in different regions of the country (Aslanoglu et al. 2011). In 2015, the potato production in Turkey reached 4.7 million tons and the plants were cultivated on 1.5 million hectares of land. Growing potato production is also facilitated thanks to the possibility to store tubers in natural caves that serve as cheap storage facilities (Caliskan et al. 2010). The aim of this research was to detect, isolate, identify and characterize the pectinolytic bacteria associated with recent outbreaks of blackleg and soft rot on potato in Turkey. With the presented study, it was verified that, indeed, the globally reported main potato pectinolytic pathogens P. atrosepticum, P. c. subsp. brasiliense, P. c. subsp. carotovorum and P. parmentieri are present in Turkey. In the current study, no bacteria from Dickeya genus were isolated, however, during the survey undertaken in 2016, bacteria from this genus were also detected on potato plants grown in Turkey (Ozturk and Aksoy 2017).
Rep-PCR fingerprinting method proved that for Pectobacterium spp. the REP primers were the most suitable, which is in agreement with the findings of Zoledowska et al. (2017). The analysis witth REP primers enabled us to distinguish three profiles among P. parmentieri strains, while at the same time with ERIC and BOX primers we obtained only one profile for the same set of P. parmentieri strains (data not shown). Low variability between P. atrosepticum strains was observed (two distinct profiles), contrary to high variability observed for P. c. subsp. carotovorum strains (five distinct profiles). Similarly, Faquihi et al. (2015) observed high variability of P. c. subsp. carotovorum strains and grouped 30 strains isolated in Morocco in nine different clades. On the other hand, P. parmentieri strains isolated in Turkey exhibited two rep-PCR profiles (1 and 2) that correspond to profile I and II described by Zoledowska et al. (2017). Profile I is also characteristic of the reference strain P. parmentieri SCC3193. The uniform REP profiles shown for Finish, Polish and Turkish strains indicated that they might have a common origin. Interesingly, in the recA sequence of six P. parmentieri strains isolated in Turkey one SNP position could be assigned, which was also described as T-A transition at 540 bp by Zoledowska et al. (2017). Here, two P. parmentieri strains with A in this position fell into profile 1 and 16 of rep-PCR analysis, while the latter four P. parmentieri strains were classified into profile 2.
When testing virulence of the strains on potato stems, slimy extended lesions mostly led to a faster collapse of the stem if plants were infected with P. atrosepticum and P. parmentieri strains than if they were inoculated with P. c. subsp. brasiliense and P. c. subsp. carotovorum. Similarly, de Boer et al. (2012) observed that strains of P. c. subsp. carotovorum and P. c. subsp. brasiliense were less virulent for stem inoculation than P. atrosepticum and P. parmentieri. Interestingly, P. c. subsp. brasiliense strains isolated in Brazil, South Africa and Poland show high tuber maceration. In our test, the P. atrosepticum isolates caused the lowest maceration, however the temperature of incubation was not optimal for the strains of this species (28 °C).
The phenotypic characterization of the tested strains revealed that for most of the assays (cellulase, protease production, swimming but not swarming) strains from different Pectobacterium species were significantly different from each other proving a large diversity in this genus. For comparison purpose also species from genus Dickeya were added. Apart from swimming motility, the reference strain D. solani IFB0099 showed higher cellulase, protease activity and swarming mobility than the reference and isolated Pectobacterium strains. The most motile in swimming were P. c. subsp. brasiliense strains, while the least motile were P. atrosepticum strains, which is in agreement with low motility phenotype observed for 23 P. atrosepticum strains isolated in Poland (Sledz, personal communication). The P. parmentieri strains showed the largest pectinase activity among Pectobacterium spp., while the activities of other plant cell wall degrading enzymes in the strains of this species were low or intermediate. Among the strains isolated in Turkey, the highest protease and cellulase activity as well as swimming ability were scored for P. c. subsp. brasiliense and P. c. subsp. carotovorum strains, while the lowest for P. atrosepticum, strains. This may lead to the conclusion, that P. c. subsp. brasiliense and P. c. subsp. carotovorum strains isolated in Turkey are better suited for spreading the infection among different plants than P. atrosepticum strains.
