Abstract
Early blight is a common disease of Solanaceae crops worldwide. The occurrence of Alternaria spp. was studied during three epidemics on tomato in northwestern Algeria. Alternaria was detected in more than 80 % of the diseased plant samples and accounted for more than 50 % of the total fungal isolates recovered from these samples. Morphological and molecular investigations revealed that small-spored isolates producing beaked conidia, i.e. belonging to the section alternaria, were prominent in most of the surveyed locations representing more than 80 % of the total Alternaria isolates in three locations (Mascara, Ain Témouchent and Sidi Belabbèsse). Based on their sporulation patterns they were recognized as A. alternata and A. tenuissima. Small-spored isolates producing conidia without beak and assigned to A. consortialis were also found at a low frequency (< 1 %). Large-spored isolates producing conidia ended by typical long beaks and identified as A. linariae (syn. A. tomatophila), A. solani and A. grandis were also recovered from all the sampled areas and represented 33.8 %, 6.3 % and 1.3 % of the total Alternaria isolates, respectively. Pathogenicity tests on tomato with a selection of 85 strains representative of the isolates collection revealed that all the tested isolates were able to produce extending lesions on inoculated leaves albeit with variable intensity. Large-spored species included the most aggressive isolates. Small-spored Alternaria, although less aggressive than large-spored Alternaria, had the ability to provoke brown necrotic spots and circumstantially developed synergistic interactions in mixed infections with moderately aggressive isolates of A. linariae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early blight is a major disease of Solanaceae and is spread worldwide (Pryor and Gilbertson 2000). Typical disease symptoms are characteristic dark necrotic lesions with concentric rings on leaves (Van der Waals et al. 2003). Severe epidemics can lead to complete defoliation and major crop losses in short periods of time (Chaerani and Voorrips 2006). In plantations in the U.S., Australia, Israel, UK, Brazil and India, these losses range from 35 to 80 % (Basu 1974; Datar and Mayee 1981; Grigolli et al. 2011). The disease is most damaging in regions with heavy rainfall, high humidity and fairly high temperatures (Chaerani and Voorrips 2006), although epidemics can also occur in semi-arid climates (Rotem et al. 1983). In the countries surrounding the Mediterranean area, tomato and potato constitute very important crops and can be cultivated throughout the year (Snoussi 2009). In northwestern regions of Algeria, early blight of tomatoes appears every year. The occurrence and damage of the disease depend mainly on frequent rain and high moisture over the month after the appearance of the first symptoms. In Algeria, as in other tropical and sub-tropical areas, early blight is considered one of the most destructive diseases in several plant species of the Solanaceae family (Abada et al. 2008) and constitutes a serious problem for the fresh market as well as for the processing industry.
Several different Alternaria spp. have been associated with early blight of tomato and potato (Simmons 2000). The causal agent of early blight of potato, tomato and other Solanaceae crops was initially considered to be A. solani Sorauer (Jones and Grout 1897). In a morphology-based taxonomic revision of Alternaria specimens from Solanaceae, Simmons (2000) described several new species like A. grandis. Besides A. solani and A. grandis, which were the dominant species from potato, a similar fungus common on tomato was described as a new species and called A. tomatophila. This morphological species has been later supported not only by inoculation studies (Fraser and Zitter 2003) but also by molecular data (Gannibal et al. 2014; Rodrigues et al. 2010) and metabolite profiling (Andersen et al. 2008). Recently specimens belonging to this species were moved to a new species named A. linariae together with other isolates from Solanaceae, Cucurbitaceae and Scrophulariaceae (Woudenberg et al. 2014). In addition to these three large-spored Alternaria species, small-spored Alternaria species such as A. alternata Keissler, A. tenuissima Nees & Nees: Fries, and A. arborescens (syn A. alternata f. sp. lycopersici) Simmons, have been repeatedly isolated from Solanaceae with symptoms resembling that of early blight at least at initial stages of infection. These species may cause brown leaf spot on potatoes (Kirk et al. 2007) and stem canker on susceptible tomato cultivars (Grogan et al. 1975), respectively.
The application of fungicides is the most common tactic used to reduce losses caused by this disease. However, timing of fungicide sprays relative to environmental conditions and potential for disease development is critical if good control is to be attained (Kemmitt 2002). In addition, visual analysis of the symptoms does not always allow to distinguish if the necrotic spots are caused by A. linariae, A. solani or even by other Alternaria species. This suggests that additional information relating to the biology of the pathogen and the etiology of disease will be necessary for the successful development of a reliable disease management program.
In this study, morphological and molecular investigations were used in order to obtain a clear differentiation and distribution of Alternaria species on early blight symptomatic tomato tissues collected in different sites in northwestern regions of Algeria. As we suspected that other solanaceous crops cultivated in the vicinity of tomato could be a source of inoculum, isolates from symptomatic potatoes and eggplants from the same areas were also included in the study. At least five different Alternaria species, including the well-recognized tomato pathogens A. solani and A. linariae, were identified with various distributions according the sampling location. Pathogenic variability of isolates representative of these Alternaria species was studied on tomato plants. This confirmed that small-spored Alternaria, albeit less aggressive than large-spored Alternaria, had the ability to provoke tomato leaf blight and circumstantially developed synergistic interactions in mixed infections with moderately aggressive isolates of A. linariae.
Materials and methods
Plant material and isolation of alternaria isolates
Sixty-five fields were sampled between 2011 and 2013 in seven geographic locations of the northwestern region corresponding to the major potato- and tomato- producing areas of Algeria. According to the intensity of early blight epidemics, one to twenty samples (diseased tomato, potato or eggplant leaves, stem or fruit) from each field were collected. A total of 944 samples from tomato, 35 from potato and 10 from eggplant were thus obtained. Ninety three samples from tomato were stored frozen (−20 °C) for further DNA extraction and the 896 remaining samples were directly processed for fungal isolation.
