Abstract
Poplars are economically important fast growing trees. They are exposed to broad range of fungal diseases like powdery mildew (PM). MLOs (mildew resistance locus O), as plant susceptibility genes, act as negative regulators and whose loss-of-functions confer complete resistance to PM disease. Herein, work identified the MLO gene family members in poplar, a woody model species. A total of 26 identified MLOs (annotated as PtMLO1–26) were distributed on 14 poplar chromosomes either individually or in groups of two to four. PtMLO genes encoded a polypeptide of 341–593 residues with a characteristic MLO domain structure. One tandem and eight segmental duplications were revealed in PtMLO genes. PtMLO proteins anchored at plasma membrane and had putative 5–9 TMDs with extracellular/cytosolic N- and C-terminuses. They were rich in leucine (9.1–12.9%), which is reported to play roles in defense response signaling. The C-terminal calmodulin-binding domain (CaMBD), reported to modulate the signaling mechanism in the defense response, was completely preserved in all PtMLOs, except PtMLO6. This domain was partially absent in PtMLO6 which is inferred to be a different MLO type or a pseudogene with a lost/impaired function in PM response. Besides, second and third cytoplasmic loops that are critical for PM-susceptibility were identified in PtMLOs. Particularly, PtMLO17, 18, 19, and 24 genes, inferred from Arabidopsis-poplar comparative phylogeny, were identified as potential candidates that may be involved in poplar-PM resistance. Notably, inductions of 14 PtMLO genes were detected in probes of microarray data such as GSE56865, GSE16417, and GSE23726 under different fungal infections indicating their involvements in plant defense. Overall, this work provided a basis for woody plant genomics for the effective and better management of poplar-PM disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poplars (Populus spp.) are economically important fast growing deciduous trees that are mainly distributed in the northern hemisphere (Pinon and Frey 2005). They are exposed to a wide range of fungal and bacterial pathogens throughout their life cycles (Newcombe 1996). For example, Melampsora spp. pathogenic to Populus are observed across the world and have important effects on plantation production in Europe and North America. Additionally, some other fungal leaf pathogens such as Venturia spp., Septoria spp. and Marssonina spp. have been also reported to affect poplars (Newcombe 1996). These bacterial and fungal pathogens are generally detected in the Populus, Aigeiros and Tacamahaca sections of poplar. Particularly, powdery mildew (PM) is a well-known damaging fungal disease that affects a wide range of plant species, ranging from the grasses to forest trees (Zheng et al. 2013; Severoglu and Ozyigit 2012).
In agricultural applications, plant resistance (R-genes) and susceptibility genes (S-genes) have an important place to combat with PM-disease (Fu et al. 2009; Parlevliet 1993). Particularly, S-genes have high significant value in agricultural practices to increase plant resistance and reduce pesticide usage (Pessina et al. 2014). S-genes, with loss-of-function, contributes to the plants a recessively inherited resistance (Pavan et al. 2010). For example, loss-of-function in a barley mildew locus O (MLO) gene confers complete PM-resistance to barley cultivars (Büschges et al. 1997). However, in Arabidopsis, loss-of-function in three closely related MLO orthologues such as AtMLO2, 6 and 12 are required for complete resistance (Consonni et al. 2006). Thus, a successful pathogen infection necessitates a complete MLO gene function in plants (Piffanelli et al. 2002).
MLO genes have been identified in some plant species, including Arabidopsis (15 genes; Devoto et al. 2003), cucumber (14 genes; Zhou et al. 2013), grape (17 genes; Feechan et al. 2009), maize (nine genes; Li and Zhu 2008), Medicago truncatula (14 genes; Rispail et al. 2013), rice (11 genes; Li and Zhu 2008), sorghum (13 genes; Singh et al. 2012), tomato (17 genes; Chen et al. 2014), wheat (seven genes; Konishi et al. 2010), soybean (39 genes; Deshmukh et al. 2014), Brachypodium distachyon (11 genes, Ablazov and Tombuloglu 2016), and Citrus sinensis (14 genes, Liu et al. 2017). In other studies, phylogenetic analyses have identified six clades in the MLO protein family. Clade V included all PM susceptibility-related proteins in dicots while clade IV had all PM susceptibility proteins in monocots (Pessina et al. 2014). In addition, seven putative transmembrane domains (TMDs) with an extracellular N- and a cytoplasmic C-terminal calmodulin-binding domain (CaMBD) were reported to characterize the MLO proteins (Devoto et al. 1999; Kim et al. 2002a, b; Reddy et al. 2003; Fig. 1).
Moreover, some highly conserved Cys and Pro residues in TMD/extracellular loops were reported to be essential for MLO function and stability (Elliott et al. 2005). The second and third cytoplasmic loops of MLO proteins have also critical importance in PM susceptibility (Reinstädler et al. 2010). MLO proteins are leucine-rich peptides (9–13%) (Singh et al. 2012) and Leu-rich proteins are thought to play significant roles in the signal transduction pathways of the defense response (Jung et al. 2004; Kêdzierski et al. 2004; Torii 2004; Osakabe et al. 2005; Kemmerling et al. 2007).
In light of above studies, MLO loss-of-function provides a potential strategy to introduce PM-resistance into the cultivated species. However, for this aim, identification of MLO genes in the plants is fundamental. Although MLO genes were identified in some plants, many are still waiting to be elucidated. In this study, Arabidopsis MLO genes were used as references to find orthologues in the poplar genome (Populus trichocarpa) and these putative genes were analyzed at nucleotide and protein sequences and 3D structure levels using bioinformatics approaches. Therefore, by identifying and analyzing the putative MLO genes, the poplar genome could contribute to the basis for future poplar-powdery mildew management studies.
