Abstract
The aim of this study was to identify the Colletotrichum species associated with anthracnose symptoms in coffee (Coffea arabica L.) plantations in northern Puebla, Mexico. In 2013, five surveys were conducted in different production areas and at different altitudes. Symptomatic leaves, shoots, and ripe and unripe fruits of the coffee variety Red Caturra were collected. Isolates were obtained and the Colletotrichum species were identified morphologically and characterized by multilocus sequence analyses of the ACT, CAL, GAPDH, and TUB2 genes and the rDNA region. Additionally, pathogenicity tests were conducted using six isolates. We identified C. gigasporum, C. gloeosporioides, C. karstii (two isolates), C. siamense, and C. theobromicola. This is the first report of these five species infecting leaves of coffee. The symptoms caused by these species were characterized, but the species causing Coffee Berry Disease was not found. This is the first report of a complex of species affecting coffee plants in the same geographical area in Mexico, and suggests that other complexes of species may be important pathogens in coffee-producing areas elsewhere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coffee (Coffea arabica L.) is a major export crop in many countries including Brazil, Vietnam, Indonesia, Colombia, and India (FAOSTAT 2013). Coffee diseases caused by Colletotrichum species are especially important as they directly affect yield (Pinkert 2004). Colletotrichum species that affect coffee have been reported from several countries as epiphytes, endophytes, or pathogens (causing anthracnose symptoms). To date, 16 species of Colletotrichum have been reported to affect coffee crops worldwide. Six species were reported in Vietnam (C. acutatum, C. boninense, C. capsici, C. gloeosporioides, C. karstii, and C. walleri), three in Thailand (C. asianum, C. fructicola, and C. siamense), two in Angola (C. cuscutae and C. fragariae), and one species in each of the following five countries: Australia (C. theobromicola), Colombia (C. gigasporum), Costa Rica (C. costarricense), Fiji (C. queenslandicum), and Kenya (C. kahawae subsp. kahawae) (Damm et al. 2012b, c; Nguyen et al. 2010; Prihastuti et al. 2009; Rakotoniriana et al. 2013; Silva et al. 2012; Weir et al. 2012). Of these 16 species, only one, C. kahawae subsp. kahawae J. M. Waller & P.D. Bridge, which is restricted to Africa, is of major concern (Waller et al. 1993). There have been reports of C. kahawae subsp. kahawae in Kenya, Angola, Cameroon, and Malawi (Weir et al. 2012). C. kahawae subsp. kahawae causes Coffee Berry Disease (CBD), which is devastating, damaging green berries and inducing premature fruit drop and/or fruit mummification (Omondi et al. 2000). In Mexico, coffee is cultivated in 15 states, among which Puebla State is a major producer (SIAP 2014) and only C. coffeanum (CNC 1952), which was considered to be C. gloeosporioides (Waller et al. 1993), was thought to infect coffee. The species has a worldwide distribution and affects several different crops: coffee in Vietnam (Nguyen et al. 2010), Brazil (Prihastuti et al. 2009), and possibly other Asian countries; and a wide range of other hosts in many other countries (Belize, Colombia, Guyana, Israel, Italy, Japan, Mexico, Nigeria, Spain, Taiwan, Thailand, South Africa, and the USA) (Cannon et al. 2008; Doyle et al. 2013; Nguyen et al. 2010). Diseases caused by Colletotrichum spp. cause symptoms of anthracnose on leaves, stems, and fruits; they have been identified based on their morphology (Sutton 1980) and, more recently, using molecular tools (Damm et al. 2012b, c).
The survey area was located in the mountains of northern Puebla, where there is a warm humid climate. In Puebla, coffee plants are cultivated between 300 and 1400 m (Evangelista et al. 2010). The aim of this study was to identify and characterize the Colletotrichum species present in coffee plantations using multilocus sequence analyses. We hypothesized that several Colletotrichum species cause anthracnose in coffee plantations in Mexico.