Fungal isolation was conducted from these samples as described in Dhingra and Sinclair (1995). Plant material showing brown or black lesions was cut into small bits measuring ca. 2 mm and surface sterilized in 0.1 % (v/v) sodium hypochlorite solution for 2 min. These fragments were then transferred into Petri dishes containing 15 ml potato sucrose agar (PSA) medium, incubated for 48 h at 25 °C. A total of 1523 discrete colonies, developing from the lesions margins, were observed under a binocular stereomicroscope (magnification: ×20 to ×40) to discriminate, based on conidia morphology, small-spored and large-spored Alternaria species from species belonging to other genera.
The fungal colonies recognized as belonging to the genus Alternaria, were individually transferred to potato carrot agar (PCA) medium (Simmons 2007) or host leaf extract agar medium (200 g l−1 healthy tomato leaves, 12 g l−1 dextrose and 18 g l−1 agar) (Kozlovski and Kvasnyuk 1984) at 25 °C in the dark. For non-sporulating isolates, four days-old colonies were wounded with a sterile scalpel and directly exposed to sunlight for 5 min to stimulate fungal sporulation (Fahim 1966) and then incubated in the dark for 24 h. Purification of Alternaria isolates was performed by the monospore culture method. The 224 pure Alternaria isolates thus obtained from sporulating colonies were stored in glycerol 30 % (w/v) at -80 °C for further study. Among this collection, a subset of isolates representing all the identified morpho-species and the different sites of sampling were used either for pathogenicity assays or for sequence analysis (Table 1). The isolation frequency (Fr) of each species was calculated according to Saleemi et al. (2012).
Morphological characterization
Morphological characters of the 224 pure Alternaria isolates, i.e. sporulation patterns and conidial features, were observed under a binocular stereomicroscope and microscope (Leica DM4500B; Leica Microsystems) as previously reported (Simmons 2007; Zhang 2003). Each isolate was transferred to Petri dishes containing PCA, and incubated at 25 °C, under cool-white fluorescent light for 7 days (light and dark cycles). Species assignments based on morphology were performed according to Simmons (2007).
Molecular characterization
DNA was extracted either from mycelium collected by scraping the surface of Petri plate cultures of the single-spore isolates (listed in Table 1) or from diseased tomato leaves. In each case, 100 μl lysis buffer (50 mM Tris-HCl pH 7.5, 50 mM EDTA, 3 % SDS, 1 % 2-mercaptoethanol) was added and the nucleic acids were isolated according to the microwave mini-prep procedure described by (Goodwin and Lee 1993). The final DNA pellet was supplemented into 100 μl TE buffer (10 mM Tris-HCl pH 8.0, 0.1 mM EDTA) and stored at −20 °C until used. Partial sequences of the ITS region were polymerase chain reaction (PCR) amplified using fungal specific primer pair (ITSF1 -ITS4) (Gardes and Bruns 1993) or universal primer pair (ITS1-ITS4) (White et al. 1990) from plant tissue or fungal DNA, respectively. Specific primers for A. solani / A. grandis (OAsF7 and OAsR6) able to amplify 164 bp fragment of the gene Alt a1 and for A. linariae (OAtF4 and OAtR2) able to amplify 438 bp fragment of the calmodulin gene were used to confirm the identification of the two groups of species (Gannibal et al. 2014). Primer pair (AAF2 and AAR3) was used for specific detection of A. alternata-related species (Konstantinova et al. 2002). To distinguish large-spored species by multilocus sequence analysis, portions of the elongation factor (EF1), glyceraldehyde-3- phosphate dehydrogenase (GAPDH) and calmodulin genes were amplified with primers pairs EF1-728F and EF1-986R (Carbone and Kohn 1999), gpd1- gpd2 (Berbee et al. 1999), and CALDF1- CALDR1 (Lawrence et al. 2013), respectively. These sequences were selected based on their support values and high resolution in separating clades within closely related species A. grandis, A. solani and A. linariae (Woudenberg et al. 2014; Gannibal et al. 2014). The sequences of these primers are given in Table 2. All amplification reactions were performed in a 50-μl reaction volume containing 75 mM Tris-HCl pH 9.0, 20 mM (NH4)2SO4, 0.01 % (w/v) Tween 20, 1.5 mM MgCl2, 200 μM each desoxyribonucleotide triphosphate, 1 unit of thermostable DNA polymerase (GoTaq, Promega) and 400 nM of each relevant oligonucleotide primer. PCR conditions for each gene were according to the references provided above. After electrophoresis in 1.2 % agarose gels in 0.5× TAE buffer (20 mM Tris-acetate pH 8, 0.5 mM EDTA), DNA was visualized by ethidium bromide staining and UV illumination. Amplified gene fragments were sequenced using automated Sequencer at GATC Lab (Germany). Sequences used for comparison included reference strains for species in section Porri recognized as causing early blight on solanaceous crops, i.e. A. solani, A. linariae and A. grandis (Woudenberg et al. 2014) and were obtained from NCBI database (http://www.ncbi.nlm.nih.gov). The phylogenetic analysis was done using the Phylogeny.fr web service (Dereeper et al. 2008). Multiple sequence alignments were generated with MUSCLE and curated using the Gblocks algorithm. Maximum-likelihood analyses were performed with PhyML (HKY85 substitution model with gamma-distributed rate variation). The robustness of the ML topologies was evaluated using the Shimodaira-Hasegawa (SH)-like test for branches.
Pathogenic variability and cross-inoculation assays
Pathogenicity tests were carried out by inoculating on tomato plants (Saint Pierre variety) 79 isolates collected from solanaceous plants (16 isolates of A. alternata, 20 isolates of A. tenuissima, 2 isolates of A. consortialis, 34 isolates of A. linariae, 5 isolates of A. solani and 2 isolates of A. grandis; Table 1), selected among large-spored and small-spored species from the different regions, and six small-spored isolates collected on non-solanaceous plants (pea, cabbage, lettuce, cucumber). These tests were conducted under glasshouse conditions in pot experiments. Tomato seeds were surface sterilized and germinated on sterile moistened filter paper in 90-mm-diameter petri dishes for 5–7 days in darkness at 20 °C. Germinating seeds were planted in pots filled with sterilized soil and were grown in a glasshouse at 28 °C with 16 h/day light. Three-weeks-old plants were used for inoculation. Conidial suspensions were prepared as described in Boedo et al. (2012). Spore density was counted using a haemocytometer and adjusted to 104 conidia per ml. Isolates from different origins have been used either in solo or in mixtures with ratio (50:50) for co-inoculation experiments. The suspensions were then liberally applied to the surface of plants in the greenhouse using a hand-sprayer without injuring the leaves. A control experiment was carried out simultaneously using sterile distilled water. Three replicates were performed for each test. For maintaining the humidity, plants were covered with plastic bags for 48 h to allow the development of the disease symptoms.