Materials and methods
Retrieval of poplar MLO sequences
15 Arabidopsis MLO proteins, MLO 1 (O49621.1), MLO 2 (Q9SXB6.1), MLO 3 (Q94KB9.1), MLO 4 (O23693.2), MLO 5 (O22815.1), MLO 6 (Q94KB7.2), MLO 7 (O22752.3), MLO 8 (O22757.2), MLO 9 (Q94KB4.2), MLO 10 (Q9FKY5.1), MLO 11 (Q9FI00.1), MLO 12 (O80961.2), MLO 13 (Q94KB2.1), MLO 14 (Q94KB1.1) and MLO 15 (O80580.1) were obtained from UniProtKB/Swiss-Prot database of NCBI (Romiti 2010; Online Resource Table 1). These sequences were used as queries for blastp analyses in Populus trichocarpa from Phytozome (phytozome.jgi.doe.gov/pz/portal.html; Goodstein et al. 2012) database with a < e−100 cut-off. Redundant sequences (isoforms of MLO genes) were then removed and remaining genes were used for subsequent analyses. Protein domains were checked in Pfam31.0 (pfam.xfam.org/; Sonnhammer et al. 1997) and Conserved Domain (ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi; Marchler-Bauer et al. 2011) databases, respectively. Also, KEGG (Kyoto Encyclopedia of Genes and Genomes) database (genome.jp/kegg/) was used to confirm MLO domain structure and biological function. These steps were crucial for identifying the correct number of MLO proteins.
Sequence analysis of poplar MLOs
Physico-chemical properties of proteins such as sequence length, molecular weight and isoelectric point (pI) were calculated by using ProtParam tool (web.expasy.org/protparam/; Gasteiger et al. 2005). Sub-cellular localizations were determined by using CELLO (subCELlular LOcalization predictor) server (cello.life.nctu.edu.tw/; Yu et al. 2006). Transmembrane domains (TMDs) were predicted using SCAMPI server (scampi.cbr.su.se/; Bernsel et al. 2008). Exon-intron organization of MLO genes was analyzed by using Gene Structure Display Server (GSDS) (gsds.cbi.pku.edu.cn/; Guo et al. 2007). For this analysis, genomic and coding sequences of MLO genes were used. Potential gene duplications were identified with the following criteria; 1) the length of alignable sequence covers >80% of the longer gene; and 2) the similarity of aligned regions >80% (Gu et al. 2002; Yang et al. 2008). Conserved motifs in proteins were analyzed by using the MEME (Multiple Em for Motif Elicitation) tool (meme.nbcr.net/meme/; Timothy et al. 2009) with parameters: max motif number to find, 10; and min-max motif width, 20–50. Protein sequences were aligned by ClustalW (Thompson et al. 1994) and visualized by BioEdit Sequence Alignment Editor (Hall 1999). The phylogenetic tree was constructed by MEGA7 (Molecular Evolutionary Genetics Analysis) (Tamura et al. 2011) with maximum likelihood (ML) method for 1000 replicates. Substitution model and tree inference option were selected as the Jones-Taylor-Thornton (JTT) model and the Nearest-Neighbor-Interchange (NNI) method, respectively. Physical map was drawn based on chromosome position obtained from Phytozome database by using chromosome diagram tool in PopGenIE (The Populus Genome Integrative Explorer) database (Sjödin et al. 2009). All of the above-mentioned analyses were performed with the aim of contributing to a better understanding of the MLO genes in the poplar genome.
Expression profiles of MLO genes
Expression profiles of Populus MLO genes in response to biotic stress were retrieved from NCBI’s Gene Expression Omnibus (GEO) (Edgar et al. 2002) to understand MLO gene regulation under biotic stress conditions. No expression data is available for P. trichocarpa and Populus species challenged with powdery mildew. However, the following data are publicly available. Affymetrix microarray data were under the accession numbers GSE56865, GSE16417 and GSE23726. Probe-set IDs were obtained using Affymetrix Poplar Genome Array (Santa Clara, CA, USA) GeneChip® oligonucleotide microarray which were utilized to prepare the transcriptome profiles for during stress response to inoculation of Laccaria bicolor (Populus tremula x Populus tremuloides; GSE56865, Populus tremula x Populus alba; GSE56865), Melampsora medusae f. sp. tremuloidae (Populus tremula x Populus alba; GSE16417), and Marssonina brunnea (Populus euphratica; GSE23726) in poplar species. In addition, control plants were used as uninoculated or mock-inoculated (MI). Data were processed using NCBI’s GEO2R tool to compare two or more groups of samples in order to identify genes that are differentially expressed across experimental conditions by using the GEOquery and limma R packages from the Bioconductor project (Smyth 2005; Sean and Meltzer 2007). The heat map was generated using the CIMminer tool that generates color-coded Clustered Image Maps (CIMs) (discover.nci.nih.gov/cimminer/home.do).
Structural analysis of MLO proteins
Secondary structural analysis was performed by using SOPMA server (npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html; Geourjon and Deleage 1995). 3D models of proteins were predicted by I-TASSER server (zhanglab.ccmb.med.umich.edu/I-TASSER/; Yang et al. 2015) and visualized by Pymol (DeLano 2002). Model quality was checked by Ramachandran plot analysis (Lovell et al. 2003). Putative binding/active sites were predicted by CASTp server (sts.bioe.uic.edu/castp/; Binkowski et al. 2003).
