Abstract
This study aims to identify and characterize species of Phaeoacremonium associated with Petri disease of table grapes in three regions in the Northeastern Brazil, to investigate the distribution of the species in these regions and to evaluate their pathogenicity and aggressiveness in excised green shoots of table grapes. Fungal identifications were made using a combination of morphology together with a phylogenetic analysis based on portions of the β-tubulin (TUB2) and actin (ACT) genes. Three species of Phaeoacremonium (Pm.) were identified: Pm. minimum, Pm. nordesticola sp. nov. and Pm. parasiticum. Phaeoacremonium minimum and Pm. parasiticum had previously been reported in grapevine. Phaeoacremonium minimum was the most prevalent species. All species of Phaeoacremonium were pathogenic on detached shoots of table grape, with Pm. minimum being the most aggressive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Table grape (Vitis spp.) is an important fresh fruit exported by Brazil. In 2013, 43,181 t of table grapes were exported and accounted for US$ 131 million (FAO 2016). The Northeastern region is responsible for 99% of Brazilian exports of table grapes, where 9600 ha are cultivated. The São Francisco Valley, located in the semi-arid region of Bahia and Pernambuco States, is the main grapevine growing area in the region, accounting for 98% of the production (Lazzarotto and Fioravanço 2013). In the Siriji Valley, located in the tropical humid region of Pernambuco State, table grapes have been grown for over 40 years with a production intended only for the local market (Araújo and Ramalho 2009). In 2011, a new production pole of table grapes was started in the Baixo Jaguaribe Valley, located in the semi-arid region of Ceará State, but the plants have not yet reached the production stage. In São Francisco and Baixo Jaguaribe Valleys are planted European cultivars (Vitis vinifera L.) grafted onto rootstock, while in Siriji Valley are planted own rooted American cultivars (Vitis labrusca L.).
Northeastern Brazil is a tropical region, thus the management systems for table grape production are adapted to the specific environmental conditions of a tropical viticulture. In both the dry and wet tropics, the growth and cropping cycle of the vine can be manipulated to extend from 5 to 12 months by a combination of pruning, modifying vine water status and the use of chemical regulators. Thus, it is possible to achieve two and a half to three vegetative cycles per year (Camargo et al. 2008; Possingham 2008; Correia et al. 2013).
A wide range of fungal diseases impact on grapevine production. Among them, grapevine trunk diseases (GTDs) are known to occur wherever grapes are grown (Úrbez-Torres et al. 2014). The sudden increase of GTD incidence worldwide has been traditionally associated with reductions in the availability of efficient fungicides, e.g. sodium arsenite, banned in 2001 (Decoin 2001), and other factors such as changes in cultural practices and vineyard management for increasing grape yield (e.g., increasing plant density, irrigation, mechanization, etc.), less protection of pruning wounds due to the increasing cost of labour or the reduced phytosanitary quality of grapevine propagating material. Petri disease is among the most destructive GTD worldwide (Mugnai et al. 1999; Gramaje and Armengol 2011). Incidence of Petri disease have been worsening in all grape-producing regions since the late 1990s, including Europe (Mugnai et al. 1999), the Near East (Arzanlou et al. 2013), North and South America (Correia et al. 2013; Úrbez-Torres et al. 2014), Oceania (Graham et al. 2009), and South Africa (Mostert et al. 2006b), and causing significant economic losses due to yield and quality reductions and vineyard replanting (Scheck et al. 1998).
The first report of Petri disease in table grapes in Northeastern of Brazil was in 2013, in the São Francisco and Siriji Valleys (Correia et al. 2013). The symptoms of this disease are characterized by reduced vigor of vine, short internodes, stunted growth, chlorotic and/or wilting leaves, occasional sudden vine collapse, and black streaking in xylem tissues and black spots in shoots and trunk (Scheck et al. 1998; Mugnai et al. 1999; Gramaje and Armengol 2011; Correia et al. 2013; Úrbez-Torres et al. 2014).
Petri disease is caused by a combination of Phaeomoniella chlamydospora (Gams, Crous, Wingf. e Mugnai) Crous & W. Gams and several species of Phaeoacremonium (Pm.) W. Gams, Crous & M. J. Wingf. (Scheck et al. 1998; Mugnai et al. 1999; Groenewald et al. 2001; Gramaje and Armengol 2011; Correia et al. 2013; Úrbez-Torres et al. 2014). The genus Phaeoacremonium was established by Crous et al. (1996), and since then, 46 species have been identified based on morphological along with molecular characters (Gramaje et al. 2015). Species delimitation within Phaeoacremonium based solely on cultural and morphological characteristics is challenging, and thus molecular analyses of part of the actin and β-tubulin gene regions have been used routinely for species delineation (Mostert et al. 2006a; Damm et al. 2008; Essakhi et al. 2008; Gramaje et al. 2009, 2012, 2014, 2015; Úrbez-Torres et al. 2014; Raimondo et al. 2014; Ariyawansa et al. 2015).
Phaeoacremonium species have worldwide distribution and wide host range, including woody plants, insect larvae and humans (Mostert et al. 2006a). Twenty-eight species have been isolated from grapevine (Gramaje et al. 2015). Of these, Pm. minimum (Tul. & C. Tul.) D. Gramaje, L. Mostert & Crous appears to be the most widely distributed and the most common in grapevines (Crous et al. 1996; Larignon and Dubos 1997; Mugnai et al. 1999; Groenewald et al. 2001; Mostert et al. 2006b; Essakhi et al. 2008; Martín et al. 2014). Other species that have also been isolated in relatively high frequencies from grapevines include Pm. parasiticum (Ajello, Georg & C.J.K Wang) W. Gams, Crous & M.J. Wingf. and Pm. viticola J. Dupont (Mostert et al. 2006b). In Brazil, three species were reported in grapevine, Pm. minimum, Pm. parasiticum (Correia et al. 2013) and Pm. angustius W. Gams, Crous & M.J. Wingf. (Gava et al. 2010).