Disease assessment
Plants were rated for disease symptoms including leaf spots with a brownish center, leaf lesions, and leaf death after 7, 15 and 21 days post inoculation (d.p.i). Disease severity was determined for each leaf (5 leaves per plant) by visual rating of disease and estimated as the percentage of necrotic leaf area (n.l.a.) as illustrated in Online resource 1. Symptom scoring data were subjected to analysis of variance (ANOVA) using strain, fungal species, incubation time or leaf number as qualitative factors. For each factor, a separate linear model was fitted, giving mean n.l.a values and standard errors. Several interactions were also investigated, including the post inoculation time * species interaction. For each ANOVA, homoscedasticity and residuals distribution were visually checked using boxplots and Q-Q plots. Multiple comparisons were performed using the Duncan multiple comparison procedure. For the co-inoculation experiments, student tests were used to compare mean n.l.a. obtained after co-inoculation with the sum of the two mean n.l.a. obtained after separate inoculations. Since 42 comparisons were made, α was set at 0.05/42 = 0.001190 following a Bonferroni correction. All statistical analyses were performed using R-3.2.4 software (R Core Team 2016).
Results
Occurrence of alternaria spp. on diseased tomatoes in northwestern Algeria
After plating on nutritive solid medium, the presence of Alternaria species was confirmed in the majority of sampled organs. These species represented up to 80 % of the isolated fungi in the Oran region (Fig. 1). It was observed that these Alternaria spp. were associated with Stemphylium spp. in more than 25 % of the analyzed samples. On average, Alternaria and Stemphylium species represented 58.3 % and 33.7 % of the 1523 fungal colonies that developed from the lesions margins, respectively. Other fungi belonging to different genera (Bipolaris sp., Fusarium sp.) were also isolated at lower frequency (less than 8 %). Morphological analysis of conidia separated the Alternaria isolates into two main groups with large and small spores respectively. The data presented in Fig. 1 revealed that the maximum incidence for large-spored Alternaria was observed at Mostaganem (52 %). In all other surveyed locations small-spored Alternaria were prominent representing more than 80 % of the total Alternaria isolates in four locations (Mascara, Ain Témouchent, Relizane and Sidi Belabbèsse).
Direct diagnostic PCR was performed on ninety-three plant samples (total tested samples) from the different locations by amplification with specific-primers. Representative results are presented on Online resource 2. Primer pair (ITSF-1/4) used to confirm presence of fungal DNA in infected leaves gave positive amplification signals with different intensity at ca. 600 bp for 82 % of the samples (total infected samples). The primer pair AAF2/R3, highly specific for A. alternata species-group, allowed the amplification of a 340 bp fragment from seventy naturally infected samples (75 % of the total tested samples and 92 % of the total infected samples). Primer set, OAsF7/R6 specific for A. solani / A. grandis amplified a 164 bp amplicon from three samples (3.2 % of the total tested samples and 3.9 % of the total infected samples) whereas, the primer set OAtF4/R2 specific for A. linariae amplified a 438 bp amplicon from 16 samples (17.2 % of total tested samples and 21 % of the total infected samples). It should be noticed that 11 % of the samples produced amplification products with both the AAF2/R3 primer set and either the OAsF7/R6 or OAtF4/R2 primer pairs suggesting co-infection of the samples with A. alternata related species and A. linariae, A. solani or A. grandis.
Morphological characterization of the alternaria isolates
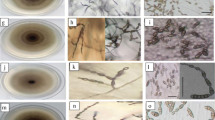
Observation of the 224 single-spored Alternaria isolates revealed substantial variability in colony morphology and sporulation when grown on (PCA) and (PSA), respectively. Close examination of morphological characters on PCA medium such as sporulation patterns, conidia shape and size, beak number and length separated both large- and small-spored isolates into two sub-groups, respectively (Fig. 2). Large-spored isolates belonging to the first sub-group produced ovoid conidia (mean spore length and width: 137.35 ± 22.14 μm and 16.45 ± 1.46 μm, respectively) with mainly a single beak measuring 66.57 ± 19.50 μm. The spores are formed in solo or in pairs from the same conidiophore, sometimes in short chains of two to three conidia when secondary conidiophores are formed at the apex or laterally. This morphotype accounted for 6.3 % of the total Alternaria isolates and was assigned to A. solani.
Morphology of small-spored Alternaria isolates with chain of conidia with short beak (morphotypes assigned to a A. alternata and b A. tenuissima), large-spored isolates with conidia ended by typical long beaks (morphotypes assigned to c A. linariae and d A. solani) and small-spored isolates with clumps of conidia without beak (morphotype assigned to e A. consortialis), Left and right panels represent microscope and binocular stereomicroscope views, respectively. Pictures were taken after 7 days at 25 °C on PCA medium. Bars =50 μM
Large-spored isolates belonging to the second sub-group formed ellipsoid conidia, slightly larger than those from the previous sub-group (mean spore length and width: 242.62 ± 38.33 μm and 16.51 ± 2.36 μm, respectively) ended by one to three beaks measuring 159.93 ± 30.53 μm. The majority of spores were born solitary. This morphotype accounted for 33.8 % of the total Alternaria isolates and was assigned to A. linariae (syn. A. tomatophila). Besides these two main sub-groups, three isolates (1.3 % of the total Alternaria isolates) from the Mostaganem region produced conidia that were slightly larger than typical A. solani (ca 150 μm mean spore length ended by a beak measuring up to 120 μm). It was difficult based exclusively on conidial morphology to assign a species name to this morphotype.