Results and discussion
Plant resistance (R) and susceptibility (S) genes are of crucial importance in agricultural practices to fight PM-disease. Especiall, S-genes, whose loss-of-function contribute recessively inherited plant resistance (Pavan et al. 2010); thereby, this may aid to improve the plant pathogen tolerance (Parlevliet 1993; Fu et al. 2009; Pessina et al. 2014). Thus, MLO genes stand as potential candidates for molecular manipulation aiming to confer PM-resistance to cultivated or economically important plants. MLOs were identified in some agricultural or grass species but studies regarding the forest trees have been rather scarce. Herein, this study attempted to identify and characterize the potential MLO genes in the tree species poplar to provide a basis for woody plant genomics in terms of better management of the poplar-powdery mildew interaction.
Identification and characterization of poplar MLOs
Using known Arabidopsis MLO1–15 sequences as references, a total of 26 MLO orthologues were identified in genome of the poplar tree (Online Resource Table 1). Then, they were sequentially numbered as PtMLO1–26 based on their chromosomal locations (Table 1). Later, genomic, coding (CDS) and protein sequences of these homologs were retrieved to be used in further analyses. Poplar MLO genes characterized with 13–15 exons encoding a polypeptide of 341–593 amino acid residues with 39.11–67.89 kDa molecular weight and with a basic 7.61–9.70 pI value. All MLO proteins contained an MLO domain (PF03094) structure and were predicted to be subcellular localized at plasma membrane with high-reliability index or high confidence measure (Table 1). They showed the physico-chemical properties similar to their Arabidopsis counterparts.
In earlier studies, MLO proteins were characterized with seven TMDs localized to the plasma membrane (Devoto et al. 1999, 2003; Ablazov and Tombuloglu 2016; Liu et al. 2017), with an extracellular N-terminal and a cytoplasmic C-terminal calmodulin-binding domain (CaMBD) (Kim et al. 2002a, b). Herein, PtMLOs were predicted to have 5–9 TMDs with a spanning range of 233–436 amino acids, of which 14 included seven putative TMDs with an outside (extracellular) N-terminus and an inside (cytosolic) C-terminus sites (Table 1). It was also implicated that various numbers of TMDs may be associated with the functional diversities of MLOs in plants. Moreover, MLO proteins were also reported to be leucine-rich peptides (9–13%) and play significant role in the signal transduction pathways of the defense response mechanisms (Singh et al. 2012; Kemmerling et al. 2007; Osakabe et al. 2005; Jung et al. 2004; Kêdzierski et al. 2004; Torii 2004). In addition, genome-wide analysis of poplar revealed that nucleotide-binding-site (NBS) leucine-rich repeat (LRR) proteins belonged to a large class of plant resistance genes (R genes) that support the pathogen effector recognition and defense response signaling (Takken et al. 2006).
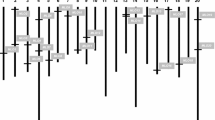
Furthermore, 26 PtMLO genes, ranging with 3583–11,682 bp were distributed on the 14 out of 19 poplar chromosomes (Fig. 2). PtMLO13, 17–19, 23 and 24 were singly located on Chr 6, 8–10, 13 and 16 respectively while other poplar MLOs were located in groups of twos or threes or fours. Chr 5 had maximum four (PtMLO9–12) MLO genes followed by three genes of Chr 7 (PtMLO14–16) and Chr 11 (PtMLO20–22), and by two genes of Chr 1 (PtMLO1–2), Chr 2 (PtMLO3–4), Chr 3 (PtMLO5–6), Chr 4 (PtMlo7–8) and Chr 17 (PtMLO25–26). Inferred from the physical map (Fig. 2), gene pairs of PtMLO3–4, PtMLO5–6, PtMLO11–12, PtMLO14–15, PtMLO20–21 and PtMLO25–26 were closely localized on their designated chromosomes. This implied that mentioning pairs may have become originated by duplication of an ancestral gene. To figure out this claim, duplication analysis was performed based on the adapted criteria (refer to Materials and methods section). The analysis showed the possibility of one tandem (PtMLO5-PtMLO6) and eight segmental duplications (PtMLO4-PtMLO11, PtMLO7-PtMLO20 or 21, PtMLO9-PtMLO15, PtMLO16-PtMLO25 or 26, PtMLO17-PtMLO19 and PtMLO22-PtMLO2). Shen et al. (2012) reported that segmental duplications may have been one of the main ways to increase the MLO gene numbers in soybean. Therefore, herein detected duplication events could cause the origination of some MLO members in poplar genome.