The regions of Samsun, Amasya, Corum and Yozgat, where the sampling of symptomatic potato plants was performed, can be assumed as areas of higher risk of potato blackleg and/or soft rot outbreaks as the number of fields devoted to potato production increases there steadily. Moreover, Pectobacterium spp. can be transmitted by latently infected seed potatoes and strains of this taxa are multi host pathogens, thus the presence of pectinolytic bacteria in potato fields may pose other crops, such as tomato, pepper, cucumber, onion, cabbage, broccoli and sugar beet that are cultivated in the same provinces, under threat. The presented study is the first step for the determination and characterization of the population of Pectobacterium and Dickeya spp. on the territory of Turkey to assess the risk of pectinolytic bacteria transition between the same and different hosts.
Change history
02 May 2018
The project “OMU-PYO. ZRT. 1901.15.011” was supported to HMA, not to MO.
References
Adeolu, M., Alnajar, S., Naushad, S., & Gupta, R. S. (2016). Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. International Journal of Systematic and Evolutionary, 66(12), 5575–5599.
Aysan, Y., Cetinkaya-Yildiz, R., Saygili, H., & Sahin, F. (2005). Present status of bacterial stem rot on tomato in Turkey. Acta Horticulturae. https://doi.org/10.17660/ActaHortic.2005.695.10.
Aysan, Y., Mirik, M., & Cetinkaya-Yildiz, R. (2009). Identification of bacterial diseases on ornamental plants, phenotypic and genotypic characterization of the strains and development of detection methods of important pathogens from plant materials. TUBITAK-TOVAG Project No: 106 O 333.
Bastas, K. K., Hekimhan, H., Maden, S., & Tör, M. (2009). First report of bacterial stalk and head rot disease caused by Pectobacterium atrosepticum on sunflower in Turkey. Plant Disease, 93(12), 1352–1352.
Benlioglu, (1991). Bolu, Nevşehir ve Niğde illerinde patates üretim alanlarında Erwinia spp.’nin yaygınlık oranları, tanılanması ve inokulum kaynakları üzerinde araştırmalar. Egean University, Institute of Natural Science, PhD, Thesis Dissertion, Izmir, Turkey.
Benlioglu, K., Oktem, Y. E., & Özakman, M. (1991). Bacterial diseases of potatoes in the major potato growing areas in Turkey. EPPO Bulletin, 21(1), 67–72.
Boyraz, N., Bastas, K. K., Maden, S., & Yasar, A. (2006). Bacterial leaf and peduncle soft rot 663 caused by Pectobacterium carotovorum on tulips in Konya, 664 Turkey. Phytoparasitica, 34(3), 272–280.
Cetinkaya-Yildiz, R., Mirik, M., Aysan, Y., & Kusek, M. (2004). An outbreak of bacterial stem rot of Dieffenbachia amoena caused by Erwinia carotovora subsp. carotovora in the eastern Mediterranean region of Turkey. Plant Disease, 88, 310–311.
Czajkowski, R., Pérombelon, M. C. M., Jafra, S., Lojkowska, E., Potrykus, M., van der Wolf, J. M., & Sledz, W. (2015). Detection, identification and differentiation of Pectobacterium and Dickeya species causing potato blackleg and tuber soft rot: A review. Annals of Applied Biology, 166(1), 18–38.
De Boer, S. H., Li, X., & Ward, L. J. (2012). Pectobacterium spp. associated with bacterial stem rot syndrome of potato in Canada. Phytopathology, 102, 937–947.
Dees, M. W., Lebecka, R., Perminow, J. I. S., Czajkowski, R., Grupa, A., Motyka, A., Zoledowska, S., Śliwka, J., Lojkowska, E., & Brurberg, M. B. (2017a). Characterization of Dickeya and Pectobacterium strains obtained from diseased potato plants in different climatic conditions of Norway and Poland. European Journal of Plant Pathology. https://doi.org/10.1007/s10658-016-1140-2.
Dees, M. W., Lysøe, E., Rossmann, S., Perminow, J., & Brurberg, M. B. (2017b). Pectobacterium polaris sp. nov., isolated from potato (Solanum tuberosum). International Journal of Systematic and Evolutionary Microbiology, 67(12), 5222–5229.