The increasing economic importance of Petri disease caused by Phaeoacremonium in grapevines and the recent discovery of several new species of this fungus associated with other woody host plants (Damm et al. 2008; Gramaje et al. 2012, 2014) led us to question what species of Phaeoacremonium may be associated with Petri disease of table grape in São Francisco, Siriji and Baixo Jaguaribe Valleys, Northeastern Brazil. The disease etiology is crucial for epidemiological studies and for a better understanding of the distribution and importance of individual species, as well as finding effective management strategies for each pathogen. Therefore, the objectives of this study were: (a) to identify and characterize species of Phaeoacremonium associated with Petri disease of table grapes in São Francisco, Siriji and Baixo Jaguaribe Valleys, (b) to investigate the distribution of the species in these regions, and (c) to evaluate their pathogenicity and aggressiveness in excised green shoots of table grapes.
Materials and methods
Field survey and fungal isolations
During 2012, samples of table grape plants of age ranging from 6 months to 10-years old showing Petri disease symptoms were obtained from 12 vineyards located in the São Francisco, Siriji and Baixo Jaguaribe Valleys (Northeastern Brazil) (Fig. 1). These regions are distant at least 500 km from each other. In each vineyard, 10 grapevines shoots exhibiting reduced vigor of vine, stunted growth, chlorotic and/or wilting leaves, sudden vine collapse, and black streaking in xylem tissues and black spots in shoots were sampled for fungal isolations. Symptomatic wood fragments taken from the margin of internal necroses and brown-black vascular streaking were washed under running tap water, surface-disinfected for 1 min in a 1.5% sodium hypochlorite solution, and washed twice with sterile distilled water. Small pieces (4–5 mm) of tissue were taken from the margin between necrotic and apparently healthy tissue and plated onto malt extract agar (MEA; Acumedia, Lansing, USA) amended with 0.5 g l−1 streptomycin sulfate (MEAS). Plates were incubated at 25 °C in the dark for 14 to 21 days. Fungal colonies emerging from plant tissue pieces, characterized by having flat slow-growing cultures on MEA and that were morphologically similar to species of Phaeoacremonium (Mostert et al. 2006a) were transferred to potato dextrose agar (PDA; Acumedia) plates and incubated at 25 °C in the dark, with observation after 14 to 21 days. Single-spore cultures were obtained using the procedure described by Goh (1999). Isolates were morphologically identified as Phaeoacremonium based on typical characteristics of the genus, namely presence of different types of phialides variable in size and shape observed in the aerial mycelium, and either discrete or integrated in conidiophores, and conidia hyaline, sporulation abundant and aseptate (Mostert et al. 2006a; Gramaje et al. 2015). Pure cultures were stored in sterilized water in Eppendorf tubes at 25 °C in the dark and stock cultures were stored in PDA slants at 5 °C in the dark.
Collection sites of Phaeoacremonium isolates from Northeastern Brazil vineyards, located in the states of Bahia (BA), Pernambuco (PE) and Ceará (CE). The names next to the dots correspond to the cities corresponding to the sampled vineyard. Dotted semicircles represent the regions defined for this study; n = number of isolates in each region
Morphological and cultural characterization
Morphological characters used to distinguish Phaeoacremonium species included conidiophore morphology, phialide type and shape size of hyphal warts and conidial size and shape (Mostert et al. 2006a). Colony characters and pigment production on MEA, PDA and oatmeal agar (OA; Difco, Detroit, USA) incubated at 25 °C in the dark were noted after 8 and 16 days. Colony colours were recorded with the colour charts of Rayner (1970). Microscopic observations for all fungi were made from mycelium of colonies cultivated on the respective medium or by using slide culture technique, as explained by Arzanlou et al. (2007) when studying the genus Mycosphaerella. Photos were captured with a Nikon DS-Ri2 camera on a Nikon Eclipse Ni-e microscope fitted with Nomarski differential interference contrast optics (Nikon Instruments Europe BV, Netherlands). The length and width of 20 conidiophores, 30 phialides and 50 conidia per isolate were measured with the Nikon measurement module. Mean and standard errors of the measurements, including mean length to width ratio (L/W) of the conidial measurements were calculated.
Isolates were also used to determine the effect of temperature on colony growth of different species. A 5-mm-diameter mycelial plug from the growing margin of a 8-day-old colony was placed in the center of a 90-mm-diameter PDA plate, and three replicates of each isolate were incubated at temperatures ranging from 10 °C to 40 °C in 5 °C intervals in the dark. After 8 and 16-days incubation periods, the colony diameters (mm) were measured in two perpendicular directions. The experiment was done twice. Colony diameters at 16 days were plotted against temperature and a curve was fitted by a cubic polynomial regression (y = a + bx + cx2 + dx3). Optimal temperature was estimated from the regression equation and numeric summary with TableCurve™ 2D v. 5.01 (SYSTAT Software Inc., Chicago, USA). Optimum temperature was defined as the temperature that produced the maximum mycelial growth. The colony diameter data at 25 °C were used to calculate the mycelial growth rate (mm day−1). One-way analyses of variance (ANOVA) were conducted with data obtained from optimum temperature and mycelial growth rate experiments, and means were compared by Fisher’s least significant difference (LSD) test at the 5% significance level using Statistix v. 9.0 (Analytical Software, Tallahassee, USA).