Conserved motifs/residues in poplar MLO proteins
To have insights about the potential conserved residues possibly associated with protein function, the most conserved 10 motifs in Arabidopsis and poplar MLO protein sequences were identified (Fig. 3). Identified motifs were distributed in a range of 21–50 amino acid residues and were detected between 21 and 41 sites in MLO sequences. Remarkably, all detected motifs were found to be related with the MLO domain structure (PF03094) (Online Resource Table 2). Besides, block diagram representation of motifs (Fig. 3) revealed that motifs 3 (FWFGRPRLLLYLIHFILFQNAFZJAFFFW) and 5 (CKKGKVPLVSAEGLHQLHIFIFVLAVFHVLYSVJTMALGRAKIRSWKKWE) are shared by all MLO sequences. The lowest number of sites was 21 for motif 10 (WYZFGLKSCFHENTELIIIRL), followed by motif 9 (ETKTLEYZFSNDPSRFRLTRZTSFVRRHL) with 24 sites, and motif 8 (LIJJLAVGTKLZAIITKMALEIQEKHAVVQGAPLVQPSDDL) with 30 sites. Thus, these 10 conserved motifs may prove the conservation of MLO genes in Arabidopsis and poplar. In the phylogenetic tree, a core cluster of Arabidopsis and poplar MLO genes related with PM-resistance consisted of AtMLO2, 6, 12, PtMLO17, 18, 19, and 24. The Arabidopsis PM-resistance genes AtMLO2, 6, 12 (Consonni et al. 2006) and probable poplar MLO genes such as PtMLO17 and 19 contained all motif types, whereas other poplar MLO genes such as MLO18 (absent as motif 1: LGVLVQFLCSYITLPLYALVTQMGSSMKKAIFDEQVAKALKGWHKAAKKK) and 24 (absent as motif 8: LIJJLAVGTKLZAIITKMALEIQEKHAVVQGAPLVQPSDDL) not contained all motif types. Motifs are commonly connected with biological functions, structures or evolutionary history of proteins. They agenerally consist of short amino acids (typically 5–25 amino acids) (Saito et al. 2004). According to these data, it can be suggested that these conserved motif structures may play vital roles for functions of MLO genes.
In this study, physico-chemical analysis showed all PtMLO proteins were rich in leucine content with 9.1–12.9% (Fig. 4), thereby suggesting possible roles of poplar MLOs in the plant defense. To further analyze the conserved sequences, localize the putative TMDs and identify the other important residues, all MLO sequences were aligned, and identical and similar residues were shaded as black and grey respectively (Fig. 4). The approximate locations of TMDs and other significant residues were determined based on the knowledge obtained from TMD predictions and similar previous studies (Devoto et al. 2003; Feechan et al. 2009; Chen et al. 2014). Aligned sequences demonstrated that residues covering TMD-3, −5 and −7 were complete in all MLO members without any disruption whereas other four TMDs were partially missing in one (TMD-4) or two (TMD-1, −2 and −6) of 26 poplar MLOs. The implication is that TMD-3, −5 and −7 may be important for proper protein function. In addition, residues covering the C-terminal calmodulin-binding domain (CaMBD) (Kim et al. 2002a, b), which has been reported to modulate the signaling mechanism in defense response (Reddy et al. 2003), was completely preserved in all 26 PtMLOs, except PtMLO6. Some residues corresponding to the CaMBD signaling domain in PtMLO6 was partially absent. It was also reported that GmMLO6 does not have any detectable CaMBD; thereby, it may be a different type of MLO gene or a pseudogene (Shen et al. 2012). In the case of an inability to bind MLO proteins with calmodulin, a partial resistance to PM has been reported (Kim et al. 2002a, b). Therefore, the PtMLO6 gene either may not be able to participate in the powdery mildew response or have impaired function.
Multiple-sequence alignment of poplar MLO proteins. Sequences were aligned by ClustalW, and identical and similar residues were shaded as black and grey respectively. Approximate locations of seven putative TMDs and C-terminal calmodulin-binding domain (CaMBD) which modulates the signaling mechanism in defense response were indicated above sequences. Potential conserved residues in MLOs were also marked with different color rectangles; LxxTPTWxVAxVC (red), LxxALxKxKxELMxLGFISLLLT (green), QLHIFIFVLA (blue), FFxQFxxSVx3DYxTLRxGFI (orange), FxFxxYxxRxLxxDFx3VGIS (purple), Px4LxVGTKL (brown), FWFxxPx3LxLIHxxLFQNxF (yellow) and CSYxTLPLYALVTQMGS (dark red)
Furthermore, the following potential residues/motifs were also identified in aligned sequences that highly characterize all PtMLOs. These residues include “LxxTPTWxVAxVC” (located in TMD-1), “LxxALxKxKxELMxLGFISLLLT” (partially in TMD-2), “QLHIFIFVLA” (located in TMD-3), “FFxQFxxSVx3DYxTLRxGFI” (loop region between TMD-3 and -4), “FxFxxYxxRxLxxDFx3VGIS” (partially in TMD-4), “Px4LxVGTKL” (located in TMD-5), “FWFxxPx3LxLIHxxLFQNxF” (partially in TMD-6) and “CSYxTLPLYALVTQMGS” (located in TMD-7). They may be also used as benchmark in characterization of the MLOs in different plant species. Second and third cytoplasmic loops of MLOs have reported to be critical for PM-susceptibility (Reinstädler et al. 2010). In this study, second and third cytoplasmic loops corresponded to the residues between TMD-3 and -4, and TMD-5 and -6 respectively when the N-terminal region was accepted as extracellularly located. Thus, motif residues, “FFxQFxxSVx3DYxTLRxGFI” and “FWFxxPx3LxLIHxxLFQNxF” in these regions may be critical for powdery mildew susceptibility in poplar tree.