Materials and methods
Colletotrichum isolates
During 2013, five coffee plantations were selected to sample the coffee cultivar Red Caturra in three municipalities in Puebla State, Mexico (Table 1). The plants at each of the five coffee plantations sampled were located at 229, 578, 810, 1060, and 1128 m above sea level, respectively. At each of the five sites, 10 leaves, stems, ripe fruit, and unripe fruit with typical anthracnose symptoms were collected (Table 1). Leaf samples usually had typical and atypical lesions of Cercospora in addition to the anthracnose symptoms (Nelson 2008), and these lesions were also used for isolations of Colletotrichum spp. The symptomatic tissues were disinfested by immersion in 1.5 % sodium hypochlorite for 2 min, rinsed three times with sterile distilled water, and dried on sterile paper. Three sections of the symptomatic leaves, stems, and fruit were plated onto 2 % malt extract agar (MEA; Oxoid, Basingstoke, England) adjusted to pH 4.86 and containing 85 % lactic acid (0.1 ml/L) (Reasol, Mexico City, Mexico) (to avoid bacterial contamination). The plates were incubated for four days under constant near ultraviolet light (nUV) at 20–22 °C. Monosporic conidial suspensions of Colletotrichum isolations (Crous et al. 2009) were stored in 25 % glycerol (Hersch Trading, Mexico) at −80 °C, and in slant tubes with potato dextrose agar (PDA; 200 g potato broth, 15 g dextrose, 20 g agar per L) covered with sterile mineral oil (Quimica Meyer, Mexico City, Mexico).
Morphological characteristics
Of the 719 symptomatic tissues plated onto MEA, 49 % developed colonies of Colletotrichum (observed colony morphology and hyaline conidia form) (Damm et al. 2012a). In a first screen, 60 (17 %) colonies were selected on the basis of different conidia morphology (Sutton 1980; Damm et al. 2012a) and/or colony morphology (Crous et al. 2009; Damm et al. 2012a). The 60 isolates were plated on PDA and incubated for 7 days under laboratory lighting conditions before evaluating the colony characteristics, conidia form and size, and the presence or absence of pseudothecia (after 25 days). In a second screen of these 60 isolates, 10 isolates were selected (Fig. 1) based on the same criteria. This double screening process allowed us to select 10 isolates that were representative of typical examples of the different morphologies among the isolations (Table 1, Fig. 1). The proportion (percentage) of identified species among the 10 selected isolates represented 17 % of the 60 colonies (Table 1).
Comparative colony morphology of 10 isolates of Colletotrichum selected in a second screen for species identification. Isolates re-selected for study from 60 initial isolates are shown in Table 1. Isolates were selected at 4 days old plate on PDA under a 12-h light photoperiod at 20–22 °C
Culture characteristics
Aliquots of monosporic isolates were plated onto four media: PDA, 2 % PDA (Damm et al. 2012c), synthetic-nutrient-poor agar (SNA; 1 g KH2PO4, 1 g KNO3, 0.5 g MgSO4.7H2O, 0.5 g KCl, 0.2 g glucose, 0.2 g saccharose per L water) plus filter paper (Crous et al. 2009), and oatmeal extract agar (Crous et al. 2009). Plates were incubated under three environments: constant white fluorescent light (Osram 40 W, Brazil), constant darkness, and nUV (General Electric, 40 W, USA) with a 12 h photoperiod. The cultures were also incubated at different temperatures depending on the requirements of each species and according to information reported in the literature for Colletotrichum species. The colony diameters were measured after 7 and 10 days of incubation.
Morphology
Structures were measured on slide mounts in lactic acid under an Eclipse E-400 compound microscope (Nikon, Tokyo, Japan) at 100×. Conidia were measured and minimum and maximum, average (av.), standard deviation (SD), rank (95 % interval) (Crous et al. 2009), and median (Mdn.) were calculated. In some species the L/W (length/width) ratio was calculated. Appressoria formation was analyzed as described by Weir et al. (2012).
Pathogenicity tests
Six isolates were selected for pathogenicity tests. Six-month-old plants of the coffee cultivar Red Caturra with and without wounds were inoculated with each isolate (three plants per isolate). The plants (60 cm tall, average of six branches and nine leaves) were grow in organic matter compost in black plastic bags (215 cm2) in a greenhouse and watered regularly.
Before inoculation, the plants were surface disinfested as described above; they were allowed to dry at room temperature in a moist chamber for 72 h at 22–29 °C, 80 %–100 % relative humidity, and 12 h photoperiod under fluorescent light (Osram 40 W, Brazil). Environmental conditions in the chamber were monitored using an Onset HOBO data logger (Onset Computer Corp., Bourne, MA, USA). A 50,000 conidia/ml suspension was prepared from cultures of each isolate grown on 2 % PDA media, the conidial density was confirmed using a hemocytometer (Bright-Line, Buffalo, NY, USA). All the leaves of the inoculated plants were sprayed (using a Mini Glass Trigger Sprayer, 230 ml; China) to run-off with the conidia suspension. The controls (three plants) were sprayed with distilled sterile water to run-off. After 3 days’ incubation (22–29 °C, 80 %–100 % relative humidity and a 12 h photoperiod), the plants were transferred to a greenhouse (21–27 °C, 45 %–60 % relative humidity, with natural light conditions). After a further 30 days, the leaf lesions were measured, photographed, and the symptoms described. Isolations were made from each lesion and the causal pathogens grown on PDA as described above so as to fulfil Koch’s postulate.