Degefu, Y., Potrykus, M., Golanowska, M., Virtanen, E., & Lojkowska, E. (2013). A new clade of Dickeya spp. plays a major role in potato blackleg outbreaks in North Finland. Annals of Applied Biology, 162(2), 231–241.
Dereeper, A., Guignon, V., Blanc, G., Audic, S., Buffet, S., Chevenet, F., Dufayard, J. F., Guindon, S., Lefort, V., Lescot, M., Claverie, J. M., & Gascuel, O. (2008). Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Research, 36, W465–W469.
Dereeper, A., Audic, S., Claverie, J. M., & Blanc, G. (2010). BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evolutionary Biology, 10, 8.
Duarte, V., De Boer, S. H., Ward, L. J., & Oliveira, A. M. R. (2004). Characterization of atypical Erwinia carotovora strains causing blackleg of potato in Brazil. Journal of Applied Microbiology, 96(3), 535–545.
Faquihi, H., Terta, M., Amdan, M., Achbani, E. H., Ennaji, M. M., & Mhand, R. A. (2015). Phenotypic and genotypic diversity of Pectobacterium carotovorum subsp. carotovorum causing soft rot disease on potatoes in Morocco. European Journal of Plant Pathology, 143, 801–818.
Gardan, L., Gouy, C., Christen, R., & Samson, R. (2003). Elevation of three subspecies of Pectobacterium carotovorum to species level: Pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov. International Journal of Systematic and Evolutionary Microbiology, 53(2), 381–391.
Harshey, R. M. (2003). Bacterial motility on a surface: Many ways to a common goal. Annual Review of Microbiology, 57, 249–273.
Hauben, L., Moore, E. R., Vauterin, L., Steenackers, M., Mergaert, J., Verdonck, L., & Swings, J. (1998). Phylogenetic position of phytopathogens within the Enterobacteriaceae. Systematic and Applied Microbiology, 21(3), 384–397.
Hélias, V., Hamon, P., Huchet, E., van der Wolf, J. M., & Andrivon, D. (2012). Two new effective semiselective crystal violet pectate media for isolation of Pectobacterium and Dickeya. Plant Pathology, 61, 339–345.
Hugouvieux-Cotte-Pattat, N., Condemine, G., & Shevchik, V. E. (2014). Bacterial pectate lyases, structural and functional diversity. Environmental Microbiology Reports, 6(5), 427–440.
Khayi, S., Cigna, J., Chong, T. M., Quêtu-Laurent, A., Chan, K. G., Hélias, V., & Faure, D. (2016). Transfer of the potato plant isolates of Pectobacterium wasabiae to Pectobacterium parmentieri sp. nov. International Journal of Systematic and Evolutionary Microbiology, 66(12), 5379–5383.
Lee, D. H., Kim, J. B., Lim, J. A., Han, S. W., & Heu, S. (2014). Genetic diversity of Pectobacterium carotovorum subsp. brasiliensis isolated in Korea. The Plant Pathology Journal, 30(2), 117.
Leite, L. N., de Haan, E. G., Krijger, M., Kastelein, P., van der Zouwen, P. S., van den Bovenkamp, G. W., Tebaldi, N. D., & van der Wolf, J. M. (2014). First report of potato blackleg caused by Pectobacterium carotovorum subsp. brasiliensis in the Netherlands. New Disease Reports, 29, 24.
Ma, B., Hibbing, M. E., Kim, H.-S., Reedy, R. M., Yedidia, I., Breuer, J., Breuer, J., Glasner, J. D., Perna, N. T., Kelman, A., & Charkowski, A. O. (2007). Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology, 97, 1150–1163.
Mansfield, J., Genin, S., Magori, S., Citovsky, V., Sriariyanum, M., Ronald, P., Dow, M., Verdier, V., Beer, S. V., Machado, M. A., Toth, I., Salmond, G., & Foster, G. D. (2012). Top 10 plant pathogenic bacteria in molecular plant pathology. Molecular Plant Pathology, 13(6), 614–629.