Comparative phylogenetic analysis of poplar MLOs
Three separate phylogenies were constructed using Arabidopsis (Fig. 5a), poplar (Fig. 5b) and Arabidopsis-poplar (Fig. 5c) MLOs. The comparative phylogenetic analysis facilitated the identification of potential MLO members involved in PM-resistance and allowed us to infer functional roles to the PtMLOs in a cross-species dependent way. All phylogenies (Fig. 5a–c) demonstrated three major clusters, namely as group I, II and III with similar topological patterns, and group I mainly was the major cluster with a broader range of phylogenetic distribution. Arabidopsis tree (Fig. 5a), which included functionally characterized sequences, was used as a benchmark to identify the MLO members involved in PM-resistance. Group I of AtMLOs contained five genes (AtMLO5, 7–10), group II had four genes (AtMLO2, 3, 6, 12) and group III included six genes (AtMLO1, 4, 11, 13–15). Arabidopsis MLOs such as AtMLO2, 6 and 12, required for complete resistance in PM (Consonni et al. 2006), were clustered in group II. In poplar (Fig. 5b), group I was further subdivided into three sub-groups (Ia-c). Group Ia included seven genes (PtMLO3, 4, 9, 11, 12, 14, 15), group Ib had seven genes (PtMLO7, 17–21, 24) and group Ic included three genes (PtMLO5, 6, 13), while group II had five genes (PtMLO1, 16, 23, 25, 26) and group III had four genes (PtMLO2, 8, 10, 22). The combined Arabidopsis-poplar tree (Fig. 5c) demonstrated that Arabidopsis MLOs such as AtMLO2, 6 and 12 required for PM-resistance were clustered with the PtMLO17–19, 24 sequences in the same clade. Thereby, inferring from the Arabidopsis MLOs, poplar PtMLO17–19 and 24 genes are implied to be potential candidates that may involve in the poplar-PM resistance.
Phylogenetic distributions of: Arabidopsis (a), poplar (b), and Arabidopsis-poplar (c) MLO proteins. Phylogenies were constructed by MEGA 6 with ML method for 1000 replicates. All trees demonstrated three major clusters, namely as group I (blue), II (red) and III (orange), with a broader range of group I. Potential MLO members that possibly involve in PM-resistance are encircled with red rectangle
Expression profiling of poplar MLO genes
In order to gain more detailed data about regulation and expression levels of of MLO genes under biotic stress conditions, the expression profiles of herein identified MLO genes were analyzed by using publicly available perturbation microarray datasets: including infection of the ectomycorrhizal fungus L. bicolor in roots of hybrid poplar P. tremula x P. tremuloides and P. tremula x P. alba, the infection of M. medusae f. sp. tremuloidae (MMT) in leaves of hybrid poplar P. tremula x P. alba, and P. euphratica leaves subjected to the infection by Marssonina pathogen. Upon exposure to fungal species, detected 14 MLO genes showed significant up and down regulation in poplar genotypes, implying their involvements in the plant defense (Fig. 6). All fungal infections apparently led to the downregulation of most MLO genes in poplars. However, the mutualistic symbiosis between L. bicolor and Populus roots also notably upregulated some MLO genes, including POPTR_0003s00970 (PtMLO5) with 3.64 folds, POPTR_0009s01710 (PTMLO18) with 2.42 folds, POPTR_0004s04960 (PtMLO7) with 2.39 folds and POPTR_0011s05790 (PtMLO20) with 2.29 folds. Thereby, the expression of MLO genes under given fungal infections did not demonstrate any particular pattern but showed a tendency to downregulation. Additionally, their considerable induction upon fungal challenge also implied that they may possibly be associated with biotic stresses.
Expression profiles of fungal-induced 14 MLO genes in different poplars based on microarray data. P. tremula x P. tremuloides and P. tremula x P. alba are inoculated by L. bicolor, P. tremula x P. alba inoculated by M. medusae f. sp. tremuloidae, and P. euphratica inoculated by M. brunnea. Red-to-green scale indicates the up-to-downregulated ranges correspondingly. 14 MLO genes in arrays are annotated herein study as POPTR_0001s11880 (PtMLO1), POPTR_0001s41280 (PtMLO2), POPTR_0003s00970 (PtMLO5), POPTR_0004s04960 (PtMLO7), POPTR_0004s22820 (PtMLO8), POPTR_0005s11350 (PtMLO10), POPTR_0005s27570 (PtMLO11), POPTR_0006s13170 (PtMLO13), POPTR_0007s08570 (PtMLO14), POPTR_0009s01710 (PTMLO18), POPTR_0011s05790 (PtMLO20), POPTR_0011s05820 (PtMLO21), POPTR_0016s08940 (PtMLO24), and POPTR_0017s00450 (PtMLO26)
Structural analysis of poplar MLOs
Finally, the secondary and tertiary structures of potential MLOs that involve in PM resistance in Arabidopsis and poplar were comparatively analyzed. In Arabidopsis AtMLO2, 6 and 12 proteins, reported to be required for complete resistance in PM disease (Consonni et al. 2006), the secondary structural elements were distributed as 38.02–39.79% α-helices, 19.62–20.77% extended strands, 8.03–8.33% beta turns and 31.41–34.03% random coils. In the herein identified poplar proteins PtMLO17–19 and 24 that may possibly be involved in PM resistance, those elements comprised of 29.60–43.44% α-helices, 17.21–24.96% extended strands, 7.87–10.33% beta turns and 29.02–36.68% random coils. Hence, Arabidopsis and poplar MLOs had not diverged too much in terms of secondary structural elements distribution; this may also suggest a possible functional relationship in the given proteins.