Molecular analysis
DNA was extracted from 10 isolates (Castellanos et al. 2013), and three genes/genetic regions were amplified: the actin gene, ACT (primers ACT − 512F/ACT − 783R; Carbone and Kohn 1999), the β-tubulin gene, TUB2 (primers Bt2a/Bt2b; Glass and Donaldson 1995), and the rDNA regions of the inner ITS regions (primers ITS1/ITS4; Gardes and Bruns 1993; White et al. 1990). Two additional genes were amplified from some isolates; the calmodulin gene, CAL (primers CL1/CL2A) and the glyceraldehyde-3-phosphate dehydrogenase gene, GAPDH (primers GDF/GDR) (Weir et al. 2012). Gene-specific amplification conditions were used for ACT (Damm et al. 2012c), TUB2 (Prihastuti et al. 2009) and ITS (Woudenberg et al. 2009). The amplified products were sequenced in both directions by Macrogen CIA (http://dna.macrogen), Korea. The sequences were analyzed by DNASTAR (2001) and Sequencher (2014), and the alignment was performed with Clustal W in MEGA 6.0 (Tamura et al. 2013). Sequences were compared using NCBI BLAST Blastx (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Accession numbers of the sequences from each isolate are listed in Table 2.
Phylogenetic analysis
Phylogeny reconstruction was implemented in MEGA 6.0 (Tamura et al. 2013), and was performed independently for each gene and region. The dataset was analyzed using the Neighbor-Joining method (NJ) and the nucleotide substitution model obtained based on Bayesian Information Criterion. Concatenated analysis was performed using T3 models (Tamura 3 parameters) with maximum likelihood; in both cases a “bootstrap” of 500 permutations was performed. Later, phylogeny reconstruction was implemented in Mr. Bayes 3.1.2 (Ronquist and Huelsenbeck 2003). The dataset was analyzed using the Bayesian inference and two MCMC (Markov chain Monte Carlo). Concatenated analysis of the two genes (ACT and TUB2) and ITS regions was performed using 250,000 generations, sampled every 10,000 generation. The tree has been deposited at TreeBase (Accession number: Tr95552).
Results
Morphological descriptions
The ten isolates, representing 17 % of the 60 colonies, corresponded to six species: C. karstii (3.4 %) from the C. boninense species complex; C. gigasporum (2.0 %) and C. sp. (1.7 %) (a possible species of this complex) from the C. gigasporum species complex; and C. gloeosporioides (3.4 %), C. siamens (4.8 %), and C. theobromicola (1.7 %) from the C. gloeosporioides species complex (Table 1 and Fig. 3). The following species descriptions are based on isolates cultured on PDA (C. gigasporum, C. gloeosporioides, C. sp., C. karstii, C. siamense, and C. theobromicola) and SNA (C. karstii and C. theobromicola) media. Growth on these media allowed us to reconfirm the culture characteristics and structural morphology reported elsewhere (Damm et al. 2012c; Rojas et al. 2010).
C. gigasporum E. F. Rakotoniriana & F. Munaut. Colonies on PDA after 7 days at 25 °C in darkness, 8.0 cm in diam., gray aerial mycelium, dense and cottony, whitish margin, colony reverse black; brown appressoria, clavate, oval, and irregular, crenate or lobed margin, and a germ pore, (10–)12.5–17.5(−22.5) × 7.5–12.5 μm; after 2 weeks abundant acervuli conidiomata in periphery of plate, conidia mass pale pinkish and pale grayish with age; abundant light to dark brown setae, septated with round or truncated apex, rough apical wall, 90–140 × 5–6 μm; phialidic conidiogenous cell, cylindrical; cylindrical conidia, hyaline, gutulate, straight, both ends rounded, 20–26(−29) × 7–8 μm [av. (±SD) 23 (±1.9) × 7 (±0.4); Mdn. 23 × 7)] (Rakotoniriana et al. 2013). Teleomorph not observed. BLAST search reached 99.6 %–100 % similarity to C. gigasporum for three genes/genetic regions (ACT, TUB2 and ITS).