DNA isolation, PCR amplification and sequencing
Using a sterile 10 μl pipette tip, a small amount of aerial mycelium was scraped from the surface of a 7 day old culture on PDA at 25 °C and genomic DNA was extracted using the AxyPrep™ Multisource Genomic DNA Miniprep Kit (Axygen Scientific Inc., Union City, USA) following the manufacturer’s instructions. DNA was viewed on 0.8% agarose gels stained with ethidium bromide (0.5 μg ml−1) for 1 min and stored at −20 °C. Portions of the β-tubulin (TUB2) and actin (ACT) genes of Phaeoacremonium isolates were amplified as described by Mostert et al. (2006a) using primer sets T1 (O’Donnell and Cigelnik 1997) and Bt2b (Glass and Donaldson 1995), and ACT-512F and ACT-783R (Carbone and Kohn 1999), respectively. Each 50 μl polymerase chain reaction (PCR) mixture included 21 μl of PCR-grade water, 1 μl of DNA template, 1.5 μM of each primer, and 1 μl of PCR Master Mix (2X) (0.05 u μl−1 de Taq DNA polimerase, reaction buffer, 4 mM MgCl2, 0.4 mM of each dNTP; Thermo Scientific, Waltham, USA). PCR reactions were carried out in a thermal cycler (Biocycler MJ 96; Applied Biosystems, Foster City, USA). The cycling parameters for ACT gene consisted of a denaturation step at 96 °C for 5 min, followed by 35 cycles at 94 °C for 30 s, 52 °C for 30 s, 72 °C for 80 s and final cycle at 72 °C for 7 min. The cycling parameters for TUB2 gene were initiated at 96 °C for 5 min followed by 36 cycles at 94 °C for 30 s, 58 °C for 30 s, 72 °C for 80 s and final cycle at 72 °C for 7 min (Graham et al. 2009). The PCR amplification products were separated by electrophoresis in 1.5% agarose gels in 1.0× Tris-acetate acid EDTA (TAE) buffer and were photographed under UV light after staining with ethidium bromide (0.5 μg ml−1) for 1 min. PCR products were purified using the AxyPrep PCR Cleanup Kit (Axygen) following the manufacturer’s instructions and sequenced in both directions using a ABI 3730 XL DNA Analyzer (Applied Biosystems) at the Macrogen Inc. (Seoul, Korea).
Phylogenetic analysis
Forward and reverse sequences were assembled using the Staden Package (Staden et al. 1998). Sequences generated in the current study were deposited in GenBank (Table 1).
Sequences of the type and reference strains of Phaeoacremonium species of each locus analyzed were downloaded from GenBank and combined with the newly generated sequences. Multiple sequence alignments for each locus independently were performed using the MEGA 7.0.14 (Kumar et al. 2016), and adjustment were manually done in where necessary. Alignment of each locus was loaded in Sequence Matrix v.1.8 (Vaidya et al. 2011) to build the concatenated matrix.
The phylogenies for each locus (ACT and TUB2) and for the concatenated matrix were inferred under the maximum likelihood (ML) criterion. The ML analyses were done in n RAxML - HPC2 (Stamatakis 2014) implemented on CIPRES Science Gateway portal (https://www.phylo.org/portal2/home.action). ML tree searches were performed under the GTRGAMMA model with 1000 pseudoreplicates.
Phylogenetic species recognition
The genealogical concordance phylogenetic species recognition (GCPSR sensu Dettman et al. 2003) criterion was employed to recognize phylogenetic species. A clade was considered an independent lineage if it satisfied the genealogical concordance criteria (the clade was present in the majority of the individual gene trees) or genealogical non-discordance criteria [the clade is strongly supported (bootstrap ≥70) in at least one gene tree, and could not be contradicted in any other individual gene tree at the same level of support].
Novel species were recognized if the clade was recognized as a phylogenetic species by the GCPSR criterion, is strongly support in the multi-locus tree, and did not nest with the type of any previously described species.
Distribution of Phaeoacremonium species
Based on the number of isolates of each Phaeoacremonium species recorded, the relative frequency of each species in relation to overall number of isolates and to the total number of isolates within each table grape population was calculated.
Pathogenicity tests
Fourteen isolates representing three different Phaeoacremonium species were selected to assess their pathogenicity on detached green shoots of cultivar “Isabel” (Table 1). Asymptomatic green shoots of plants not sprayed with fungicides were collected in a commercial vineyard in São Vicente Férrer (Siriji Valley). The shoots were immediately placed into large plastic containers filled with sterile water, with the shoots placed over a plastic grid. The plastic containers were partially sealed with plastic bags and transported to Universidade Federal Rural de Pernambuco. The cut ends were dipped in wax and in the center of each shoot (30 cm long) a superficial wound (~4-mm length, 2-mm deep) was made using a sterilized scalpel. A 4-mm mycelium plug from a 12-day-old PDA culture of each isolate was placed into the wound. Non-colonized PDA agar plugs were used as negative controls. The inoculated area was wrapped with Parafilm (Pechiney Co., Chicago, USA) to prevent rapid dehydration. Inoculated shoots were placed in large plastic containers, as described above, and incubated at 25 °C and 12-h photoperiod in a growth chamber. After 23 days, the Parafilm was removed, the shoots were sliced through lengthwise and the internal lesions visually observed. The isolates were considered pathogenic when the lesioned area advanced beyond the 4-mm diameter inoculated area. The aggressiveness of the isolates was evaluated by measurement of the lesion lengths with a digital calliper (Mitutoyo Co., Kanagawa, Japan). The experiment was arranged in a completely randomized design with ten replicates per treatment (isolate) and one shoot per replicate. The experiment was conducted twice. Differences in aggressiveness of Phaeoacremonium species were determined by one-way ANOVA and means were compared by LSD test at the 5% significance level using Statistix v. 9.0.
Results
Morphological and cultural characterization
Twenty-two isolates of Phaeoacremonium were obtained from table grape plants showing Petri disease symptoms in Northeastern Brazil. All species showed morphological features typical of the genus Phaeoacremonium (Mostert et al. 2006a), namely presence of different types of phialides observed in the aerial mycelium, and either discrete or integrated in conidiophores, and conidia hyaline and aseptate (Fig. 3). No teleomorph structure was observed during this study.