Moreover, 3D models of those Arabidopsis and poplar MLOs were generated, and the quality of models were verified by Ramachandran plot analysis in which ≥90% residues were in allowed region (Fig. 7). This indicates the fairly good fit of the predicted models. The models of PtMLO17–19, 24 and AtMLO6 showed similar topological conformations. Supportably, the p-blast search of protein sequences (refer to “Homolog/E-value” column in Table 1) also corroborated that PtMLO17–19 and 24 are the homologs of AtMLO6. This shows that those sequences are well conserved at primary and tertiary structural levels. Furthermore, binding sites of those proteins in generated models were predicted. The various binding patterns were detected in poplar PtMLOs compared to that of the Arabidopsis proteins (Online Resource Fig. 1-2). This may contribute to the divergence of MLOs as well as to increase the flexibility in a species-dependent manner.
3D models of known Arabidopsis and predicted poplar MLOs involved in PM resistance. Arabidopsis and poplar models are visualized with red and green respectively, with yellow N- and C-terminal tags. AtMLO2, 6 and 12 are reported to be required for complete PM-resistance. PtMLO17–19 and 24, identified in this study, are thought to involve in poplar PM resistance
Conclusion
Poplars are fast growing deciduous trees with important economic value. However, they are exposed to broad range of fungal pathogens, with powdery mildew (PM) one of the well-known fungal diseases that damage poplar. Plant susceptibility genes like MLOs have great importance in agricultural practice to manage this disease. For this purpose, identification of MLO genes in the plants has been a fundamental step. Thus, this work identified the putative MLO genes in a tree species poplar. A total of 26 putative MLO orthologues were identified in the poplar genome of which 14 adhered to the criteria of fully functional MLO proteins. Identified homologs were investigated from primary sequences to the tertiary structural level. Particularly, four genes PtMLO17, 18, 19 and 24 were revealed as potential candidates that may be involved in the poplar PM resistance. Overall, this work provides a basis for woody plant genomics in future studies aimed at the better management of poplar-PM disease.
References
Ablazov, A., & Tombuloglu, H. (2016). Genome-wide identification of the mildew resistance locus O (MLO) gene family in novel cereal model species Brachypodium distachyon. European Journal of Plant Pathology, 145, 239–253.
Bernsel, A., Viklund, H., Falk, J., Lindahl, E., von Heijne, G., & Elofsson, A. (2008). Prediction of membrane-protein topology from first principles. Proceedings of the National Academy of Sciences USA, 105, 7177–7181.
Binkowski, T. A., Naghibzadeh, S., & Liang, J. (2003). CASTp: Computed atlas of surface topography of proteins. Nucleic Acids Research, 31, 3352–3355.
Büschges, R., Hollricher, K., Panstruga, R., Simons, G., Wolter, M., Frijters, A., van Daelen, R., van der Lee, T., Diergaarde, P., Groenendijk, J., Topsch, S., Vos, P., Salamini, F., & Schulze-Lefert, P. (1997). The barley MLO gene: A novel control element of plant pathogen resistance. Cell, 88, 695–705.
Chen, Y., Wang, Y., & Zhang, H. (2014). Genome-wide analysis of the mildew resistance locus o (MLO) gene family in tomato (Solanum lycopersicum L.) Plant Omics Journal, 7, 87–93.
Consonni, C., Humphry, M. E., Hartmann, H. A., Livaja, M., Durner, J., Westphal, L., Vogel, J., Lipka, V., Kemmerling, B., Schulze-Lefert, P., Somerville, S. C., & Panstruga, R. (2006). Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nature Genetics, 38, 716–720.
Davis, S., & Meltzer, P. S. (2007). GEOquery: A bridge between the gene expression omnibus (GEO) and BioConductor. Bioinformatics, 23, 1846–1847.
DeLano, W. L. (2002). The PyMOL molecular graphics system. Palo Alto: DeLano Scientific LLC.
Deshmukh, R., Singh, V. K., & Singh, B. D. (2014). Comparative phylogenetic analysis of genome-wide Mlo gene family members from Glycine max and Arabidopsis thaliana. Molecular Genetics & Genomics, 289, 345–359.
Devoto, A., Piffanelli, P., Nilsson, I., Wallin, E., Panstruga, R., Heijne, G. V., & Schulze-Lefert, P. (1999). Topology, subcellular localization, and sequence diversity of the MLO family in plants. Journal of Biological Chemistry, 274, 34993–35004.
Devoto, A., Hartmann, H. A., Piffanelli, P., Elliott, C., Simmons, C., Taramino, G., Goh, C. S., Cohen, F. E., Emerson, B. C., Schulze-Lefert, P., & Panstruga, R. (2003). Molecular phylogeny and evolution of the plant specific seven-transmembrane MLO family. Journal of Molecular Evolution, 56, 77–88.
Edgar, R., Domrachev, M., & Lash, A. E. (2002). Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Research, 30, 207–210.
Elliott, C., Uller, J. M., Miklis, M., Bhat, R. A., Schulze-Lefert, P., & Panstruga, R. (2005). Conserved extracellular cysteine residues and cytoplasmic loop–loop interplay are required for functionality of the heptahelical MLO protein. Biochemical Journal, 385, 243–254.
Feechan, A., Jermakow, A. M., Torregrosa, L., Panstruga, R., & Dry, I. B. (2009). Identification of grapevine MLO gene candidates involved in susceptibility to powdery mildew. Functional Plant Biology, 35, 1255–1266.
Fu, X. L., Lu, Y. G., Liu, X. D., & Li, J. Q. (2009). Crossability barriers in the interspecific hybridization between Oryza sativa and O. meyeriana. Journal of Integrative Plant Biology, 51, 21–28.