Small-spored isolates all produced dark pigmented multiseptate conidia that never exceeded 60 μm in length and beared very short beak. The two sub-groups were distinguished mainly based on their sporulation patterns. Isolates from the first sub-group produced conidial chains of 2–6 units long and typically produce branches (1 to 5 conidia) having a well-defined primary conidiophore with few terminal and sub terminal branches. This morphotype was assigned to A. alternata and accounted for 16.2 % of the total Alternaria isolates. Isolates from the second sub-group formed chains of conidia with more than nine units, branching of chains usually was minor (1 to 2 conidia) or lacking. This morphotype was assigned to A. tenuissima and accounted for 39.6 % of the total Alternaria isolates.
Two other isolates produced obovoid non-beaked conidia (mean spore length and width: 25.4 ± 3.69 μm and 13.4 ± 2.16 μm, respectively) with a narrow base formed singly on a short conidiophore or in chains and may form secondary conidiophores at the apex and could be assigned to the genus Ulocladium.
Molecular characterization of the alternaria isolates
Preliminary molecular characterization of the Alternaria isolates based on sequence data was obtained using the internal transcribed spacer region (ITS) amplified from a subset of 41 Alternaria isolates (listed in Table 1). Four isolates morphologically identified as belonging to the Bipolaris and Stemphylium genera were also included. Based on cluster analysis of ITS data, large- and small-spored Alternaria spp. isolates were clearly separated into two major groups (Fig. 3), and the two isolates recognized as belonging to the Ulocladium genus based on morphological data were found to cluster with Alternaria consortialis (Thüm.) syn. Ulocladium consortialis belonging to the Ulocladioides section of Alternaria. However as anticipated from previous reports, it was neither possible to distinguish species within the small-spored isolates cluster (i.e. species belonging to the alternaria section) nor to discriminate A. linariae from other species pathogenic to Solanaceae (i.e. A. solani and A. grandis) within the large-spored isolates cluster. To ensure that isolates listed in Table 1 and morphologically recognized as A. solani or A. linariae indeed belong to these two species, diagnostic PCR with the specific primer sets OAsF7/R6 and OAtF4/R2 were realized on DNA extracted from these fungi. In all cases identification of the fungal species was confirmed.
Phylogenetic tree reconstructed by the maximum likehood method from the alignment of ITS rDNA sequences of 41 Alternaria isolates and four isolates belonging to two different genera all isolated from diseased solanaceous plant samples. Bootstrap values greater than 50 % are indicated. The ITS sequences of A. alternata EGS 34–016 (GenBank acc. no. AY438647), A.arborescens EGS 39–128 (GenBank acc no. AF347033), A. tenuissima EGS34–015 (GenBank acc no. AF347032), A. consortialis CBS104.31 (GenBank acc no. KC584247), A. solani CBS116.651 (GenBank acc no. KC584217), A. linariae EGS44–074 (GenBank acc no. KJ18188), A. grandis CBS 109158 (GenBank acc no. KJ718239), Stemphylium lycopersici isolate SSN-L (GenBank acc no. KF483120) and Bipolaris sorokiniana strain XFI (GenBank acc no. JX136715) were included as references
In addition, a multilocus analysis was performed by comparing elongation factor, calmodulin and glyceraldehyde-3- phosphate dehydrogenase genes sequences from a subset of 20 isolates identified as A. solani or A. linariae (listed in Table 1) based on morphological data. The resulting phylogenetic tree obtained from the PhyML analysis of concatenated datasets divided large-spored isolates into two major groups (Fig. 4). All isolates identified as belonging to the A. linariae species based on morphological characters and the reference strain for this species (CBS 109156) were found in Group I. Group II could further be divided into two subgroups; one (group II.2) that clustered the isolates morphologically identified as A. solani with a reference strain of this species (CBS 109157) and a second subgroup (II.1) comprising three morphologically similar isolates from the Mostaganem region and a reference strain (CBS 109158) for the species A. grandis.
Phylogenetic tree reconstructed by the maximum likehood method from the alignment of concatenated sequences of portions of the EF1a, CAL and GPD genes from 20 large-spored Alternaria isolates. Bootstrap values greater than 50 % are indicated. Corresponding sequences from A. solani CBS109157, A. linariae CBS 109156 and A. grandis CBS 109158 were included as references. The tree was rooted with sequences from A. limicola CBS 483.90
Pathogenic variability of the alternaria isolates
In our greenhouse conditions, all the tested isolates were able to produce extending lesions on tomato leaves albeit with variable intensity (Figs. 5 and 6). When disease severity, expressed as the percentage of necrotic leaf area (n.l.a), was subjected to ANOVA, strain and fungal species effects were found to be highly significant (p < 2.10−16). Differences in symptom expression were observed between large- and small-spored strains. The lesion progression was slow during the first week post inoculation with isolates of the section alternaria. Circular spots were narrow (≤ to 4 mm in diameter) and produced sparsely mainly on the border of the leaves. Up to the third week post inoculation symptoms appeared like brown to dark spots on leaf surface occasionally surrounded by a yellow halo. Older leaves usually turned yellow, sometime dying while remaining attached to the plant. By contrast, under the same glasshouse conditions, the majority of inoculations with A. solani, A. grandis and A. linariae resulted in disease symptoms already observable at four days after inoculation and covering more than 60 % of the leaf area in the third week post inoculation. Concentric rings with dark and light brown color were observed on plants as a result of irregular growth patterns of the pathogen within the leaf and stem tissues; yellow halos around each spot and lesions were usually bordered by veins. Severely infected leaves may turn yellow to brown and fall off. Diagnostic PCR on DNA extracted from inoculated plants tissues showed that only isolates belonging to the phylogenetic groups I or II were detected in the leaf samples not infected with the alternaria species group and vice versa, confirming the absence of cross-contamination between inoculated plants.