The morphological characteristics observed in Pm. minimum were: colony colour on MEA honey-brown or beige; yellow pigment not produced on MEA and PDA; mycelium texture mostly verruculose; conidiophore structure mostly short and usually unbranched, 18.9–36.5 × 1.9–2.9 μm (\( \overline{x} \) = 23.9 ± 1.7 × 2.4 ± 0.4 μm; n = 32); conidiogenous cells phialides predominant type II and III; type I phialides cylindrical, 2.5–4.9 × 0.6–1.5 μm (\( \overline{x} \) = 4.1 ± 0.7 × 1 ± 0.3 μm; n = 38); type II phialides mostly elongate-ampulliform and attenuated at the base, 6.6–11 × 1.3–2.7 μm (\( \overline{x} \) = 9.6 ± 1.7 × 1.8 ± 0.5 μm; n = 66); type III phialides subcylindrical or elongate-ampulliform and attenuated at the base, 11–19.5 × 1.7–2.6 μm (\( \overline{x} \) = 14.9 ± 2.1 × 2.1 ± 0.4 μm; n = 52); conidia hyaline, mostly oblong-ellipsoid or cylindrical, occasionally reniform, 3.1–7× 1.3–3.4 μm (\( \overline{x} \) = 4.8 ± 0.9 × 2.1 ± 0.3 μm; n = 165), L/W ratio = 2.3.
The morphological characteristics observed in Pm. parasiticum were: colony colour on MEA brown with medium brown center; yellow pigment produced on MEA and PDA; mycelium texture verrucose; conidiophore structure mostly long and branched, 27.2–56.1× 2–3.4 μm (\( \overline{x} \) = 39.1 ± 8.2 × 2.6 ± 0.5 μm; n = 34); conidiogenous cells phialides predominant type III; type I phialides cylindrical, occasionally widened at the base, 5.7–6.4 × 1.1–2.2 μm (\( \overline{x} \) = 6.0 ± 0.3× 1.6 ± 0.5 μm; n = 32); type II phialides subcylindrical, tapering toward the apex,12–24.3 × 1.4–2.6 μm (\( \overline{x} \) = 17.4 ± 3.0 × 2.1 ± 0.4 μm; n = 42); type III phialides mostly cylindrical to subulate, 22–40.7× 2.4–3.6 μm (\( \overline{x} \) = 29.9 ± 6.3× 2.9 ± 0.4 μm; n = 62); conidia hyaline, mostly oblong-ellipsoid, sometimes allantoid to broadly oblong, 2.7–6.3× 1.3–2.8 μm (\( \overline{x} \) = 4 ± 0.7× 1.8 ± 0.3 μm; n = 134), L/W ratio = 2.2.
All species of Phaeoacremonium used in this study grew at temperatures ranging from 10 °C to 35 °C. Only one isolate of Pm. minimum (CMM 4322) and two isolates of P. parasiticum (CMM 4315 and CMM 4321) grew at 40 °C. There were no significant differences (P > 0.05) among the Phaeoacremonium species in relation to mycelial growth rate (0.22–0.25 mm day−1) and optimum temperature for mycelial growth (29.6–30.1 °C).
Phylogenetic analyses
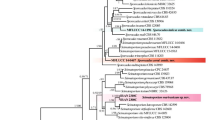
Three distinct haplotypes were found among the twenty-two isolates based on the DNA sequences. The topologies of each single locus tree were not discordant among them. Alignments of ACT and TUB2 provided topologies with great resolution, and clades of all Phaeoacremonium species were recovered with significant support. The individual gene trees resolved the same clades recovered in the multi locus tree (Fig. 2).
Maximum likelihood tree of Phaeoacremonium species inferred from a concatenated alignment of ACT and TUB2. Significant supports for the Maximum Likelihood analysis (≥70) are shown above the nodes. “-” indicates no-significant support. Branches crossed by diagonal lines are shortened by 50%. Types are emphasized in bold font. Isolates from grapevine are highlighted in red. Wuestneaia molokaiensis CBS114877 was used as outgroup. The scale bar indicates the average number of substitutions per site
Phylogenetic species recognition
The 22 Phaeoacremonium isolates from grapevine included in the multi locus analyses were resolved in three independent lineages according to the GCPSR criterion, and could be recognized as phylogenetic species. Fifteen isolates were assigned to the species P. minimum. Three isolates were placed within the P. parasiticum clade and were assigned to this species. Four isolates nested together in a clade closely related with P. luteum, and is described in the present study as a novel species named Phaeoacremonium nordesticola. The species description is presented in the taxonomic section.
Taxonomy
Based on the DNA sequence analyses and morphological characters, one species of Phaeoacremonium proved distinct from known species, and is described below.
Phaeoacremonium nordesticola M.A. Silva, M.P.S. Câmara & S.J. Michereff sp. nov. MycoBank register = 820710; Figs. 3a–p.
Phaeoacremonium nordesticola holotype (culture CMM 4312). Sixteen-day-old colony grown on MEA (a), PDA (b) and OA (c); (d-o) Aerial structures on MEA (D-G) Type III phialides; (h-i) Type III and Type II phialides (indicated by arrow); (j) Type III and Type I phialides (indicated by arrow); (k) Type II and Type I phialides (indicated by arrow); (l-n) Type I phialides; (o) Single conidiophore; (p) Structures on the surface of and in MEA: conidia. Scale bars: d – 10 μm; p – 5 μm. Scale bar for d applies to d-o
Etymology:
The name refers to Northeastern Brazilian region, where this species was first found.