Gasteiger, E., Hoogland, C., Gattiker, A., Duvaud, S., Wilkins, M. R., Appel, R. D., & Bairoch, A. (2005). In M. W. John (Ed.), Protein identification and analysis tools on the ExPASy server (pp 571–607). The Proteomics Protocols Handbook: Humana.
Geourjon, C., & Deleage, G. (1995). SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Computer Applications in the Biosciences, 11, 681–684.
Goodstein, D. M., Shu, S., Howson, R., Neupane, R., Hayes, R. D., Fazo, J., Mitros, T., Dirks, W., Hellsten, U., Putnam, N., & Rokhsar, D. S. (2012). Phytozome: A comparative platform for green plant genomics. Nucleic Acids Research, 40, D1178–D1186.
Gu, Z., Cavalcanti, A., Chen, F. C., Bouman, P., & Li, W. H. (2002). Extent of gene duplication in the genomes of drosophila, nematode, and yeast. Molecular Biology and Evolution, 19, 256–262.
Guo, A. Y., Zhu, Q. H., Chen, X., & Luo, J. C. (2007). [GSDS: a gene structure display server]. Yi chuan= Hereditas/Zhongguo yi chuan xue hui bian ji 29, 1023–1026.
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Jung, E. H., Jung, H. W., Lee, S. C., Han, S. W., Heu, S., & Hwang, B. K. (2004). Identification of a novel pathogen-induced gene encoding a leucine-rich repeat protein expressed in phloem cells of Capsicum annuum. Biochimica et Biophysica Acta, 1676, 211–222.
Kêdzierski, L., Montgomery, J., Curtis, J., & Handman, E. (2004). Leucinerich repeats in host–pathogen interactions. Archivum Immunologiae et Therapiae Experimentalis, 52, 104–112.
Kemmerling, B., Schwedt, A., Rodriguez, P., Mazzotta, S., Frank, M., Qamar, S. A., Mengiste, T., Betsuyaku, S., Parker, J. E., Müssig, C., Thomma, B. P., Albrecht, C., de Vries, S. C., Hirt, H., & Nürnberger, T. (2007). The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Current Biology, 17, 1116–1122.
Kim, M. C., Lee, S. H., Kim, J. K., Chun, H. J., Choi, M. S., Chung, W. S., Moon, B. C., Kang, C. H., Park, C. Y., Yoo, J. H., Kang, Y. H., Koo, S. C., Koo, Y. D., Jung, J. C., Kim, S. T., Schulze-Lefert, P., Lee, S. Y., & Cho, M. J. (2002a). MLO, a modulator of plant defense and cell death, is a novel calmodulin-binding protein. Journal of Biological Chemistry, 277, 19304–19314.
Kim, M. C., Panstruga, R., Elliott, C., Müller, J., Devoto, A., Yoon, H. W., Park, H. C., Cho, M. J., & Schulze-Lefert, P. (2002b). Calmodulin interacts with MLO protein to regulate defense against mildew in barley. Nature, 416, 447–450.
Konishi, S., Sasakuma, T., & Sasanuma, T. (2010). Identification of novel Mlo family members in wheat and their genetic characterization. Genes & Genetic Systems, 85, 167–175.
Li, Q., & Zhu, H. (2008). Molecular evolution of the Mlo gene family in Oryza sativa and their functional divergence. Gene, 409, 1–10.
Liu, L. P., Qu, J. W., Yi, X. Q., & Huang, H. H. (2017). Genome-wide identification, classification and expression analysis of the mildew resistance locus O (MLO) gene family in sweet orange (Citrus sinensis). Brazilian Archives of Biology and Technology, 60, e17160474.
Lovell, S. C., Davis, I. W., Arendall, W. B., de Bakker, P. I., Word, J. M., Prisant, M. G., Richardson, J. S., & Richardson, D. C. (2003). Structure validation by Cα geometry: ɸ, ψ and Cβ deviation. Proteins: Structure, Function, and Bioinformatics, 50, 437–450.
Marchler-Bauer, A., Lu, S., Anderson, J. B., Chitsaz, F., Derbyshire, M. K., DeWeese-Scott, C., et al. (2011). CDD: A conserved domain database for the functional annotation of proteins. Nucleic Acids Research, 39, D225–D229.
Newcombe, G. (1996). In: Stettler, R., Bradshaw, T., Heilman, P., Hinckley, T. (Eds.). The specificity of fungal pathogens of Populus (pp 223–246). Biology of populus and its implications for management and conservation. Canada: NRC Research Press.
Osakabe, Y., Maruyama, K., Seki, M., Satou, M., Shinozaki, K., & Yamaguchi-Shinozaki, K. (2005). Leucine-rich repeat receptor-like Kinase1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis. Plant Cell, 17, 1105–1119.
Parlevliet, J. E. (1993). What is durable resistance, a general outline. In T.H. Jacobs & J.E. Parlevliet (Eds.), Durability of Disease Resistance (23–29) Dordrecht: Kluwer.
Pavan, S., Jacobsen, E., Visser, R. G., & Bai, Y. (2010). Loss of susceptibility as a novel breeding strategy for durable and broad-spectrum resistance. Molecular Breeding, 25, 1–12.
Pessina, S., Pavan, S., Catalano, D., Gallotta, A., Visser, R. G., Bai, Y., Malnoy, M., & Schouten, H. J. (2014). Characterization of the MLO gene family in Rosaceae and gene expression analysis in Malus domestica. BMC Genomics, 15, 618.