C. gloeosporioides (Penz.) Penz. & Sacc. Colonies on PDA at 20 °C, fluorescent light and nUV, 12-h dark/12-h light photoperiod, 4.8–5.0 cm in diam., after 7 days and 7.0–7.5 cm in diam., after 10 days; abundant cottony mycelia, dark gray with white margins and conidiomata at center of colony, salmon-colored conidia mass, reverse colony beige with blackish areas in zonate arrangement. On same media and in same environmental conditions, colonies after 7 days 6.4–6.6 cm in diam., flat, center of colony with dense cottony aerial mycelium, rest of colony slightly cottony, whitish to slightly grayish, fine erose and whitish margin; reverse colony pale salmon to orange with dark gray flecks at center, beige to white margin; abundant orange conidiomata, few brown setae, brown oval, lobulated and clavate appressoria, one germ pore, (4.6–)7.6–9.7(−13.7) × 4.4–7.9(9.8) μm; cylindrical and sub-cylindrical conidia with rounded ends, some with slightly truncated base, gutulate, 13–16 × 5–6 μm [av. (±SD) 14.8 (±1.1) × 5 (±0.3); Mdn. 15 × 5)], L/A: 2.6. Teleomorph not observed. BLAST search matched 100 % to C. gloeosporioides for five genes (ACT, ITS, TUB2, CAL, and GAPDH). Morphology consistent with that described by Cannon et al. (2008) and Liu et al. (2013).
Colletotrichum sp. Colonies on PDA after 7 days growth at 25 °C in darkness, 8.0 cm in diam., gray woolly aerial mycelia (less dense and shorter than those of isolate number 41; Table 1) with abundant scattered and semi-immersed structures; colonies rounded, and formed by a center of hyphae with a hard compact mass and covered with abundant cottony mycelia; colony with whitish margin and blackish reverse; brown appressoria, ovoid, crenate, with germ pore, (6–)8–17.5(−20) × 4–7(−10) μm after 2 weeks, some conidiogenous cells and conidia in black mucilaginous mass on surface of compact or hardened structures, no setae observed; hyaline conidia, gutulate, cylindrical and some clavate, straight and some curved, with both ends rounded and some truncated with marked hilum, aseptate, one or two septa in some aged conidia, (11–)15–27(−28) × (5–)6–8(−9) μm [av. (±SD) 22 (±3.9) × 7 (±0.7); Mdn. 23 × 7)]; under white fluorescent light/nUV with 12-h light/12-h dark photoperiod, same described morphology but larger conidia, (19–)20–32(−33) × 6–8 μm [av. (±SD) 26 (±3.7) × 7 (±0.4); Mdn. 27 × 7)]. Teleomorph not observed. In BLAST search, only ACT sequence differed (by four nucleotides) from that of C. gigasporum (98.8 %; difference was reflected in conidiomata and conidia size and shape). Other two sequences (ITS and TUB2) had 99.6 %–100 % similarity. This isolate should be further studied under different incubation conditions.
C. karstii Youlian, Y., Cai, L., Yu, Z., Liu, Z., & K. D. Hyde. Colonies on PDA at 20 °C and fluorescent light and nUV with 12-h light/12-h dark photoperiod, 3.7–4.3 cm in diam., after 7 days and 5.4–6.5 cm in diam., after 10 days; white cottony mycelia abundant in center and moderate along the rest of the colony, zonated; colony reverse pale salmon to pale orange with dark areas and zonate; abundant acervular conidiomata and conidia mass from salmon to pale orange; conidia with granulated cytoplasm, straight, cylindrical with rounded ends and some with rounded apex and truncated base, prominent hilum, (12–)13–17(−18) × 5–6 μm [av. (±SD) 15 (±1.4) × 5 (±0.4); Mdn. 15 × 5)]; on SNA, under nUV with 12-h light/12-h dark photoperiod at 20 °C, smaller conidia, (11–)12–14 × 5–6 μm [av. (±SD) 13 (±0.8) × 5 (±0.5); Mdn. 13 × 6)]; appressoria brown ovoid, ellipsoidal, subglobose and germ pore, (4.9–)6.9–12.6(−18) × (3.4–)4.4–7.5 μm; setae absent, and perithecia present after 30 days in PDA. BLAST search showed 99.6 %–100 % similarity to C. karstii for three sequences (ACT, ITS, TUB2), except for one isolate (number 42) showed 98.6 % similarity to ACT sequence. The description agreed with those of Damm et al. (2012c) and Youlian et al. (2011).