Pathogenicity of Alternaria linariae, A. solani and Alternaria section alternaria on tomatoes (from the left to the right). Three-weeks-old tomato plants inoculated with spore suspensions from: a isolate 260 (A. linariae), b isolate 266 (A. solani), c isolate 125 (A. tenuissima). The plants on the left part of each panel were inoculated with water and used as controls. Pictures have been taken at 7 days after inoculation
Disease severity on tomato plants inoculated with 85 Alternaria isolates collected in northwestern Algeria. Disease severity was estimated as the mean percentage of necrotic leaf surface (n.l.a.). For each inoculated isolate, fives leaves from three plants were scored at 7, 14 and 21 dpi. Data was fitted to a linear model with the strain as a qualitative factor. Means and standard errors were calculated from the model
Among the small-spored Alternaria isolates none produced a mean n.l.a. above 36.6 %, and mean n.l.a. above 25 % was observed with only five isolates, all of them being recognized as belonging to the morpho-species A. tenuissima. The vast majority of the small-spored isolates, as well as the two tested A. consortialis isolates, were found weakly aggressive. The minimum lesion size (i.e. n.l.a below 2.5 %) was recorded for the isolate 150. On average the small-spored isolates from non-solanaceous plants were as aggressive as those from tomato, potato or eggplant. Maximum mean n.l.a levels (above 75 %) were recorded for nine A. linariae isolates and two A. solani isolates. The mean aggressiveness of A. solani was significantly higher than that of the two other large-spored Alternaria species, although this observation will have to be confirmed with more isolates. There was a great variability in aggressiveness among these large-spored isolates and some of the more aggressive small-spored isolates were found more aggressive than some isolates of A. linariae. It should be noticed that disease progression between 7 and 21 dpi was significantly higher in leaves inoculated with A. linariae isolates than with any other isolates (Fig. 7).
Disease progression on tomato plants inoculated with Alternaria isolates belonging to the six different species collected on solanaceous plants in northwestern Algeria. Disease severity was estimated as the mean percentage of necrotic leaf surface (n.l.a). For each inoculated isolate, fives leaves from three plants were scored at 7, 14 and 21 dpi. Six groups of isolates were assigned to A. alternata (black circle), A. tenuissima (open circle), A. consortialis (black square), A. grandis (open square), A. linariae (black triangle) and A. solani (open triangle) species. Data was fitted to a linear model with the species and post inoculation time as two qualitative factors. Interaction of the two factors was found to be significant. Mean and standard errors were calculated from the model. Asterisks indicate significant species * post inoculation times interactions with p < 5 % (*), p < 1 % (**) or p < 0.01 % (***)
As we observed that some necrotic lesions from samples collected in the fields were contaminated by both large- and small-spored Alternaria species, we performed co-inoculations experiments by mixing in all possible combinations three small-spored isolates, selected to represent different levels of aggressiveness, with three isolates of A. solani, one isolate of A. grandis and ten isolates of A. linariae. Data shown on Table 3 revealed that the type of interaction was not dependent of the small-spored isolates used in combination with the A. solani, A. grandis and A. linariae isolates. Indeed all the small-spored strains developed either additive (i.e. no interaction), synergistic or antagonistic interactions depending on their large-spored partner isolate. Of the 42 tested combinations, seven were found synergistic and 26 were found antagonistic. Some of the large-spored isolates produced synergistic (e.g. A. linariae isolates 223 and 254), antagonistic (e.g. A. grandis and A. solani isolates 248 and 292, respectively) or additive (e.g. A. linariae isolates 307) interactions with all their small-spored partners (Fig. 8). However it seemed that the most important parameter that might explain the type of interaction was the aggressiveness of the large-spored isolate with synergistic effects mostly found for weakly aggressive A. linariae isolates.
Typical interactions between large-spored and small-spored Alternaria on tomato plants co-inoculated with different combinations of isolates. Disease severity (+/− standard errors) was estimated as the mean percentage of necrotic leaf surface (n.l.a) at 7 dpi (circles), 14 dpi (squares) and 21 dpi (triangles)
Discussion
Early blight is becoming an increasing threat for tomato production in the Mediterranean Basin. We report here, for the first time, the occurrence of different Alternaria spp. that may cause this disease in the northwest of Algeria. The association of the morphological characteristics and molecular analyses on fungal isolates from diseased tomato samples allowed for the identification of four species (A. consortialis, A. linariae, A. solani and A. grandis) and one species-group (section. Alternaria) of the genus Alternaria.
The distinction between small-spored isolates was mainly based on their sporulation patterns as the data from the ITS rRNA genes were not able to resolve closely related isolates. Indeed it was recently shown, using whole genomes sequencing, that A. alternata and A. tenuissima might not correspond to distinct phylogenetic species and thus should be synonymized (Woudenberg et al. 2015).
By contrast, the other species were recognized based on both morphological characteristics of conidia and nucleotide sequence polymorphisms. Two groups of isolates produced filament-beaked and large-conidia and were assigned to A. linariae (syn. A. tomatophila) and A. solani. These two Alternaria species, which are common on solanaceous crops, were morphologically differentiated mainly based on the beak length. However, as already mentioned by Gannibal et al. (2014), this parameter was found highly variable within each group and ranges of variation overlapped, precluding unambiguous assignment of all the isolates. Indeed, based on morphological characters, these species have long been considered as a single species called A. solani. A. tomatophila and A. grandis were described as a new species by Simmons (2000) to distinguish isolates from tomato and isolates from potato in addition to A. solani that preserved the name. In accordance with their close phylogenetic relationship (Lawrence et al. 2013) we were unable to distinguish between the three species based on the analysis of the ITS rRNA gene sequences. However, using species-specific primers designed by Gannibal et al. (2014) we were able to quickly assign each filament-beaked isolate to A. linariae and to A. solani or A. grandis. Further distinction between these three species came from the analysis of the partial sequence of the EF-1α, calmodulin and Gpd genes where single nucleotide polymorphisms between members of A. linariae, A. solani and A. grandis were observed. Isolates corresponding to the latter species had never been isolated from tomato until recently in Algeria (Bessadat et al. 2016) and produced conidia with body and beak slightly longer than those of A. solani (Rodrigues et al. 2010). In the same vein, A. subcylindrica and A. cretica represent other tomato-pathogenic Alternaria species cross-reacting with the specific primer pair OAtF4-OAtR2 designed by Gannibal et al. (2014). However, these two species have been recently synonymized to A. linariae together with A. tomatophila (Woudenberg et al. 2014). Two of our tomato isolates were placed in a sixth morphological group as they produced non-beaked obovoid conidia. Based on these morphological traits that distinguish Ulocladium from Alternaria and other related genera (Simmons 1967) these isolates were initially recognized as Ulocladium sp. However, the taxonomy of this genus evolved with considerable historical controversy (Runa et al. 2009) and fungi formerly included in this genus were recently re-assigned to the Alternaria genus and separated into three morphologically similar sections: Ulocladioides, Pseudoulocladium and Ulocladium (Woudenberg et al. 2013).