Nabhan, S., Wydra, K., Linde, M., & Debener, T. (2012). The use of two complementary DNA assays, AFLP and MLSA, for epidemic and phylogenetic studies of pectolytic enterobacterial strains with focus on the heterogeneous species Pectobacterium carotovorum. Plant Pathology, 61, 498–508.
Ngadze, E., Brady, C. L., Coutinho, T. A., & Van der Waals, J. E. (2012). Pectinolytic bacteria associated with potato soft rot and blackleg in South Africa and Zimbabwe. European Journal of Plant Pathology, 134(3), 533–549.
Nykyri, J., Niemi, O., Koskinen, P., Nokso-Koivisto, J., Pasanen, M., Broberg, M., Plyusnin, I., Toronen, P., Holm, L., Pirhonen, M., & Palva, E. T. (2012). Revised phylogeny and novel horizontally acquired virulence determinants of the model soft rot phytopathogen Pectobacterium wasabiae SCC3193. PLoS Pathology, 8(11), e1003013.
Onkendi, E. M., & Moleleki, L. N. (2014). Characterization of Pectobacterium carotovorum subsp. carotovorum and brasiliense from diseased potatoes in Kenya. European Journal of Plant Pathology, 139(3), 557–566.
Ozturk, M., & Aksoy, H. M. (2017). First report on Dickeya solani associated with potato blackleg and soft rot in Turkey. Journal of Plant Pathology, 99(1), 287–304.
Ozturk, M., Aksoy, H. M., Ozturk, S., Potrykus, M., & Lojkowska, E. (2016). First report of potato blackleg and soft rot caused by Pectobacterium wasabiae in Turkey. New Disease Reports, 34, 17–17.
Panda, P., Fiers, M. A. W. J., Armstrong, K., & Pitman, A. R. (2012). First report of blackleg and soft rot of potato caused by Pectobacterium carotovorum subsp. brasiliensis in New Zealand. New Disease Reports, 26, 15.
Pérombelon, M. C. M., & Kelman, A. (1980). Ecology of the soft rot erwinias. Annual Review of Phytopathology, 18, 361–387.
Potrykus, M., Sledz, W., Golanowska, M., Slawiak, M., Binek, A., Motyka, A., Zoledowska, S., Czajkowski, R., & Lojkowska, E. (2014). Simultaneous detection of major blackleg and soft rot bacterial pathogens in potato by multiplex polymerase chain reaction. Annals of Applied Biology, 165(3), 474–487.
Potrykus, M., Golanowska, M., Sledz, W., Zoledowska, S., Motyka, A., Kolodziejska, A., Butrymowicz, J., & Lojkowska, E. (2016). Biodiversity of Dickeya spp. isolated from potato plants and water sources in temperate climate. Plant Disease, 100(2), 408–417.
Samson, R., Legendre, J. B., Christen, R., Achouak, W., & Gardan, L. (2005). Transfer of Pectobacterium chrysanthemi (Brenner et al. 1973) Hauben et al. 1998 and Brenneria paradisiaca to the genus Dickeya gen. nov. as D. chrysanthemi comb. nov. and D. paradisiaca comb. nov. and delineation of four novel species: D. dadantii sp. nov., D. dianthicola sp. nov., D. dieffenbachiae sp. nov. and D. zeae sp. nov. International Journal of Systematic and Evolutionary Microbiology, 54, 1415–1427.
Schaad, N. W., Jones, J. B., & Chun, W. (2001). Laboratory guide for the identification of plant pathogenic bacteria (No. Ed. 3). American Phytopathological Society (APS Press).
Slawiak, M., Lojkowska, E., & Van der Wolf, J. M. (2009). First report of bacterial soft rot on potato caused by Dickeya sp. (syn. Erwinia chrysanthemi) in Poland. Plant Pathology, 58(4), 794–794.
Slawiak, M., van Doorn, R., Szemes, M., Speksnijder, A. G. C. L., Waleron, M., van der Wolf, J. M., Lojkowska, E., & Schoen, C. D. (2013). Multiplex detection and identification of bacterial pathogens causing potato blackleg and soft rot in Europe, using padlock probes. Annals of Applied Biology, 163(3), 378–393.