Description
colonies on PDA at first hazel (17″‘i) to olivaceous buff (21″‘d), becoming greenish glaucous (23″‘i) and olivaceous (21”k) at the surface with the reverse side of the colonies olivaceous buff (21″‘d) after 8 days in the dark at 25 °C. Colonies reaching a radius of 16.7–1.7 mm after 8 days in the dark at 25 °C on MEA. No pigment produced on MEA and PDA. Minimum temperature for growth 10 °C, optimum 29.7 °C, maximum 37 °C. The mycelial growth rate at 25 °C was 0.22 ± 0.02 mm day−1. Aerial mycelium consisting of branched, septate hyphae that occurs singly or in bundles of up to 8; hyphae without warts. Conidiophores mostly short, usually unbranched, 18.9–36.5 × 1.90–2.9 μm (\( \overline{x} \) = 23.9 ± 1.7 × 2.4 ± 0.4 μm; n = 46). Conidiogenous cells phialides predominant type II, terminal or lateral, mostly monophialidic; type I phialides cylindrical, 2.5–4.9 × 0.6–1.5 μm (\( \overline{x} \) = 4.0 ± 0.4 × 1.1 ± 0.2 μm; n = 36); type II phialides mostly elongate-ampulliform and attenuated at the base, 6.6–11 × 1.3–2.7 μm (\( \overline{x} \) = 9.4 ± 1.0 × 1.9 ± 0.4 μm, n = 58); type III phialides cylindrical to subcylindrical, 11–19.5 × 1.6–2.6 μm (\( \overline{x} \) = 14.9 ± 21 × 1.9 ± 0.4 μm; n = 42); Conidia hyaline, mostly allantoid, few reniform, 2.9–7.5 × 1.1–2.4 μm (\( \overline{x} \) = 4.2 ± 0.9 × 1.7 ± 0.3 μm; n = 178), L/W ratio = 2.6.
-
Sexual morph: not produced in culture.
-
Substrate: Vitis vinifera.
-
Known Distribution: Brazil (Ceará, Pernambuco).
-
Type: Brazil, Pernambuco, Petrolina, on Vitis vinifera tree, 2012, coll. M. A. Silva (holotype URM register = 89963 dry culture produced on pine needles, ex-type culture URM register = 7391 = CMM 4312).
Additional cultures examined
Brazil, Pernambuco, Petrolina, isolated from Vitis vinifera trees, 2012, coll. M.A. Silva, CMM 4313 and CMM 4314; Brazil, Ceará, Russas, isolated from Vitis vinifera tree, 2012, coll. M.A. Silva, CMM 4334.
Notes – Phylogenetically Pm. nordesticola is closely related to Pm. luteum D. Gramaje, T.I. Burgess & J. Armengol, but cultures of Pm. luteum produced yellow pigment on MEA, PDA and OA, which did not occur with Pm. nordesticola. Phialides type I (4–6 × 1.5–2 μm), type II (10.5–17 × 2–3.5 μm) and type III (20–30 × 2–3 μm), and conidia (4–6 × 2–3 μm; \( \overline{x} \) = 5 × 2.5 μm) of Pm. luteum are longer and wider than those of Pm. nordesticola (type I phialides: 2.5–4.9× 0.6–1.5 μm; type II phialides: 6.6–11 × 1.3–2.7 μm; type III phialides: 11–19.5 × 1.6–2.6 μm; conidia: 2.9–7.5 × 1.1–2.4 μm,\( \overline{x} \) = 4.23 × 1.65 μm). The conidia L/W ratio of Pm. luteum (2.2) is smaller that of Pm. nordesticola (2.6). Phaeoacremonium nordesticola differs from its closest phylogenetic neighbor, Pm. luteum, by unique fixed alleles in one loci based on alignments of the separate loci deposited in TreeBase as study S16135: alignments and β-tubulin positions 118 (T), 242 (G), 361 (C), 508 (A) and Actin positions 2 (A), 13 (C), 59 (C), 102 (A), 745 (T) and 259 (A).
Distribution of Phaeoacremonium species
Phaeoacremonium minimum was the predominant species isolated from table grape trees (15 isolates) followed by Pm. nordesticola (four isolates) and Pm. parasiticum (three isolates). The distribution of Phaeoacremonium species differed between the three regions of the Northeastern Brazil. The three Phaeoacremonium species were found only in São Francisco Valley. Pm. minimum and Pm. parasiticum were found in Siriji Valley (Fig. 4). A single isolate of Pm. nordesticola was obtained in Baixo Jaguaribe Valley.
Pathogenicity tests
All isolates of Phaeoacremonium were pathogenic on detached green shoots of grapevine, resulting in visible lesions 23 days after inoculation. The symptoms observed both on the surface and internally were brown dark necrotic lesions which extended upward and downward from the point of inoculation. There were significant (P ≤ 0.05) differences in aggressiveness among the species. Phaeoacremonium minimum was the most aggressive, causing the largest lesion (10.9 ± 1.1 mm), while Pm. nordesticola was the less aggressive, causing the smaller lesion (8.6 ± 0.6 mm). Phaeoacremonium parasiticum showed intermediate aggressiveness, causing lesion of 9.5 ± 0.9 mm.
Discussion
Table GTDs and the associated pathogens have been little studied worldwide. This study constitutes the first attempt to assess the diversity of Phaeoacremonium species on table grapes showing Petri disease symptoms in Brazil. Species identification was based on morphological characters and analysis of partial sequences of actin and β-tubulin genes. Three species were identified, namely Pm. minimum, Pm. parasiticum, and the new species Pm. nordesticola. The colony characteristics and conidiophores, phialides and conidia dimensions of the first two species obtained in this study were similar to those previously described in the literature (Mostert et al. 2006a).
Since 1996, when the genus Phaeoacremonium was first established (Crous et al. 1996), 28 species of Phaeoacremonium had been isolated from grapevines and identified based on their cultural, morphological, and molecular characters (Crous et al. 1996; Dupont et al. 2000; Groenewald et al. 2001; Mostert et al. 2005; Mostert et al. 2006b; Essakhi et al. 2008; Graham et al. 2009; Gramaje et al. 2009; Úrbez-Torres et al. 2014; Raimondo et al. 2014). Interestingly, the majority of these species (22 out of 28) have been identified within the last 8 years, which may be a response to a significant upsurge in the number of field surveys conducted since the early 2000s spurred by increases in Petri disease incidences in grape growing regions worldwide. Discovery of a novel species in the present study increases to 29 and 47, respectively, the total number of Phaeoacremonium species occurring in grapevine and in this genus.