C. siamense Prihastuti, L., Cai & K. D. Hyde. PDA cultures at 28 °C in darkness, 8.0 cm in diam., after 7 days; cottony aerial mycelia ranging from moderate to abundant among isolates, colonies slightly grayish-whitish or brownish; reverse colony pale beige-yellowish or orangeish, abundant yellowish or orange conidiomata, fusiform conidia, some oblong, straight, hyaline, 12–18(−19) × 4–5 μm [av. (±SD) 15 (±1.9) × 4 (±0.4); Mdn. 15 × 4)]; appressoria brown, ovoid and clavate, some subglobose, and germ pore, 5.3–9.0(−13.4) × 3.9–5.9(−7.7) μm; setae present at the center of colony or absent. Teleomorph not observed. BLAST search showed 99.1 %–100 % similarity to C. siamense for analyzed genes except for ACT and GAPDH, with two isolates (numbers 22 and 27) showing 98.5 % and 98.9 %, respectively. The description agreed with that of Prihastuti et al. (2009).
C. theobromicola Delacr. Colonies on PDA at 25 °C in 12-h light/12-h photoperiod, 4.61 cm in diam., after 4 days, and after 10 days dense cottony mycelia dark gray and zonate; abundant conidiomata with orange conidia mass, reverse colony dark gray; in SNA after 10 days: hyaline, immersed, and light aerial hyaline hyphae, scattered conidiomata in agar and huge on filter paper with orange conidia mass; conidia on SNA at 10 days and mounted in 3 % KOH were straight 13–19 × (3–)4–5 μm [av. (±SD) 16 (±1.6) × 4 (±0.4); Mdn. 16 × 4)], L/A: 3.25–3.8, sub-cylindrical, some clavate; brown appressoria on SNA, irregular with lobed margin, some proliferated, (7–)9–11 × 6–8(−9) μm; no setae and no teleomorph observed. The description agreed with that of Rojas et al. (2010). The BLAST search showed 99.6 %–100 % similarity to C. theobromicola for ACT, ITS, and TUB2 sequences.
Cercospora lesions. Only three species, Colletotrichum karstii, C. siamense (two isolates) and Colletotrichum sp. were isolated from these lesions. Isolates number 13, 22 and 27, and 51 respectively (Table 1).
Pathogenicity tests
The six isolates (one of each of the five identified species, except for Colletotrichum sp.) were pathogenic to coffee leaves. The leaves developed first signs of symptoms at 14 days after inoculation. Lesions on uninjured plants were brown and formed mainly at the leaf apexes and borders (Fig. 2a–f); initially small circular lesions of up to 1.0 cm in diameter formed. Plants inoculated with C. karstii (Fig. 2c and d) and C. siamense (Fig. 2e) showed symptoms of collapse (area, 3 × 2–5 cm) along the leaf edges. Wounded leaves developed small circular lesions of 0.5–1.5 cm in diameter around the wound site, which were light brown-olivaceous and later extended over the entire leaf area causing the lamina to turn dark brown. Sometimes the center of the lesions was grayish, and some leaves became distorted (Figs. 2g, l).
Anthracnose symptoms on leaves of the coffee cultivar Red Caturra inoculated with Colletotrichum isolates. Isolates were inoculated onto unwounded (a–f) and wounded (g–l) leaves. Unwounded: b, d, f, necrosis at leaf apex and edges at 14 days after inoculation; a, c, e, symptoms at 30 days after inoculation. Wounded: g, h, i, k, l showing symptoms on and around wounds (individual lesions) at 14 days after inoculation; j, necrosis (fused lesions) around and on leaf at 30 days after inoculation; a, g, Colletotrichum gloeosporioides (21*); b, h, Colletotrichum gigasporum (41); c, i, Colletotrichum karstii (13); d, j, C. karstii (42); e, k, Colletotrichum siamense (38); f, l, Colletotrichum theobromicola (8); Colletotrichum sp. (51), not inoculated. * Isolate number
Although the leaf lesions caused by the five Colletotrichum species were similar, the symptom development differed among species. With wounded plants, those inoculated with C. siamense (Fig. 2k) developed symptoms 10 days before those inoculated with the remaining four species. Unwounded leaves inoculated with C. gloeosporioides, C. karstii (isolate 13), and C. siamense developed symptoms in approximately 30 days (Fig. 2a, c, e; respectively), while wounded plants developed symptoms 16 days earlier (Fig. 2g, i, k; respectively). The isolates of C. karstii developed symptoms within 30 days, whether or not the plants were wounded (Fig. 2c, j). The other two species, C. gigasporum and C. theobromicola, caused similar sized symptoms whether or not the leaves were wounded (Fig. 2b, f, h, l l). Acervuli formed in the lesions. The same species that had been inoculated were recovered from the symptoms and grew in pure culture. No Colletotrichum colonies were isolated from the controls.