Sledz, W., Jafra, S., Waleron, M., & Lojkowska, E. (2000). Genetic diversity of Erwinia carotovora strains isolated from infected plants growing in Poland. Eppo Bulletin, 30(3/4), 403–408.
Tardy, F., Nasser, W., Robert-Baudouy, J., & Hugouvieux-Cotte- Pattat, N. (1997). Comparative analysis of the five major Erwinia chrysanthemi pectate lyases: Enzyme characteristics and potential inhibitors. Journal of Bacteriology, 179, 2503–2511.
Toth, I. K., Van Der Wolf, J. M., Saddler, G., Lojkowska, E., Hélias, V., Pirhonen, M., & Elphinstone, J. G. (2011). Dickeya species: An emerging problem for potato production in Europe. Plant Pathology, 60(3), 385–399.
Van der Merwe, J. J., Coutinho, T. A., Korsten, L., & van der Waals, J. E. (2010). Pectobacterium carotovorum subsp. brasiliensis causing blackleg on potatoes in South Africa. European Journal of Plant Pathology, 126(2), 175–185.
Van der Wolf, J.M., Nijhuis, E. H.,Kowalewska, M. J., Saddler, G. S., Parkinson, N., Elphinstone, J. G., Pritchard, L., Toth, I. K., Lojkowska, E., Potrykus, M., Waleron, M., de Vos, P., Cleenwerck, I., Pirhonen, M., Garlant, L., Helias, V., Pothier, J. F., Pfluger, V., Duffy, B., Tsror, L., & Manulis, S. (2014). Dickeya solani sp. nov., a pectinolytic plant pathogenic bacterium isolated from potato (Solanum tuberosum). International Journal of Systematic and Evolutionary Microbiology, 64(3), 768–774.
Van der Wolf, J. M., Haan, E. G., Kastelein, P., Krijger, M., Haas, B. H., Velvis, H., Mendes, O., Kooman-Gersmann, M., & Zouwen, P. S. (2017). Virulence of Pectobacterium carotovorum subsp. brasiliense on potato compared with that of other Pectobacterium and Dickeya species under climatic conditions prevailing in the Netherlands. Plant Pathology, 66(4), 571–583.
Waleron, M., Waleron, K., Podhajska, A. J., & Lojkowska, E. (2002). Genotyping of bacteria belonging to the former Erwinia genus by PCR-RFLP analysis of recA gene fragment. Microbiology, 148, 583–595.
Waleron, M., Waleron, K., & Lojkowska, E. (2013). Occurrence of Pectobacterium wasabiae in potato field samples. European Journal of Plant Pathology, 137(1), 149–158.
Waleron, M., Waleron, K., & Lojkowska, E. (2015). First report of Pectobacterium carotovorum subsp. brasiliense causing soft rot on potato and other vegetables in Poland. Plant Disease, 99(9), 1271.
Waleron, M., Misztak, A., Waleron, M., Franczuk, M., Wielgomas, B., & Waleron, K. (2017). Transfer of Pectobacterium carotovorum subsp. carotovorum strains isolated from potatoes grown at high altitudes to Pectobacterium peruviense sp. nov. Systematic and Applied Microbiology. https://doi.org/10.1016/j.syapm.2017.11.005.
Weisburg, W. G., Barns, S. M., Pelletier, D. A., & Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology, 173, 697–703.
Zoledowska, S., Motyka, A., Zukowska, D., Sledz, W., & Lojkowska, E. (2017). Population structure and biodiversity of Pectobacterium parmentieri isolated from potato fields in temperate climate. Plant Disease, PDIS-05.
Acknowledgments
This work was supported by project grant from OMU-PYO. ZRT. 1901.15.011. to MO and NCN UMO-2014/14/M/NZ8/00501 to EL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Human and/or animals rights
Not applied.
Informed consent
Not applied.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ozturk, M., Aksoy, H.M., Potrykus, M. et al. Genotypic and phenotypic variability of Pectobacterium strains causing blackleg and soft rot on potato in Turkey. Eur J Plant Pathol 152, 143–155 (2018). https://doi.org/10.1007/s10658-018-1459-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1459-y