Piffanelli, P., Zhou, F., Casais, C., Orme, J., Schaffrath, U., Collins, N., Panstruga, R., & Schulze-Lefert, P. (2002). The barley MLO modulator of defense and cell death is responsive to biotic and abiotic stress stimuli. Plant Physiology, 129, 1076–1085.
Pinon, J., & Frey, P. (2005). Interactions between poplar clones and Melampsora populations and their implications for breeding for durable resistance. In M. H. Pei & A. R. McCracken (Eds.), Rust diseases of willow and poplar (pp. 139–154). Wallingford: CAB International.
Reddy, V. S., Ali, G. S., & Reddy, A. S. N. (2003). Characterization of a pathogen-induced calmodulin binding protein: Mapping of four Ca2+−dependent calmodulin-binding domains. Plant Molecular Biology, 52, 143–159.
Reinstädler, A., Müller, J., Czembor, J. H., Piffanelli, P., & Panstruga, R. (2010). Novel induced MLO mutant alleles in combination with site-directed mutagenesis reveal functionally important domains in the heptahelical barley MLO protein. BMC Plant Biology, 10, 31–43.
Rispail, N., Merino, P., & Rubiales, D. (2013). Identification and characterization of mlo gene family members in the model legume M. truncatula. https://digital.csic.es/handle/10261/97322.
Romiti, M. (2010). Entrez Nucleotide and Entrez Protein FAQs. Gene, 1, 270.
Saito, H., Honma, T., Minamisawa, T., Yamazaki, K., Noda, T., Yamori, T., & Shiba, K. (2004). Synthesis of functional proteins by mixing peptide motifs. Chemistry & Biology, 11, 765–773.
Severoglu, Z., & Ozyigit, I. I. (2012). Powdery mildew disease in some natural and exotic plants of Istanbul, Turkey. Pakistan Journal of Botany, 44, 387–393.
Shen, Q., Zhao, J., Du, C., Xiang, Y., Cao, J., & Qin, X. (2012). Genome-scale identification of MLO domain-containing genes in soybean (Glycine max L. Merr.) Genes & Genetic Systems, 87, 89–98.
Singh, V. K., Singh, A. K., Chand, R., & Singh, B. D. (2012). Genome wide analysis of disease resistance MLO gene family in sorghum (Sorghum bicolor L. Moench). Journal of Plant Genomics, 2, 18–27.
Sjödin, A., Street, N. R., Sandberg, G., Gustafsson, P., & Jansson, S. (2009). The populus genome integrative explorer (PopGenIE): A new resource for exploring the populus genome. New Phytologist, 182, 1013–1025.
Smyth, G. K. (2005). Limma: Linear models for microarray data. In R. Gentleman, V. Carey, S. Dudoit, R. Irizarry, & W. Huber (Eds.), Bioinformatics and computational biology solutions using R and Bioconductor (pp. 397–420). New York: Springer.
Sonnhammer, E. L., Eddy, S. R., & Durbin, R. (1997). Pfam: A comprehensive database of protein domain families based on seed alignments. Proteins-Structure Function and Genetics, 28, 405–420.
Takken, F. L., Albrecht, M., & Tameling, W. I. (2006). Resistance proteins: Molecular switches of plant defense. Current Opinion of Plant Biology, 9, 383–390.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739.
Thompson, J. D., Higgins, D. G., & Gibson, T. J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22, 4673–4680.
Timothy, L., Mikael, B. B., Buske, F. A., Frith, M., Grant, C. E., Clementi, L., Ren, J., Li, W. W., & Noble, W. S. (2009). MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Research, 37, 202–208.
Torii, K. U. (2004). Leucine-rich repeat receptor kinases in plants: Structure, function, and signal transduction pathways. International Review of Cytology, 234, 1–46.
Yang, S., Zhang, X., Yue, J. X., Tian, D., & Chen, J. Q. (2008). Recent duplications dominate NBS-encoding gene expansion in two woody species. Molecular Genetics & Genomics, 280, 187–198.
Yang, J., Yan, R., Roy, A., Xu, D., Poisson, J., & Zhang, Y. (2015). The I-TASSER suite: Protein structure and function prediction. Nature Methods, 12, 7–8.
Yu, C. S., Chen, Y. C., Lu, C. H., & Hwang, J. K. (2006). Prediction of protein subcellular localization. Proteins: Structure, Function, and Bioinformatics, 64, 643–651.
Zheng, Z., Nonomura, T., Appiano, M., Pavan, S., Matsuda, Y., Toyoda, H., Wolters, A. M. A., Visser, R. G. F., & Bai, Y. (2013). Loss of function in Mlo orthologs reduces susceptibility of pepper and tomato to powdery mildew disease caused by Leveillula taurica. PLoS One, 8, e70723.
Zhou, S. J., Jing, Z., & Shi, J. L. (2013). Genome-wide identification, characterization, and expression analysis of the MLO gene family in Cucumis sativus. Genetics and Molecular Research, 12, 6565–6578.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 9430 kb)
Rights and permissions
About this article
Cite this article
Filiz, E., Vatansever, R. Genome-wide identification of mildew resistance locus O (MLO) genes in tree model poplar (Populus trichocarpa): powdery mildew management in woody plants. Eur J Plant Pathol 152, 95–109 (2018). https://doi.org/10.1007/s10658-018-1454-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1454-3