Phylogenetic analysis
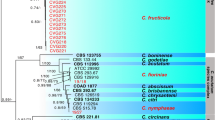
The ACT gene differentiated the isolates into six phylogenetic species, with one clade per species. Of the 10 isolates analyzed, three C. karstii isolates clustered together and two C. siamense isolates clustered together in different clades. The C. sp. was closely related to C. gigasporum, even though these two isolates had different conidia morphology and colony characteristics. The six species described in this study were confirmed to be different from the highly regulated species of Colletotrichum associated with coffee, C. kahawae subsp. kahawae (tree root) (Fig. 3).
Phylogenetic tree based on Bayesian Inference of concatenated ACT, ITS, and TUB2 gene sequences of 10 isolates (numbers on the right) of Colletotrichum species identified in this study. Values indicate percentage based on 250,000 MCMC (Markov chain Monte Carlo). C. kahawae subsp. kahawae (concatenated accession numbers: JQ071912, FJ907446, FJ972608) was the outgroup. Tree Accession number: Tr95552 (TreeBase)
Discussion
Based on the results of the inoculation experiments, several Colletotrichum species were found to be pathogenic to coffee in Mexico. A similar diversity of pathogenic species has been observed on coffee plants in Asia (C. gloeosporioides, C. karstii and C. siamense) (Damm et al. 2012b; Nguyen et al. 2010; Prihastuti et al. 2009). In South America, three species had previously been reported to be associated with coffee plants: C. gigasporum, an endophyte (Colombia) (Rakotoniriana et al. 2013), C. gloeosporioides and C. siamense (Brazil) (Prihastuti et al. 2009). In Mexico, coffee anthracnose was first reported in 1952 (CNC 1952), and the causal pathogen was considered to be C. gloeosporioides (Waller et al. 1993). However, the present study is the first formal report of C. gloeosporioides infecting coffee plants in Mexico. Among the six species identified, the fact that only C. karstii, C. siamense and C. sp. were isolated from typical and atypical Cercospora lesions could be an indication of pathogenic interaction between Cercospora and Colletotrichum species. Colletotrichum was reported to affect coffee plants in Hawaii (Nelson 2008), and was associated with Cercospora lesions, although it was not specified which Colletotrichum species was involved. Moreover, from field observations, it can be inferred that atypical Cercospora symptoms (Nelson 2008) and the predominance of anthracnose increase leaf damage, indicated a possible synergistic effect of these two pathogens on coffee. Furthermore, C. karstii is known to have a wide host range (Sharma and Shenoy 2013), as well as being the most common species of Colletotrichum and the species with the greatest geographical diversity within the C. boninense complex (Damm et al. 2012c). C. karstii was isolated as a pathogen from the Orchidaceae and as an endophyte in roots (Youlian et al. 2011). Other hosts of C. karstii include grape (Vitis vinifera), chili (Capsicum spp.), and tomato (Lycopersicon esculentum) (Youlian et al. 2011). On coffee, C. karstii has been isolated from fruit, but it was not reported to be pathogenic (Damm et al. 2012c). In the present study, it was pathogenic to coffee leaves and was associated with Cercospora lesions.
Another species in this study, C. gigasporum, was isolated as an endophyte from C. arabica in Colombia (Rakotoniriana et al. 2013) and from Coffea sp. in Vietnam (Liu et al. 2014). In Mexico, it was isolated as a foliar endophyte from Stylosanthes guianensis (Rakotoniriana et al. 2013) and it was associated with Musa sp. (Liu et al. 2014). C. gigasporum is mentioned in the literature as a potential coffee pathogen (Rakotoniriana et al. 2013), and was shown to be a pathogen of coffee fruit (Prihastuti et al. 2009). In the present study, C. siamense was pathogenic on coffee and was isolated from leaves and symptomatic unripe fruit.
C. theobromicola is a pathogen of Stylosanthes spp., Fragaria spp., and Theobroma cacao (Rojas et al. 2010; Weir et al. 2012) and it is mentioned in the literature as a possible pathogen of coffee. This species was only isolated from low altitudes, where the temperatures are higher (tropical monsoon climate) (Am) (Köppen 1936) (Table 1); an environment characterized by wet and dry seasons. C. theobromicola is generally restricted to warm tropical environments (Rojas et al. 2010), and its pathogenicity to coffee was demonstrated for the first time in this study. C. gigasporum, C. gloeosporioides, C. kartii, C. siamense, and C. sp. were isolated from a tropical rainforest climate (Af) (Köppen 1936) (Table 1), an environment lacking a dry season and where most Colletotrichum species diversity was found. An intensive and exhaustive sampling could reveal species diversity higher than in Asia (Damm et al. 2012a) and elsewhere in coffee plantations.