Phaeoacremonium nordesticola is recognized as a new species in the genus Phaeoacremonium, closely related to Pm. luteum. Considering the phylogenetic data, the isolates of Pm. nordesticola formed a clade strongly supported in the maximum-parsimony analysis (100%). Phaeoacremonium nordesticola can also be distinguished from Pm. luteum based on colony characteristics, mycelial growth and phialides dimensions described for this species (Gramaje et al. 2014). Phaeoacremonium nordesticola did not produce yellow pigment on MEA, PDA or OA, which occurs with from Pm. luteum. Phialides type I, II and III of Pm. luteumare longer and wider than those of Pm. nordesticola. The conidia L/W ratio of Pm. luteum is smaller that of Pm. nordesticola.
In this work, Pm. minimum was the most frequently isolated species associated with Petri disease of table grape, and also the most widespread species in vineyards of Northeastern Brazil. This species had been reported in table grapes in Northeastern Brazil (Correia et al. 2013), being recognized as the most common species on grapevines worldwide (Mostert et al. 2006b; Essakhi et al. 2008; Martín et al. 2014; Úrbez-Torres et al. 2014). This species has been isolated from other host plants than grapevine, including Actinidia chinensis (Crous and Gams 2000), Cydonia oblonga (Sami et al. 2014), Diospyros kaki (Moyo et al. 2016), Malus domestica (Cloete et al. 2011), Olea europea (Crous and Gams 2000), Phoenix dactylifera (Mohammadi 2014), Prunus armeniaca (Damm et al. 2008), Prunus persica (Damm et al. 2008), Prunus salicina (Damm et al. 2008), Prunus pennsylvanica (Hausner et al. 1992), Pyrus communis (Cloete et al. 2011) and Salix sp. (Hausner et al. 1992).
The other two species isolated in this work, Pm. parasiticum and Pm. nordesticola, were isolated with similar frequency (18.2%) from table grapes with Petri disease symptoms. Phaeoacremonium parasiticum is commonly isolated from grapevines in relatively high frequencies (Dupont et al. 2002; Mostert et al. 2006b) and had been reported in table grapes in Northeastern Brazil (Correia et al. 2013). Besides Brazil, Pm. parasiticum was also isolated in Algeria (Berraf-Tebbal et al. 2011), Argentina (Dupont et al. 2002), Australia (Mostert et al. 2005), Chile (Auger et al. 2005), Iran (Mostert et al. 2006a), Italy (Essakhi et al. 2008), Peru (Romero-Rivas et al. 2009), South Africa (Crous et al. 1996), Spain (Aroca et al. 2006), Turkey (Dupont et al. 2000) and USA (Mostert et al. 2006a). This species was also found on other woody hosts as an endophyte or as agent of plant disease, including A. chinensis (Di Marco et al. 2004), Aquilaria agallocha (Mostert et al. 2006b), Cupressus sp. (Mostert et al. 2006b ), Cydonia oblonga (Sami et al. 2014), Diospyros kaki (Moyo et al. 2016), Nectandra sp. (Hawksworth et al. 1976), O. europea (Nigro et al. 2013), P. dactylifera (Hawksworth et al. 1976), P. armeniaca (Hawksworth et al. 1976), Prunus avium (Rumbos 1986), P. communis (Sami et al. 2014), Quercus virginiana (Halliwell 1966) and Santalum album (Gramaje et al. 2014). Phaeoacremonium parasiticum have also been associated with human infections, often causing phaeohyphomycosis (lumps of fungal growth under the skin) (Crous et al. 1996; Mostert et al. 2005; Mostert et al. 2006a). Species of Phaeoacremonium are opportunistic pathogens needing a subcutaneous traumatic inoculation or predisposed host to be able to infect and cause disease (Mostert et al. 2006b).
Regarding cultural characteristics, the optimum temperature for mycelial growth for Phaeoacremonium species from table grape varied between 29.6 and 30.1 °C, and some isolates of Pm. minimum and Pm. parasiticum grew at 40 °C, corroborating with information from the literature (Mostert et al. 2006a). The growth of Pm. parasiticum at 10 °C observed in this study contrast to information that the minimum temperature for this species is 15 °C (Mostert et al. 2006a). As can be observed, cultural characteristics may vary among isolates of the same species and therefore are of limited value in the determination of species.
Although many new Phaeoacremonium species have been identified from Petri disease infected plants, there is a lack of evidence for pathogenicity in most studies (Dupont et al. 2000; Groenewald et al. 2001; Mostert et al. 2006b; Mostert et al. 2006b; Essakhi et al. 2008; Gramaje et al. 2009), making it difficult to evaluate whether the majority of Phaeoacremonium species are involved in disease development. All isolates of Phaeoacremonium species in this study were able to infect, colonize, and produce lesions in detached grapevine shoots, confirming their status as Petri disease pathogens, including the new species Pm. nordesticola. Differences in aggressiveness among Phaeoacremonium species inoculated in grapevine, as observed in this study, have been reported previously (Mostert et al. 2006b; Halleen et al. 2007; Aroca and Raposo 2009; Úrbez-Torres et al. 2014).
This article reports three species of the genus Phaeoacremonium associated with Petri disease of table grape in Northeastern Brazil. All the species found in Northeastern Brazil have potential to cause disease in table grapes, but Pm. minimum was the most aggressive species. Studies are needed on the epidemiology and impact on table grape production together with information referring to ecology, distribution, host range and fungicide sensitivity of all species of Phaeoacremonium found in this study.
The results of this study will certainly be crucial to a better formulation of Petri disease control strategies and genetic improvement programs in tropical viticulture.
References
Araújo, J. L. P., & Ramalho, P. J. P. (2009). Custos de produção. In J. M. Soares & P. C. S. Leão (Eds.), A vitivinicultura no semi-árido brasileiro (pp. 727–736). Brasília: Embrapa Informação Tecnológica.