Using nucleotide sequence analysis at the ITS rDNA locus we showed that these isolates could be considered as belonging to A. consortialis, a plant pathogenic species already reported as infecting tomatoes.
Despite this great diversity of Alternaria spp. recovered from diseased tomatoes, small-spored Alternaria species were almost always the most prevalent morphotype in all the prospected locations of northwestern Algeria, excepted in Mostaganem. This observation was not surprising as A. alternata and A. tenuissima constitute worldwide distributed species commonly isolated from a wide variety of plant species (Rotem, 1994) and in particular from solanaceous crops (Bessadat et al. 2014; Orina et al. 2010; Weir et al. 1998). For instance, in a three year survey on potato plots in Germany, Leiminger et al. (2010) reported that the isolation frequency of small-spored Alternaria reached almost 100 % while large-spored species were isolated from 55 to 60 % of the plant samples. However, the impact of these small-spored species on early blight disease development has been controversially discussed and they were sometimes considered either as strictly saprophytic or secondary invaders (Stammler et al. 2014) or as causal agents. The pathogenicity trials we performed in the present study revealed that, under our greenhouse conditions, none of the small-spored isolate could be considered as highly aggressive on tomato plants but some, all assigned to the morpho-species A. tenuissima, were able to produce severe progressive lesions when inoculated singly. Small-spored Alternaria isolates that accounted 50–85 % of the total Alternaria isolated from tomato in northwest Algeria should thus be considered as potential causal agents of leaf blight on tomato. Members of the A. arborescens species complex, in which tomato-pathogenic toxin-producing populations (also known as A. alternata f. sp. lycopersici) have been described (Akagi et al. 2009), were not identified based on morphological criteria in our specimen collection. This corroborates our previous investigation focusing on small-spored Alternaria from tomato showing that the morphospecies A. arborescens only represented 1.5 % of the isolates collected in northwestern Algeria (Bessadat et al. 2014). However, as members of the A. arborescens species-complex share the same nucleotide sequence than other members of the section alternaria at the ITS rDNA locus, further sequence analyses of other loci that allow to distinguish species within this section (Woudenberg et al. 2015) are needed to validate these observations. Similarly, A. consortialis isolates represented less than 1 % of the total Alternaria collection and these two species have probably a low incidence on the early blight disease in Algeria.
Not surprisingly, large-spored Alternaria that represented 15–50 % of the total Alternaria isolates and were in average highly aggressive constituted the most threatening species for the development of early blight epidemics on tomato in Algeria. A. linariae was isolated at a much higher frequency than A. solani, in accordance with the previous statement that A. solani is the dominant species on potato while A. linariae (syn. A. tomatophila) is more common on tomato (Simmons 2000). In several countries like the United States, Australia, New Zealand, Venezuela and Brazil, A. linariae and A. grandis have been associated with early blight in tomato and potato respectively (Lourenço et al. 2009; Simmons 2000), but they had not yet been reported in Algeria. The situation in Algeria is comparable to that described in Russia where A. solani could be isolated from both tomato and potato while A. linariae was restricted to tomato (Gannibal et al. 2014) but slightly different from that in Brazil where only A. linariae was isolated from diseased tomato and only A. grandis from diseased potato (Rodrigues et al. 2010). A. solani was therefore detected for the first time on different combinations of host and geographical locations of Algeria. This plant pathogen is probably able to infect a broad range of hosts under distinct environmental conditions. Such set of alternative host plant species may provide field inoculum all year round and the presence of A. solani and A. grandis on tomato might result of the proximity of potato and tomato fields and the close phylogenetic relationship between this two cultures. However in contrast with the Russian situation (Gannibal et al. 2014), and despite their prevalence on tomato, on average, isolates of A. linariae were not found more aggressive on tomato than those of A. solani.
We showed here that both large-spored and small-spored Alternaria species could co-exist within a single symptomatic tissue sample. This does not constitute a new finding as, more than one hundred years ago, Jones and Grout (1897) reported similar observation in their original description of A. solani as causal agent of potato blight. However, most of the authors that have reported similar findings considered that small-spored Alternaria species were saprophytic and had no influence on the disease. To check whether interactions between the two morphological groups of Alternaria were likely to occur during tomato tissue colonization, co-inoculations of small-spored species and A. linariae, A. grandis or A. solani isolates were performed. Both synergistic and antagonistic effects were observed. The type of interaction was not dependent of the small-spored isolates used in combination with isolates of the porri section and synergistic effects were mainly observed when the small-spored isolate is mixed with a moderately aggressive large-spored isolate. It was however difficult to accurately predict the outcome of the interaction. This might explain why results found in the literature seem contradictory. For instance Spoelder et al. (2014) and Stammler et al. (2014) reported that mixtures of A. solani and A. alternata have comparable pathogenicity than A. solani used in solo when inoculated on potato and tomato, respectively. By contrast, cross protection as well as synergistic effect between small-spored and pathogenic Alternaria species have also been demonstrated (Bashan et al. 1991; Spurr 1977; Van den Heuvel 1971).
In conclusion, this study provides data on the diversity and pathogenic fitness of Alternaria spp. isolated from Solanaceae and highlights the complexity of the etiology of early blight. Such data might be helpful to identify appropriate control measures for a better management of this disease in Algeria and in other Mediterranean tomato producing areas.
References
Abada, K. A., Mostafa, S. H., & Mervat, R. (2008). Effect of some chemical saltson suppressing the infection by early blight disease of tomato. Egyptian Journal of Applied Science, 23, 47–58.