Anthracnose affecting coffee leaves is a novel observation, because most of the species identified elsewhere cause damage to coffee fruit. Hence, foliar damage by Colletotrichum species may affect coffee production due to interference with photosynthesis, which would ultimately affect fruit production and yield. Also, leaves with anthracnose could be a source of inoculum for fruit. More research regarding the incidence and severity of each species of Colletotrichum, their aggressiveness, and ability to infect different plant organs (leaves and fruit) will be valuable to ascertain the importance of these pathogens.
The regulated species C. kahawae subsp. kahawae was not among those identified in this study. This is an encouraging result, because it has caused serious damage in other countries. The results of this study show that there is a complex of at least six Colletotrichum species that are foliar pathogens of coffee in Mexico. This information is a useful baseline for developing sustainable management strategies for anthracnose of coffee.
References
Cannon, P. F., Buddie, A. G., & Bridge, P. D. (2008). The typification of Colletotrichum gloeosporioides. Mycotaxon, 104, 189–204.
Carbone, I., & Kohn, L. M. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia, 91, 553–556.
Castellanos, G., Jara, C., & Mosquera, G. (2013). Producción de micelio en medio líquido para extracción de ADN. CIAT. Centro Internacional de Agricultura Tropical (Mycelium production in liquid medium for DNA extraction. CIAT. International Center of Tropical Agriculture). http://ciat.cgiar.org/wp-content/uploads/2013/04/guia_practica9.pdf. Accessed 15 February 2015.
CNC (1952). Comisión Nacional del Café. Manual Práctico del Cultivo de Cafeto en México (Practical Handbook of Coffee Cultivars in Mexico). Mexico.
Crous, P. W., Verkley, G. J. M., Groenewald, J. Z., & Samson, R. A. (Eds.). (2009). Fungal Biodiversity. CBS, Laboratory Manual Series. Utrecht: CBS-KNAW Fungal Biodiversity Centre.
Damm, U., Cannon, P. F., & Crous, P. W. (Eds.) (2012a). Colletotrichum: complex species or species complexes? Studies in Mycology, 73, 1–215.
Damm, U., Cannon, P. F., Woudenberg, J. H. C., & Crous, P. W. (2012b). The Colletotrichum acutatum species complex. Studies in Mycology, 73, 37–114.
Damm, U., Cannon, P. F., Woudenberg, J. H. C., Johnston, P. R., Weir, B. S., Tan, Y. P., Shivas, R. G., & Crous, P. W. (2012c). The Colletotrichum boninense species complex. Studies in Mycology, 73, 1–36.
DNASTAR (2001). Lasergene expert sequence analysis software, User manual. Version 5. Wisconsin, USA: DNASTAR Inc. Madison.
Doyle, V. P., Oudemans, P. V., Rehner, S. A., & Litt, A. (2013). Habitat and host indicate lineage identity in Colletotrichum gloeosporioides s.l. from wild and agricultural landscapes in North America. PLoS One, 8(5), e62394. doi:10.1371/journal.pone.0062394.
Evangelista, O. V. J., López, B. J., Caballero, N. J., & Martínez, A. M. A. (2010). Patrones espaciales de cambio de cobertura y uso del suelo en el área cafetalera de la sierra norte de Puebla (Spatial patterns of change in coverage and land use in the coffee area of the mountains of northern Puebla). Investigaciones Geográficas. Boletín del Instituto de Geografía. Universidad Nacional Autónoma de México. UNAM. Mexico, 72, 23–38. http://www.redalyc.org/articulo.oa?id=56919174003. Accessed 4 May 2015.
FAOSTAT (2013). Food and Agriculture Organization of the United Nations Statistics .http://faostat.fao.org/. Accessed 12 February 2016.
Gardes, M., & Bruns, T. D. (1993). ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Molecular Ecology, 2, 113–118.
Glass, N. L., & Donaldson, G. (1995). Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology, 61, 1323–1330.
Köppen, W. (1936). Das Geographische System der Klimate. In W. Köppen & R. Geiger (Eds.), Handbuch der Klimatologie. Bd., I, Teil C. Balul I, Teil C. Berlin: Verlag von Gebrüder Borntl’aeger. http://koeppen-geiger.vu-wien.ac.at/pdf/Koppen_1936.pdf. Accessed 7 Jul 2016.
Liu, F., Damm, U., Cai, L., & Crous, P. (2013). Species of the Colletotrichum gloeosporioides complex associated with anthracnose diseases of Proteaceae. Fungal Diversity, 61, 89–105.
Liu, F., Cai, L., Crous, P. W., & Damm, U. (2014). The Colletotrichum gigasporum species complex. Persoonia, 33, 83–97.