Ariyawansa, H. A., et al. (2015). Fungal diversity notes 111–252 – Taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity, 75, 27–274.
Aroca, A., García-Figueres, F., Bracamonte, L., Luque, J., & Raposo, R. (2006). A survey of trunk disease pathogens within rootstocks of grapevines in Spain. European Journal of Plant Pathology, 115, 195–202.
Aroca, A., & Raposo, R. (2009). Pathogenicity of Phaeoacremonium species on grapevines. Journal of Phytopathology, 157, 413–419.
Arzanlou, M., Groenewald, J. Z., Gams, W., Braun, U., Shin, H. D., & Crous, P. W. (2007). Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology, 58, 57–93.
Arzanlou, M., Moshari, S., Salari, M., & Badali, H. (2013). Molecular characterization and pathogenicity of Phaeoacremonium spp. associated with esca disease of grapevines in northern Iran. Archives of Phytopathology and Plant Protection, 46, 375–388.
Auger, J., Perez, I., Esterio, M., Navia, V., Gubler, W. D., & Eskalen, A. (2005). Fungi associated with grapevine wood decay and young vine decline in Chile. Phytopathologia Mediterranea, 44, 89–90.
Berraf-Tebbal, A., Bouznad, Z., Santos, J. M., Coelho, M. A., Péros, J. P., & Phillips, A. J. L. (2011). Phaeoacremonium species associated with Eutypa dieback and esca of grapevines in Algeria. Phytopathologia Mediterranea, 50, S86–S97.
Camargo, U. A., Protas, J. F. S., & Mello, L. M. R. (2008). Grape growing and processing in Brazil. Acta Horticulturae, 785, 51–57.
Carbone, I., & Kohn, L. M. (1999). A method for designing primers sets for speciation studies in filamentous ascomycetes. Mycologia, 91, 553–556.
Cloete, M., Fourie, P. H., Damm, U., Crous, P. W., & Mostert, L. (2011). Fungi associated with die-back symptoms of apple and pear trees, a possible inoculum source of grapevine trunk disease pathogens. Phytopathologia Mediterranea, 50, S176–S190.
Correia, K. C., Câmara, M. P. S., Barbosa, M. A. G., Sales Jr., R., Agustí-Brisach, C., Gramaje, D., García-Jiménez, J., Abad-Campos, P., Armengol, J., & Michereff, S. J. (2013). Fungal trunk pathogens associated with table grape decline in northeastern Brazil. Phytopathologia Mediterranea, 52, 380–387.
Crous, P. W., & Gams, W. (2000). Phaeomoniella chlamydospora gen. Et comb. Nov., a causal organism of petri grapevine decline and esca. Phytopathologia Mediterranea, 39, 112–188.
Crous, P. W., Gams, W., Wingfield, M. J., & Van Wyk, P. S. (1996). Phaeoacremonium gen. Nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia, 88, 786–796.
Damm, U., Mostert, L., Crous, P. W., & Fourie, P. H. (2008). Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Persoonia, 20, 87–102.
Decoin, M. (2001). Grapevine products: News on withdrawals and restrictions. Phytoma, 543, 28–33.
Dettman, J. R., Jacobson, D. J., & Taylor, J. W. (2003). A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution, 57, 2703–2720.
Di Marco, S., Calzarano, F., Osti, F., & Mazzullo, A. (2004). Pathogenicity of fungi associated with a decay of kiwifruit. Australasian Plant Pathology, 33, 337–342.
Dupont, J., Laloui, W., Magnin, S., Larignon, P., & Roquebert, M. F. (2000). Phaeoacremonium viticola, a new species associated with esca disease of grapevine in France. Mycologia, 92, 499–504.
Dupont, J., Magnin, S., Césari, C., & Gatica, M. (2002). ITS and β-tubulin markers help delineate Phaeoacremonium species, and the occurrence of P. parasiticum in grapevine disease in Argentina. Mycological Research, 106, 1143–1150.
Essakhi, S., Mugnai, L., Crous, P. W., Groenewald, J. Z., & Surico, G. (2008). Molecular and phenotypic characterization of novel Phaeoacremonium species associated with petri disease and esca of grapevine. Persoonia, 21, 119–134.
FAO (2016). FAOSTAT. Resource database. http://faostat3.fao.org/home/E. Accessed on 24 October 2016.
Gava, R., Urben, A. F., & Garrido, L. R. (2010). Influence of culture media and temperature on mycelial growth of Phaeoacremonium angustius. Phytopathologia Mediterranea, 49, 110–111.
Glass, N. L., & Donaldson, G. C. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous infection due to Phaeoacremonium spp. Journal of Clinical Microbiology, 41, 1332–1336.
Goh, T. K. (1999). Single-spore isolation using a handmade glass needle. Fungal Diversity, 2, 47–63.
Graham, A. B., Johnston, P. R., & Weir, B. S. (2009). Three new Phaeoacremonium species on grapevines in New Zealand. Australasian Plant Pathology, 38, 505–513.
Gramaje, D., Agustí-Brisach, C., Pérez-Sierra, A., Moralejo, E., Olmo, D., Mostert, L., Damm, U., & Armengol, J. (2012). Fungal trunk pathogens associated with wood decay of almond trees on Mallorca (Spain). Persoonia, 28, 1–13.
Gramaje, D., & Armengol, J. (2011). Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification, and management strategies. Plant Disease, 95, 1040–1055.
Gramaje, D., Armengol, J., Mohammadi, H., Banihashemi, Z., & Mostert, L. (2009). Novel Phaeoacremonium species associated with petri disease and esca of grapevines in Iran and Spain. Mycologia, 101, 920–929.
Gramaje, D., León, M., Pérez-Sierra, A., Burgess, T., & Armengol, J. (2014). New Phaeoacremonium species isolated from sandalwood trees in Western Australia. IMA Fungus, 5, 67–77.