Akagi, Y., Akamatsu, H., Otani, H., & Kodama, M. (2009). Horizontal chromosome transfer, a mechanism for the evolution and differentiation of a plant-pathogenic fungus. Eukaryotic Cell, 8, 1732–1738.
Andersen, B., Dongo, A., & Pryor, B. M. (2008). Secondary metabolite profiling of Alternaria dauci, A. porri, A. solani, and A. tomatophila. Mycological Research, 2(2), 241–250.
Bashan, Y., Levanony, H., & Or, R. (1991). Association between Alternaria macrospora and Alternaria alternata, causal agents of cotton leaf blight. Canadian Journal of Botany, 69, 2603–2607.
Basu, P. K. (1974). Measuring early blight, its progress and influence on fruit losses in nine tomato cultivars. Canadian Plant Disease Survey, 54, 45–51.
Berbee, M. L., Pirseyedi, M., & Hubbard, S. (1999). Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia, 91, 964–977.
Bessadat, N., Simoneau, P., Benichou, S., Setti, B., Kihal, M., & Henni, J. E. (2014). Morphological, physiological and pathogenic variability of small-spore Alternaria causing leaf blight of Solanaceae in Algeria. African Journal of Microbiology Research, 8(37), 3422–3434.
Bessadat, N., Hamon, B., Henni, J. E., & Simoneau, P. (2016). First report of tomato early blight caused by Alternaria grandis in Algeria. Plant Disease, 100, 533.
Boedo, C., Benichou, S., Berruyer, R., Bersihand, S., Dongo, A., Simoneau, P., et al. (2012). Evaluating aggressiveness and host range of Alternaria dauci in a controlled environment. Plant Pathology, 61, 63–75.
Carbone, I., & Kohn, L. M. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia, 91, 553–556.
Chaerani, R., & Voorrips, R. E. (2006). Tomato early blight (Alternaria solani ): the pathogen, genetics, and breeding for resistance (review). Journal of General Plant Pathology, 72, 335–347.
Datar, V. V., & Mayee, C. D. (1981). Assessment of loss in tomato yield due to early blight. Indian Phytopathology, 34, 191–195.
Dereeper, A., Guignon, V., Blanc, G., Audic, S., Buffet, S., Chevenet, F., et al. (2008). Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research, 2008 Jul 1; 36 (Web Server Issue):W465–9. Epub 2008 Apr 19, from http://www.phylogeny.fr/.
Dhingra, O. D., & Sinclair, J. B. (1995). Basic plant pathology methods. Boca Raton, FL: CRC Lewis.
Fahim, M. M. (1966). The effect of light and other factor in the sporulation of Alternaria porri. Transactions of the British Mycological Society, 49(1), 73–78.
Fraser, J. T., & Zitter, T. A. (2003). Two species of Alternaria cause early blight of potato (Solanum tuberosum) and tomato (Lycopersicon esculentum). Phytopathology, 93, (supplement) S3.
Gannibal, P. B., Orina, A. S., Mironenko, N. V., & Levitin, M. M. (2014). Differentiation of the closely related species, Alternaria solani and A. tomatophila, by molecular and morphological features and aggressiveness. European Journal of Plant Pathology, 139, 609–623.
Gardes, M., & Bruns, T. D. (1993). ITS primers with enhanced specificity for basidiomycetes- application to the identification of Mycorrhizae and rusts. Molecular Ecology, 2, 113–118.
Goodwin, D. C., & Lee, S. B. (1993). Microwave miniprep of total genomic DNA from fungi, plants, protists and animals for PCR. BioTechniques, 15, 438–444.
Grigolli, J. F. J., Kubota, M. M., Alves, D. P., Rodrigues, G. B., Cardoso, C. R., Henriques da Silva, D. J., & Mizubuti, E. S. G. (2011). Characterization of tomato accessions for resistance to early blight. Crop Breeding and Applied Biotechnology, 11, 174–180.
Grogan, R. G., Kimble, K. A., & Misaghi, I. (1975). A stem canker disease of tomato caused by Alternaria alternate f.sp. lycopersici. Phytopathology, 65, 880–886.
Jones, L. R., & Grout, A. J. (1897). Notes on two species of Alternaria. Bulletin of the Torrey Botanical Society, 24, 254–258.
Kemmitt, G. (2002). Early blight of potato and tomato. The Plant Health Instructor. doi:10.1094/PHI-I-2002-0809-01.
Kirk, W. W., Schafer, R. L., Tumbalan, P., & Wharton, P. (2007). Evaluation of fungicide programs for potato early blight and brown leaf spot control. Plant Disease Management Report, 2(V065), 1–2.
Konstantinova, P., Bonants, P. J. M., van Gent-Pelzer, M. P. E., van der Zouwen, P., & van den Bulk, R. (2002). Development of specific primers for detection and identification of Alternaria spp. in carrot material by PCR and comparison with blotter and plating assays. Mycological Research, 106, 23–33.
Kozlovski, B. E., & Kvasnyuk, N. Y. (1984). Stimulation of sporulation of Macrosporium solani ell. Mart. on nutrient media with potato leaf extracts. Mikologiya i Fitopatologiya, 18(2), 139–140.
Lawrence, D. P., Gannibal, P. B., Peever, T. L., & Pryor, B. M. (2013). The sections of Alternaria: formalizing species-groups concepts. Mycologia, 105, 530–546.
Leiminger, J., Bahnweg, G., & Hausladen, H. (2010). Population genetics – consequences on early blight disease. Twelfth Euro Blight workshop -PPO-Special Report, 14, 171–178.
Lourenço Jr., V., Moya, A., González-Candelas, F., Carbone, I., Maffia, L. A., & Mizubuti, E. S. G. (2009). Molecular diversity and evolutionary processes of Alternaria solani in Brazil inferred using genealogical and coalescent approaches. Phytopathology, 99, 765–774.
Orina, A. S., Gannibal, P. B., & Levitin, M. M. (2010). Species diversity, biological characters and geography of Alternaria Fungi associated with solanaceous plants. Mikologiya i Fitopatologiya, 44, 150–159.