Nelson, S. C. (2008). Cercospora leaf spot and berry blotch of coffee. USA: Department of Plant and Environmental Protection Sciences Plant Disease. College of Tropical Agriculture and Human Resource. Cooperative Extension Service. University of Hawai'i at Manoa http://www.ctahr.hawaii.edu/oc/freepubs/pdf/PD-41.pdf. Accessed 23 February 2015.
Nguyen, P. T. H., Pettersson, O. V., Olsson, P., & Liljeroth, E. (2010). Identification of Colletotrichum species associated with anthracnose disease of coffee in Vietnam. European Journal of Plant Pathology, 127, 73–87.
Omondi, C. O., Ayiecho, P. O., Mwang'Ombe, A. W., & Hindorf, H. (2000). Reaction of some Coffea arabica genotypes to strains of Colletotrichum kahawae, the cause of coffee berry disease. Journal of Phytopathology, 148, 61–63.
Pinkert, C. J. (2004). Nutrient and quality analysis of coffee cherries in Huong Hoa district, Vietnam (No. 280, p. 47). Plant Research International.
Prihastuti, H., Cai, L., Chen, H., McKenzie, E. H. C., & Hyde, K. D. (2009). Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Diversity, 39, 89–109.
Rakotoniriana, E. F., Scauflaire, J., Rabemanantsoa, C., Urveg-Ratsimamanga, S., Corbisier, A. M., Quetin-Leclercq, J., Declerck, S., & Munaut, F. (2013). Colletotrichum gigasporum sp. nov., a new species of Colletotrichum producing long straight conidia. Mycological Progress, 12, 403–412.
Rojas, E. I., Rehner, S. A., Samuels, G. J., Van Bael, S. A., Herre, E. A., Cannon, P. F., Chen, R., Pang, J., Wang, R., Zhang, Y. Q., & Sha, T. (2010). Colletotrichum gloeosporioides s.l. associated with Theobroma cacao and other plants in Panama: multilocus phylogenies distinguish pathogen and endophyte clades. Mycologia, 102, 1318–1338.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics Applications Note, 19, 1572–1574.
Sequencher (2014). Sequence analysis software Version 5.3. Ann Arbor, MI, USA: Gene Codes Corporation.
Sharma, G., & Shenoy, B. D. (2013). Multigene sequence-based identification of Colletotrichum cymbidiicola, C. karstii and C. phyllanthi from India. Czech Mycology, 65, 79–88.
SIAP (2014). Servicio de información agroalimentaria y pesquera. Cierre de la producción agrícola por estado (SIAP. Agrifood and Fisheries Information Service. Agricultural production by state). http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-estado/. Accessed 10 Feb 2015.
Silva, D. N., Várzea, V., Cai, L., Salgueiro, P. O., & Batista, D. (2012). Application of the Apn2/MAT locus to improve the systematics of the Colletotrichum gloeosporioides complex: an example from coffee (Coffea spp.) hosts. Mycologia, 104, 396–409.
Sutton, B. C. (1980). The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Kew, Surrey, England: Commonwealth Mycological Institute.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Waller, J. M., Bridge, P. D., Black, B., & Hakiza, G. (1993). Characterization of the coffee berry disease pathogen, Colletotrichum kahawae sp. nov. Mycological Research, 97, 989–994.
Weir, B. S., Johnston, P. R., & Damm, U. (2012). The Colletotrichum gloeosporioides species complex. Studies in Mycology, 73, 115–180.
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR Protocols: a guide to methods and applications. San Diego: Academic Press.
Woudenberg, J. H. C., Aveskamp, M. M., Gruyter, J. de, Spiers, A. G., & Crous, P. W. (2009). Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia, 22, 56–62.
Youlian, Y., Cai, L., Yu, Z., Liu, Z., & Hyde, K. D. (2011). Colletotrichum species on Orchidaceae in southwest China. Cryptogamie, Mycologie, 32, 229–253.
Acknowledgments
The authors wish to thank the National Council for Science and Technology (CONACYT) in Mexico for support (grant no. 1131132). The authors also thank the following farmers in Puebla State: Evaristo López Lugo, Rufino Vargas Castillo, and Sostenes Olivares S., for allowing us to collect coffee tree samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cristóbal-Martínez, A.L., de Jesús Yáñez-Morales, M., Solano-Vidal, R. et al. Diversity of Colletotrichum species in coffee (Coffea arabica) plantations in Mexico. Eur J Plant Pathol 147, 605–614 (2017). https://doi.org/10.1007/s10658-016-1029-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-1029-0