Gramaje, D., Mostert, L., Groenewald, J. Z., & Crous, P. W. (2015). Phaeoacremonium: From esca disease to phaeohyphomycosis. Fungal Biology, 119, 759–783.
Groenewald, M., Kang, J.-C., Crous, P. W., & Gams, W. (2001). ITS and beta-tubulin phylogeny of Phaeoacremonium and Phaeomoniellaspecies. Mycological Research, 105, 651–657.
Halleen, F., Mostert, L., & Crous, P. W. (2007). Pathogenicity testing of lesser- known vascular fungi of grapevines. Australasian Plant Pathology, 36, 277–285.
Halliwell, R. S. (1966). Association of Cephalosporium with a decline of oak in Texas. Plant Disease Reporter, 50, 75–78.
Hausner, G., Eyjólfsdóttir, G., & Reid, J. (1992). Two additional species of the genus Togninia. Canadian Journal of Botany, 70, 724–734.
Hawksworth, D. L., Gibson, I. A. S., & Gams, W. (1976). Phialophora parasiticaassociated with disease conditions in various trees. Transactions of the British Mycological Society, 66, 427–431.
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874.
Larignon, P., & Dubos, B. (1997). Fungi associated with esca disease in grapevine. European Journl of Plant Pathology, 103, 147–157.
Lazzarotto, J. J., & Fioravanço, J. C. (2013). Tendências e sazonalidades nas exportações e importações brasileiras de uva de mesa. Informações Econômicas, 43, 43–58.
Martín, L., Miera, L. E. S., & Martín, M. T. (2014). AFLP and RAPD characterization of Phaeoacremonium aleophilum associated with Vitis vinifera decline in Spain. Journal of Phytopathology, 162, 245–257.
Mohammadi, H. (2014). Phaeoacremonium spp. and Botryosphaeriaceae spp. associated with date palm (Phoenix dactylifera L.) decline in Iran. Journal of Phytopathology, 162, 575–581.
Mostert, L., Groenewald, J. A., Summerbell, R. C., Gams, W., & Crous, P. W. (2006a). Taxonomy and pathology on Togninia (Diaporthales) and its Phaeoacremonium anamorphs. Studies in Mycology, 54, 1–115.
Mostert, L., Groenewald, J. Z., Summerbell, R. C., Robert, V., Sutton, D. A., Padhye, A. A., & Crous, P. W. (2005). Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. Journal of Clinical Microbiology, 43, 1752–1767.
Mostert, L., Halleen, F., Fourie, P. H., & Crous, P. W. (2006b). A review of Phaeoacremonium species involved in petri disease and esca of grapevines. Phytopathologia Mediterranea, 45, S12–S29.
Moyo, P., Mostert, L., Bester, M., & Halleen, F. (2016). Trunk disease fungi associated with Diospyros kaki in South Africa. Plant Disease. accepted. doi:10.1094/PDIS-02-16-0245-RE.
Mugnai, L., Graniti, A., & Surico, G. (1999). Esca (black measles) and brown wood streaking: Two old and elusive diseases of grapevines. Plant Disease, 83, 404–418.
Nigro, F., Boscia, D., Antelmi, I., & Ippolito, A. (2013). Fungal species associated with a severe decline of olive in southern Italy. Journal of Plant Pathology, 95, 668.
O’Donnell, K., & Cigelnik, E. (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution, 7, 103–116.
Possingham, J. V. (2008). Developments in the production of table grapes, wine and raisins in the tropical regions of the world. Acta Horticulturae, 785, 45–50.
Raimondo, M. L., Lops, F., & Carlucci, A. (2014). Phaeoacremonium italicum sp nov., a new species associated with esca of grapevine in southern Italy. Mycologia, 106, 1119–1126.
Rayner, R. W. (1970). A mycological colour chart. Kew: Commonwealth Mycological Institute & British Mycological Society.
Romero-Rivas, L. C., Álvarez, L. A., Gramaje, D., & Armengol, J. (2009). First report of Phaeoacremonium parasiticum causing petri disease of grapevine in Perú. Plant Disease, 93, 200.
Rumbos, I. (1986). Phialophora parasitica, causal agent of cherry dieback. Journal of Phytopathology, 117, 283–287.
Sami, S., Mohammadi, H., & Heydarnejad, J. (2014). Phaeoacremonium species associated with necrotic wood of pome fruit trees in Iran. Journal of Plant Pathology, 96, 487–495.
Scheck, H. J., Vasquez, S. J., Fogle, D., & Gubler, W. D. (1998). Grape growers report losses to black-foot and grapevine decline. California Agriculturist, 52, 19–23.
Staden, R., Beal, K. F., & Bonfield, J. K. (1998). The Staden package, 1998. In S. Misener & S. A. Krawetz (Eds.), Bioinformatics methods and protocols (pp. 115–130). Humana Press: New York.
Stamatakis, A. (2014). RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312–1313.
Úrbez-Torres, J. R., Haag, P., Bowen, P., & O’Gorman, D. T. (2014). Grapevine trunk diseases in British Columbia: Incidence and characterization of the fungal pathogens associated with esca and petri diseases of grapevine. Plant Disease, 98, 469–482.
Vaidya, G., Lohman, D. J., & Meier, R. (2011). SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics, 27, 171–180.
Acknowledgements
This work was financed by Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco - FACEPE (APQ 137-5.01/12) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES (Project 203/2009 - International Cooperation CAPES-Brazil/DGU-Spain). M. P. S. Câmara and S. J. Michereff are recipients of Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq research fellowships. D. Gramaje was supported by the DOC-INIA program from the National Institute for Agronomic Research (INIA), co-funded by the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, M.A., Correia, K.C., Barbosa, M.A.G. et al. Characterization of Phaeoacremonium isolates associated with Petri disease of table grape in Northeastern Brazil, with description of Phaeoacremonium nordesticola sp. nov.. Eur J Plant Pathol 149, 695–709 (2017). https://doi.org/10.1007/s10658-017-1219-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1219-4