Pryor, B. M., & Gilbertson, R. L. (2000). Molecular phylogenetic relationships amongst Alternaria species and related fungi based upon analysis of nuclear ITS and mt SSU rDNA sequences. Mycological Research, 104, 1312–1321.
R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Rodrigues, T. T. M. S., Berbee, M. L., Simmons, E. G., Cardoso, C. R., Reis, A., Maffia, L. A., et al. (2010). First report of Alternaria tomatophila and A. grandis causing early blight on tomato and potato in Brazil. New Disease Reports, 22, 28. doi:10.5197/j.2044-0588.2010.022.028.
Rotem, J. (1994). The Genus Alternaria: biology, epidemiology, and pathology. St Paul, MN: American Phytopathological Society Press.
Rotem, J., Bashi, E., & Kranz, J. (1983). Studies of crop loss in potato blight caused by Phytophthora infestans. Plant Pathology, 32(2), 117–122.
Runa, F., Soo Park, M., & Pryor, B. (2009). Ulocladium systematics revisited: phylogeny and taxonomic status. Mycological Progress, 8, 35–47.
Saleemi, M. K., Khan, M. Z., Khan, A., Javed, I., Ul Hasan, Z., Hameed, M. R., et al. (2012). Occurrence of toxigenic fungi in maize and maize-gluten meal from Pakistan. Phytopathologia Mediterranea, 5(1), 219–224.
Simmons, E. G. (1967). Typification of Alternaria, Stemphylium, and Ulocladium. Mycologia, 59, 67–92.
Simmons, E. G. (2000). Alternaria themes and variations (244-286) species on Solanaceae. Mycotaxon, 75, 1–115.
Simmons, E. G. (2007). Alternaria. An identification manual: CBS Biodiversity Series No. 6. CBS Fungal Biodiversity Centre, Utrecht, the Netherlands. 775 pp.
Snoussi, S. A. (2009). Etude de base sur la tomate en Algérie. Programme régeional de gestion intégrée des ravageurs pour le Proche-Orient. from http://www.ipm-neareast.com.
Spoelder, J., Ellens, R., & Turkensteen, L. (2014). Comparing pathogenicity of Alternaria alternata and Alternaria solani on potato. Fourteenth Euroblight Workshop. PPO – Special Report, 16, 97–102.
Spurr Jr., H. W. (1977). Protective applications of conidia of non-pathogenic Alternaria alternata isolates for control for brown tobacco brown spot disease. Phytopathology, 67, 128–132.
Stammler, G., Bohme, F., Philippi, J., Miessner, S., & Tegge, V. (2014). Pathogenicity of alternaria-species on potatoes and tomatoes. Fourteenth Euroblight Workshop PPO – Special Report, 16, 85–96.
Van den Heuvel, J. (1971). Antagonism between pathogenic and saprophytic Alternaria species on bean leaves. In T. E. Preece & C. H. Dickinson (Eds.), Ecology of leaf surface micro-organisms. New York: Academic Press.
Van Der Waals, J. E., Korsten, L., Avelino, T. A. S., & Denner, F. D. N. (2003). Influence of environmental factors of Alternaria solani conidia above a south African potato crop. Phytoparasitica, 31(4), 353–364.
Weir, T. L., Huff, D. R., Christ, B. J., & Romaine, C. P. (1998). RAPD-PCR analysis of genetic variation among isolates of Alternaria solani and Alternaria alternata from potato and tomato. Mycologia, 90, 813–821.
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: a guide to methods and applications (pp. 315–322). San Diego, CA: Academic Press.
Woudenberg, J. H. C., Groenewald, J. Z., Binder, M., & Crous, P. W. (2013). Alternaria redefined. Studies in Mycology, 75, 171–212.
Woudenberg, J. H. C., Truter, M., Groenewald, J. Z., & Crous, P. W. (2014). Large spore Alternaria pathogens in section Porri disentangled. Studies in Mycology, 79, 1–47.
Woudenberg, J. H. C., Seidi, M. F., Groenewald, J. Z., de Vries, M., Stielow, J. B., Thomma, B. P. H. J., et al. (2015). Alternaria section Alternaria: species, formae speciales or pathotypes. Studies in Mycology, 82, 1–21.
Zhang, T. Y. (2003). Flora Fungorum Sinicorum. vol. 16, The genus Alternaria (pp. 1–281). Beijing: Science Press.
Acknowledgments
Part of this work was done during a stay at the University of Angers- France, financed by a grant from the University of Oran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Online resource 1
Scale used to estimate disease severity on tomato leaves inoculated by Alternaria spp. (TIFF 1141 kb)
Online resource 2
Representative results of the analysis of PCR products obtained after amplification on DNA extracted from symptomatic tomato tissues with primer sets (A) ITSF1–4, (B) AAF2-R3, (C) OAtF4-R2, and (D) OAtF7-R6 specific for fungi, Alternaria spp. of the alternata section, A. linariae, and A. solani, respectively. Lanes 1 correspond to positive controls and were loaded with PCR products obtained after amplification with relevant primer sets from DNA of either A. alternata CBS916.96 (A, B) or A. linariae EGS-44-024 (C) or A. solani (CBS110.41). Infected tomato samples were collected in Oran (lanes 2, 8, 12), Mostaganem (lanes 3, 5), Tlemcen (lanes 4, 11), Ain Témouchent (lanes 3, 6, 10), Mascara (lane 7) and Sidi Bel Abbèsse (lane 9). Lanes M were loaded with the molecular marker. Approximate sizes (in bp) of the amplification products are indicated on the left. (TIFF 494 kb)
Online resource 3
Effect of cross inoculations on disease severity: Student t-test associated p-values. (DOCX 62 kb)
Rights and permissions
About this article
Cite this article
Bessadat, N., Berruyer, R., Hamon, B. et al. Alternaria species associated with early blight epidemics on tomato and other Solanaceae crops in northwestern Algeria. Eur J Plant Pathol 148, 181–197 (2017). https://doi.org/10.1007/s10658-016-1081-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-1081-9